Published online Jan 27, 2022. doi: 10.4240/wjgs.v14.i1.12
Peer-review started: May 2, 2021
First decision: June 27, 2021
Revised: July 20, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 27, 2022
Processing time: 262 Days and 6.3 Hours
Gastroparesis is a chronic disease of the stomach that causes a delayed gastric emptying, without the presence of a stenosis. For 30 years the authors identified pylorospasm as one of the most important pathophysiological mechanisms determining gastroparesis. Studies with EndoFLIP, a device that assesses pyloric distensibility, increased the knowledge about pylorospasm. Based on this data, several pyloric-targeted therapies were developed to treat refractory gastroparesis: Surgical pyloroplasty and endoscopic approach, such as pyloric injection of botulinum and pyloric stenting. Notwithstanding, the success of most of these techniques is still not complete. In 2013, the first human gastric per-oral endoscopic myotomy (GPOEM) was performed. It was inspired by the POEM technique, with a similar dissection method, that allows pyloromyotomy. Therapeutical results of GPOEM are similar to surgical approach in term of clinical success, adverse events and post-surgical pain. In the last 8 years GPOEM has gained the attention of the scientific community, as a minimally invasive technique with high rate of clinical success, quickly prevailing as a promising therapy for gastroparesis. Not surprisingly, in referral centers, its technical success rate is 100%. One of the main goals of recent studies is to identify those patients that will respond better to the therapies targeted on pylorus and to choose the better approach for each patient.
Core Tip: Many studies tried to identify the factors that may predict the response to pyloric targeted therapies in gastroparesis according to etiology, prevalent symptoms, antroduodenal manometric study and EndoFLIP. Unfortunately, it is still difficult to reach an accurate determination of the optimal candidates for each treatment. Currently, surgical and endoscopic approach has been compared in term of safety and the results seem encouraging for endoscopic method. In this review we summarize indications, side effects and outcome of gastric per-oral endoscopic myotomy compared to surgical pyloroplasty.
- Citation: Verga MC, Mazza S, Azzolini F, Cereatti F, Conti CB, Drago A, Soro S, Elvo B, Grassia R. Gastric per-oral endoscopic myotomy: Indications, technique, results and comparison with surgical approach. World J Gastrointest Surg 2022; 14(1): 12-23
- URL: https://www.wjgnet.com/1948-9366/full/v14/i1/12.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i1.12
Epidemiology: Gastric retention > 60% at two hours and/or > 10% after four hours from a meal is considered pathological[1], in absence of organic strictures[2]. Gastroparesis (GP) is a chronic alteration of the gastric motility that leads to a delay in stomach emptying. Mainly, it is an idiopathic condition; however it can be also caused by diabetes and post-surgical conditions, such as fundoplicatio, vagotomy, bariatric surgery and esophagectomy. Less frequent etiologies are: Post-infectious gastroparesis and neurological or autoimmune diseases[3]. The related symptoms are often dyspepsia-like. Thus, gastroparesis is an underdiagnosed condition. The prevalence is estimated around 3% in United States (mean age of 37.7 years, with an F:M ratio of 4:1)[4] and American data showed a large increase in hospitalizations between 1997 and 2013 for gastroparesis, estimating a related increase in costs of 1026%[5].
Pathophysiology: The current knowledges of the pathophysiology of GP remain partial[6]. This explains the delay in the diagnosis and the lack of a reference therapy, that is still an open challenge.
Histologically, loss of interstitial cells of Cajal (ICC) is the most important finding. Indeed, these cells show ultrastructural modification such as intracytoplasmatic vacuoles and apoptotic features. However, up to now, no definitive explanations are available[7].
Gastroparesis may be characterized by two different patterns at antroduodenal manometry study: Waves of contraction of reduced amplitude (< 40 mmHg), suggestive for myopathy, or reduced and disorganized gastric motility. This latter pattern is more frequent, but not exclusive, in neurogenic alterations[8,9]. Moreover, pylorospasm appears to be one of the crucial components[10].
However, antroduodenal manometry is a complex procedure and it is unfortunately little available in daily clinical practice.
The patient with a suspicion of gastroparesis should always undergo a thoroughly evaluation of the previous medical history coupled with a complete physical examination. EGDS is mandatory in order to exclude organic lesions.
The second step consists in calculating a validated score, the gastroparesis cardinal symptoms index (GCSI), that evaluates symptoms in the previous two weeks from the patient evaluation. GCSI has shown to be reliable and reproducible[11]. It is based on three subscales (post-prandial fullness/early satiety-4 items; nausea/vomiting-3 items; bloeating-2 items) and each item ranges from 0 (none) to 5 (severe). GCSI is not a diagnostic tool but it is useful to measure the severity of the disease and the post treatment improvement. Most of the available studies exclude the patients who have GCSI < 2.0 from both endoscopic and surgical therapy (Table 1). Importantly, the psychometric evidence of the GCSI was also found to be consistent with European guidelines and the Food and Drugs Administration (FDA)[12,13].
| Are you suffering of | None | Very mild | Mild | Moderate | Severe | Very severe |
| Nausea | 0 | 1 | 2 | 3 | 4 | 5 |
| Retching | 0 | 1 | 2 | 3 | 4 | 5 |
| Vomiting | 0 | 1 | 2 | 3 | 4 | 5 |
| Stomach fullness | 0 | 1 | 2 | 3 | 4 | 5 |
| Inability to finish a normal sized meal | 0 | 1 | 2 | 3 | 4 | 5 |
| Feeling excessively full after meals | 0 | 1 | 2 | 3 | 4 | 5 |
| Loss of appetite | 0 | 1 | 2 | 3 | 4 | 5 |
| Bloating | 0 | 1 | 2 | 3 | 4 | 5 |
| Belly visibly larger | 0 | 1 | 2 | 3 | 4 | 5 |
Overall, the severity of GCSI appears to properly correlate with the objective measurements of the gastric emptying time at 2 h, but not at 4 h[14]. This is particular true when considering nausea, vomiting, and premature satiety
Moreover, the patient should undergo to a gastric emptying study by scintigraphy or stable isotope breath test, using for example octanoid acid: This is an easy test and do not expose patient to ionizing radiation.
The study of gastric emptying time and GCSI[11] are the most commonly used tools to define the severity of the disease and evaluate the treatment response.
Nevertheless, the evaluation of pyloric sphincter by means of EndoFLIP seems promising. EndoFLIP is a cylindrical bag placed through the pylorus that uses impedance planimetry to determine cross sectional areas (CSA). It allows the measurement of the intrabag pressure and CSA/pressure response (distensibility) of the pylorus.
A study examined 114 patients, showing that the gastric emptying time correlated better with the reduced pyloric distensibility assessed by EndoFLIP than with the basal pyloric pressure assessed by using manometry[15].
However, not all the studies show the same results. A study evaluated the diagnostic accuracy of the EndoFLIP in 54 patients diagnosed with GP. The pyloric diameter and the CSA resulted inversely proportional to the key symptoms of GCSI. However, the study did not find a direct correlation between the pyloric diameter and the CSA and the gastric emptying at two and four hours[16].
A study published by Fathalizadeh and colleagues in December 2020 investigated the feasibility and the safety of intraprocedural EndoFLIP during gastric per-oral endoscopic myotomy (GPOEM). The authors examined 14 patients. 12 of 14 had pre and post procedure measurement. Median GCSI decreased from pre procedural assessment (3.1), to post procedural one, after one month (2.2); they also found an improvement of pyloric diameter and pyloric distensibility (respectively P = 0.0012 and P = 0.007). The authors concluded that EndoFLIP during pyloromyotomy (pre procedural and immediately post procedural) can be useful to determine if further myotomy is needed and it may also predict the clinical response to GPOEM[17].
Recently, Conchillo et al[18] published a very interesting study with 24 patients (100% technical success rate) to investigate the role of antroduodenal motility pattern and EndoFLIP in predicting the outcome after GPOEM: Clinical response was not correlated with motility pattern, whereas was associated with the pyloric distensibility improvement. However, there are no yet parameters that can surely predict the clinical response after GPOEM[18,19].
The present review aims to present indications, technical aspects, advantages and limitations of GPOEM.
All studies mentioned in this article have been searched by PubMed using key words as ‘GPOEM’, ‘gastro peroral endoscopic myotomy’, ‘POP’, ‘gastroparesis’, ‘refractory gastroparesis’, ‘pyloromyotomy’, ‘pyloroplasty’, ‘GCSI’, ‘gastroparesis cardinal symptom index’ ‘EndoFLIP’. Only English papers with available abstract and full text were considered.
In our manuscript we firstly presented the indication and the technical aspects of GPOEM. Secondly, we evaluated the criteria for the ideal candidate for GPOEM procedure, based on GCSI and gastric electrical stimulator (GES) analysis. Then we highlighted the pros and cons of GPOEM, compared to the other existing techniques to treat GP.
Patients with mild symptoms can be referred for hygienic and dietary correction coupled with medical therapy with prokinetics, especially metoclopramide. However, response to prokinetics decreases over the time. Moreover, these drugs are burdened with important side effects, such as extrapyramidal symptoms and amenorrhea, in case of long term use[20,21].
On the contrary, patients with severe and persistent symptoms require advanced interventional therapies. The use of pyloric-targeted therapies, such as pyloric myotomy, have recently increased. However, when a severe impairment of antral and or duodenal contractile activity is present, even pyloric myotomy can be ineffective[21,22].
The available pyloric targeted procedures can be divided in two categories: Surgical and endoscopic ones.
Surgical pyloroplasty: This technique is mainly performed by using laparascopic approach and the most famous technique is Heineke Mikulicz, which is characterized by a longitudinal incision of the pyloric ring and transverse suture. Almost 90% of patients reached an improvement or the normalization of the gastric emptying. Also the robotic pyloroplasty has been recently proposed as a safe and effective approach[23].
Placement of an electrical stimulator: A small stimulator characterized by high frequency (12 cycles/min) and low stimulation energy can be placed on the greater curvature of the stomach, 10 cm far from pylorus, with a laparoscopic or laparotomic approach.
Gastrectomy: Subtotal or total gastrectomy with Roux en y gastric bypass can be proposed as the ultimate surgical option.
Injection of botulinum toxin: This approach was firstly described by Pasricha et al[19] in 1995 and subsequently adapted by Sharma et al in 1998[23]. This is an endoscopic procedure where a small dose of botulinic toxin is injected around the pyloric ring in 4 points with a sclerosis needle. No studies support the efficacy of this technique.
Pyloric stenting: Temporary deployment of a fully covered self-expanding metal stents was firstly described in 2013 by Clark[24]. Sometimes the stent can be fixed by using Apollo or clips to avoid its migration, which is the main complication of this technique.
This technique was introduced in 2013 by Khashab[25]. It was developed starting from the technical and physio pathological basis of the already established esophageal POEM, experimented by Inoue[26].
The post procedure results, collected from the available literature, seem particularly promising.
Malik et al[27] and Jacques et al[28] firstly evaluated EndoFLIP data before and after the treatment. Pyloric distensibility index was found as the only predictive parameter for the outcome of GPOEM in both studies[27,28]. Hedberg et al[29] analyzed pre and post procedure EndoFLIP data in 13 out of 17 patients who underwent to GPOEM. This study confirmed an increase in pyloric distensibility from 5.6 (± 1.7) to 10.8 (± 5.0) cm2 post procedure[29]. The association between cross sectional pyloric area after treatment, the clinical response and the gastric emptying was confirmed even in a recent study by Vosoughi et al[30], that analyzed the outcome of GPOEM on thirty-seven patients analyzed in 5 centers[30].
To date, it is not clear whether the effectiveness of GPOEM depends on the physical destruction of the pyloric musculature itself or if it triggers further changes in gastric pathophysiology (Table 2).
| PRO | CONS | |
| Surgical options | ||
| Pyloromyotomy | (1) High technical success rate; and (2) Improvement in GCSI and GES | (1) Risk of gastric outlet obstruction and leakage; (2) Invasive; and (3) Time consuming |
| Electrical stimulator | (1) Test response with temporary device; and (2) Predictive features are male sex, diabetic etiology and short duration of disease | High rate of long term complications (infection, erosion, migration, perforation and chronic pain) |
| Endoscopic options | ||
| Botulinum toxin | (1) Easy and tolerable procedure; (2) Repeatable; and (3) Predictive for response to other pyloric techniques | (1) Moot in literature; and (2) Can induce sclerosis and anatomic alteration of pyloric region |
| Pyloric stent placement | (1) Temporized technique; and (2) Predictive for response to other pyloric targeted techniques | Risk of stent migration and duodenal perforation |
General recommendations: Generally, GPOEM procedure is performed in supine position with the patient under general anesthesia. However, sometimes the patient is placed on the left lateral position, in order to reduce the loop of the endoscope in the gastric cavity.
Major complications of the procedure are: Pneumoperitoneum, intra and postprocedural bleeding, perforation of the mucosa overlying the tunnel and, rarely, gastric ulcers and pyloric stenosis (6.8%)[31] (Table 3).
| GPOEM | ||
| PRO | High clinical success rate (71%-100%) | |
| High technical success rate (100%) | ||
| Less perioperative morbidity and operating time than surgery pyloromyotomy | ||
| Minimally invasive | ||
| Short hospitalization time | ||
| Positive predictive factors | Lower starting GCSI | |
| Fewer symptoms | ||
| Idiopathic and post-surgical GP | ||
| CONS | Limited to tertiary care center and very expert physicians | |
| Risk of pneumoperitoneum and abdominal pain | ||
| Poorer results for diabetic GP and female | ||
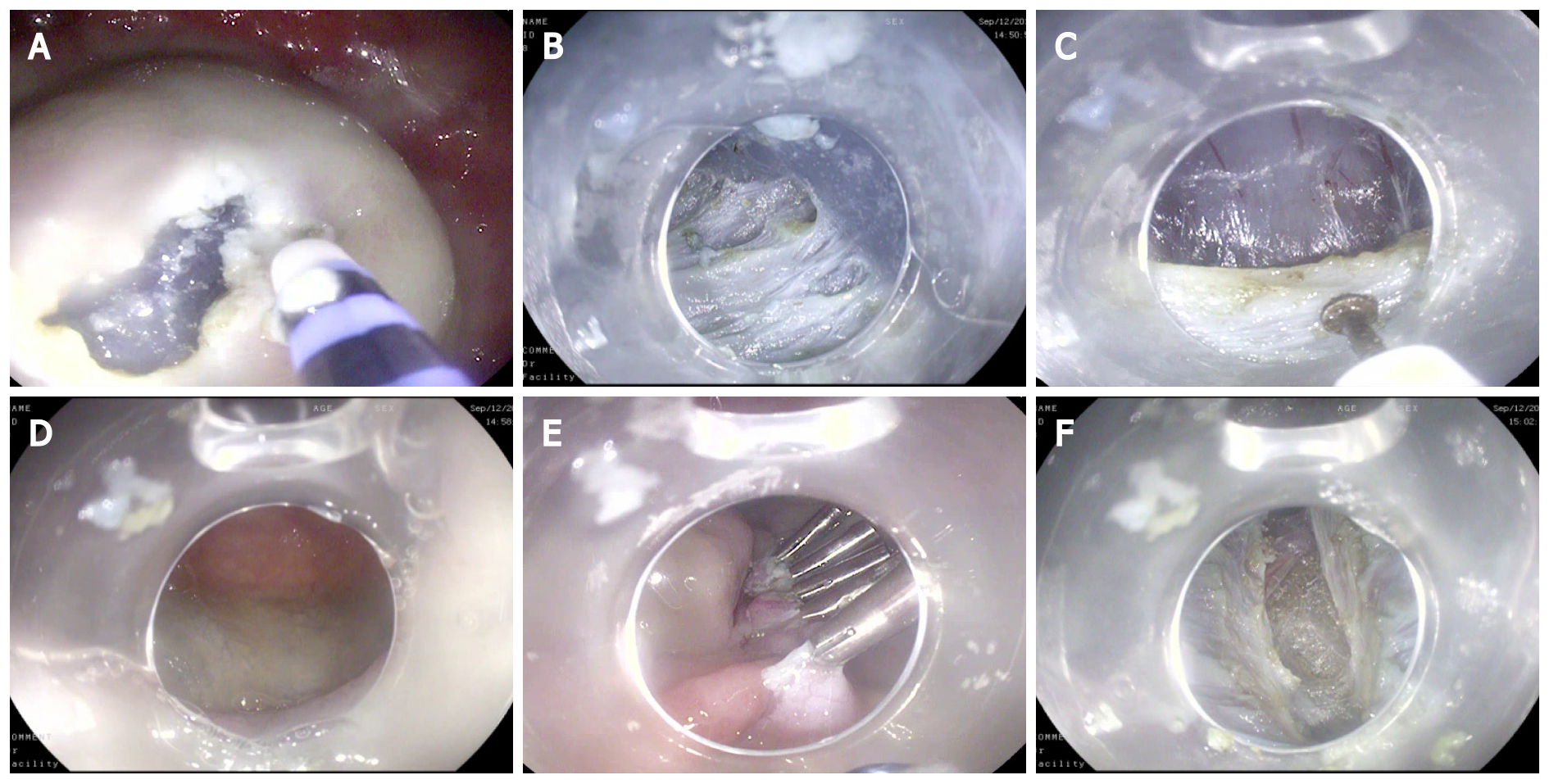
Technical aspects of GPOEM: The procedure follows the same technical steps as an esophageal POEM: (1) Mucosal incision about 5 cm from the pylorus with creation of an access to the submucosal plane after detaching the planes by injection of lifting solution (Figure 1A); (2) Creation of the submucosal tunnel with dissection technique up to the duodenal bulb and exposure of the pylorus (Figure 1B and C); (3) Verification of the integrity of the mucosal surface (Figure 1D); (4) Myotomy (Figure 1E); and (5) Closure of the mucosal flap with multiple endoclips (Figure 1F).
From a technical point of view, the access is generally chosen on the greater gastric curvature, with the endoscope kept in neutral position. Nonetheless, some operators choose the access on the small curvature and rarely on the anterior wall or posterior wall[23,31].
An important step of the procedure is to correctly identify the pyloric muscular ring. Generally it is performed visualizing the muscular ring across the blue dyed submucosa of the pyloric area. Nonetheless, sometimes its identification may be cumbersome. Xue et al[32], proposed the use of endoclip to facilitate muscular ring location. The study compared Fluoroscopy-guided G POEM vs GPOEM on 14 patients. The authors proved in seven patients that this approach was feasible, safe and not time consuming. However, no statistical differences between the two groups were found[32].
There is no unanimity regarding the proper depth of the myotomy. However, it has been shown that selective circular myotomy, including full-thickness, can be successfully achieved without increasing too much the risk of perforation[25].
The length of the myotomy should be between 2 cm and 3.5 cm[26] and the closure of the mucosal access can be carried out either with hemostatic clips or by endoscopic suture[31,33].
A recent study, from a referral center, suggested a possible superiority of a double myotomy: The authors analyzed two groups of patients (single vs double myotomy) showing that the patients who underwent a double pyloromyotomy had higher rate of clinical response (86% vs 67% P = 0.04). Double myotomy could be an interesting and effective approach in the near future. However, due to the study limitations, such as the prospective single center nature, the short term follow-up and the absence of data on the acquired expertise of operators in the double myotomy group, further studies are required[34].
Regarding the accessories used during the procedure, the choice is entrusted to the operator: Triangle tip knife (KD 640 L Olympus), Hybrid Knife (ERBE), Hook Knife (KD 620 LR) are used according operator’s choice.
Technical differences between POEM and GPOEM: The crucial difference between POEM and GPOEM lies in the in the large knowledge of the pathophysiology of achalasia compared to the little information available regarding the role of gastric motility in GP. There are also some technical and anatomical differences. Although the length of the antral tunnel is shorter than the esophageal one, some anatomical characteristics of the target zone make it more demanding from a technical point of view. The reasons that make GPOEM more difficult than POEM are many. Firstly, the cardial area is not anatomically represented by a real muscle, whereas in GPOEM there is the need to identify the pyloric muscle with the highest precision. Moreover, the curved direction of the submucosal tunnel, the presence of antral contractility, the reduced thickness of the duodenal mucosa increases the difficulty and the risk of perforation[27].
Post procedural management of the patient undergoing GPOEM: GPOEM is usually performed in inpatient setting, but no difference in terms of complications was found in non-hospitalized patients. Moreover, most of the centers use a contrast study after the procedure, before the patient dischargement. However, it has been proposing to avoid the routine post-operative contrast study, unless intraoperative complications occur.
Regarding the antibiotic prophylaxis, the Standards of Practice Committee of the American Society of Gastrointestinal Endoscopy in the 2015 guidelines for antibiotic prophylaxis in endoscopy did not give a precise indications for the procedures of the third space[35]. However, it is routinely performed, even if no high level of evidence is available.
Mostly, prolonged fasting (almost 24 h), and liquid diet are required in the days following the procedure[33].
The use of carbon dioxide for insufflation is mandatory.
Some randomized studies on ESD and POEM did not show statistically significant differences in terms of infections or sepsis in patients who did not undergo antibiotic prophylaxis[36]: To date, however, the vast majority of centers favor the administration of antibiotic prophylaxis. Usually with a single shot of a third generation cephalosporin.
Outcome of GPOEM: In 2018, Kahaleh et al[37] published a large international multi-center retrospective study on GPOEM. This study was conducted on 33 patients with refractory GP between America and France. The study demonstrated an excellent response to GPOEM, with 85% of patients achieving both symptom improvement, assessed by GCSI, and a reduction of the gastric emptying time.
In 2019, Mekaroonkamol et al[38] performed a systematic review on GPOEM. Between January 2013 and September 2018, 13 publications were collected (12 retrospective studies) for a total of 291 patients undergone to endoscopic pyloro
In the largest reported GPOEM published review[39] a 100% technical success was achieved on a total of 325 patients. Major complications were noted in 8.3% of cases. Clinical success ranged from 68% to 90%, with an improvement in GCSI of up to 90% and an improvement in stomach emptying time of up to 66%.
Xu et al[40] showed a statistically significant improvement for both GCSI and voiding time, hypothesizing that the former has a negative predictive value (< 30), whereas the second has a positive predictive value (emptying time < 221.6 min and retention at 2 h < 78.6%)
The relationship between gastric emptying time and the clinical manifestations of GP is very controversial. None of the symptoms of GCSI, considered either individually or in the score, correlated well with gastric emptying at baseline. Nonetheless, good responders to any treatment (medical, invasive or minimally invasive) show a linear correlation between symptoms improvement and reduction of gastric emptying time.
One of the main goals of the recent studies is to identify those patients that respond better to the therapies targeted on pylorus. Available knowledge showed that GP related to prior foregut surgery and idiopathic ones respond better to the therapy than the diabetic ones[14].
Another important key factor for clinical success seems to be the disease duration before the treatment. Uemura et al[14] demonstrated that the longer duration of the disease is related to a lower reduction in GCSI at 12 mo post procedure, stressing therefore the importance of early intervention to obtain long-term benefits[14].
The overall emptying time alone is therefore not yet an optimal post-procedure evaluation parameter[41]. Malik et al[27] showed a significant improvement of symptoms after GPOEM that was not corroborated by a clear reduction of the emptying time: 8 patients had symptoms improvements 6 patients had completed GES post procedure and 4 achieved a normal emptying time, 1 had stable value and 1 reported a worsening of gastric emptying time[27]. This findings were similar to other studies reporting an improvement of gastric emptying time after GPOEM, ranging from 34% to 100%[38].
It could be considered to add the study of the retention pattern with GES to predict the response to GPOEM; the possible role of this test in the pre-procedure diagnostic work up was proposed by Spandorfer et al[42]. They used the proximal-to-distal gastric T1/2 ratio. It found no differences in the pattern between idiopathic and diabetic GP and a correlation between more proximal retention pattern and response to GPOEM. Unfortunately, the sample with complete data before and after GES study was very little[42].
Symptoms that seem to respond better to GPOEM are nausea and vomiting, whereas abdominal pain and swelling responded less to the treatment. One possible explanation is that these latter symptoms are mainly related to visceral individual sensitivity and therefore they are difficult to evaluate.
Strong et al[43] reported their experience of GPOEM in 177 patients. 38 patients (21.5%) presented a post-surgical GP. The most frequent procedures were anti-reflux and hiatal hernia surgery. However, other surgical procedures that may induce iatrogenic vagotomy (esophagectomy, heart-lung transplant, excision of bronchial cyst or large hepatic adenoma) were included. This study demonstrated that, in the post-surgical subgroup, GPOEM induced both a clear symptom improvement but also a normalization of emptying time in at least half of the patients. The authors confirmed both the efficacy of GPOEM for post-surgical patients and the role of vagotomy as a suppressor of the propulsive antral component, thus clarifying the pathophysiological reasons for a better response to pyloromyotomy in this subgroup.
Similarly, a case report from John Hopkins University[44] also confirmed the excellent results of the technique in patients undergoing sleeve gastrectomy. Indeed, it is a procedure that may induce important mechanical motility impairment in the proximal stomach. The study highlighted an improvement of symptoms coupled with an enlargement of pylorus diameter and CSA, leading to a better compliance and a reduced pyloric pressure.
A recent systematic review aggregated the results of 10 studies published between 2015 and 2019. A total of 292 patients treated with GPOEM for refractory GP were evaluated[31]. GP etiology was as follow: 26.7% postsurgical, 26.7% diabetes-associated, 5.1% other underlying conditions, 41.5% idiopathic. The mean follow up period was 7.8 ± 5.5 mo Clinical success was achieved in all patients. Significant symptomatic improvement was achieved after 83.9% (95%CI: 78.5–89.3; I2: 0%; P = 0.928) of the procedures. The results of meta-regression analysis showed no significant relationships between clinical success rate and patients characteristics, GP etiology, preprocedural GCSI score, GES evaluation and previous pylorus-directed treatment. The mean post procedural follow up time was 7.8 ± 5.5 mo.
We have limited data concerning long term outcomes: Abdelfatah et al[45] in 2020 demonstrated a clinical improvement in 81.1% at initial follow up ( 73/90 patients at 6 mo) while 7.1% had recurrence. One year after procedure, the overall clinical response was 69.1%. The strength of the study is a large size with a very long follow up (until 36 mo): Among 7 patients with follow up of at least three years, 14% had recurrence and 86% of them maintained a clinical response.
Even if few data are available about the long term outcomes, a certain number of patients has been observing to lose clinical response, with a recurrence of refractory symptoms. Therefore, one of the most challenging issues that should be addressed in the future is how to treat them. A recent case report described two patients affected by idiopathic GP. It showed that the redo of GPOEM is feasible and promising, with a good clinical response. However, as underlined by the authors, this procedure needs a very experienced operator, due to the existing fibrosis coming from the first treatment. The main limitation of this interesting case report consists in the short term outcomes (the first loss of response was observed after 18 and 15 mo respectively, but the follow up after redo GPOEM was 6 mo only in one case and unknown in the other)[46].
Comparison between GPOEM and GES: GPOEM has also been compared with GES by Shen et al[47]. They hypothesize that GPOEM could be superior to GES. They analyzed with a propensity score two groups, 23 patients each, who underwent respectively GES or GPOEM for refractory GP. This study observed a similar clinical response in non-idiopathic GP between the two techniques, but significant better response to GPOEM for idiopathic GP. Moreover, they observed recurrence (with 12 mo. follow up) in 26.1% of patients in GPOEM group and in 56.5% of patients in GES group, without higher adverse events rate in GPOEM group.
Comparison between GPOEM and surgical pyloroplasty: A large meta-analysis comparing GPOEM (332 patients) vs surgical pyloroplasty (375 patients) showed that the two procedures are comparable in terms of technical success and clinical success[48]. Indeed, the emptying time was reduced to 4 h, the length of hospitalization was reduced, post-procedural pain and complication rate decreased (GES improvement 84% for pyloroplasty and 85% for GPOEM, adverse events 11% each, P = 0.95). However, GPOEM showed a shorter mean procedural time compared to surgical pyloroplasty. Moreover, idiopathic GP or previous pyloric treatment (botulin toxin and gastric stimulator) seem to be positive predictors to GES improvement after GPOEM
One of the most important challenges in the therapeutic scenario of GP is to identify the features of the ideal patient for GPOEM vs pyloroplasty, in order to obtain the best clinical result.
The pyloric spasm could be one of the keys to select the patients with the higher probability of being therapy responders. Indeed, it has been widely demonstrated that pylorus motility is only one of the possible factors responsible for GP.
Furthermore, concerning the available tools used to assess GP severity, it would be useful to validate cut-off values to standardize the treatment indications. Up to now, few authors proposed cut offs, such as GCSI baseline of at least 2.0 and emptying time at 4h greater than at least 20% of normal as cut off for proceeding with GPOEM[14] . However, many studies suggest a better response to GPOEM in patients with lower baseline GCSI and little symptoms[14,27].
Interestingly, the literature data show that non-diabetic GP is more responsive to GPOEM and the shorter duration of symptoms seems to be a predictor for the maintenance of the clinical response at 12 mo.
Overall, the studies[7] show that GPOEM seem to reduce more nausea and vomiting than the abdominal pain and the distension. A possible explanation could be that nausea and vomiting are more related to a delayed gastric emptying; whereas, the pain and abdominal distension could be mainly dependent from altered fundic adaptation and individual visceral hypersensitivity[39]. However, it seems that, like the distension of the gastric fundus, also the destruction of the pyloric muscle ring is able to activate the antroduodenal phasic motor activity.
Undoubtedly, the results of GPOEM are promising[14,31] and the experience gained from POEM has made it possible to achieve high technical success with few complications from the first procedures. Indeed, first multicenter study by Khashab et al[25] shows a technical success of 100%, with 86% of clinical response and 7% of complication rate.
However, further literature data on GPOEM are needed to standardize the indications and optimize the results.
For both surgical procedures and the endoscopic approach, it would be extremely useful to add informations on the probability of pre-procedural success by stratifying the patients using a score. In this direction, objective and reproducible tests such as the EndoFLIP or electrogastrography with their scores should be routinely used. This would allow to offer to each patient a targeted therapy, based on their clinical condition. Petrov et al[49] proposed a decision flowchart, according to both the main symptom pattern and the result of the gastric emptying study. The authors proposed three different therapeutic approaches: Gastric stimulation, gastric stimulation coupled with pyloromyotomy or GPOEM.
GPOEM is a procedure available only in tertiary endoscopic centers with experienced endoscopist, already trained on “third space” procedures. Indeed, the procedure outcomes are strictly dependent on the operator's experience. Furthermore, importantly, there is a lack of procedural and managerial standardization.
Finally, given its recent introduction, the available follow-up is limited and strong data about the maintenance of benefits are lacking. Indeed, the follow up available in literature ranges from 3 to 24 mo[14,45].
Further studies in larger series with longer follow up are thus needed to corroborate the available results.
GPOEM is a safe and promising technique for the treatment of refractory gastroparesis. Thus, the interest for this procedure is increasing. Nevertheless, further studies are needed to standardize the technique and to create the selection criteria to define the optimal candidates for GPOEM. We propose a diagnostic and therapeutic flowchart (Supplementary Figure 1).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cai Q S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-37; quiz 38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 748] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 2. | Parkman HP. Idiopathic gastroparesis. Gastroenterol Clin North Am. 2015;44:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Usai-Satta P, Bellini M, Morelli O, Geri F, Lai M, Bassotti G. Gastroparesis: New insights into an old disease. World J Gastroenterol. 2020;26:2333-2348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (5)] |
| 4. | Jung HK, Choung RS, Locke GR 3rd, Schleck CD, Zinsmeister AR, Szarka LA, Mullan B, Talley NJ. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 404] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 5. | Wadhwa V, Mehta D, Jobanputra Y, Lopez R, Thota PN, Sanaka MR. Healthcare utilization and costs associated with gastroparesis. World J Gastroenterol. 2017;23:4428-4436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (2)] |
| 6. | Camilleri M, Chedid V, Ford AC, Haruma K, Horowitz M, Jones KL, Low PA, Park SY, Parkman HP, Stanghellini V. Gastroparesis. Nat Rev Dis Primers. 2018;4:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 7. | Grover M, Farrugia G, Stanghellini V. Gastroparesis: a turning point in understanding and treatment. Gut. 2019;68:2238-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (1)] |
| 8. | Camilleri M, Bharucha AE, di Lorenzo C, Hasler WL, Prather CM, Rao SS, Wald A. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20:1269-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Desipio J, Friedenberg FK, Korimilli A, Richter JE, Parkman HP, Fisher RS. High-resolution solid-state manometry of the antropyloroduodenal region. Neurogastroenterol Motil. 2007;19:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90:1919-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 288] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJ, Tack J. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13:833-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Chassany O, Sagnier P, Marquis P, Fullerton S, Aaronson N. Patient-Reported Outcomes: The Example of Health-Related Quality of Life—a European Guidance Document for the Improved Integration of Health-Related Quality of Life Assessment in the Drug Regulatory Process. Ther Innov Regul Sci. 2002;36:209-238. [RCA] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Leidy NK, Revicki DA, Genesté B. Recommendations for evaluating the validity of quality of life claims for labeling and promotion. Value Health. 1999;2:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 297] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Uemura KL, Chaves D, Bernardo WM, Uemura RS, de Moura DTH, de Moura EGH. Peroral endoscopic pyloromyotomy for gastroparesis: a systematic review and meta-analysis. Endosc Int Open. 2020;8:E911-E923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Snape WJ, Lin MS, Agarwal N, Shaw RE. Evaluation of the pylorus with concurrent intraluminal pressure and EndoFLIP in patients with nausea and vomiting. Neurogastroenterol Motil. 2016;28:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Malik Z, Sankineni A, Parkman HP. Assessing pyloric sphincter pathophysiology using EndoFLIP in patients with gastroparesis. Neurogastroenterol Motil. 2015;27:524-531. [RCA] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Fathalizadeh A, Klingler M, Landreneau J, Allemang M, Rodriguez J, Ponsky J, El-Hayek K. Real-time intraoperative functioning lumen imaging probe during endoscopic per-oral pyloromyotomy (pop). Surg Endosc. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Conchillo JM, Straathof JWA, Mujagic Z, Brouns JH, Bouvy ND, Keszthelyi D, Masclee AAM. Gastric peroral endoscopic pyloromyotomy for decompensated gastroparesis: comprehensive motility analysis in relation to treatment outcomes. Endosc Int Open. 2021;9:E137-E144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Pasricha PJ, Colvin R, Yates K, Hasler WL, Abell TL, Unalp-Arida A, Nguyen L, Farrugia G, Koch KL, Parkman HP, Snape WJ, Lee L, Tonascia J, Hamilton F. Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol. 2011;9:567-76.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther. 2010;31:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Lacy BE, Parkman HP, Camilleri M. Chronic nausea and vomiting: evaluation and treatment. Am J Gastroenterol. 2018;113:647-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Bajpai S, Khan A, Rutledge KM, Stahl RD. Impact of Robotic Versus Laparoscopic Pyloroplasty on Short- and Long-term Outcomes in Patients with Gastroparesis. J Gastrointest Surg. 2021;25:2679-2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Lacy BE, Crowell MD, Schettler-Duncan A, Mathis C, Pasricha PJ. The treatment of diabetic gastroparesis with botulinum toxin injection of the pylorus. Diabetes Care. 2004;27:2341-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Clarke JO, Sharaiha RZ, Kord Valeshabad A, Lee LA, Kalloo AN, Khashab MA. Through-the-scope transpyloric stent placement improves symptoms and gastric emptying in patients with gastroparesis. Endoscopy. 2013;45 Suppl 2 UCTN:E189-E190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Khashab MA, Stein E, Clarke JO, Saxena P, Kumbhari V, Chander Roland B, Kalloo AN, Stavropoulos S, Pasricha P, Inoue H. Gastric peroral endoscopic myotomy for refractory gastroparesis: first human endoscopic pyloromyotomy (with video). Gastrointest Endosc. 2013;78:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 26. | Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1232] [Article Influence: 82.1] [Reference Citation Analysis (1)] |
| 27. | Malik Z, Kataria R, Modayil R, Ehrlich AC, Schey R, Parkman HP, Stavropoulos SN. Gastric Per Oral Endoscopic Myotomy (G-POEM) for the Treatment of Refractory Gastroparesis: Early Experience. Dig Dis Sci. 2018;63:2405-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 28. | Jacques J, Pagnon L, Hure F, Legros R, Crepin S, Fauchais AL, Palat S, Ducrotté P, Marin B, Fontaine S, Boubaddi NE, Clement MP, Sautereau D, Loustaud-Ratti V, Gourcerol G, Monteil J. Peroral endoscopic pyloromyotomy is efficacious and safe for refractory gastroparesis: prospective trial with assessment of pyloric function. Endoscopy. 2019;51:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 29. | Hedberg HM, Carbray J, Ujiki MB. Initial Experience with Endoscopic Pyloromyotomy, with Description and Video of Technique. J Gastrointest Surg. 2019;23:1706-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Vosoughi K, Ichkhanian Y, Jacques J, Aadam AA, Benias PC, Law R, Hasler WL, Canakis A, Ragi O, Triggs J, Bowers N, Brewer Gutierrez OI, Kumbhari V, Kalloo AN, Bulat RS, Pandolfino JE, Khashab MA. Role of endoscopic functional luminal imaging probe in predicting the outcome of gastric peroral endoscopic pyloromyotomy (with video). Gastrointest Endosc. 2020;91:1289-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 31. | Spadaccini M, Maselli R, Chandrasekar VT, Anderloni A, Carrara S, Galtieri PA, Di Leo M, Fugazza A, Pellegatta G, Colombo M, Palma R, Hassan C, Sethi A, Khashab MA, Sharma P, Repici A. Gastric peroral endoscopic pyloromyotomy for refractory gastroparesis: a systematic review of early outcomes with pooled analysis. Gastrointest Endosc. 2020;91:746-752.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Xue HB, Fan HZ, Meng XM, Cristofaro S, Mekaroonkamol P, Dacha S, Li LY, Fu XL, Zhan SH, Cai Q. Fluoroscopy-guided gastric peroral endoscopic pyloromyotomy (G-POEM): a more reliable and efficient method for treatment of refractory gastroparesis. Surg Endosc. 2017;31:4617-4624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Tao J, Patel V, Mekaroonkamol P, Luo H, Li B, Guan Q, Shen S, Chen H, Cai Q. Technical Aspects of Peroral Endoscopic Pyloromyotomy. Gastrointest Endosc Clin N Am. 2019;29:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Abdelfatah MM, Li B, Kapil N, Noll A, Li L, Luo H, Chen H, Xia L, Chen X, Patel V, Mekaroonkamol P, Massaad J, Keilin S, Cai Q. Short-term outcomes of double vs single pyloromyotomy at peroral endoscopic pyloromyotomy in the treatment of gastroparesis (with video). Gastrointest Endosc. 2020;92:603-609. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | ASGE Standards of Practice Committee, Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Cash BD. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81-89. [RCA] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (2)] |
| 36. | Kato M, Kaise M, Obata T, Yonezawa J, Toyoizumi H, Yoshimura N, Yoshida Y, Kawamura M, Tajiri H. Bacteremia and endotoxemia after endoscopic submucosal dissection for gastric neoplasia: pilot study. Gastric Cancer. 2012;15:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Kahaleh M, Gonzalez JM, Xu MM, Andalib I, Gaidhane M, Tyberg A, Saumoy M, Baptista Marchena AJ, Barthet M. Gastric peroral endoscopic myotomy for the treatment of refractory gastroparesis: a multicenter international experience. Endoscopy. 2018;50:1053-1058. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Mekaroonkamol P, Shah R, Cai Q. Outcomes of per oral endoscopic pyloromyotomy in gastroparesis worldwide. World J Gastroenterol. 2019;25:909-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 39. | Podboy A, Hwang JH, Nguyen LA, Garcia P, Zikos TA, Kamal A, Triadafilopoulos G, Clarke JO. Gastric per-oral endoscopic myotomy: Current status and future directions. World J Gastroenterol. 2019;25:2581-2590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Xu J, Chen T, Elkholy S, Xu M, Zhong Y, Zhang Y, Chen W, Qin W, Cai M, Zhou P. Gastric Peroral Endoscopic Myotomy (G-POEM) as a Treatment for Refractory Gastroparesis: Long-Term Outcomes. Can J Gastroenterol Hepatol. 2018;2018:6409698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 41. | Janssen P, Harris MS, Jones M, Masaoka T, Farré R, Törnblom H, Van Oudenhove L, Simrén M, Tack J. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108:1382-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 42. | Spandorfer R, Zhu Y, Abdelfatah MM, Mekaroonkamol P, Dacha S, Galt JR, Halkar R, Cai Q. Proximal and Distal Gastric Retention Patterns in Gastroparesis and the Impact of Gastric Per-Oral Endoscopic Myotomy: A Retrospective Analysis Using Gastric Emptying Scintigraphy. J Nucl Med Technol. 2020;48:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Strong AT, Landreneau JP, Cline M, Kroh MD, Rodriguez JH, Ponsky JL, El-Hayek K. Per-Oral Pyloromyotomy (POP) for Medically Refractory Post-Surgical Gastroparesis. J Gastrointest Surg. 2019;23:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Farha J, Fayad L, Kadhim A, Şimşek C, Badurdeen DS, Ichkhanian Y, Itani MI, Kalloo AN, Khashab MA, Kumbhari V. Gastric Per-Oral Endoscopic Myotomy (G-POEM) for the Treatment of Gastric Stenosis Post-Laparoscopic Sleeve Gastrectomy (LSG). Obes Surg. 2019;29:2350-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Abdelfatah MM, Noll A, Kapil N, Shah R, Li L, Nustas R, Li B, Luo H, Chen H, Xia L, Mekaroonkamol P, Shahnavaz N, Keilin S, Willingham F, Christie J, Cai Q. Long-term Outcome of Gastric Per-Oral Endoscopic Pyloromyotomy in Treatment of Gastroparesis. Clin Gastroenterol Hepatol. 2021;19:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 46. | Li B, Calderon, LF, Chen H, Xu J, Cai Q. Redo GPOEM for Refractory Gastroparesis: is it Feasible and Useful? J Gastroenterol Hepatol Res. 2020;9:3253-3254. [DOI] [Full Text] |
| 47. | Shen S, Luo H, Vachaparambil C, Mekaroonkamol P, Abdelfatah MM, Xu G, Chen H, Xia L, Shi H, Keilin S, Willingham F, Christie J, Lin E, Cai Q. Gastric peroral endoscopic pyloromyotomy vs gastric electrical stimulation in the treatment of refractory gastroparesis: a propensity score-matched analysis of long term outcomes. Endoscopy. 2020;52:349-358. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Mohan BP, Chandan S, Jha LK, Khan SR, Kotagiri R, Kassab LL, Ravikumar NPG, Bhogal N, Chandan OC, Bhat I, Hewlett AT, Jacques J, Ponnada S, Asokkumar R, Adler DG. Clinical efficacy of gastric per-oral endoscopic myotomy (G-POEM) in the treatment of refractory gastroparesis and predictors of outcomes: a systematic review and meta-analysis using surgical pyloroplasty as a comparator group. Surg Endosc. 2020;34:3352-3367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 49. | Petrov RV, Bakhos CT, Abbas AE, Malik Z, Parkman HP. Endoscopic and Surgical Treatments for Gastroparesis: What to Do and Whom to Treat? Gastroenterol Clin North Am. 2020;49:539-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |