INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer by diagnosis and the fourth highest cause of cancer-related death worldwide[1]. Surgical resection or liver transplantation are curative options, but unfortunately less than 40% of patients are eligible due to advanced stage at diagnosis[2].
Liver resection remains the mainstay among the curative treatments, but it is affected by a recurrence rate of up to 60% at 5 years, even for early stage tumours[3]. The route of recurrence is still a matter of debate. The relapse of HCC may be driven by precancerous status of the remaining diseased liver: namely “multicentric de-novo occurrence”, these tumours are always primitive[4].
However, the majority of recurrence is attributed to intra-hepatic metastasisation, driven by the acquisition of the cancer hallmark of invasiveness[5]. Clinically speaking, no tools have been developed to recognise the two different patterns before treatment, although basic and pre-clinical studies have identified several genetic signatures[6,7]. While multicentric occurrence cannot be controlled by liver resection alone, intra-hepatic metastasisation could be avoided by an appropriate resection: almost thirty years ago, Makuuchi et al[8] stasisation could be avoided by an appropriate resection: almost thirty years ago, Makuuchi et al[8] reported a high rate of recurrence after surgery when microvascular invasion and satellitosis were present in the histological specimen. This evidence and that from other experiments[8-10], ed portal vein dissemination to be considered to be the main route of intra-hepatic metastasis, developing the notion of anatomical resection that relies on the complete removal of the whole segmental portal-flow area of the liver segment hosting the tumour. This technical approach was expected to allow better control of the area with the highest risk of tumour spread, reducing recurrence rates. However, several authors compared recurrence rates among anatomical and non-anatomical resections, without a clear conclusion[11]. In recent years, the challenge of recurrence even after liver transplantation, a better knowledge of the tumour blood flow area[12], and the modern knowledge derived from molecular studies, have forced us to rethink the portal theory of HCC recurrence. In fact, intra-hepatic metastasis seems to be caused by local dissemination among the tumour blood flow, or by the systemic dissemination of tumour cells. These circulating tumour cells (CTCs) have also been identified in other types of tumours[13], and may have the ability to rehome themselves in the liver[14], and consequently could explain cases of relapse even after organ transplantation. In the present paper, we aimed to critically review the literature regarding HCC recurrence and CTC identification, and their role in surgery. We interpret our previous data in light of results from other studies, aiming to suggest a possible general picture to inform future research in the field.
RECONSIDERING THE ROUTE OF RECURRENCE: EVIDENCE-DRIVEN HYPOTHESIS
Nakashima et al[15] have proposed that the portal vein (PV) may act as the efferent vessel during the oncoprogression of HCC, particularly in the setting of cirrhotic patients, where the hepatic veins are compromised. In this theory, the hepatic artery is the feeding vessel, and the PV, as an efferent vessel, penetrates the tumour capsule, and becomes the path of minor resistance for tumour infiltration or expansion[9] and the drainage pathway of the neoplasm. This mechanism was described to explain the high rate of tumour thrombi observed, and the presence of satellitosis near the primitive tumour. Those considerations led to the proposal of the anatomic resection (AR) to completely remove the parenchymal area fed by the portal branch (namely, the liver segment), in which there may be an increased risk of recurrence. However, the superiority of AR has been never proven, and several reports are available in favour or against this hypothesis[11,16]. More importantly, according to the theory, AR should completely eliminate the risk of local recurrence (relapse at the surgical edge), by eliminating the area where the tumour may have spread. However, our and others data[17,18] have reported a comparable rate of local recurrence among AR and non-anatomical resections, questioning the ability of a radical segment resection to control the oncological burden. Thus, the highest rate of intra-hepatic recurrence occurred in other liver segments than the one carrying the primitive nodule, suggesting a different or at least a concomitant route of the tumour cells. More recently, we tried to identify the risk factors for either local or intra-hepatic distant recurrence in a large European series[19], observing that local relapse occurred frequently in cases of positive surgical margin (and consequently as a kind of surgical failure), while the presence of microvascular invasion and satellitosis were hallmarks of increased risk of intra-hepatic distant relapse. These data suggest that, when those histological features occurred, the tumour may have already invaded the blood circulation, with a metastasisation potential in other locations that may not be explained by the local portal flow, and that cannot be controlled by modifying the extent of surgery. In this sense, the tumour micro-thrombi assessed by histology near the primitive nodule could not be considered only a local extension of the disease (as supposed by the portal flow theory), but a sign of systemic dissemination. Another ‘brick in the wall’ was suggested by the clinical data: recently, Hidaka et al[20] reported that the complete removal of the portal-bearing area did not modify the risk of recurrence in cases of microvascular invasion, and this data was confirmed in our recent meta-analysis[11].
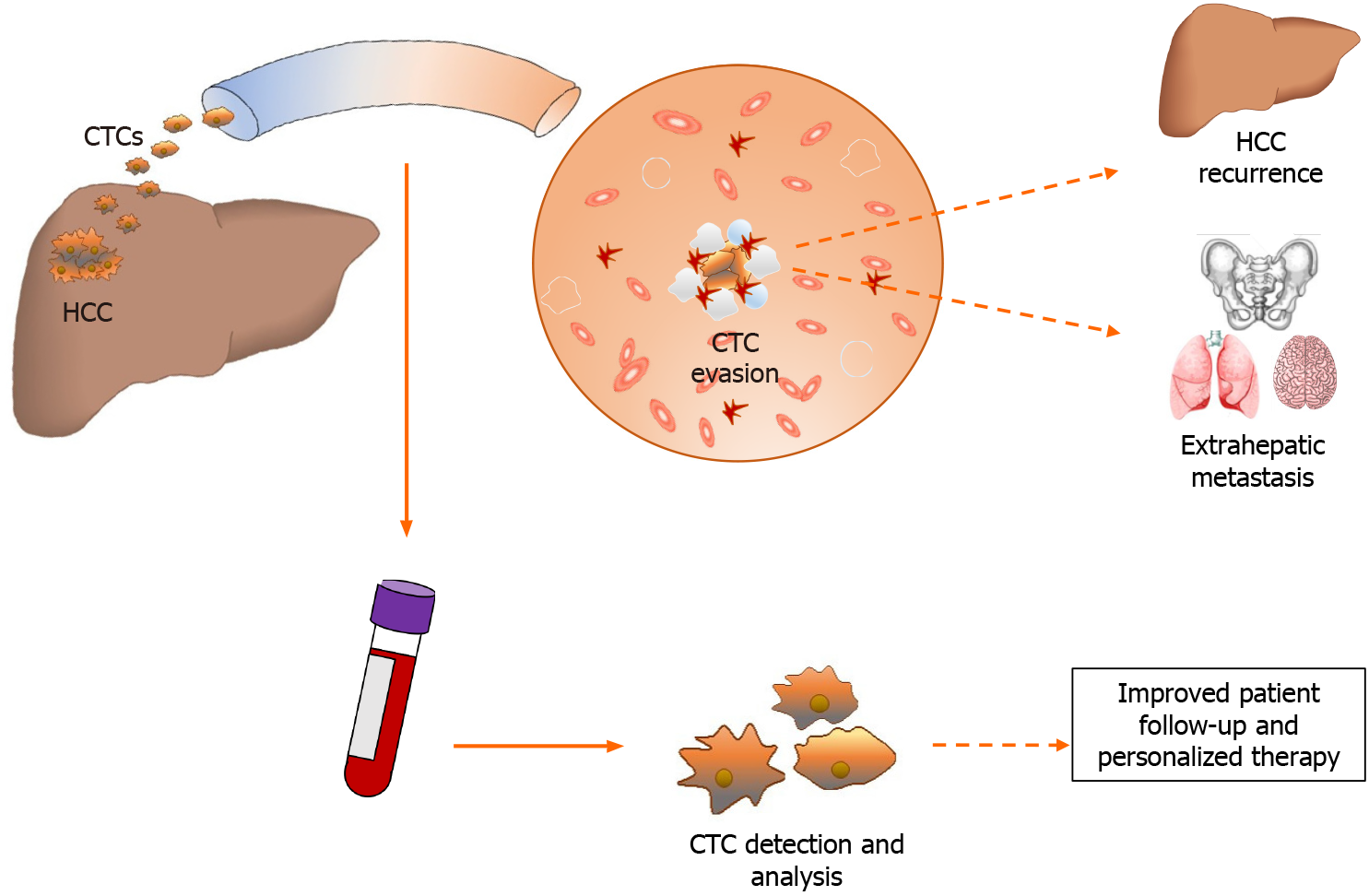
Sakon et al[12,21] studied the tumour blood flow (TBF) area, discovering that this coincided with the segmental portal area only in 18% of their cohort. In up to 75% of cases, the TBF was independent of the PV area, and the rate of recurrence was reduced only in cases where the TBF was completely included in the resection area, regardless of the removal of the liver segment. The authors proposed a subclassification of HCC recurrence based on two different mechanisms: local recurrence, which is driven by the invasion of the local tumour blood flow with a peritumoural dissemination, and a systemic dissemination driven by the spread of CTCs derived from the primitive nodule, which may be able to “rehome” after passing through the systemic circulation. While the first mechanism could be controlled by an effective radical resection, the second relies on the oncological progression of the tumour, and could explain cases of intra-hepatic relapse at a distance from the original site, but also recurrence after transplantation. In 2018, a very interesting study was conducted by Sun et al[22], who tested the spatial heterogeneity of phenotypic and molecular characteristics of CTCs within the circulatory system, discovering that a higher number of CTCs were detected in sites other than the PV. In particular, the percentages of CTCs detected in blood sampled from a peripheral vein, peripheral artery, hepatic veins, infrahepatic inferior vena cava, and PV before HCC resection were 68.5%, 45.2%, 80.8%, 39.7%, and 58.9%, respectively. Moreover, CTC and circulating tumour microemboli burden detected in hepatic veins and peripheral circulation, but not in the PV, were associated with postoperative lung metastasis and intrahepatic recurrence, respectively. These pieces of evidence suggest that the classical recurrence theory for HCC cannot explain many real-scenario observations. A novel approach, integrating the discovery of CTCs and their role in tumour biology with clinical experience, will allow a novel and tailored approach to select the best candidates for curative strategies, but will also be able to provide a novel biomarker with the ability to summarise the biological data of the tumour, using a very simple blood sample analysis (Figure 1). The detection of these cells and their possible role in surgery will be further explored in the following sections.
Figure 1 Hepatocellular carcinoma releases tumor cells into circulation where they become circulating tumor cells.
Circulating tumor cells after evading the immune system can cause hepatocellular carcinoma recurrence or distant metastasis. The liquid biopsy intercepts these cells into the bloodstream and allows to study the tumor characteristics and guarantee personalized therapy. CTCs: Circulating tumor cells; HCC: Hepatocellular carcinoma.
CTCS MOLECULAR CHARACTERISTICS
In the context of cancer pathogenesis, and especially for carcinomas, the “epithelial-mesenchymal transition” (EMT) is a fundamental mechanism playing a key role in the metastatic proces[23]. Several authors agree that this rearrangement of cell status is neither stable nor binary, and neoplastic epithelial cells that have activated an EMT program very rarely advance to a fully mesenchymal state[24]. Also, the reverse process known as mesenchymal-epithelial transition (MET) is required for metastatic colonisation in the same or other tissues[25]. Both of the above-mentioned mechanisms can actively operate in the generation of CTCs. Since CTCs are a phenotypically distinct subpopulation that originate from the tumour microenvironment, the idea behind the identification of CTCs is to discover characteristic markers of both EMT/MET transition and of the primary tumour.
CTC identification is technically difficult due to the low concentration of these cells in blood[26]. In recent years, research has focused on improving the specificity and sensitivity of CTC detection and facilitating accurate molecular characterisation[14]. Based on physical and/or biological properties of the cells, several strategies and systems have been developed to improve CTC enrichment. Filter membranes, such as the CanPatrolTM system, and microfluidic devices, such as CTC-iChip and Labyrinth-chip, allow separation of cells based on their sizes[14,27]. Alternatively, Ficoll-type density gradient methods make it easier to separate blood cells, exploiting their different density[28].
One of the most used methods is the Cell Search® system, which is based on immunomagnetic enrichment[29]. This CTC isolation strategy exploits the expression on the cell surface of the protein EpCAM, which is the most accredited marker for positive affinity-selection of CTCs. The Cell Search® system is the only system approved by the Food and Drug Administration (FDA) to predict the outcome of patients affected by breast cancer[30]. Nevertheless, enumeration of EpCAM+ CTCs alone has demonstrated modest clinical sensitivity and, for instance, in cancers with low EpCAM expression, the Cell Search® system showed a lower CTC recovery rate compared to microfluidic devices[31]. In 2018, Pang et al[32] developed a method which exploits the surface-enhanced Raman scattering (SERS) technology and nanoparticles linked to antibodies directed against the specific hepatic proteins asialoglycoprotein receptor (ASGPR) and glypican-3 (GPC3), allowing isolation of EpCAM- CTCs. Moreover, not all CTCs have metastatic or relapsing potential, so simple quantification without better molecular characterisation could lead to incorrect clinical conclusions. The use of isolation and enrichment devices is supported by other laboratory techniques such as immunofluorescence staining of different markers [fluorescence-activated cell sorting (FACS) and fluorescent in situ hybridization (FISH)] and/or gene expression analysis [real time PCR (qPCR) and single cell RNA sequencing (scRNA-seq)] in order to obtain in depth CTC characterisation[33]. Considering all of these biological /phenotypic and experimental issues, the application of this method in common clinical practice has proved to be difficult. Making this strategy even harder is the heterogeneity of the tumour itself and, among protein markers, cytokeratins (CKs), vimentin, CD44, CD133 and CD90 are the most used so far[34]. CKs, like EpCAM, are epithelial markers, but unlike the latter, they are intracellular proteins, thus they are identified mainly using immunocytochemistry. Cells are usually stained for CK8, 18 and 19, but recently other markers such as human epidermal growth factor receptor 2 (HER2) and the estrogen receptor (ER) have been examined to facilitate detection of CTCs with metastatic potential[35].
As mentioned above, the major drawback of using epithelial markers is their inability to detect CTCs that no longer express them after undergoing EMT, a process which is strongly associated with overexpression of vimentin and CD44[36,37]. CD44 is often used as a marker in combination with the stem-like markers CD133 and CD90[38]. However, plasma membrane and cytoplasmic proteins are not the only markers used to detect potential CTCs; complex studies have tried to generate the mRNA expression profiles of CTCs in different diseases[39]. In particular, D’Avola et al[33] recently developed a new method that sequentially combines image flow cytometry and high density scRNA-seq in order to identify CTCs in patients with HCC. The authors suggest the advantages of genome-wide transcriptome profiling to confidently detect CTCs and its potential role in monitoring HCC heterogeneity and detecting HCC driver genes, which could ultimately help customize therapeutic interventions in these patients.
CTCS IDENTIFICATION IN HCC PATIENTS
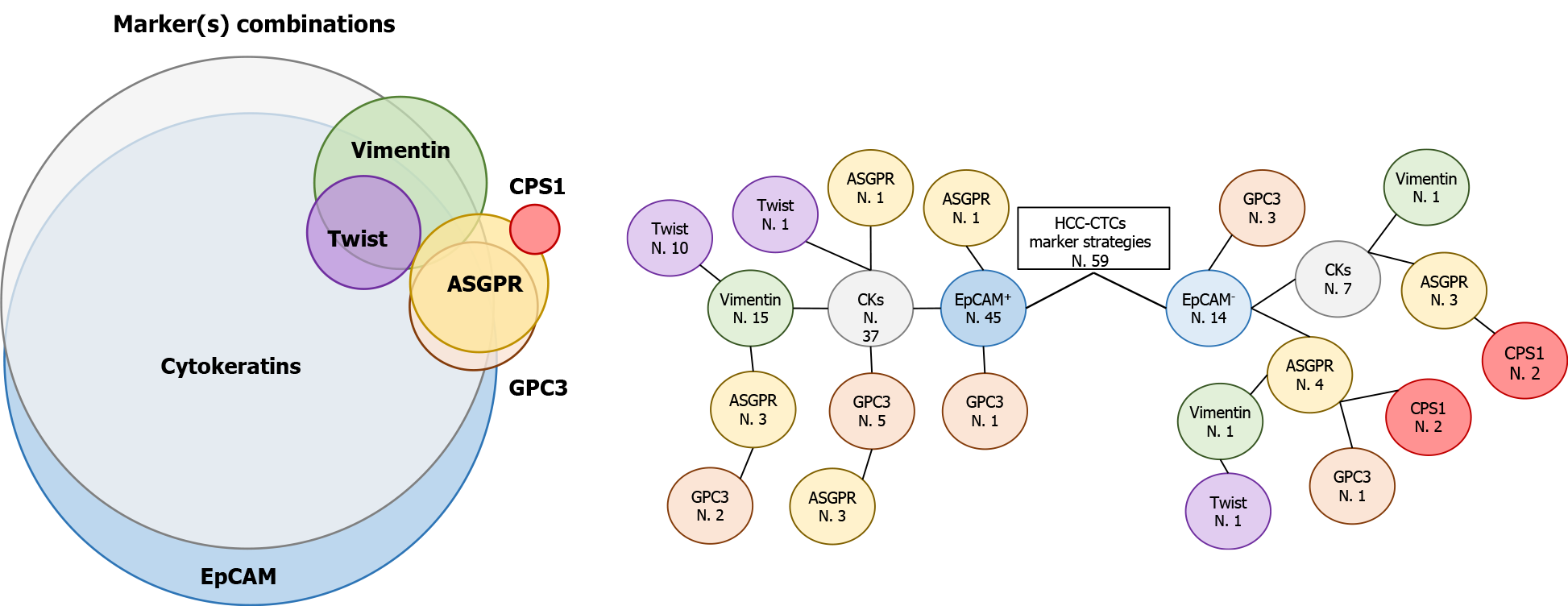
To date, there are no specific or accredited CTC-related protocols for detection of HCC that are agreed upon by the scientific community. Several studies have been performed to deeply investigate and introduce the use of CTC enumeration/characterisation in HCC monitoring in clinical practice. Most of these studies are primarily based on the previously validated EpCAM/CK markers, with secondary examination of other markers or features (Figure 2). In recent years, several authors in the HCC field have taken advantage of combining the markers vimentin and twist; as mentioned above, these mesenchymal markers have followed the common epithelial markers EpCAM/CKs. Ou et al[40] observed that the presence of mesenchymal CTCs tended to occur in advanced stage patients and was associated with earlier recurrence in a large cohort of HCC patients. ASGPR and carbamoyl-phosphate synthetase 1 (CPS1) are interesting in the context of HCC. Liu et al[41] demonstrated that CTC enrichment, combined with identification using an antibody cocktail against ASGPR and CPS1, not only significantly improves sensitivity for CTC enrichment, but also provides high specificity for CTC detection in patients with HCC, thereby minimising false negative/positive results. The combination of ASGPR and CPS1 was used also by Li et al[42], confirming the increased sensitivity for HCC CTC detection.
Figure 2 Frequently used markers for circulating tumor cell detection hepatocellular carcinoma-related.
Epithelial cell adhesion molecule+ circulating tumor cell identification is often combined with cytokeratins, vimentin, twist, Glypican-3 and asialoglycoprotein receptor (ASGPR). In some cases, ASGPR is also used with the hepatocellular marker carbamoyl-phosphate synthetase 1 (Venn diagram). Tree diagram show the number of articles (N.) that use different marker combinations for circulating tumor cell isolation. Bibliography counts articles related to hepatocellular carcinoma field and published from 2009 to 2020. EpCAM: Epithelial cell adhesion molecule; CKs: Cytokeratins; ASGPR: Asialoglycoprotein receptor; GPC3: Glypican-3; CPS1: Carbamoyl-phosphate synthetase 1.
In 2016, Zhang et al[43] isolated ASGPR+/CPS1+ CTCs from HCC patients, which were then cultured and expanded to form spheroid-like structures in a 3D cell culture assay. They suggested that this method could aid physicians in the selection of appropriate drug therapies for HCC patients. The role of CTCs expressing mesenchymal features in predicting HCC early recurrence was confirmed in the same year by Qi et al[44], in a monocentric study with 112 enrolled patients. However, mesenchymal CTC use in clinical practice is controversial, since their analysis in a different cohort of HCC patients who underwent liver transplantation was not able to predict HCC recurrence[45]. Conversely, in liver transplantation, the entire organ is replaced with a healthy liver deriving from a donor. Thus, the HCC recurrence is likely due to circulating and/or dormant tumour cells, which have acquired the ability to escape from the host’s immune system. Clusters of CTCs were first predicted and then observed as intravascular tumour microemboli, represented by multicellular epithelial tumour cells. In a mouse model experiment, in which human-derived CTCs were used, it was observed that CTC clusters are not derived from intravascular aggregation of single CTCs or from the progeny of a single primary tumour cell that proliferates in the vascular space, but instead, evidence showed that CTC clusters derive from groupings of primary tumour cells that enter the bloodstream together[46].
CTCS AS A MOLECULAR SIGNATURE OF THE HISTOLOGICAL CHARACTERISTICS OF THE PRIMITIVE HCC
Recently it has been reported that CTCs positive for EpCAM, N-Cadherin and CD90 expression (triple positive CTCs) are more frequently associated with microvascular invasion (MVI), as detected in a histological specimen after liver resection[47]. The histopathological finding of MVI is a feature of advanced HCC, associated with a higher probability of recurrence and metastasis[48]; however, with the imaging tests and biomarkers currently available, the preoperative identification of MVI remains difficult[49]. Rodríguez-Perálvarez et al[50] showed that MVI incidence was between 15.0% and 57.1% at histopathological examination after liver resection and transplantation, in a systematic review. Thus, different tumour stages and HCC invasive characteristics affect MVI incidence. The possibility given by the triple positive CTCs, associated with the actual diagnostics tool, to pre-operatively identify MVI may play a role in the use of preoperative predictive models in therapeutic decision-making in patients with HCC.
CTCS VARIATIONS AFTER LIVER RESECTION
After surgical tumor excision, CTC levels drop dramatically and post-operative CTC levels can be used as tools to verify surgical resection as a monitor for tumor burden [14]. Yu et al[51] evaluated the effect of surgical liver resection on CTCs in patients with HCC, demonstrating that a lower CTC level after surgical resection is an independent prognostic factor for better disease-free-survival (HR 0.620; 95%CI: 0.479–0.803; P < 0.001) and overall-survival (HR 0.608; 95%CI: 0.443–0.834; P = 0.002). Ou et al[40] demonstrated that increased CTC numbers were observed in patients with high levels (> 400 mcg/L) of alpha fetoprotein (AFP), advanced TNM and BCLC stage, and the presence of embolus or microembolus. They also investigated CTC heterogeneity, noting a significant correlation between mesenchymal CTCs and high AFP levels, multiple tumours, advanced TNM and BCLC stage, presence of embolus or microembolus, and earlier recurrence.
These CTCs could be considered as a very early sign of tumour migration: invisible micro-metastasis, impossible to detect with standard methods but playing a fundamental role in patients’ clinical evolution[52]. Sun et al[53] analysed the diagnostic value of CTCs in HCC patients, performing a meta-analysis on 20 studies of a total of 998 HCC patients. From their work, it emerges that CTC positivity is associated with a lower overall survival (HR 2.417; 95%CI: 1.421–3.250; P < 0.001) and disease free survival (HR 3.59; 95%CI: 1.984–6.495; P < 0.001). CTC analysis determines the tumour molecular characteristics before any treatment, evaluating cancer differentiation and identifying markers as possible molecular therapy targets or mechanisms of resistance to therapy[54]. The selective pressure that develops over time since starting the treatment leads to increased cellular heterogeneity of the tumour, production of drug resistant subclones, and the selection of rare mutants[55], essentially the tumour is characterised by different genetic backgrounds at different times. Therefore, the tumour genome during follow-up could differ significantly from its initial state, and this difference cannot be assessed unless repeated sampling is performed. However, repeat biopsy is rarely feasible and, without knowledge of the genetic changes, complete treatment personalisation and targeted therapy is impossible[56]. In comparison, liquid biopsy is easily repeatable during follow-up, making knowledge of all tumour genome changes possible. In the future, it will be desirable to use quantitative and qualitative analysis of CTCs to develop personalised therapy for each patient. The phenotyping of those cells, and their quantification in the peripheral blood, may allow identification of patients with a more severe and more aggressive disease, who could be the target population in which adjuvant therapies as Sorafenib may play a role. Currently, it is not evident what features are associated with response to such treatments[57]. The presence and characteristics of the CTCs identified in peripheral blood may become a molecular marker to decide the follow-up schedule, and estimations of risk could be updated at each visit by repeating the test. In other words, CTCs could become a new predictive marker to better stratify patients and assign them to the best individual treatment plan, improving long-term cancer outcomes.
CTCS AND LIVER TRANSPLANT FOR HCC
Orthotopic liver transplantation (OLT) is the most favourable option for the treatment of HCC, with a 5-year overall survival rate of 75% and disease-free survival rate of 83%[58]. Despite stringent criteria in patient selection for transplantation, HCC recurrence still remains a significant problem, with a rate of 15%–20%[59,60]. Due to organ shortage and recurrence risk even after transplantation, it is important to be able to select patients for LT in order not to misallocate a limited resource.
Tumour size, AFP levels, and micro- and macro-vascular invasion are the main prognostic factors for recurrence risk after transplantation[61]. The aim of patient selection criteria should be to prevent transplantation in those patients with an expected HCC recurrence and to improve transplantation for those patients who have a high likelihood of being cured. The present parameters are based on morphology, but in the modern molecular era, new information could be available to better understand the patient’s tumour biology in a tailored fashion.
Xu et al[62] highlighted how the CTC-positive rate and number of CTCs present is higher in patients beyond the Milan criteria than in patients within the criteria (91% vs 69%, P = 0.009; and 27 ± 27 vs 6 ± 9, P < 0.001; respectively). This suggests that including the CTC count in pre-transplant evaluation could revolutionise the eligibility criteria for transplantation. Chen et al[63] analysed preoperative CTCs in HCC patients who underwent LT and followed them up for at least one year, or until relapse or death occurred. They found that recurrence is associated with presence of preoperative CTCs (P = 0.013); multivariate analysis confirmed that CTCs are an independent risk factor for the onset of recurrence after LT (HR: 5.411; 95%CI: 1.132–25,874; P = 0.034). These data reflect the 1-year DFS rate, which is 91.6% for the CTC-negative and 61.5% for the CTC-positive group (P = 0.020). On the other hand, the 1-year overall survival rate for the CTC-negative and CTC-positive group is 91.7% and 88.5%, respectively, with no significant difference. Very few data are available about the potential role of CTCs as preoperative predictors of HCC recurrence after LT, and it is still a controversial issue. However, their application could drastically change the allocation protocols, enabling a more tailored algorithm with potentially better ability to predict the risk of relapse and, consequently, differentiate the cases that could benefit from transplant from the ones that could not.
CTCS IDENTIFICATION IN A REAL-CLINICAL SCENARIO: THE FINDINGBIOREC PROTOCOL
In light of the previously mentioned data, the University of Milano-Bicocca, the University of Piemonte Orientale and Humanitas University have decided to collaborate by creating a study with the aim of "finding the seeds of recurrence", using liquid biopsy to detect CTCs as markers of disease and prognosis in HCC.
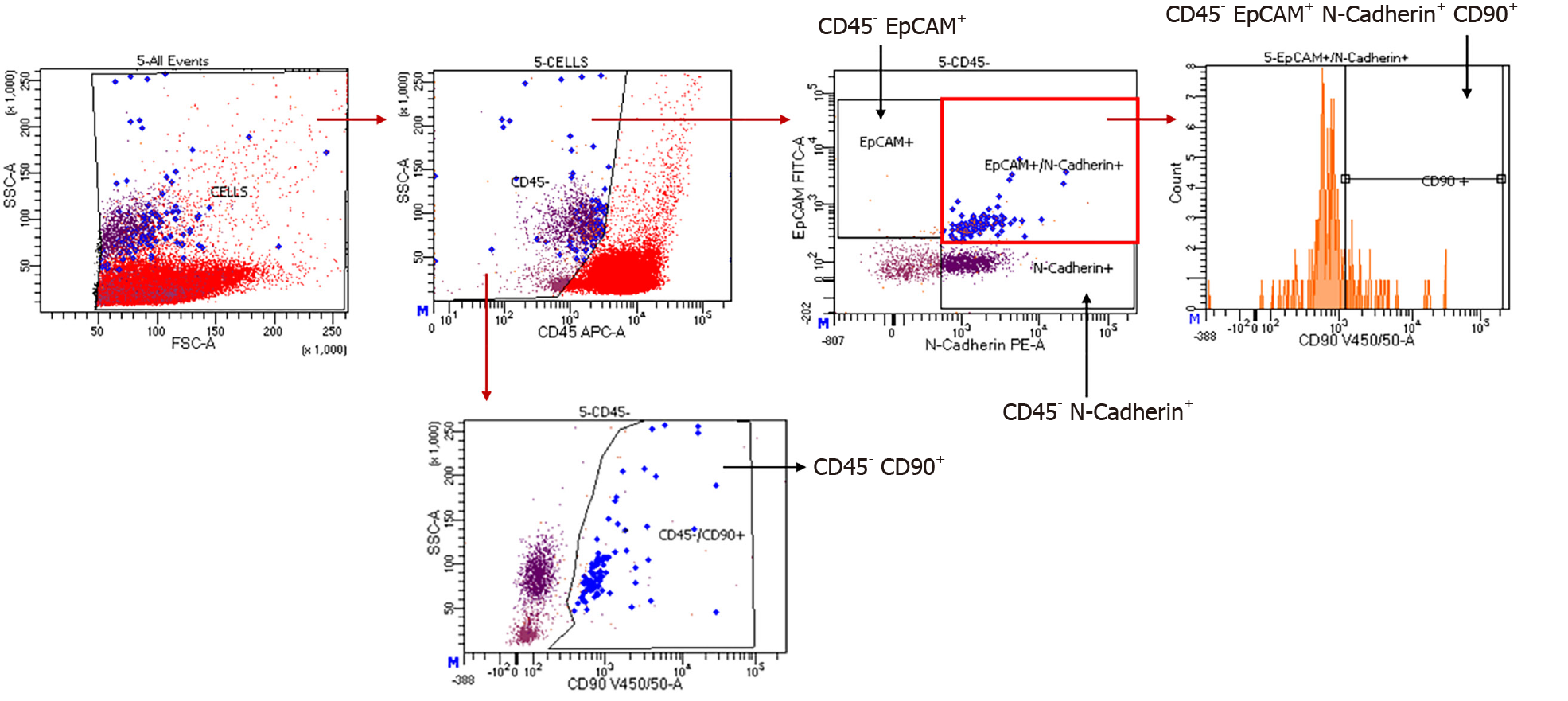
Our hypothesis is that CTCs may spread from the original tumours as a hallmark of advanced cancer, which has already developed the characteristic of invasiveness. It is our opinion that early stage tumours do not release CTCs into the bloodstream at the same rate or quality as advanced tumours. Patients with a positive CTC liquid biopsy may have a worse prognosis, due to an increased relapse rate. Finally, from a pathophysiological point of view, we want to demonstrate that recurrence is due to CTC seeding, in order to gain a better understanding of HCC carcinogenesis. The “FINDINGIBIOREC” study (clinicaltrial.gov ID: NCT04800497) was developed: a prospective, observational cohort study, conducted in two tertiary referral centres for liver cancer, in which each enrolled patient is submitted to liquid biopsy prior to surgery and then every 3 mo during the follow-up schedules, for 3 years. Patients with a first diagnosis of HCC, no previous treatment for this condition, no other oncological history, and BCLC stage 0-A-B are prospectively enrolled. The samples are processed and the CTCs are detected using FACSymphony™ with subsequent identification of the following markers: EpCAM, N-cadherin (N-cad) and CD90 (Figure 3). Patients are followed up with clinical assessments; CT or, where necessary, MRI and AFP level, together with liquid biopsy. With this protocol, we aim to better highlight the trends of CTCs at different time-points and their correlation with the oncologic prognosis in very early and early HCC. The study is currently enrolling, and it will be closed in 2023.
Figure 3
Representative example of a patient’s PBMC analysis within the FINDINGBIOREC protocol.
CONCLUSION
HCC may produce early CTCs, which seem to be the seed of the recurrence. Their presence in the blood stream has been correlated with the presence of MVI, suggesting that the latter is a surrogate sign of a systemic disease that cannot be controlled by classical liver segment resection alone. Those cells could be detected and studied by liquid biopsy, which is a safe method to obtain information on the patient's disease status. This allows tumour molecular characterisation during different disease phases, and could become a new method for patient stratification. The study of CTCs allows selection of patients and the type of treatment they will receive in order to optimize HCC therapy. During the follow-up, an increase in CTCs makes it possible to identify tumour recurrence and implement further therapy early. In future, liquid biopsy could be implemented in the pre- and post-operative routine of HCC patients in order to gain more accurate information on tumour type and stage, and guarantee the most personalised therapy possible for the patients.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fernandes SA S-Editor: Ma YJ L-Editor: A P-Editor: Wu RR