Published online Sep 27, 2021. doi: 10.4240/wjgs.v13.i9.941
Peer-review started: February 7, 2021
First decision: April 6, 2021
Revised: April 15, 2021
Accepted: July 27, 2021
Article in press: July 27, 2021
Published online: September 27, 2021
Processing time: 222 Days and 22.4 Hours
In recent years, the incidence of gastrointestinal cancer has remained high. Currently, surgical resection is still the most effective method for treating gastrointestinal cancer. Traditionally, radical surgery depends on open surgery. However, traditional open surgery inflicts great trauma and is associated with a slow recovery. Minimally invasive surgery, which aims to reduce postoperative complications and accelerate postoperative recovery, has been rapidly developed in the last two decades; it is increasingly used in the field of gastrointestinal surgery and widely used in early-stage gastrointestinal cancer. Nevertheless, many operations for gastrointestinal cancer treatment are still performed by open surgery. One reason for this may be the challenges of minimally invasive technology, especially when operating in narrow spaces, such as within the pelvis or near the upper edge of the pancreas. Moreover, some of the current literature has questioned oncologic outcomes after minimally invasive surgery for gastrointestinal cancer. Overall, the current evidence suggests that minimally invasive techniques are safe and feasible in gastrointestinal cancer surgery, but most of the studies published in this field are retrospective studies and case-matched studies. Large-scale randomized prospective studies are needed to further support the application of minimally invasive surgery. In this review, we summarize several common minimally invasive methods used to treat gastrointestinal cancer and discuss the advances in the minimally invasive treatment of gastrointestinal cancer in detail.
Core Tip: The incidence of gastrointestinal tumors is high. Minimally invasive surgery has changed the traditional treatment of these patients. Minimally invasive surgery is a revolutionary treatment for gastrointestinal tumors that can reduce surgical complications and accelerate postoperative recovery. Here, we discuss the role and prospect of minimally invasive surgery in the treatment of gastrointestinal tumors.
- Citation: Ye SP, Zhu WQ, Huang ZX, Liu DN, Wen XQ, Li TY. Role of minimally invasive techniques in gastrointestinal surgery: Current status and future perspectives. World J Gastrointest Surg 2021; 13(9): 941-952
- URL: https://www.wjgnet.com/1948-9366/full/v13/i9/941.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i9.941
With the development of science and technology, minimally invasive surgery is a new option for the radical treatment of tumors. Minimally invasive surgery is gaining increasing popularity for the treatment of gastrointestinal cancer, including endoscopic resection, laparoscopic resection, and da Vinci surgical system resection. Minimally invasive techniques have resulted in less blood loss and fewer complications than conventional surgery.
Minimally invasive surgery is not just about minimizing trauma but also about achieving a complete radical tumor removal. To achieve this goal, high-definition, high-magnification devices have been developed for use in gastrointestinal cancer surgery, allowing surgeons to perform more accurate resection and avoid unnecessary damage compared with traditional surgery because the tumor and surrounding structures can be better visualized.
For any minimally invasive technique, there is always a learning curve to overcome and sufficient evidence to substantiate its effectiveness; equally important is whether the benefits of these techniques are worth the added cost and time.
Endoscopic resection may be presently thought of as an option for the majority of early gastric malignancy cases and could be recognized as a definitive treatment unless it is thought that there is a significant risk of lymph node metastasis[1-3]. The most risky component of lymph hub metastasis is lymphatic vessels in the vicinity of the tumor. Other risk factors include submucosal intrusion (T1b), poor differentiation, ulceration, and a large tumor[1]. Several studies have reported no significant difference in long-term overall survival or tumor-specific survival between patients with early gastric cancer treated endoscopically and those who underwent conventional surgical resection[4,5].
There are currently two primary endoscopic resection techniques: Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). EMR is robust and technically reproducible with a short learning curve, whereas ESD is technically more demanding and therefore has a much longer learning curve. However, ESD normally brings about en bloc specimens, higher extent of complete resections, and fewer nearby recurrences[6,7]. Asian and European guidelines recommend ESD as the endoscopic resection method of choice for early gastric cancer[1,8,9].
The detection rate of early colorectal cancer has increased due to the improvements in quality of life and the emphasis on medical check-ups. Early (T1) colorectal cancers with a low risk of lymphatic metastasis can be treated by endoscopic techniques[10-12]. Unfortunately, most patients with early-stage colorectal cancer do not receive adequate endoscopic treatment evaluation and still undergo surgical treatment[13,14]. Endoscopic resection of stage T1 colorectal cancer depends on the tumor size and the depth of invasion. When submucosal invasion is highly suspected, ESD and endoscopic full-thickness resection are better choices than EMR[15-17]. A number of studies have shown that endoscopic treatment of patients with stage T1 colorectal cancer is safe and feasible, and there is no significant difference between the results of endoscopic treatment and surgical treatment[18-21]. Although endoscopic treatment requires adequate physician proficiency and proper assessment of the tumor stage, the advantages of endoscopic treatment in terms of a lower cost and faster postoperative recovery are enormous. Therefore, doctors should properly recognize the advantages of endoscopic treatment and should consider whether endoscopic treatment can benefit their patients with early colorectal cancer.
Laparoscopy is a landmark advance in the history of minimally invasive surgery, and its use is intended to help minimize surgical trauma, reduce pain, and accelerate recovery of bowel function and general mobility after surgery. All of these factors have the potential to shorten the length of hospital stay and reduce patient suffering.
Since Kitano et al[22] first reported laparoscopic-assisted gastrectomy for early gastric cancer in 1994, laparoscopic gastric cancer surgery has developed rapidly, especially in East Asian countries with a high incidence of gastric cancer, such as China, Japan, and Korea. Despite the rapid development of laparoscopic gastric cancer surgery, the clinical issues surrounding laparoscopic gastric cancer surgery still require more solid medical evidence, mainly due to insufficient evidence of its long-term oncologic efficacy and the optimal extent of lymph node dissection[23].
The KLASS-02-Randomized Clinical Trial of Korean[24] followed and observed 1050 patients in terms of the 3-year relapse-free survival rate. A total of 492 patients underwent laparoscopic surgery and 482 patients underwent open surgery. The 3-year relapse-free survival rate of the laparoscopy group was 80.3%, and this rate of the open group was 81.3%. It was concluded that for patients with locally advanced gastric cancer, the recurrence-free survival rate of laparoscopic distal gastrectomy combined with D2 lymphadenectomy is similar to that of open surgery.
The Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group established the largest multicenter cohort of laparoscopic gastric cancer, the CLASS-01 Randomized Clinical Trial Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer[25]. This study showed that the 3-year disease-free survival rate of laparoscopic distal gastrectomy was not less than that of open distal gastrectomy.
These studies are inadequate and have limitations, such as geographical differences between the East and the West, but they provide a scientific basis and clinical experience for the promotion of laparoscopic gastric cancer surgery[24-32] (Table 1).
| Ref. | Study type | Comparison | Group | Endpoints | Results |
| Kim et al[31], KLASS-01-RCT, 2019 | Randomized clinical trial | Laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer | LADG (n = 706); ODG (n = 711) | 5-yr overall survival rate and 5-yr cancer-specific survival rate | No significant difference between the two groups in the 5-yr overall survival rate (94.2% vs 93.3%) or 5-yr cancer-specific survival rate (97.1% vs 97.2%) |
| Lee et al[30], KLASS-02-RCT, 2019 | Multicenter randomized controlled trial | Laparoscopic distal gastrectomy (LDG) vs open distal gastrectomy (ODG) for D2 lymphadenectomy | LADG (n = 526); ODG (n = 524) | Thirty-day morbidity, 90-d mortality, postoperative pain, and recovery | Laparoscopic distal gastrectomy was associated with a lower complication rate, faster recovery, and less pain (P < 0.05), and there was no significant difference in mean number of totally retrieved lymph nodes (46.6 vs 47.4, P = 0.451) |
| Hyung et al[24], KLASS-02-RCT, 2020 | Randomized clinical trial | Laparoscopic distal gastrectomy surgery vs open distal gastrectomy surgery for locally advanced gastric cancer | LADG (n = 492); ODG (n = 482) | 3-yr relapse-free survival rate | No significant difference between the two groups in the 3-yr relapse-free survival rate (80.3% vs 81.3%) |
| Yu et al[25], The CLASS-01 RCT, 2019 | Randomized clinical trial | Laparoscopic distal gastrectomy surgery vs open distal gastrectomy for early-stage gastric cancer | LADG (n = 519); ODG (n = 520) | 3-year disease-free survival rate | No significant difference between the two groups in 3-year disease-free survival rate (83.1% vs 85.2%) |
| Liu et al[27], The CLASS-02, 2020 | Multicenter randomized clinical trial | Laparoscopic total gastrectomy (LTG) vs open total gastrectomy (OTG) for patients with clinical stage I gastric cancer | LTG (n = 105); OTG (n = 109) | Morbidity and mortality within 30 d following surgeries; recovery courses; postoperative hospital stays | No significant difference in morbidity and mortality within 30 d following surgeries |
| Katai et al[26], JCOG0912, 2020 | A multicenter, non-inferiority, phase 3 randomized controlled trial | Laparoscopy-assisted distal gastrectomy (LADG) vs open distal gastrectomy (ODG) for patients with clinical stage I gastric cancer | LADG (n = 462); ODG (n = 459) | Relapse-free survival | LADG was non-inferior to ODG for relapse-free survival (94% vs 95.1%, P < 0.05), and LADG should be considered a standard treatment option |
| Kinoshitaet al[28], LOC-A Study, 2019 | Multicenter cohort study | Laparoscopic gastrectomy (LC) vs open gastrectomy (OP) for locally advanced gastric cancer | LC (n = 305); Op (n = 305) | 5-yr overall survival; recurrence rate; hazard ratio for recurrence (HR) | No significant difference between the two groups in the 5-yr overall survival (53.0% vs 54.2%) and recurrence rate (30.8% vs 29.8%) |
| Park et al[29], COACT 1001, 2018 | Randomized phase II multicenter clinical trial | Laparoscopy-assisted distal gastrectomy (LADG) with D2 lymph node dissection vs open distal gastrectomy (ODG) for the treatment of advanced gastric cancer | LADG (n = 105); ODG (n = 99) | Noncompliance rate of the lymph node dissection; 3-yr disease-free survival (DFS), 5-yr overall survival, complications, and surgical stress response | No significant difference between the two groups in the noncompliance rate of lymph node dissection (47.0% vs 43.2%) and 3-yr DFS (80.1% vs 81.9%) |
Surgery is the main treatment for colorectal cancer, and minimally invasive surgery is the mainstream developmental direction of surgery in recent years. Laparoscopic colorectal cancer surgery has become the standard technique for the treatment of colon cancer in many countries around the world and has been shown to be safe and feasible in randomized trials and population-based studies due to its short-term efficacy[33-44]. However, more evidence is needed to determine its long-term efficacy, especially for advanced colorectal tumors[45] (Table 2).
| Ref. | Study type | Comparison | Group | Endpoints | Results |
| Bonjer et al[44], 2015 | Randomized clinical trial | Laparoscopic vs open surgery for rectal cancer | LC (n = 699); OP (n = 345) | Locoregional recurrence 3 yr after index surgery, and disease-free and overall survival | No significant difference between the two groups in locoregional recurrence 3 yr after index surgery, or disease-free and overall survival (86.7% vs 83.6%) |
| Fleshman et al[43], ACOSOG Z6051 Randomized Controlled Trial, 2019 | Randomized clinical trial | Laparoscopic-assisted resection vs open resection of stage II or III rectal cancer | LC (n = 243); OP (n = 243) | Disease-free survival and local recurrence | No significant difference between the two groups in disease-free survival and local recurrence |
| Park et al[39], 2020 | Multicenter comparative study | Laparoscopic vs open surgery for small T4 colon cancer | LC (n = 149); OP (n = 300) | Blood loss, length of hospital stay, postoperative morbidity, and overall survival or disease-free survival | No significant difference between the two groups in overall survival or disease-free survival, and LC was associated with favorable short-term oncologic outcomes in patients with tumors ≤ 4.0 cm |
| Li et al[40], 2021 | Multicenter comparative study | Laparoscopic vs open surgery for transverse colon cancer | LC (n = 181); OP (n = 235) | Operation time, postoperative hospitalization, lymph node retrieval, 5-yr overall survival | LC was associated with statistically longer operation time (209.96 vs 173.31 min, P = 0.002) and shorter postoperative hospitalization (12.05 vs 14.44 d, P = 0.001), but there was no significant difference in lymph node retrieval and 5-yr overall survival |
| Garbarino et al[42], 2021 | Propensity score-matched analysis | Laparoscopic vs open surgery for rectal resection | LC (n = 181); OP (n = 2 35) | Operative time, postoperative morbidity, hospital stay, safe oncological adequateness | LC was associated with shorter hospital stay (P < 0.001), but there was no significant difference in safe oncological adequateness |
The operation for rectal cancer is very complicated and is related to the accessibility of the pelvis and its complex anatomical structure. The surgical treatment of rectal cancer has a greater technical challenge than colon cancer, mainly due to the anatomical limitations of the pelvis and the protection by the pelvic plexus[46]. However, laparoscopic surgery has significant advantages compared to open surgery. Although most studies show no difference in short- and long-term outcomes between laparoscopic and open surgery for rectal cancer, it is still a debated issue. Some studies suggest that the long-term efficacy of laparoscopic rectal cancer resection is yet to be determined and is not superior to that of open surgery[47]. In general, an increasing number of studies have confirmed the efficacy and advantages of laparoscopy in colorectal cancer surgery, and it has been widely used.
Minimally invasive laparoscopic techniques are now rapidly gaining popularity, but conventional laparoscopy provides only a two-dimensional (2D) view. Three-dimensional (3D) laparoscopy overcomes this disadvantage and offers the advantage of a greater field of view[48]. Some studies have shown that 3D laparoscopic surgery provides better depth perception, significantly reduces the operative time and intraoperative blood loss, and shortens the surgeon's learning curve[48-51]. However, there is a lack of prospective evidence on the safety and efficacy of 3D technology in the long term. Despite the controversy, the benefits of 3D laparoscopy are undeniable and it has a promising future.
To overcome the shortcomings of laparoscopic techniques, especially when working in confined spaces such as the pelvis, da Vinci robotic surgery system robots, which are precise, stable, and flexible and can be operated remotely and gradually, are becoming a new option for minimally invasive surgery. The da Vinci robotic surgery system developed by the US Intuitive Surgical Company received US FDA marketing approval in July 2000 and began to be used in clinical applications. In 2002, Weber et al[52] reported the first robotic system-assisted surgery for benign colonic disease, and in the same year, Hashizume et al[53] also reported robotic colorectal surgery for malignant disease. With the development of the technology, it has been widely used in gastrointestinal surgery, hepatobiliary surgery, urology, gynecology, etc.[54]. However, the high cost and a lack of evidence of efficacy are limitations.
Despite the lack of more robust multicenter evidence, robotic surgery has been increasingly used as a minimally invasive means for treating gastric cancer because of the potential surgical advantages that it may have over conventional laparoscopy. However, Kim et al[55] showed that there was no significant difference between the two in terms of surgical blood loss, number of intermediate openings, time to oral feeding, or the length of hospital stay. At the same time, Uyama et al[56] showed that robotic gastric cancer surgery is safe and effective for stage I/II gastric cancer and can reduce the incidence of early postoperative complications compared to laparoscopic surgery. The short-term efficacy of robotic gastric cancer surgery is therefore good, but more evidence is still needed to prove it.
Current evidence suggests that the short-term efficacy of robotic-assisted colorectal cancer surgery is good and it may have potential minimally invasive advantages[57-61]. Robotic surgery for rectal cancer has been promoted as an improved minimally invasive procedure due to the flexibility of the da Vinci robot for operating in confined spaces such as the pelvis. Some prospectively randomized studies have shown that the clinical outcomes of robotic surgical resection of rectal cancer are similar to those of laparoscopic and open surgery[62-69]. There is also literature confirming that robotic rectal cancer surgery is closely associated with better short-term outcomes than laparoscopic surgery, and it has advantages in protecting the pelvic nerves, resulting in fewer short-term postoperative complications and shorter hospital stays[70,71].
Crippa et al[71] analyzed 600 patients. The number of patients undergoing robotic surgery was 317 (52.8%), and the laparoscopic group consisted of 283 (47.2%) patients. Both groups were similar in terms of age, sex, and body mass index (BMI). The overall incidence of short-term complications in patients undergoing robotic surgery was lower than that in the laparoscopic group (37.2% vs 51.2%; P < 0.001). However, larger prospectively randomized trials are needed to support its use. There is no denying that robotic flexibility may be more promising than laparoscopy in rectal cancer surgery.
The aim of minimal invasiveness is to reduce trauma. To avoid the need for an auxiliary abdominal incision, natural orifice specimen extraction surgery (NOSES) is a newly developed method that extracts specimens through natural orifices via the trans-anal or transvaginal route to reduce trauma and to avoid auxiliary abdominal incisions[72]. Trans-anal removal of specimens is mainly used for left-sided colectomies and rectal procedures, and transvaginal removal is used for all colonic procedures, especially right-sided colectomies and large specimens[73,74]. It is seldom used for operations on the stomach, but the study by Jeong et al[73] concluded that in carefully selected elderly women with early gastric cancer, transvaginal specimen collection may be a safe and feasible procedure.
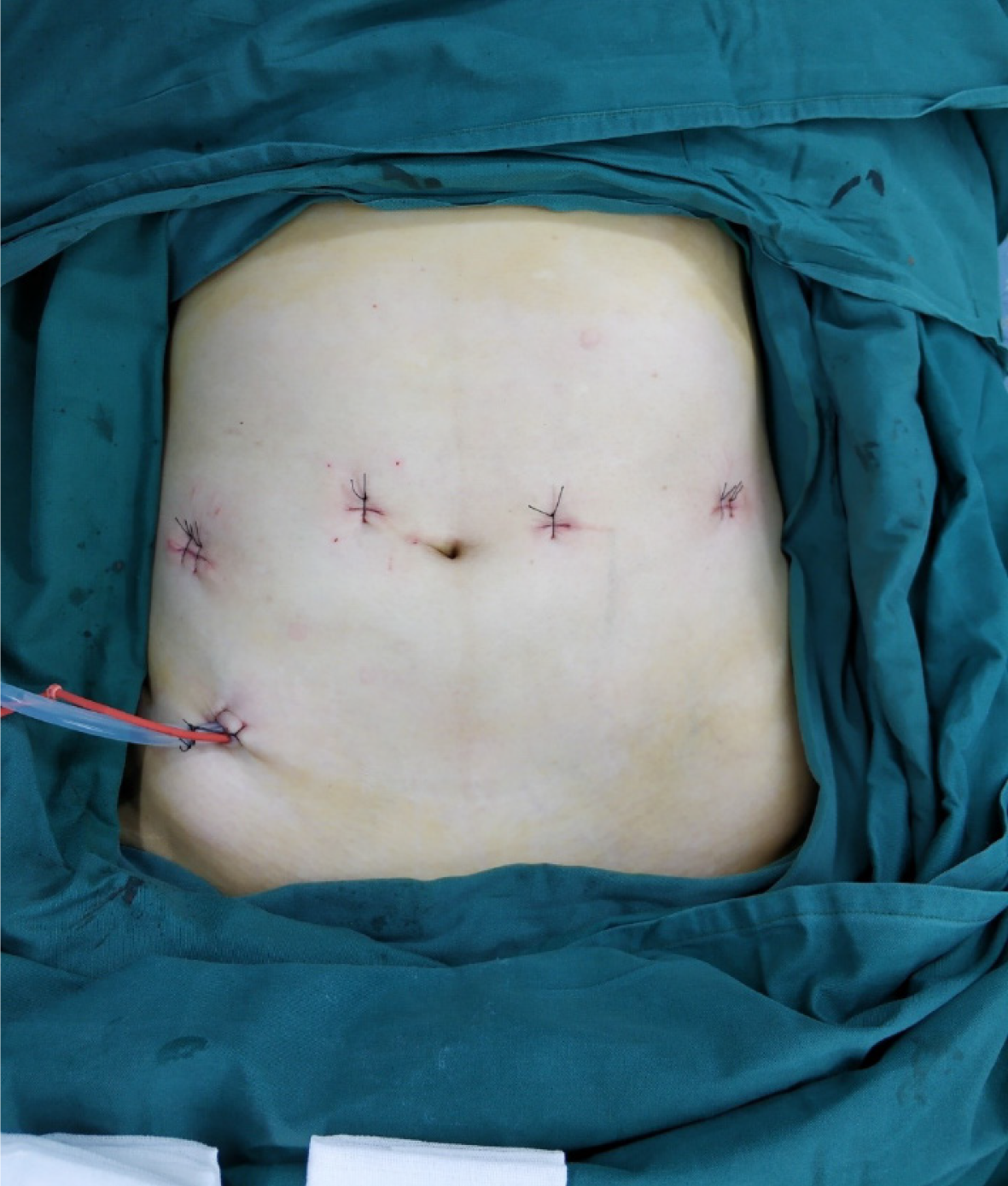
The NOSES technique is currently used mainly in colorectal cancer surgery, especially rectal surgery. Many studies have shown that the NOSES technique is safe and feasible for colorectal cancer; although it may increase the probability of contamination of the surgical area, this does not appear to translate into a higher incidence of infection[75-81]. Colorectal resection with NOSES is more advantageous in terms of postoperative recovery, postoperative pain, esthetics, and complications (Figure 1). However, not every patient is suitable for NOSES. Patients with stage T4 tumors and large tumors should not undergo NOSES. Trans-anal specimens are suitable for both men and women, but the tumor size should be less than 3 cm, whereas transvaginal specimens are suitable for women and the tumor size should be no larger than 5 cm. In addition, the BMI of the patient should be less than 30 kg/m2 for anal specimens and less than 35 kg/m2 for transvaginal specimens[82,83]. Hence, the NOSES procedure indications should be strictly observed.
Advances in minimally invasive techniques have opened a new era in gastrointestinal treatment. For gastrointestinal tumors, the most important treatment is surgical resection. However, it is often overlooked that early-stage gastrointestinal cancer can be treated endoscopically with a good result. To obtain the best prognosis and minimal trauma, it is very important to choose an appropriate surgical method.
For advanced tumors, total resection including regional lymph nodes should be performed. The emergence of laparoscopic surgery has brought innovation to minimally invasive surgery. As laparoscopic techniques continue to mature and surgeons become more skilled, surgeons can do even more with a laparoscopic view. There are many reported studies showing that the efficacy of total laparoscopic surgery is positive; completely laparoscopic surgery reduces the size of the secondary incision and reduces trauma[84-90].
A 3D laparoscopic imaging system is a further improvement on conventional laparoscopic techniques, and with improved laparoscopic views, it may help to shorten the learning curve of surgeons. Laparoscopic surgery has been recognized in the early treatment of gastrointestinal cancer, and its use in the treatment of most advanced tumors has also been affirmed. We look forward to international multicenter research evidence.
To improve the inadequacy of laparoscopic techniques, especially when operating in a narrow space, such as the pelvis and at the superior margin of the pancreas, surgeons started using robotic surgery systems. Among them, the da Vinci robot surgery system is used most often, and its technology is relatively mature, which offers the advantages of anti-shaking, three-dimensional vision, and operational flexibility, taking minimally invasive surgery to new levels of precision[91,92]. At present, the research on da Vinci robots is mainly retrospective. From the results, some short-term curative effects are better than those of laparoscopy, and the long-term curative effect is equivalent. However, these results need further confirmation in randomized clinical trial results. Additionally, one of the greatest drawbacks of the da Vinci robotic surgical system is its cost. The da Vinci surgical system is the only surgical robot available on the market today, and it has a high upfront cost. At the same time, surgeons need to go through a long learning curve to use the robotic system, meaning that the da Vinci system costs considerable time and money upfront, which is a major reason for its need for further development. Hence, before large-scale randomized clinical trial research is confirmed, we recommend that gastrointestinal surgery with rich experience in laparoscopy be carried out.
The future of surgical robots will move toward miniaturization and intelligence, and with the maturity of 5G technology, artificial intelligence technology and 5G technology have the potential to be combined with robotic surgical systems to help surgeons operate remotely, improve medical conditions, reduce healthcare costs, and benefit more patients.
In summary, minimally invasive surgery is the goal of surgeons. Combined with our experience, robotic surgery systems may be used increasingly widely. As interest and research in minimally invasive surgery continue to grow, the role of minimally invasive techniques in gastrointestinal surgery will become increasingly important.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cazorla E S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Li JH
| 1. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1323] [Article Influence: 330.8] [Reference Citation Analysis (2)] |
| 2. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 926] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 3. | Hatta W, Gotoda T, Koike T, Masamune A. History and future perspectives in Japanese guidelines for endoscopic resection of early gastric cancer. Dig Endosc. 2020;32:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, Kim DH, Lee JH, Kim MY, Kim BS, Oh ST, Yook JH, Jang SJ, Yun SC, Kim SO, Kim JH. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011;73:942-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Choi IJ, Lee JH, Kim YI, Kim CG, Cho SJ, Lee JY, Ryu KW, Nam BH, Kook MC, Kim YW. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc. 2015;81:333-41.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc. 2014;6:555-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (2)] |
| 7. | Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 8. | Shahidi N, Bourke MJ. ESD, not EMR, should be the first-line therapy for early gastric neoplasia. Gut. 2020;69:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Smyth E, Schöder H, Strong VE, Capanu M, Kelsen DP, Coit DG, Shah MA. A prospective evaluation of the utility of 2-deoxy-2-[(18) F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer. 2012;118:5481-5488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Hassan I, Wise PE, Margolin DA, Fleshman JW. The Role of Transanal Surgery in the Management of T1 Rectal Cancers. J Gastrointest Surg. 2015;19:1704-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Aepli P, Criblez D, Baumeler S, Borovicka J, Frei R. Endoscopic full thickness resection (EFTR) of colorectal neoplasms with the Full Thickness Resection Device (FTRD): Clinical experience from two tertiary referral centers in Switzerland. United European Gastroenterol J. 2018;6:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 764] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 13. | Moss A, Nalankilli K. Completing the circle of informed consent for EMR versus surgery for nonmalignant large or complex colorectal polyps. Gastrointest Endosc. 2016;84:304-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Keswani RN, Law R, Ciolino JD, Lo AA, Gluskin AB, Bentrem DJ, Komanduri S, Pacheco JA, Grande D, Thompson WK. Adverse events after surgery for nonmalignant colon polyps are common and associated with increased length of stay and costs. Gastrointest Endosc. 2016;84:296-303.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Backes Y, Schwartz MP, Ter Borg F, Wolfhagen FHJ, Groen JN, de Vos Tot Nederveen Cappel WH, van Bergeijk J, Geesing JMJ, Spanier BWM, Didden P, Vleggaar FP, Lacle MM, Elias SG, Moons LMG; Dutch T1 CRC Working Group. Multicentre prospective evaluation of real-time optical diagnosis of T1 colorectal cancer in large non-pedunculated colorectal polyps using narrow band imaging (the OPTICAL study). Gut. 2019;68:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, Saunders BP, Rex DK, Soetikno RM. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013;78:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 17. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 400] [Article Influence: 44.4] [Reference Citation Analysis (1)] |
| 18. | van de Ven SEM, Backes Y, Hilbink M, Seerden TCJ, Kessels K, de Vos Tot Nederveen Cappel WH, Groen JN, Wolfhagen FHJ, Geesing JMJ, Borg FT, van Bergeijk J, Spanier BWM, Mundt MW, Pullens HJM, Boonstra JJ, Opsteeg B, van Lent AUG, Schrauwen RWM, Laclé MM, Moons LMG, Terhaar Sive Droste JS; Dutch T1 CRC Working Group. Periprocedural adverse events after endoscopic resection of T1 colorectal carcinomas. Gastrointest Endosc. 2020;91:142-152.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Kuellmer A, Mueller J, Caca K, Aepli P, Albers D, Schumacher B, Glitsch A, Schäfer C, Wallstabe I, Hofmann C, Erhardt A, Meier B, Bettinger D, Thimme R, Schmidt A; FTRD study group. Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc. 2019;89:1180-1189.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 20. | Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 296] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 21. | Dang H, de Vos Tot Nederveen Cappel WH, van der Zwaan SMS, van den Akker-van Marle ME, van Westreenen HL, Backes Y, Moons LMG, Holman FA, Peeters KCMJ, van der Kraan J, Langers AMJ, Lijfering WM, Hardwick JCH, Boonstra JJ. Quality of life and fear of cancer recurrence in T1 colorectal cancer patients treated with endoscopic or surgical tumor resection. Gastrointest Endosc. 2019;89:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [PubMed] |
| 23. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2809] [Article Influence: 561.8] [Reference Citation Analysis (5)] |
| 24. | Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kim MC, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Han SU; Korean Laparoendoscopic Gastrointestinal Surgery Study Group. Long-Term Outcomes of Laparoscopic Distal Gastrectomy for Locally Advanced Gastric Cancer: The KLASS-02-RCT Randomized Clinical Trial. J Clin Oncol. 2020;38:3304-3313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 263] [Article Influence: 52.6] [Reference Citation Analysis (1)] |
| 25. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 522] [Article Influence: 87.0] [Reference Citation Analysis (1)] |
| 26. | Katai H, Mizusawa J, Katayama H, Morita S, Yamada T, Bando E, Ito S, Takagi M, Takagane A, Teshima S, Koeda K, Nunobe S, Yoshikawa T, Terashima M, Sasako M. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 27. | Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, Du X, Huang H, Hu J, Li G, Yu P, Li Y, Suo J, Zhao N, Zhang W, Li H, He H, Sun Y; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol. 2020;6:1590-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 28. | Kinoshita T, Uyama I, Terashima M, Noshiro H, Nagai E, Obama K, Tamamori Y, Nabae T, Honda M, Abe T; LOC-A Study Group. Long-term Outcomes of Laparoscopic Versus Open Surgery for Clinical Stage II/III Gastric Cancer: A Multicenter Cohort Study in Japan (LOC-A Study). Ann Surg. 2019;269:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 29. | Park YK, Yoon HM, Kim YW, Park JY, Ryu KW, Lee YJ, Jeong O, Yoon KY, Lee JH, Lee SE, Yu W, Jeong SH, Kim T, Kim S, Nam BH; COACT group. Laparoscopy-assisted versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: Results From a Randomized Phase II Multicenter Clinical Trial (COACT 1001). Ann Surg. 2018;267:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 30. | Lee HJ, Hyung WJ, Yang HK, Han SU, Park YK, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Kim MC; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg. 2019;270:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 334] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 31. | Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Hyung WJ; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-term Survival Among Patients With Stage I Gastric Cancer: The KLASS-01 Randomized Clinical Trial. JAMA Oncol. 2019;5:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 374] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 32. | Hyung WJ, Yang HK, Han SU, Lee YJ, Park JM, Kim JJ, Kwon OK, Kong SH, Kim HI, Lee HJ, Kim W, Ryu SW, Jin SH, Oh SJ, Ryu KW, Kim MC, Ahn HS, Park YK, Kim YH, Hwang SH, Kim JW, Cho GS. A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer. 2019;22:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 33. | Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy AM; COlon cancer Laparoscopic or Open Resection Study Group (COLOR). Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1677] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 34. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM; MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2292] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 35. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 2976] [Article Influence: 496.0] [Reference Citation Analysis (3)] |
| 36. | Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 737] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 37. | Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Nelson H; Clinical Outcomes of Surgical Therapy Study Group. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655-62; discussion 662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 798] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 38. | Bagshaw PF, Allardyce RA, Frampton CM, Frizelle FA, Hewett PJ, McMurrick PJ, Rieger NA, Smith JS, Solomon MJ, Stevenson AR; Australasian Laparoscopic Colon Cancer Study Group. Long-term outcomes of the australasian randomized clinical trial comparing laparoscopic and conventional open surgical treatments for colon cancer: the Australasian Laparoscopic Colon Cancer Study trial. Ann Surg. 2012;256:915-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 39. | Park SS, Lee JS, Park HC, Park SC, Sohn DK, Oh JH, Han KS, Lee DW, Lee DE, Kang SB, Park KJ, Jeong SY; Seoul Colorectal Research Group (SECOG). Favorable short-term oncologic outcomes following laparoscopic surgery for small T4 colon cancer: a multicenter comparative study. World J Surg Oncol. 2020;18:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Li Z, Zou Z, Lang Z, Sun Y, Zhang X, Dai M, Mao S, Han Z. Laparoscopic versus open radical resection for transverse colon cancer: evidence from multi-center databases. Surg Endosc. 2021;35:1435-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Hewett PJ, Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Rieger NA, Smith JS, Solomon MJ, Stephens JH, Stevenson AR. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg. 2008;248:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 42. | Garbarino GM, Canali G, Tarantino G, Costa G, Ferri M, Balducci G, Pilozzi E, Berardi G, Mercantini P. Laparoscopic versus open rectal resection: a 1:2 propensity score-matched analysis of oncological adequateness, short- and long-term outcomes. Int J Colorectal Dis. 2021;36:801-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, Peters WR Jr, Maun DC, Chang GJ, Herline A, Fichera A, Mutch MG, Wexner SD, Whiteford MH, Marks J, Birnbaum E, Margolin DA, Larson DW, Marcello PW, Posner MC, Read TE, Monson JRT, Wren SM, Pisters PWT, Nelson H. Disease-free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer: Follow-up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann Surg. 2019;269:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 44. | Bonjer HJ, Deijen CL, Haglind E; COLOR II Study Group. A Randomized Trial of Laparoscopic versus Open Surgery for Rectal Cancer. N Engl J Med. 2015;373:194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 45. | Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 821] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 46. | van der Sijp MP, Bastiaannet E, Mesker WE, van der Geest LG, Breugom AJ, Steup WH, Marinelli AW, Tseng LN, Tollenaar RA, van de Velde CJ, Dekker JW. Differences between colon and rectal cancer in complications, short-term survival and recurrences. Int J Colorectal Dis. 2016;31:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 47. | Cleary RK, Morris AM, Chang GJ, Halverson AL. Controversies in Surgical Oncology: Does the Minimally Invasive Approach for Rectal Cancer Provide Equivalent Oncologic Outcomes Compared with the Open Approach? Ann Surg Oncol. 2018;25:3587-3595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Zheng CH, Lu J, Zheng HL, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Huang CM. Comparison of 3D laparoscopic gastrectomy with a 2D procedure for gastric cancer: A phase 3 randomized controlled trial. Surgery. 2018;163:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Storz P, Buess GF, Kunert W, Kirschniak A. 3D HD versus 2D HD: surgical task efficiency in standardised phantom tasks. Surg Endosc. 2012;26:1454-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 50. | Kanaji S, Suzuki S, Harada H, Nishi M, Yamamoto M, Matsuda T, Oshikiri T, Nakamura T, Fujino Y, Tominaga M, Kakeji Y. Comparison of two- and three-dimensional display for performance of laparoscopic total gastrectomy for gastric cancer. Langenbecks Arch Surg. 2017;402:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Chiu CJ, Lobo Prabhu K, Tan-Tam CC, Panton ON, Meneghetti A. Using three-dimensional laparoscopy as a novel training tool for novice trainees compared with two-dimensional laparoscopy. Am J Surg. 2015;209:824-827.e1; discussion 827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Weber PA, Merola S, Wasielewski A, Ballantyne GH. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum. 2002;45:1689-94; discussion 1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 323] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 53. | Hashizume M, Shimada M, Tomikawa M, Ikeda Y, Takahashi I, Abe R, Koga F, Gotoh N, Konishi K, Maehara S, Sugimachi K. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc. 2002;16:1187-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 54. | Braumann C, Jacobi CA, Menenakos C, Ismail M, Rueckert JC, Mueller JM. Robotic-assisted laparoscopic and thoracoscopic surgery with the da Vinci system: a 4-year experience in a single institution. Surg Laparosc Endosc Percutan Tech. 2008;18:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, Park JM, An JY, Kim MC, Park S, Song KY, Oh SJ, Kong SH, Suh BJ, Yang DH, Ha TK, Kim YN, Hyung WJ. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg. 2016;263:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 56. | Uyama I, Suda K, Nakauchi M, Kinoshita T, Noshiro H, Takiguchi S, Ehara K, Obama K, Kuwabara S, Okabe H, Terashima M. Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: a multi-institutional prospective single-arm study. Gastric Cancer. 2019;22:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 57. | Zarak A, Castillo A, Kichler K, de la Cruz L, Tamariz L, Kaza S. Robotic versus laparoscopic surgery for colonic disease: a meta-analysis of postoperative variables. Surg Endosc. 2015;29:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | deSouza AL, Prasad LM, Park JJ, Marecik SJ, Blumetti J, Abcarian H. Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum. 2010;53:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 59. | Xu H, Li J, Sun Y, Li Z, Zhen Y, Wang B, Xu Z. Robotic versus laparoscopic right colectomy: a meta-analysis. World J Surg Oncol. 2014;12:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 60. | Solaini L, Bazzocchi F, Cavaliere D, Avanzolini A, Cucchetti A, Ercolani G. Robotic versus laparoscopic right colectomy: an updated systematic review and meta-analysis. Surg Endosc. 2018;32:1104-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 61. | Cheng CL, Rezac C. The role of robotics in colorectal surgery. BMJ. 2018;360:j5304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | Feroci F, Vannucchi A, Bianchi PP, Cantafio S, Garzi A, Formisano G, Scatizzi M. Total mesorectal excision for mid and low rectal cancer: Laparoscopic vs robotic surgery. World J Gastroenterol. 2016;22:3602-3610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 63. | Park JS, Choi GS, Lim KH, Jang YS, Jun SH. S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc. 2011;25:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 64. | D'Annibale A, Pernazza G, Monsellato I, Pende V, Lucandri G, Mazzocchi P, Alfano G. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc. 2013;27:1887-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 65. | Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol. 2012;19:2485-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 264] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 66. | Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, Kim H. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol. 2009;16:1480-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 67. | Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam BH, Sohn DK, Oh JH. Robot-assisted Versus Laparoscopic Surgery for Rectal Cancer: A Phase II Open Label Prospective Randomized Controlled Trial. Ann Surg. 2018;267:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 68. | Kang J, Yoon KJ, Min BS, Hur H, Baik SH, Kim NK, Lee KY. The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison--open, laparoscopic, and robotic surgery. Ann Surg. 2013;257:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 69. | Ye SP, Zhu WQ, Liu DN, Lei X, Jiang QG, Hu HM, Tang B, He PH, Gao GM, Tang HC, Shi J, Li TY. Robotic- vs laparoscopic-assisted proctectomy for locally advanced rectal cancer based on propensity score matching: Short-term outcomes at a colorectal center in China. World J Gastrointest Oncol. 2020;12:424-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP. Short-term clinical outcome of robot-assisted intersphincteric resection for low rectal cancer: a retrospective comparison with conventional laparoscopy. Surg Endosc. 2013;27:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 71. | Crippa J, Grass F, Dozois EJ, Mathis KL, Merchea A, Colibaseanu DT, Kelley SR, Larson DW. Robotic Surgery for Rectal Cancer Provides Advantageous Outcomes Over Laparoscopic Approach: Results From a Large Retrospective Cohort. Ann Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 72. | Palanivelu C, Rangarajan M, Jategaonkar PA, Anand NV. An innovative technique for colorectal specimen retrieval: a new era of "natural orifice specimen extraction" (N.O.S.E). Dis Colon Rectum. 2008;51:1120-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 73. | Jeong SH, Lee YJ, Choi WJ, Paik WY, Jeong CY, Park ST, Choi SK, Hong SC, Jung EJ, Joo YT, Ha WS. Trans-vaginal specimen extraction following totally laparoscopic subtotal gastrectomy in early gastric cancer. Gastric Cancer. 2011;14:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Zeng WG, Zhou ZX. Mini-invasive surgery for colorectal cancer. Chin J Cancer. 2014;33:277-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Zhuang CL, Zhang FM, Wang Z, Jiang X, Wang F, Liu ZC. Precision functional sphincter-preserving surgery (PPS) for ultralow rectal cancer: a natural orifice specimen extraction (NOSE) surgery technique. Surg Endosc. 2021;35:476-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | He J, Hu JF, Shao SX, Yao HB, Zhang XF, Yang GG, Shen Z. The Comparison of Laparoscopic Colorectal Resection with Natural Orifice Specimen Extraction versus Mini-Laparotomy Specimen Extraction for Colorectal Tumours: A Systematic Review and Meta-Analysis of Short-Term Outcomes. J Oncol. 2020;2020:6204264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 77. | Zhou S, Wang X, Zhao C, Zhou H, Pei W, Liang J, Zhou Z. Can transanal natural orifice specimen extraction after laparoscopic anterior resection for colorectal cancer reduce the inflammatory response? J Gastroenterol Hepatol. 2020;35:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Chang SC, Chen HC, Chen YC, Ke TW, Tsai YY, Wang HM, Fingerhut A, Chen WT. Long-term Oncologic Outcomes of Laparoscopic Anterior Resections for Cancer with Natural Orifice Versus Conventional Specimen Extraction: A Case-Control Study. Dis Colon Rectum. 2020;63:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Costantino FA, Diana M, Wall J, Leroy J, Mutter D, Marescaux J. Prospective evaluation of peritoneal fluid contamination following transabdominal vs. transanal specimen extraction in laparoscopic left-sided colorectal resections. Surg Endosc. 2012;26:1495-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 80. | Zhou S, Wang X, Zhao C, Pei W, Zhou H, Liu Q, Liang J, Zhou Z. Comparison of short-term and survival outcomes for transanal natural orifice specimen extraction with conventional mini-laparotomy after laparoscopic anterior resection for colorectal cancer. Cancer Manag Res. 2019;11:5939-5948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 81. | Park JS, Choi GS, Kim HJ, Park SY, Jun SH. Natural orifice specimen extraction versus conventional laparoscopically assisted right hemicolectomy. Br J Surg. 2011;98:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 82. | Guan X, Liu Z, Longo A, Cai JC, Tzu-Liang Chen W, Chen LC, Chun HK, Manuel da Costa Pereira J, Efetov S, Escalante R, He QS, Hu JH, Kayaalp C, Kim SH, Khan JS, Kuo LJ, Nishimura A, Nogueira F, Okuda J, Saklani A, Shafik AA, Shen MY, Son JT, Song JM, Sun DH, Uehara K, Wang GY, Wei Y, Xiong ZG, Yao HL, Yu G, Yu SJ, Zhou HT, Lee SH, Tsarkov PV, Fu CG, Wang XS; International Alliance of NOSES. International consensus on natural orifice specimen extraction surgery (NOSES) for colorectal cancer. Gastroenterol Rep (Oxf). 2019;7:24-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 83. | Izquierdo KM, Unal E, Marks JH. Natural orifice specimen extraction in colorectal surgery: patient selection and perspectives. Clin Exp Gastroenterol. 2018;11:265-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 84. | Chen K, Pan Y, Cai JQ, Xu XW, Wu D, Yan JF, Chen RG, He Y, Mou YP. Intracorporeal esophagojejunostomy after totally laparoscopic total gastrectomy: A single-center 7-year experience. World J Gastroenterol. 2016;22:3432-3440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 85. | Xu X, Huang C, Mou Y, Zhang R, Pan Y, Chen K, Lu C. Intra-corporeal hand-sewn esophagojejunostomy is a safe and feasible procedure for totally laparoscopic total gastrectomy: short-term outcomes in 100 consecutive patients. Surg Endosc. 2018;32:2689-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Ko CS, Gong CS, Kim BS, Kim SO, Kim HS. Overlap method versus functional method for esophagojejunal reconstruction using totally laparoscopic total gastrectomy. Surg Endosc. 2021;35:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 87. | Kumagai K, Hiki N, Nunobe S, Sekikawa S, Chiba T, Kiyokawa T, Jiang X, Tanimura S, Sano T, Yamaguchi T. Totally laparoscopic pylorus-preserving gastrectomy for early gastric cancer in the middle stomach: technical report and surgical outcomes. Gastric Cancer. 2015;18:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 88. | Sun Z, Zheng X, Chen G, Wang L, Sang Q, Xu G, Zhang N, Aminbuhe. Technical details of and prognosis for the "China stitch", a novel technique for totally laparoscopic hand-sewn esophagojejunostomy. Biosci Trends. 2020;14:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Umemura A, Koeda K, Sasaki A, Fujiwara H, Kimura Y, Iwaya T, Akiyama Y, Wakabayashi G. Totally laparoscopic total gastrectomy for gastric cancer: literature review and comparison of the procedure of esophagojejunostomy. Asian J Surg. 2015;38:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 90. | Matsuda T, Iwasaki T, Mitsutsuji M, Hirata K, Tsugawa D, Sugita Y, Shimada E, Kakeji Y. A Simple and Reliable Method for Intracorporeal Circular-Stapled Esophagojejunostomy Using a Hand-Sewn Over-and-Over Suture Technique in Laparoscopic Total Gastrectomy. Ann Surg Oncol. 2015;22 Suppl 3:S355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 91. | Sharma NL, Shah NC, Neal DE. Robotic-assisted laparoscopic prostatectomy. Br J Cancer. 2009;101:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Gao C, Yang M, Wu Y, Wang G, Xiao C, Liu H, Lu C. Hybrid coronary revascularization by endoscopic robotic coronary artery bypass grafting on beating heart and stent placement. Ann Thorac Surg. 2009;87:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |