Published online Jan 27, 2022. doi: 10.4240/wjgs.v14.i1.36
Peer-review started: October 4, 2021
First decision: November 18, 2021
Revised: November 29, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: January 27, 2022
Processing time: 106 Days and 16.3 Hours
As a new digital holographic imaging technology, mixed reality (MR) technology has unique advantages in determining the liver anatomy and location of tumor lesions. With the popularization of 5G communication technology, MR shows great potential in preoperative planning and intraoperative navigation, making hepatectomy more accurate and safer.
To evaluate the application value of MR technology in hepatectomy for hepatocellular carcinoma (HCC).
The clinical data of 95 patients who underwent open hepatectomy surgery for HCC between June 2018 and October 2020 at our hospital were analyzed retrospectively. We selected 95 patients with HCC according to the inclusion criteria and exclusion criteria. In 38 patients, hepatectomy was assisted by MR (Group A), and an additional 57 patients underwent traditional hepatectomy without MR (Group B). The perioperative outcomes of the two groups were collected and compared to evaluate the application value of MR in hepatectomy for patients with HCC.
We summarized the technical process of MR-assisted hepatectomy in the treatment of HCC. Compared to traditional hepatectomy in Group B, MR-assisted hepatectomy in Group A yielded a shorter operation time (202.86 ± 46.02 min vs 229.52 ± 57.13 min, P = 0.003), less volume of bleeding (329.29 ± 97.31 mL vs 398.23 ± 159.61 mL, P = 0.028), and shorter obstructive time of the portal vein (17.71 ± 4.16 min vs 21.58 ± 5.24 min, P = 0.019). Group A had lower alanine amino
MR has some application value in three-dimensional visualization of the liver, surgical planning, and intraoperative navigation during hepatectomy, and it significantly improves the perioperative outcomes of hepatectomy for HCC.
Core Tip: Mixed reality (MR) is a new digital holographic imaging technology that enables real-world and virtual three-dimensional images to be displayed and interacted in the same visual space. MR has some application value in three-dimensional visualization of the liver, surgical planning, and intraoperative navigation during hepatectomy. We performed a retrospective study to evaluate the application value of MR technology in hepatectomy for hepatocellular carcinoma (HCC). MR significantly improved the perioperative outcomes of hepatectomy for HCC compared to hepatectomy with traditional methods, demonstrating the potential value of clinical application.
- Citation: Zhu LY, Hou JC, Yang L, Liu ZR, Tong W, Bai Y, Zhang YM. Application value of mixed reality in hepatectomy for hepatocellular carcinoma. World J Gastrointest Surg 2022; 14(1): 36-45
- URL: https://www.wjgnet.com/1948-9366/full/v14/i1/36.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i1.36
Primary liver cancer (PLC) is a common malignant tumor of the digestive system worldwide. According to the new data released by GLOBOCAN2020, the annual number of new cases of liver cancer has reached 841000 worldwide, ranking seventh among malignant tumors[1]. Hepatocellular carcinoma (HCC) accounts for a large proportion (85%-90%) of PLCs[2]. Surgery remains the most important treatment for HCC, and radical resection significantly improves the patients prognosis[3]. With the in-depth understanding of the anatomical structure of the liver and the rapid development of surgical techniques, precise hepatectomy and anatomical hepatectomy have been widely performed. Three-dimensional (3D) visualization, indocyanine green fluorescence imaging, intraoperative ultrasound, augmented reality (AR), and virtual reality (VR) have been used to determine the location of the tumor and the boundary of the liver segment, which play important roles in hepatectomy[4-7]. In recent years, with the rapid development of mixed reality (MR) technology, it has been preliminarily applied in hepatectomy for HCC[8].
MR is a new digital holographic imaging technology that enables real-world and virtual 3D images to be displayed in an interactive fashion in the same visual space[9]. Given its unique advantages, MR technology not only changes the situation of separation of traditional two-dimensional (2D) images from surgery but also compensates for the shortcomings of AR and VR technology. Microsoft released its first MR head-mounted display (MR-HMD) in 2016; HoloLens allows surgeons to interact with 3D holograms and manipulate images from their point of view using MR-HMDs[10]. MR technology makes image-guided surgery possible, especially by plastically presenting 3D holograms on or above the surgical site.
MR has been proven to be a practical tool for intraoperative surgical guidance in the operating room[11]. Previous studies have shown that MR has been gradually applied to neurosurgery, orthopedics, and urology, yielding improvements in perioperative outcomes for patients[12-14]. In hepatectomy for patients with HCC, MR also exhibit great potential in preoperative planning and intraoperative navigation, which makes hepatectomy more accurate and personalized[15]. However, to our knowledge, few studies have evaluated the application value of MR in hepatectomy. In this study, 95 patients with HCC who underwent hepatectomy were retrospectively analyzed to evaluate the application value of MR.
We retrospectively collected the clinical data of 132 patients who underwent hepatectomy between June 2018 and October 2020 in the Department of Hepatobiliary Surgery of Tianjin First Central Hospital. Patients who underwent resection of additional organs (except for the gallbladder), received immunotherapy or targeted therapy, had Child-Pugh C liver function or indocyanine green 15 min retention > 20%, or distant metastasis were excluded. All patients were confirmed to have HCC by postoperative pathology. Finally, 95 patients were enrolled in the study, including 38 patients who underwent MR-assisted hepatectomy in Group A and 57 patients who underwent hepatectomy with traditional methods in Group B. The general clinical data of the 95 patients are shown in Table 1. This study was approved by the hospital ethics committee, and informed consent was obtained from all the patients.
| Characteristic | Patient (n = 95) | P value | |
| Group A (n = 37) | Group B (n = 58) | ||
| Age (yr), n (%) | 57.62 ± 9.16 | 60.22 ± 9.19 | 0.819 |
| Sex (female/male), n (%) | 13/24 | 15/43 | 0.334 |
| BMI | 23.91 ± 3.66 | 23.82 ± 3.42 | 0.471 |
| History of abdominal surgery (yes/no), n (%) | 9/28 | 11/47 | 0.532 |
| Tumor size (cm) | 5.52 ± 1.95 | 5.20 ± 1.88 | 0.428 |
| Tumor number, n (%) | 0.948 | ||
| 1 | 24 (64.86) | 38 (65.52) | |
| ≥ 2 | 13 (35.14) | 20 (34.48) | |
| Tumor location, n (%) | 0.637 | ||
| Right lobe | 17 (45.95) | 23 (39.66) | |
| Left lobe | 14 (37.84) | 21 (36.21) | |
| Bilateral lobes | 6 (16.22) | 14 (24.14) | |
| Liver cirrhosis (yes/no), n (%) | 31/6 | 51/7 | 0.566 |
| HBV infection (yes/no), n (%) | 29/8 | 44/14 | 0.777 |
| AFP, n (%) | 0.532 | ||
| < 400 (ng/mL) | 28 (75.68) | 47 (81.03) | |
| ≥ 400 (ng/mL) | 9 (24.32) | 11 (18.97) | |
| Liver function, n (%) | 1.000 | ||
| Child-Pugh A | 34 (91.89) | 54 (93.10) | |
| Child-Pugh B | 3 (8.11) | 4 (6.90) | |
| Preoperative lab examination | |||
| ALB (g/L) | 41.38 ± 5.75 | 40.89 ± 5.30 | 0.675 |
| TBIL (μmol/L) | 12.75 ± 3.57 | 13.88 ± 4.87 | 0.198 |
| PT (s) | 12.39 ± 1.27 | 12.18 ± 1.19 | 0.424 |
| ALT (U/L) | 27.87 ± 9.69 | 29.58 ± 12.12 | 0.469 |
| AST (U/L) | 30.56 ± 10.25 | 33.42 ± 11.72 | 0.229 |
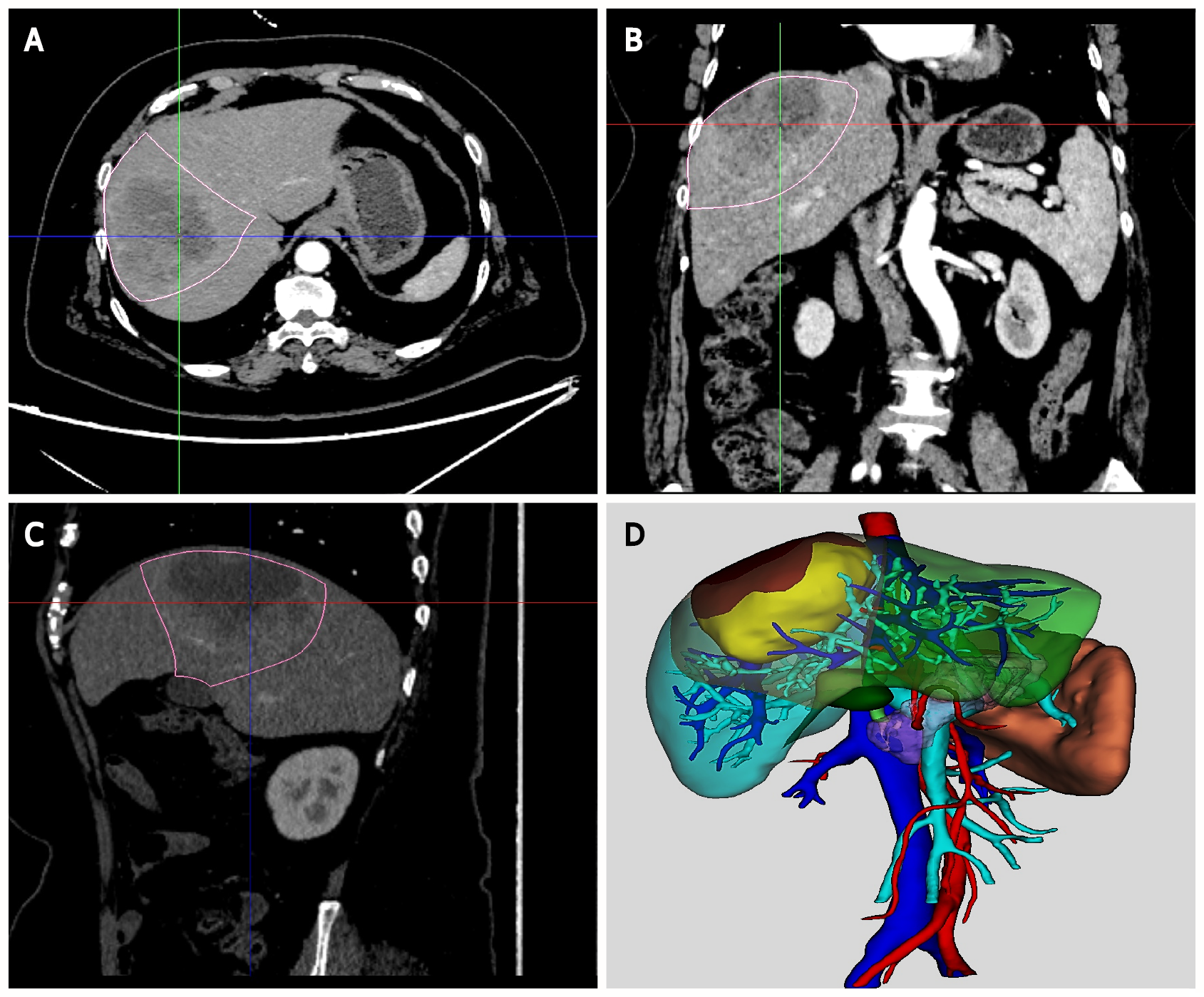
Computed tomography (CT) images of the two groups were obtained using a 128-slice spiral CT system, including three-phase enhanced images and nonenhanced images. The CT images of 38 patients in Group A were stored in the format of Digital Imaging and Communications in Medicine and imported into MR diagnostic imaging processing software (TM-MIS 1.0, Tuomeng Science and Technology Ltd, Heilongjiang, China) for 3D reconstruction. MR software could depict liver, tumor, blood vessels, and other normal tissues automatically, which were distinguished by different colors. The 3D holograms were generated and optimized by the radiologist and surgeon with reference to the original CT images. Finally, they were uploaded to the web server.
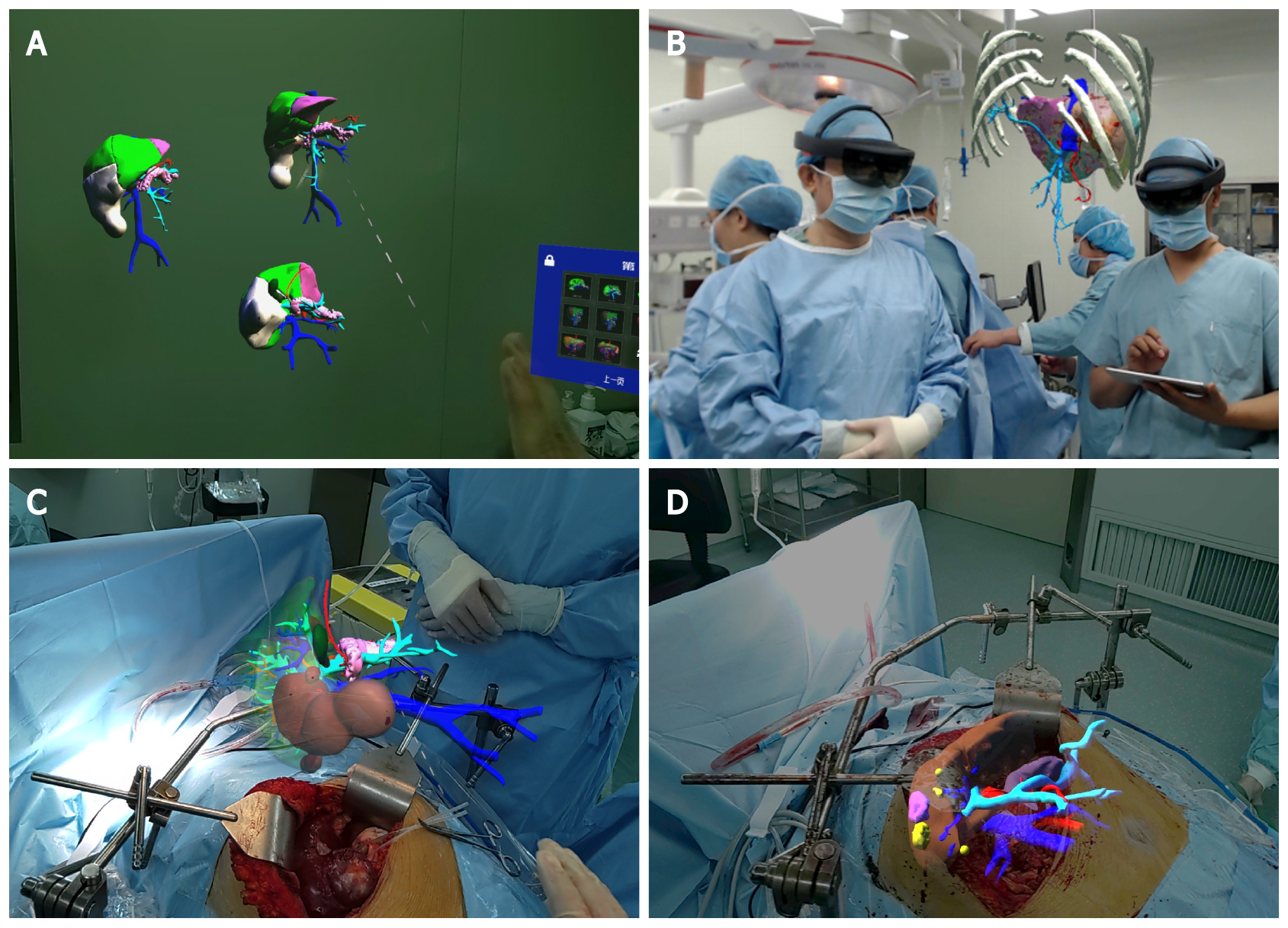
In Group A, the hologram of each patient was downloaded to the MR-HMD from the web server. After wearing the MR-HMD, the surgeon could observe the liver anatomy and tumor location through the 3D hologram. Virtual surgery was performed on the 3D hologram, and the resection and residual liver volume were calculated in real time to evaluate the feasibility of the proposed surgical strategy. Surgical planning was performed to ensure the complete removal of the tumor while retaining a larger volume of the liver. During hepatectomy, the surgeon and assistant wore MR-HMDs, and the hologram was adjusted to fuse with the patient's liver or located above the surgical visual field to relocate the tumor location and guide the operation. In Group B, 2D CT images of the patient were used for surgical planning, and hepatectomy was performed based on the operator's clinical experience and spatial imagination. All operations were performed by laparotomy. The Pringle maneuver was used for hepatic vascular exclusion during hepatectomy, and abdominal drainage was routinely placed.
All patients received the same symptomatic treatment strategy before and after the operation. Various perioperative results, including operation time, volume of bleeding, implementation of the Pringle maneuver, obstructive time of the portal vein, laboratory examination at postoperative day 3, postoperative complications within 30 days, and hospitalization days, were collected and compared between the two groups. Postoperative complications included perioperative mortality, hepatic failure, abdominal bleeding, bile leakage, abdominal infection, pleural effusion, pulmonary infection, and wound infection, and these complications were assessed based on the Clavien-Dindo classification system[16].
Data were analyzed using SPSS version 25.0 (IBM, United States). All measurement data are expressed as the mean ± SD or percentage. The data of patients before, during, and after surgery were compared by Student’s t test, chi-square test, and Fisher’s exact test to compare data from patients in Groups A and B. P < 0.05 was considered statistically significant.
A total of 95 patients with HCC were included in this study. Patients were divided into Group A (with MR, n = 37) and Group B (without MR, n = 58) based on whether MR technology was used. We collected basic patient information (age, sex, body mass index, and history of abdominal surgery), tumor data (tumor size, tumor number, and tumor location), Child-Pugh classification, liver cirrhosis, hepatitis B virus infection, and preoperative laboratory data (alpha fetoprotein, albumin, total bilirubin, prothrombin time, alanine aminotransferas, aspartate aminotransferase). All the data are summarized in Table 1. No statistically significant differences in the baseline characteristics were noted between the two groups.
To describe the process of MR-assisted hepatectomy in more detail, we presented a typical case in Group A. The 3D hologram was reconstructed from the preoperative CT image of the patient and downloaded to the MR-HMD (Figure 1), which could be brought into the operating room. Surgical planning was performed and evaluated before the operation, and it was reconfirmed in the operating room. The 3D hologram was placed above the surgical field or fused with the patient's liver to determine the location of the tumor and important blood vessels, which is of great help to guide the operation (Figure 2).
The intraoperative results of the two groups of patients are shown in Table 2. The operation time of Group A patients, who underwent MR-assisted hepatectomy, was significantly shorter than that of Group B (202.86 ± 46.02 min vs 229.52 ± 57.13 min, P = 0.003). Furthermore, patients in Group A had a lower intraoperative volume of bleeding than those in Group B (329.29 ± 97.31 mL vs 398.23 ± 159.61 mL, P = 0.028). Although there was no significant difference in the intraoperative Pringle maneuver between the two groups (P = 0.148), the obstructive time of the portal vein of Group A was shorter than that of Group B (17.71 ± 4.16 min vs 21.58 ± 5.24 min, P = 0.019).
| Variable | Group A (n = 37) | Group B (n = 58) | P value |
| Surgical procedure, n (%) | |||
| Extended left hepatectomy1 | 4 (10.81) | 7 (12.07) | 1.000 |
| Extended right hepatectomy2 | 2 (5.41) | 5 (8.62) | 0.855 |
| Left hepatectomy | 8 (21.62) | 12 (20.69) | 0.913 |
| Right hepatectomy | 5 (13.51) | 8 (13.79) | 0.969 |
| Sectionectomy | 8 (21.62) | 9 (15.52) | 0.449 |
| Segmentectomy | 7 (18.92) | 8 (13.79) | 0.505 |
| Partial resection | 3 (8.11) | 9 (15.52) | 0.457 |
| Operative time (min) | 202.86 ± 46.02 | 229.52 ± 57.13 | 0.003 |
| Volume of bleeding (mL) | 329.29 ± 97.31 | 398.23 ± 159.61 | 0.010 |
| Pringle maneuver (yes/no), n (%) | 14/23 | 31/27 | 0.148 |
| Obstructive time of portal vein (min) | 17.71 ± 4.16 | 21.58 ± 5.24 | 0.019 |
The postoperative laboratory results, postoperative complications, and hospitalization days of the two groups were collected and are shown in Table 3. Group A exhibited both lower alanine aminotransferas (ALT) and albumin (ALB) levels on the third day after the operation (119.74 ± 29.08 U/L vs 135.53 ± 36.68 U/L, P = 0.029 and 33.60 ± 3.21 g/L vs 31.80 ± 3.51 g/L, P = 0.014, respectively), but no significant differences in aspartate aminotransferase and TB were noted between the two groups (P = 0.343 and P = 0.557, respectively). The total postoperative complications within 30 d and hospitalization days in Group A were significantly lower than those in Group B [14 (37.84%) vs 35 (60.34%), P = 0.032 and 12.05 ± 4.04 d vs 13.78 ± 4.13 d, P = 0.049, respectively].
| Variable | Group A (n = 37) | Group B (n = 58) | P value |
| ALT at postoperative day 3 (U/L) | 119.74 ± 29.08 | 135.53 ± 36.68 | 0.029 |
| AST at postoperative day 3 (U/L) | 106.20 ± 20.99 | 110.91 ± 24.99 | 0.343 |
| ALB at postoperative day 3 (g/L) | 33.60 ± 3.21 | 31.80 ± 3.51 | 0.014 |
| TB at postoperative day 3 (μmol/L) | 43.07 ± 8.60 | 44.33 ± 11.04 | 0.557 |
| Perioperative complications, n (%) | |||
| Perioperative mortality | 0 (0) | 1 (1.72) | 1.000 |
| Hepatic failure | 0 (0) | 2 (3.45) | 0.519 |
| Abdominal bleeding | 1 (2.70) | 2 (3.45) | 1.000 |
| Bile leakage | 0 (0) | 2 (3.45) | 0.519 |
| Abdominal infection | 1 (2.70) | 3 (5.17) | 0.952 |
| Pleural effusion | 2 (5.41) | 6 (10.34) | 0.641 |
| Pulmonary infection | 1 (2.70) | 3 (5.17) | 0.952 |
| Wound infection | 2 (5.41) | 4 (6.90) | 1.000 |
| Total complications | 7 (18.92) | 23 (39.66) | 0.034 |
| CDC, n (%) | 0.339 | ||
| 0-2 | 35 (94.59) | 50 (86.21) | |
| ≥ 3 | 2 (5.41) | 8 (13.79) | |
| Hospitalization days (d) | 12.05 ± 4.04 | 13.78 ± 4.13 | 0.049 |
Hepatectomy for liver cancer is still a high-risk operation with numerous postoperative complications, high mortality, and high risk for postoperative recurrence[17]. With the development of MR, it has been gradually applied to hepatectomy. We have established a complete technical process of MR-assisted hepatectomy in our center. To the best of our knowledge, this is the first study to explore the application value of MR in hepatectomy for HCC. The results suggested that MR-assisted hepatectomy yielded better perioperative outcomes than traditional hepatectomy.
Traditional hepatectomy mainly depends on the subjective “3D reconstruction” of CT, MRI, and other 2D images by surgeons, which requires extensive experience and long-term surgical practice. The development of 3D reconstruction technology makes the anatomy of the liver clearer, which in turn makes hepatectomy more efficient and safer[4,18]. MR allows 3D holograms to be downloaded to the MR-HMD, whereas traditional 3D reconstruction images are limited to flat screens. Furthermore, the spatial understanding of patient-specific liver anatomy is improved by MR[19]. Before the operation, surgeons could manipulate the 3D holograms to observe the anatomy of the liver and tumor location. The resection plane of the surgical plan was determined more accurately to retain sufficient residual liver volume and improve the safety of the operation[20]. On the other hand, 3D holograms could be used for virtual hepatectomy. Mise et al[21] reviewed and analyzed 1194 cases of hepatectomy for liver cancer and living donor liver transplantation and found that virtual hepatectomy with 3D reconstruction improved the vein reconstruction rate of transplantation and reduced the operation time, and the 5-year disease-free survival rate of patients with virtual hepatectomy was higher[21].
In the present study, MR-assisted hepatectomy significantly reduced the operation time and obstructive time of the portal vein, although it may take 10 min or more to adjust the hologram for intraoperative navigation. This advantage was probably the result of a better understanding of the tumor location and hepatic vascular anatomy through 3D holograms. In addition, the operative approach and resection plane were clearer with the help of intraoperative navigation by fusing the 3D hologram with the liver. In addition, this was also one of the main reasons for reducing the volume of bleeding. Moreover, the recovery of ALT and ALB in patients with MR-assisted hepatectomy was faster, indicating better recovery of liver function. It has been suggested that a shorter operation time and shorter obstructive time of the portal vein could promote the recovery of liver function after the operation[22]. The operation time and volume of bleeding during the operation have an important influence on the incidence of postoperative complications. In our study, we found that there were fewer postoperative complications within 30 d in the MR-assisted hepatectomy group compared with the traditional hepatectomy group. This procedure also shortened the hospital stays of the patients undergoing MR-assisted hepatectomy.
In summary, MR-assisted hepatectomy significantly improved the perioperative outcomes of patients with HCC. MR technology gives surgeons a pair of “perspective eyes” to penetrate the liver, especially during the preoperative “last minute” and intraoperative navigation during hepatectomy[23]. Some studies have found that the “last minute” simulation before liver surgery can relieve the pressure on surgeons and help them operate more safely and accurately[15]. MR may also have certain application potential for laparoscopic and robotic hepatectomy, and it will be explored in the future. On the other hand, according to our center's experience in MR-assisted hepatectomy, MR technology has a great advantage in the localization of small liver cancers, and we will explore this advantage in the next step of studies.
In the teaching of surgery, MR technology significantly improves the surgeon’s perception of the liver and provides a more realistic 3D virtual learning environment for junior surgeons[24]. After wearing the MR-HMD, surgeons can share computer-generated 3D holograms of the liver and observe the anatomical structure from all angles. Given that the real environment is not necessary, some studies have noted that VR may be better than MR for teaching[25]. However, the emergence of MR-HMD may change this concept. The virtual hepatectomy software developed by Uchida et al[26] simulates various types of anatomical hepatectomy, and its virtual hepatectomy process increases the interactive experience of surgery[26]. Similarly, MR technology can also achieve virtual hepatectomy by using 3D holograms. In summary, virtual MR teaching is of great significance in promoting the progress of liver surgeons. On the other hand, patients could understand the operation plan more intuitively through MR, which is beneficial to the communication between doctors and patients.
However, this study has some limitations. First, this was a single-center retrospective study, and more cases from multiple centers are needed to further evaluate the value of MR. Second, the choice of MR-assisted hepatectomy was mixed with factors, such as the surgeon's preference and patient's financial status, rather than by defined indication. Third, it was still challenging to fuse 3D holograms directly into the liver due to the morphological changes of the liver caused by dissociating the liver, surgical operation, and respiratory movements of patients.
MR has some application value in 3D visualization of the liver, surgical planning, and intraoperative navigation during hepatectomy, and it significantly improves the perioperative outcomes of hepatectomy for HCC.
As a new digital holographic imaging technology, mixed reality (MR) it has been preliminarily applied in hepatectomy for hepatocellular carcinoma (HCC). In this study, 95 patients with HCC who underwent hepatectomy were retrospectively analyzed to evaluate the application value of MR.
MR has been gradually applied to neurosurgery, orthopedics, and urology with an improvement in perioperative outcomes. MR may also have great potential in hepatectomy by preoperative planning and intraoperative navigation.
The aim of this study was to explore the application value of MR technology in hepatectomy for HCC.
Total 95 patients with HCC were enrolled in the study, including 38 patients who underwent MR-assisted hepatectomy in Group A and 57 patients who underwent hepatectomy with traditional methods in Group B. Perioperative variables of the two groups of patients were collected and compared.
MR-assisted hepatectomy could significantly reduce the operation time, obstructive time of the portal vein, and the volume of bleeding. And the recovery of alanine aminotransferas and albumin in patients with MR-assisted hepatectomy was faster.
MR significantly improved the perioperative outcomes of hepatectomy for HCC.
MR may also have a certain application potential for laparoscopic and robotic hepatectomy, and it will be explored in future.
The authors thank all the doctors in the Department of Hepatobiliary surgery of Tianjin first Central Hospital for their efforts and contributions to this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Broering DC, Kim BS S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64646] [Article Influence: 16161.5] [Reference Citation Analysis (176)] |
| 2. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4108] [Article Influence: 586.9] [Reference Citation Analysis (6)] |
| 3. | Chen Q, Shu C, Laurence AD, Chen Y, Peng BG, Zhen ZJ, Cai JQ, Ding YT, Li LQ, Zhang YB, Zheng QC, Xu GL, Li B, Zhou WP, Cai SW, Wang XY, Wen H, Peng XY, Zhang XW, Dai CL, Bie P, Xing BC, Fu ZR, Liu LX, Mu Y, Zhang L, Zhang QS, Jiang B, Qian HX, Wang YJ, Liu JF, Qin XH, Li Q, Yin P, Zhang ZW, Chen XP. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67:2006-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 4. | He YB, Bai L, Aji T, Jiang Y, Zhao JM, Zhang JH, Shao YM, Liu WY, Wen H. Application of 3D reconstruction for surgical treatment of hepatic alveolar echinococcosis. World J Gastroenterol. 2015;21:10200-10207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Vávra P, Roman J, Zonča P, Ihnát P, Němec M, Kumar J, Habib N, El-Gendi A. Recent Development of Augmented Reality in Surgery: A Review. J Healthc Eng. 2017;2017:4574172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 6. | Quero G, Lapergola A, Soler L, Shahbaz M, Hostettler A, Collins T, Marescaux J, Mutter D, Diana M, Pessaux P. Virtual and Augmented Reality in Oncologic Liver Surgery. Surg Oncol Clin N Am. 2019;28:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Majlesara A, Golriz M, Hafezi M, Saffari A, Stenau E, Maier-Hein L, Müller-Stich BP, Mehrabi A. Indocyanine green fluorescence imaging in hepatobiliary surgery. Photodiagnosis Photodyn Ther. 2017;17:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Lungu AJ, Swinkels W, Claesen L, Tu P, Egger J, Chen X. A review on the applications of virtual reality, augmented reality and mixed reality in surgical simulation: an extension to different kinds of surgery. Expert Rev Med Devices. 2021;18:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 9. | Gao Y, Tan K, Sun J, Jiang T, Zou XW. Application of Mixed Reality Technology in Visualization of Medical Operations. Chin Med Sci J. 2019;34:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Tepper OM, Rudy HL, Lefkowitz A, Weimer KA, Marks SM, Stern CS, Garfein ES. Mixed Reality with HoloLens: Where Virtual Reality Meets Augmented Reality in the Operating Room. Plast Reconstr Surg. 2017;140:1066-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 11. | Cartucho J, Shapira D, Ashrafian H, Giannarou S. Multimodal mixed reality visualisation for intraoperative surgical guidance. Int J Comput Assist Radiol Surg. 2020;15:819-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Koike T, Kin T, Tanaka S, Takeda Y, Uchikawa H, Shiode T, Saito T, Takami H, Takayanagi S, Mukasa A, Oyama H, Saito N. Development of Innovative Neurosurgical Operation Support Method Using Mixed-Reality Computer Graphics. World Neurosurg X. 2021;11:100102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Verhey JT, Haglin JM, Verhey EM, Hartigan DE. Virtual, augmented, and mixed reality applications in orthopedic surgery. Int J Med Robot. 2020;16:e2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 14. | Li G, Dong J, Wang J, Cao D, Zhang X, Cao Z, Lu G. The clinical application value of mixed-reality-assisted surgical navigation for laparoscopic nephrectomy. Cancer Med. 2020;9:5480-5489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Saito Y, Sugimoto M, Imura S, Morine Y, Ikemoto T, Iwahashi S, Yamada S, Shimada M. Intraoperative 3D Hologram Support With Mixed Reality Techniques in Liver Surgery. Ann Surg. 2020;271:e4-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24843] [Article Influence: 1183.0] [Reference Citation Analysis (0)] |
| 17. | Chen L, Wang YB, Zhang YH, Gong JF, Li Y. Effective prediction of postoperative complications for patients after open hepatectomy: a simplified scoring system based on perioperative parameters. BMC Surg. 2019;19:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Li P, Wang M, Yang Y, Liu H, Pan Z, Jiang B, Lau WY, Huang G, Zhou W. Preoperative three-dimensional vs two-dimensional evaluation in assessment of patients undergoing major liver resection for hepatocellular carcinoma: a propensity score matching study. Ann Transl Med. 2020;8:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Pelanis E, Kumar RP, Aghayan DL, Palomar R, Fretland ÅA, Brun H, Elle OJ, Edwin B. Use of mixed reality for improved spatial understanding of liver anatomy. Minim Invasive Ther Allied Technol. 2020;29:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Nakayama K, Oshiro Y, Miyamoto R, Kohno K, Fukunaga K, Ohkohchi N. The Effect of Three-Dimensional Preoperative Simulation on Liver Surgery. World J Surg. 2017;41:1840-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Mise Y, Hasegawa K, Satou S, Shindoh J, Miki K, Akamatsu N, Arita J, Kaneko J, Sakamoto Y, Kokudo N. How Has Virtual Hepatectomy Changed the Practice of Liver Surgery? Ann Surg. 2018;268:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (4)] |
| 22. | Famularo S, Giani A, Di Sandro S, Sandini M, Giacomoni A, Pinotti E, Lauterio A, Gianotti L, De Carlis L, Romano F. Does the Pringle maneuver affect survival and recurrence following surgical resection for hepatocellular carcinoma? J Surg Oncol. 2018;117:198-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Kumar RP, Pelanis E, Bugge R, Brun H, Palomar R, Aghayan DL, Fretland ÅA, Edwin B, Elle OJ. Use of mixed reality for surgery planning: Assessment and development workflow. J Biomed Inform. 2020;112S:100077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Barteit S, Lanfermann L, Bärnighausen T, Neuhann F, Beiersmann C. Augmented, Mixed, and Virtual Reality-Based Head-Mounted Devices for Medical Education: Systematic Review. JMIR Serious Games. 2021;9:e29080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 166] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 25. | Andolfi C, Plana A, Kania P, Banerjee PP, Small S. Usefulness of Three-Dimensional Modeling in Surgical Planning, Resident Training, and Patient Education. J Laparoendosc Adv Surg Tech A. 2017;27:512-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Uchida Y, Taura K, Nakao M, Uemoto S. A clinical pilot study of Resection Process Map: A novel virtual hepatectomy software to visualize the resection process, case series. Int J Surg. 2019;71:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |