Published online Sep 27, 2021. doi: 10.4240/wjgs.v13.i9.923
Peer-review started: February 14, 2021
First decision: March 16, 2021
Revised: March 22, 2021
Accepted: August 6, 2021
Article in press: August 6, 2021
Published online: September 27, 2021
Processing time: 215 Days and 15.2 Hours
The onset and manifestations of irritable bowel syndrome (IBS) is associated with several factors, and the pathophysiology involves various central and peripheral mechanisms. Most studies indicate that the management of gut microbiota could significantly affect the improvement of subjective disorders in patients with IBS. Numerous clinical trials have assessed the efficacy of probiotics for IBS with controversial conclusions. Several clinical trials have suggested that probiotics can improve global IBS symptoms, while others only improve individual IBS symptoms, such as bloating scores and abdominal pain scores. Only a few clinical trials have found no apparent effect of probiotics on IBS symptoms. Generally, probiotics appear to be safe for patients with IBS. However, the question of which probiotics should be used for certain IBS subtypes remains unresolved. In everyday practice, the dose of the recommended probiotic remains questionable, as well as how long the probiotic should be used in therapy. The use of probiotics in the M subtype and non-classified IBS is particularly problematic, in which combination therapy should be recommended due to the change in symptoms. Therefore, new approaches are needed in the design of clinical studies that should address certain subtypes of IBS.
Core Tip: The onset and manifestations of irritable bowel syndrome (IBS) is associated with a number of factors, and the pathophysiology involves various central and peripheral mechanisms. The results of most studies indicate that influencing the gut microbiota could significantly affect the improvement of subjective disorders in patients with IBS. The most important open questions are the design of a clinical study in which the IBS subgroup is not initially defined and whether all IBS subtypes can be treated with the same probiotic or combination of probiotics. IBS subtype-designed clinical studies are urgently needed as a good foundation to define recommendations and guidelines for the use of probiotics in IBS.
- Citation: Benjak Horvat I, Gobin I, Kresović A, Hauser G. How can probiotic improve irritable bowel syndrome symptoms? World J Gastrointest Surg 2021; 13(9): 923-940
- URL: https://www.wjgnet.com/1948-9366/full/v13/i9/923.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i9.923
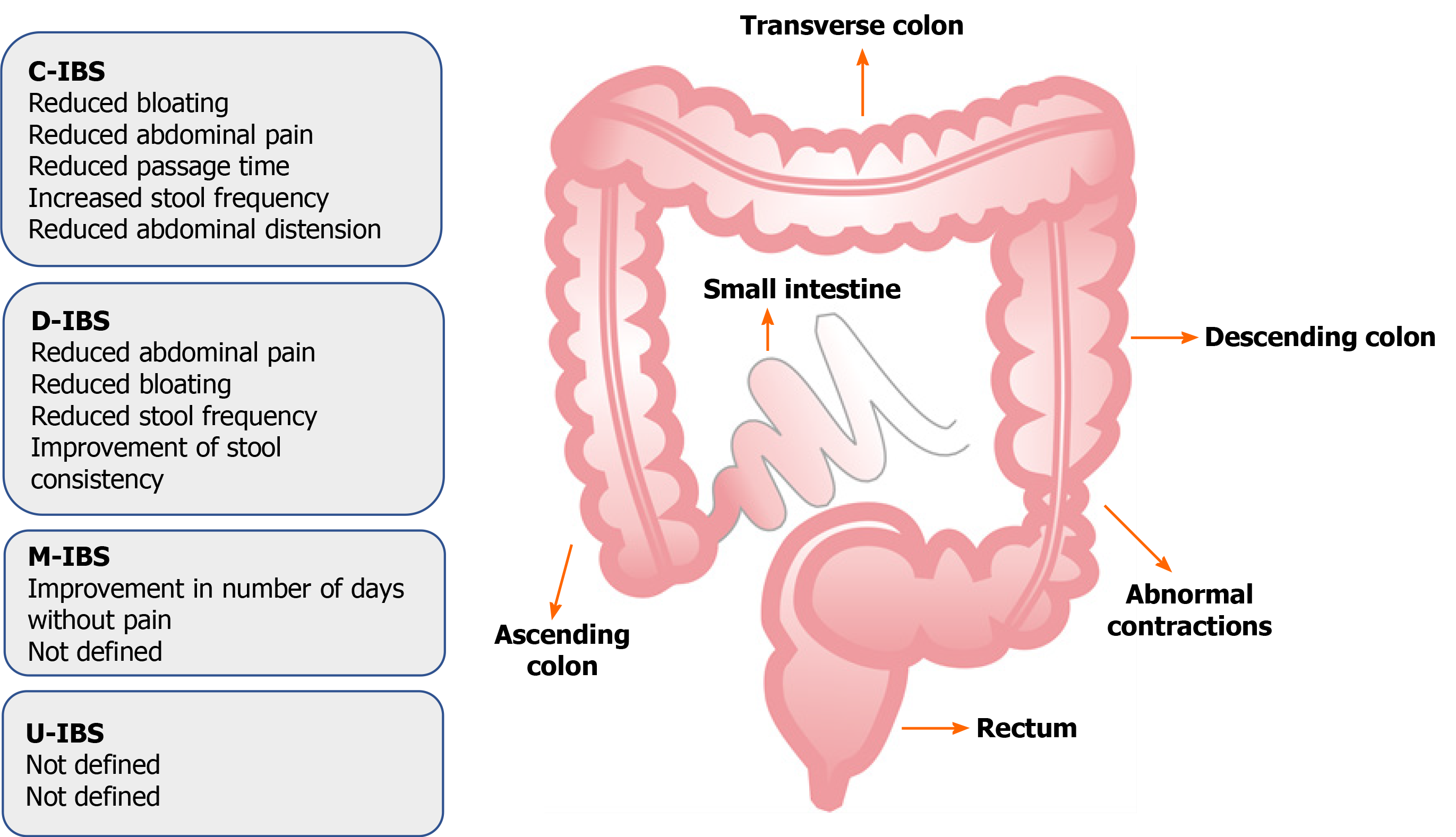
Irritable bowel syndrome (IBS) is a brain-gut disorder characterised by chronic abdominal pain and discomfort that involves a change in the bowel habits and includes the absence of an organic pathological process. Other related symptoms include abdominal distension, bloating, flatulence, diarrhoea, constipation, or a combination of two symptoms. According to these bowel habit patterns, the disease is divided into subtypes: C-IBS (IBS with predominant constipation), D-IBS (IBS with predominant diarrhoea), and M-IBS (IBS with mixed bowel habits) and U-IBS (IBS unclassified). Patients with U-IBS meet the diagnostic criteria for IBS, but bowel habits cannot be accurately categorised into the above explained three subtypes[1,2].
There are no objective tests used to diagnose the disease; therefore, diagnosis is based on symptoms taken as criteria for determining IBS. These symptoms were adopted in 1988 in Rome at the World Congress of Gastroenterologists and revised several times, and based on basic science research and clinical trials, Roman IV criteria were adopted and have been in force since 2016[3,4] (Table 1).
| Diagnostic criteria | Symptoms included in criteria |
| Rome 1 (1990) | Abdominal pain or discomfort relived with defecation; Abdominal pain or discomfort associated with a change in stool frequency or consistency; In addition, two or more of the following on at least 5% of occasions or days for 3 mo: (1) Altered stool frequency and form; (2) Altered stool passage; (3) Passage of mucus; and (4) Bloating or distension |
| Rome II (1999) | Abdominal discomfort or pain that has two or three features for 1 wk (need to be consecutive) in the last year; Relieved with defecation; Onset associated with a change in the frequency of stools; Onset associated with a change in the form of stools |
| Rome III (2006) | Recurrent abdominal pain or discomfort three days per month in the last 3 mo associated with two or more of: (1) Improvement in defecation; (2) Onset associated with a change in the frequency of stools; and (3) Onset associated with a change in the form of stools |
| Rome IV (2016) | Recurrent abdominal pain on average at least 1 d/wk in the last 3 mo, associated with 2 or more of the following1: (1) Related to defecation (i.e., either increasing or improving pain); (2) Associated with a change in stool frequency; and (3) Associated with a change in stool form (appearance) |
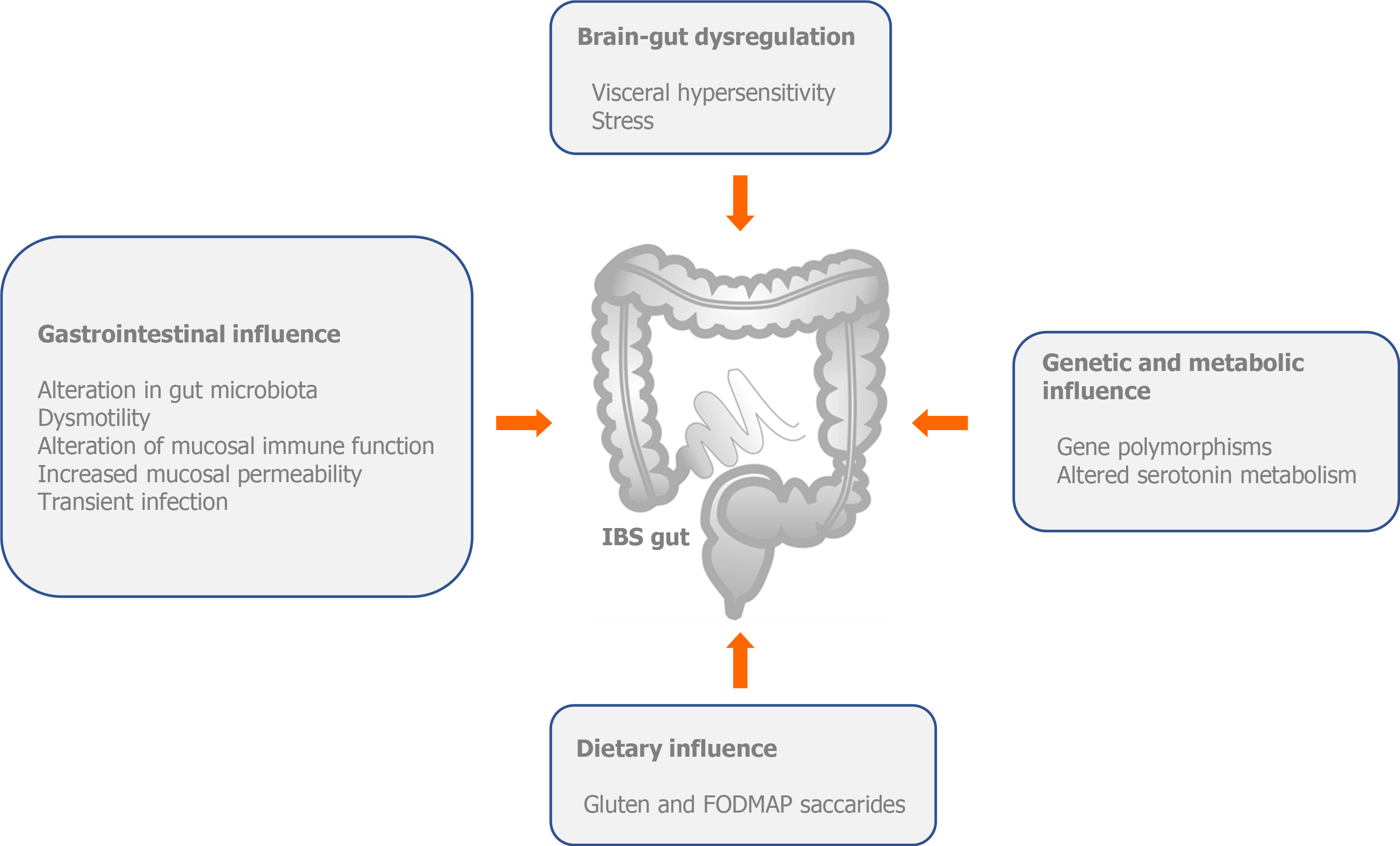
Although the pathophysiology of IBS has not been fully elucidated, nowdays, we can claim with certainty that IBS is an unexplained brain–gut disorder (Figure 1).
The pathophysiology of IBS includes central and peripheral mechanisms. Central mechanisms involve a number of factors, including genetic (mutation of SCN5A, which belongs to a family of genes that provide instructions for making sodium channels) and altered serotonin metabolism; alterations in brain-gut function (stress and visceral hypersensitivity) and dietary influence [gluten and fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs)]. Peripheral mechanisms involve changes in gastrointestinal motility, intestinal permeability, local immune response disorder, low-grade inflammation, disordered bile salt metabolism, post-infectious changes, chronic infections and disturbances in the intestinal microbiota[5,6] (Figure 1).
Intestinal microbiota has been associated with numerous syndromes and thus, with IBS; therefore, there is a growing interest in modulating the microbiota as one of the treatment options. Because microbiota is connected with the central nervous system across the axis referred to as the gut-brain axis, additional changes in this relationship are imposed as a major factor in the pathophysiology of IBS, which acts through central and peripheral mechanisms and metabolic products of microbes in the gastrointestinal system. This, in turn, causes an altered perception of visceral events, so the individual perceives them as hyperalgesia or allodynia[7-10].
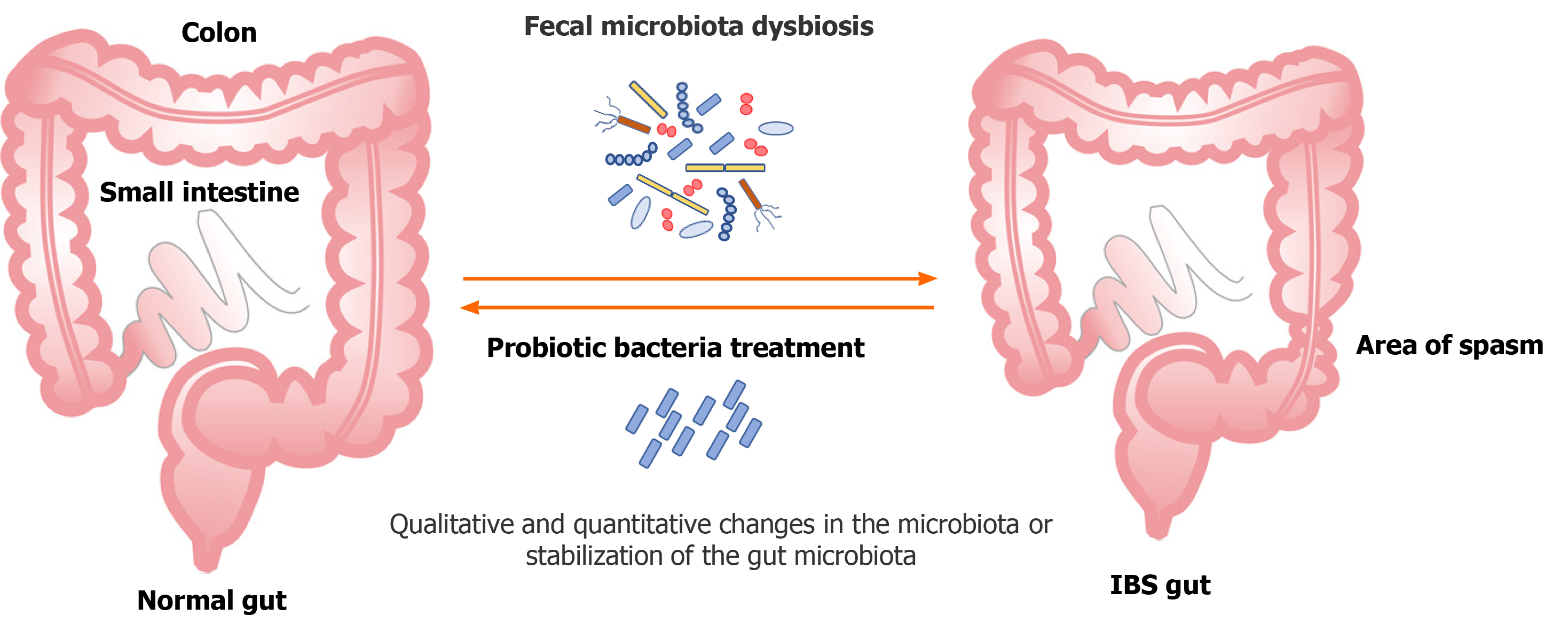
It is estimated that there are more than 100 trillion bacteria in the body of an adult; 80% of which are in the digestive system, which, in turn, contains more than 100 species of bacteria[11]. Bacteroidetes and Firmicutes predominate, and the amounts of Proteobacteria, Actinomyces, Fusobacterium and Verrucomicrobia are relatively small[12]. During life, and due to a number of environmental factors, the diversity and numerical proportion of individual strains change and there is a possibility that antibiotics and probiotics may affect the intestinal dysbiosis and microbial imbalance that may exist in IBS. Previous studies indicate a high percentage of dysbiosis in IBS patients compared to the general population[13,14]. Generally, the composition and activities of Lactobacillus and Bifidobacterium are heavily compromised in IBS patients[15]. Tap et al[16] reported that the severity of IBS was positively correlated with low microbial richness, absence of Methanobacteriales and the number of Bacteroides enterotypes. Pozuelo et al[17] found a lower abundance of butyrate-producing and methane-producing bacteria in IBS-D and IBS-M patients. Lower counts of methanogens may explain the symptoms of flatulence or excess gas in the abdomen. Dysbiosis in IBS patients is presented with an increase in abundance of Proteobacteria (Veillonella) and Firmicutes (Lactobacillus and Ruminococcus) and with decreased Bifidobacterium, Faecalibacterium, Erysipelotrichaceae and methanogens[18,19].
One of the approaches of treating IBS is the rationale use of probiotics due to their potential to correct dysbiosis (qualitative and quantitative changes in the gut microbiota) or stabilise the host microbiota (Figure 2).
There are more evidence and assumptions regarding how the gut microbiota is associated with IBS formation, either directly or indirectly. It is known that 10% of patients who develop some forms of IBS previously had an episode of infectious diarrhoea (postinfectious IBS), during which changes in the normal gut microbiota occur[20-22]. An association between broad-spectrum antibiotics and IBS is also described[23]. The microbiota interacts extensively with external factors, which occur due to some forms of diet[24].
Patients with IBS are more likely than healthy populations to develop depression and anxiety, and it is well known that gut microbiota even affects mood and behaviour in humans[25,26]. The microbiota is a separate variable and the axis is called the microbiota-brain axis. The most important communication pathway in this relationship is the tenth cerebral nerve, the vagus nerve. The observed benefits, which arose due to the ingestion of Lactobacillus rhamnosus (L. rhamnosus) JB-1, resulted in a reduction in anxiety and depression-like behaviour, disappeared after vagotomy in mice. At the brain level, probiotic-induced changes in GABA receptor (receptor for neurotransmitter gamma-aminobutyric acid) expression are also involved in the pathogenesis of anxiety and depression, and disappear in vagotomised mice[27].
After fecal transplantation of microbiota from depressed patients into animals, certain characteristics of depression began to manifest in the recipients (rodents), such as anhedonia and anxiety-like behaviour, and the door to a wide range of assumptions to be investigated opened[28]. An experiment was performed on healthy young students taking probiotic supplements and a reduction in cognitive response to sadness in the form of decreased aggressive thoughts was found after four weeks[29]. As a stress index in some experiments, cortisol level were considered a sign of stress, and levels decreased with improved emotional response in those taking probiotics[30]. These findings, as well as the results of many other studies in the field, were the inspiration for transferring this information to the model of patients with IBS, given the association of the gastrointestinal system, microbiota, brain, and neurotransmitters, which is formed, in part, depending on the composition of microorganisms in the intestine and disturbed axis in these patients.
The high ratio of Firmicutes:Bacteroides in patients with IBS correlates with depression and anxiety[31], and the result of an additional study shows that the use of prebiotics (defined as selectively fermented carbohydrate ingredients that cause specific changes in the composition and/or activity of the gut microbiota, and thus contribute to host health[32]) and galactooligosaccharides reduce anxiety for four weeks and has a positive effect on quality of life. Another study included the species Bifidobacterium longum (B. longum) and measured anxiety, depression, IBS symptoms, somatisation, and quality of life in the first, sixth, and tenth weeks. As early as the sixth week, subjects reported a reduction in depressive symptoms and improvement in quality of life, but there was no effect on IBS symptoms or anxiety. Functional magnetic resonance imaging showed a reduced response to negative emotional stimuli in multiple areas of the brain, including the amygdala and frontolimbic area. Decreased levels of methylamine and aromatic amino acid metabolites were found in the urine of these subjects[33]. Nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), hydrogen, methane, and ammonia may be of microbial origin and are normally created in our body, but also imported with various external factors, such as a red meat-enriched diet. H2S gas has been recognised as a neuromodulator/ neurotransmitter that influences intestinal inflammation and sensitivity and is a product of the intestinal microbiota. Therefore, it plays an important role in modulating visceral pain[34-39].
It has already been mentioned that there is evidence of direct modulation of several systems involved in visceral hypersensitivity; for example, via local expression of cannabinoid receptor type 2 and tryptophan hydrolase 1 isoform, and that patients with IBS, in whom hypersensitivity exists, have functional dysbiosis. Probiotics (Lactobacillus reuteri) directly alter the visceral perception of nociceptive stimulants[40]. Lactobacillus reuteri inhibits the autonomic nervous system response to colorectal distension in mice[41]. Only a few studies have been performed in humans to confirm these results in animal models. By importing unfermented dairy products containing Bifidobacterium animalis, Streptococcus thermophilus, Lactobacillus bulgaricus and Lactococcus lactis, the possibility of influencing the activity of areas of the brain, which control the central processing and processing of emotions and sensations, is opened[42].
There are many variables that affect the survival of gut microbiota, especially high oxygen, pH, salt and bile contents, which are all under the influence of intestinal motility.
The change in motility in patients with IBS is manifested by stronger and faster postprandial intestinal muscle contractions in IBS-D and faster passage through the gastrointestinal system and irregular luminal contractions. Bacteroides thetaiotaomicron has been shown to alter the expression of a gene involved in neurotransmission and smooth muscle function[43], Escherichia coli Nissle 1917[44] improves contractility of colon, and L. rhamnosus causes a disorder of contractility stimulated by acetilcolin[45]. Therefore, we can ask ourselves whether the import of probiotics or prebiotics could affect the above mentioned functions via bacteria already present in our bowels.
Recent findings suggest that probiotics have a good effect on the stabilisation of gut microbiota in patients with IBS[46] and modulation of the immune response in the form of normalisation of the interleukin (IL)-10/IL-12 ratio produced by mononuclear cells[47]. In patients with diarrhoeal disease, there are indications of disorders of the function of the intestinal mucosal barrier, which is measured by an increase in intestinal permeability. This leads to an increase in the number of T lymphocytes, mast cells, and enterochromaffin cells[48]. These changes indicate that IBS could have a low-grade inflammatory component in pathophysiology. Several sources report the ability of probiotics to modulate the innate and acquired immune responses with a tendency to achieve a balance between proinflammatory and anti-inflammatory cytokines[46]. A possible therapeutic option would be to use probiotics that interact with the host epithelium to resolve possible inflammation and preserve barrier function. It has been shown that, in adults, Lactobacillus gasseri KS-13, B. longum MM2 175, and Bifidobacterium bifidum G9-1 change the profile of cytokines by stimulating the production of less inflammatory cytokines[49]; and Saccharomyces boulardii reduces pro-inflammatory IL-8 and tumor necrosis factor alpha and increases the level of anti-inflammatory IL-10[50]. The authors of one study[51] concluded that the use of probiotics resulted in reduced intestinal barrier permeability, which may be consistent with these claims.
One of the generally accepted definitions of probiotics is that they are living microorganisms that contribute to the well-being and health of a host when administered in an adequate dose[52]. Lactobacillus and Bifidobacterium are the most common species that are put in the center of studies in the context of IBS because of their numerical superiority over the rest, as well as the number of aerobes vs anaerobes[53,54]. In 2007, Rousseaux et al[55] demonstrated that direct contact of certain probiotic bacteria [Lactobacillus acidophilus (L. acidophilus)] with epithelial cells induces the expression of opioid and cannabinoid receptors in the gut and contributes to the modulation and restoration of the normal perception of visceral pain. Lactic acid bacteria (LAB) are currently the most widely studied, and this group of probiotics consists of approxi
Several studies had their limitations in the form of inconsistencies in reports, variable treatment periods, small number of subjects and heterogeneous groups of patients, according to the form of the syndrome (diarrhoeal/constipation). In vitro and in vivo studies have shown that the probiotic combination VSL#3 [L. acidophilus, Lactobacillus plantarum (L. plantarum), Lactobacillus casei (L. casei), and Lactobacillus delbrueckii subspecies bulgaricus (L. delbrueckii spp. bulgaricus), Bifidobacterium breve (B. breve), B. longum, and Bifidobacterium infantis (B. infantis) and Streptococcus salivarius ssp. thermophiles] is likely to modulate the host immune response, intestinal microbiota, anti-inflammatory pathways, responses to visceral pain, and epithelial barrier function[58-62]. It also has different effects on different types of disease. Kim et al[63] found that the combination of probiotics VSL#3 slowed intestinal passage compared to placebo, indicating that the aforementioned probiotic is likely to have a better effect on the diarrhoeal form of the disease. The diversity and richness of gut microbiota has been shown to be associated with slower intestinal passage[64], whereas in softer stools, this diversity is significantly reduced[65].
Several studies have included a prepared, specific combination of eight different strains, consisting mainly of LAB and Bifidobacterium (including B. longum, B. infantis, B. breve, L. acidophilus, L. casei, L. delbrueckii spp. bulgaricus, L. plantarum and Streptococcus salivarius), that showed efficacy in patients with IBS in the form of reduction of bloating and abdominal symptoms[66-70].
The most commonly used probiotic bacteria in studies are Lactobacillus, Bifidobacterium, Enterococcus and Streptococcus, and in most studies that included these probiotics, there was a marked improvement in the reduction of abdominal pain and discomfort. Individual studies and the applied probiotic species/strains and the results are shown in Table 2. Diagnostic criteria of IBS were Rome III and IV, with duration of therapy of at least six weeks.
| Ref. | Type of IBS (%) | Sample size | Probiotic | Outcome by the type of IBS (probiotic group) | Common outcome (probiotic group) |
| Sinn et al[71], 2008 | D: 20; C: 27; M: 62.5 | 40 | L.acidophillus SDC 2012, 2013 | Not specified | Reduction of abdominal pain (28%), bowel habit satisfaction (18.2%), reduction of straining at stool (25.4%) |
| Hong et al[72], 2009 | D: 45.7; C: 20; M: 8.6; Non classified: 25.7 | 70 | Bifidobacterium bifidum BGN4, B. lactis AD011, Lactobacillus acidophilus AD031, L. casei IBS041 | Not specified | Reduction of pain score (-31.9), defecation and discomfort (-29.2), no significant change in QOL and bowel habits (defecation frequency and stoll consistency) |
| Guglielmetti et al[73], 2011 | D: 21.3; C: 19.7; M: 58.2; NC: 0.8 | 122 | Bifidobacterium bifidum MIMBb75 | Not specified | Improved global IBS symptoms by -0.88 points, reduction in pain/discomfort by -0.82 points, distention/bloating by -0.92 points, urgency by -0.76 points (Likert scale) |
| Cui and Hu[74], 2012 | D: 48.3; C: 20; M: 11.7; NC: 10 | 60 | Bifidobacterium longum and Lactobacillus acidophilus | Not specified | Improvement in frequency of abdominal pain (23% vs 6%), abdominal distension (27% vs 7%), bowel habits (26% vs 8%), dissatisfaction with defecation (20% vs 10%). |
| Dapoigny et al[75], 2012 | D: 30; C: 22; M: 34; NC: 14 | 50 | Lactobacillus casei rhamnosus LCR35 | D: significant reduction in abdominal pain; M: no relevant difference between groups | No clinicaly relevant changes overall |
| Ducrottéet al[76], 2012 | All types | 214 | Lactobacillus plantarum 299v | Not specified | Mean frequency of abdominal pain was reduced significantly by 51.9%, reductions in stool frequency, bloating and feeling of incomplete emptying, significant reduction of the daily number of stools |
| Amirimani et al[77], 2013 | All types | 102 | Lactobacillus reuteri | Not specified | Increased frequency of defecation, no significat difference in bloating, urgency,abdominal pain, stool shape. Study did not clasiffy between D and C subtype |
| Begtrup et al[78], 2013 | D: 40; C: 19; M: 38; NC: 2 | 131 | L. paracasei ssp paracasei F19, L. Acidophilus; La5 and Bifidobacterium Bb12 | Not specified | Adequate relief of symptoms at least 50% of the time (52% vs 41%), No difference in diarrhea, bloating and satiety |
| Roberts et al[79], 2013 | D and C | 179 | Bifidobacterium lactis CNCM I-2494, S.thermophilus, L.bulgaris | Not specified | Improvements in symptoms scores, bloating, flatulence, ease of bowel movement and quality of life (48% vs 33%) |
| Jafari et al[80], 2014 | All types | 108 | Probio-Tec® Quatro-cap-4 | Not specified | Decrease in VAS score for abdominal pain and bloating, decrease in feeling incomplete defecation |
| Ludidi et al[81], 2014 | All types | 40 | Bifidobacterium lactis W52, Lactobacillus casei W56, L. salivarius W57, Lactococcus lactis W58, L. acidophilus NCFM, and L. rhamnosus W71 | Not specified | Decrease in visceral hypersensitivity in both groups,decreased pain in both groups, no significat difference in overal symptom improval |
| Pedersen et al[82], 2014 | D: 38; C: 17.3; M: 40.7; NC: 4 | 81 | Lactobacillus rhamnosus GG | Not specified | Improvement in IBS-SSS score nad QOL score. Low FODMAP diet showed efficient in IBS-C, and probiotic in IBC-D |
| Sisson et al[83], 2014 | D: 37.6; C: 21.5; M: 35.5; NC: 5.4 | 186 | Lactobacillus rhamnosus NCIMB 30174, L. plantarum NCIMB 30173, L. Acidophilus NCIMB 30175, Enterococcus faecium NCIMB 30176 | Not specified | Reduction in IBS-SSS score (abdominal paion, bloating, bowel habit satisfaction)-63.3 vs -28.3. No difference in QOL score |
| Yoon et al[84], 2014 | D: 53.1; C: 40.8; M: 6.1 | 49 | Bifidobacterium bifidum (KCTC 12199BP), B. lactis (KCTC 11904BP), B. longum (KCTC 12200BP), L. acidophilus (KCTC 11906BP), L. rhamnosus (KCTC 12202BP) and Streptococcus thermophilus (KCTC 11870BP) | Not specified | Global relief of IBS symptoms (68% vs 37.5%), reduced abdominal pain and discomfort. No difference in stool consistency. Changes in the fecal microbiota genome (detected by PCR test) |
| Pineton de Chambrun et al[85], 2015 | D: 28.5; C: 46.9; M: 24.6 | 179 | Saccharomyces cerevisiae CNCM I-3856 | Not specified | Same results regarding abdominal pain and discomfort in both groups, but probiotic group showed improvement in during the second month of use |
| Yoon et al[86],2015 | D: 48.1; C: 18.5; M: 21; NC: 12.4 | 80 | Bifidobacterium bifidum (KCTC 12199BP), B. lactis (KCTC11904BP), B. longum (KCTC 12200BP), Lactobacillus acidophilus (KCTC 11906BP), L. rhamnosus (KCTC 12202BP), Streptococcusthermophilus | Not specified | Increase in probiotic strains in stool samples, higher adequate symptom relief (but not statisticaly relevant), improvement in the diarhea symptom score |
| Lyra et al[87], 2016 | D: 38.9; C: 16.6; M: 44; NC: 0.5 | 391 | Lactobacillus acidophilus NCFM (ATCC 700396) | Not specified | No difference in both groups in IBS-SSS score |
| Spiller et al[88], 2016 | D: 20.8; C: 47.4; M: 31.7 | 379 | Saccharomyces cerevisiase I-3856 | Reduced abdominal pain and bloating in IBS-C | No overall benefit in all subtypes, but significant improvement in C subtype |
| Preston et al[89], 2018 | D: 46.4; C: 35.7; M: 18.6 | 113 | Lactobacillus acidophilus CL1285, L. casei LBC80R, L. rhamnosus CLR2 | Improvement of IBS-SSS score for female D subtype by 50% to 144%. Better satisfation with bowel habits in C subtype. Better QOL in IBS-D females. Impruvment in number of days without pain M subtype | No improvement in IBS-SSS score overall or QOL overall |
The results of several dozen examined studies showed a reduction in abdominal distension and bloating. In a meta-analysis of 42 randomised controlled trials, 34 reported improvements in at least one symptom[90]. No significant difference was observed in the individual groups of probiotics used: Lactobacillus, Bifidobacterium, Streptococcus, or in a combination of the above[47,49,91,92]. The main limitation of most of the clinical studies is that the patient groups were heterogeneous; however, the overall result of all the analyses was the alleviation of general symptomatology.
A meta-analysis of the efficacy of B. infantis 35624 in the IBS was performed. As in the studies already mentioned, the efficacy targets were symptoms related to abdominal pain, bloating and bowel emptying habits, and respondent satisfaction with the management of these symptoms. The analysis included three studies conducted based on the use of B. infantis and two additional probiotics. The results showed a significant improvement in all examined parametres in terms of the mixture of probiotics together with B. infantis, but not equally effective if B. infantis was solely used. According to that analysis, treatment with a mixture of probiotics that also contain this bacterium could have an effect in treating the disease. However, it should be considered that the number of participants in the examination was too small, and certainly, the stated claims should be further examined[50].
An interesting fact is the analysis of several studies that show that the use of one probiotic, rather than a combination of several, taken in a short period and in a low dose, proved to be better in the final general condition, general feeling of patients after treatment (testing) and improvement in their quality of life. Yoon et al[84] hypothesised that multi-strain-containing probiotics may result in different effects and benefits on IBS symptoms, as each bacterial species produces a different effect in the gastrointestinal system, and two or more probiotic species in combination have a synergistic effect. However, research has also shown that competition between introduced combined species or strains is possible, which can lead to negative effects. Analysis of gut microbiota before and after probiotic administration showed that different strains have different viabilities and overdoses can disturb living conditions by competition[81].
The question of the dosage of the individual probiotics that needs to be applied to achieve the final desired effects was raised. Initially, an answer is not offered; significant is that an adequate dose is needed for the desired effect. There are several variables that could affect the effective dose of probiotics: Desired effect, specific strain, probiotic carrier, and the mode of application. In a unique study, the combination of two strains of L. plantarum and one strain of Pediococcus acidilactici (confirmed to reduce inflammation and frequency of diarrhoea in animal models of intestinal inflammation) were applied in two doses: 1-3 × 1010 CFU (colony forming unit) per capsule and 3-6 × 109 CFU per capsule, in equal representation of each probiotic. The results are such that all patients, regardless of the dose of I.31 (as the combination of probiotics is called), indicating that the achievement effect is attained even at lower values, reported a better quality of life after three weeks of intolerance to mixtures, while reduction of anxiety was reported only after six weeks. Interestingly, the effect was achieved earlier when a higher dose was administered[93]. We must, however, emphasise that although the authors claim they tested high and low doses of probiotics, 109 bacteria per capsule can in no way be considered low dose. The difference between these two doses is too small and the authors should have used a slightly lower dose to examine whether probiotic dose influences IBS.
Liang et al[94] analyzed several clinical trials, with a primary goal to clarify the effective dose of applied probiotics which, in this case, is a combination of Lactobacillus and Bifidobacterium. Their conclusion is comparable to previous studies, with an observed improvement in global symptoms that was achieved even at low doses. In most studies, a dose of 109 CFU/d to 1010 CFU/d of the tested strains is the recom
According to Lorenzo-Zúñiga et al[93], probiotics do not follow pharmacological rules in achieving the effect of saturation, but this effect is attained according to the principle of synergism or antagonism, in which negative effects are caused. High doses of probiotics can cause short-term discomfort in the gastrointestinal system due to excessive fermentation of carbohydrates, which is a feature of the most studied and represented strains in patients with IBS[95].
In other medical cases, conditions and diseases, the doses of specific probiotic strains have been studied. Namely, the results of one study showed that L. rhamnosus GG has a greater effect in acute gastroenteritis in children when administered at a dose greater than 1010 CFU/d[96]. S. boulardii administered in patients with diarrhoea, after low and high doses, achieved an equal effect[97]. In addition, no difference was found in the dose-dependent effect for Lactobacillus reuteri DSM17938 on diarrhoea[98].
In 2006, in a meta-analysis of antibiotic-related diarrhoea and necrotising enterocolitis, a result based on 25 studies involving 13 probiotic products reported that probiotic doses less than 1010 CFU did not result in treatment success. The results were confirmed in later meta-analyses[99].
Significantly less research regarding the effectiveness of probiotics have been conducted in patients with C-IBS. Based on the Bristol stool scale, study participants described their stool as hard or lumpy (≥ 25% of all stools) and fluffy or watery (< 25% of all stools).
It is known that in these patients, there exists an increased number of bacteria that produce methane[100] and the amount of gas released is directly correlated with the severity of severe constipation[101], which is consistent with the slower passage through the intestine in these cases, with reduced segmental contraction and attenuated propulsion. Given these facts, the effect of B. lactis DN-173 010 on distension, bloating, and other IBS symptoms was examined[102]. Patients complained of a visible increase in abdominal volume at least twice a week and met other Roman III. The dose of B. lactis was 1.25 × 1010 CFU/g, and S. thermophilus and L. bulgaricus (1.2 × 109 CFU/g) were added; in fact, fermented milk and yogurts were found to contain these probiotics. The results showed that fermented dairy products reduced abdominal distension and accelerated intestinal passage. Reduced bloating was also reported, as were other IBS symptoms. There are fewer studies involving subjects who have constipation-like problems, and one of these studies was published in 2014. The results are impressive and show that probiotics have significantly reduced the passage time by 12.4 h and increased stool frequency by 1.3 wkly bowel movements. Success is related to the administration of B. lactis (increasing weekly bowel movements by 1.5 movements), but not to L. casei Shirota (recorded decreased weekly bowel movements per week to 0.2). Stool consistency was better during intake of B. lactis, but not L. casei Shirota strain[103]. Health-related quality of life was also a frequently examined aspect in patients, making it the primary subject of the study by Guyonnet et al[104], because they believed that the patient's perception of symptoms and the impact of difficulties on daily life are extremely important. In general, patients with more severe disease and frequent symptomatology felt relief, but were reluctant to report it. This was in contrast to those subjects who had moderate or mild disease and did not experience significant improvement, but reported a change in symptom severity. Interestingly, in a number of studies, placebo groups also reported positive effects, which is an increasingly central point of the study (Table 3).
| Ref. | Type of IBS | Sample size | Probiotic | Outcome by the type of IBS (probiotic group) |
| Agrawal et al[102], 2009 | C | 34 | Bifidobacterium lactis DN-173 010 | Reduction in bloating and distension (-1.52 cm), reduction of orocaecal (-1.2 h) and colonic (-12.2 h) transit times, reduction of pain and discomfort (-0.5) |
| Michail et al[105], 2011 | D | 24 | VSL#3: Lactobacillus acidophilus, L. plantarum, L. casei, and L. delbrueckii ssp. bulgaricus, Bifidobacterium breve, B. longum, and B. infantis and Streptococcus salivarius ssp. thermophilus | Significant decreases in the bloating, diarrhea, satiety and QOL in both groups (placebo and probiotic) |
| Ki Cha et al[106], 2012 | D | 50 | Lactobacillus acidophilus, L. plantarum, L. rhamnosus, Bifidobacterium breve, B. lactis, B. longum and Streptococcus thermophilus | Symptoms (abdominal pain, abdominal discomfort, loose/watery stool, urgency, mucus in stool, bloating, and passage of gas) relief was higher (> 50%), improved stool consistency |
| Abbas et al[50], 2014 | D | 72 | Saccharomyces boulardii | Decrease in the blood and tissue levels of proinflammatory cytokines IL-8 and TNF-a and and increase in the anti-inflammatory IL-10, improvement in body image and food avoidance |
| Lorenzo-Zúñiga et al[93], 2014 | D | 84 | Lactobacillus plantarum (CECT7484 and CECT7485), Pediococcus acidilactici (CECT7483) | Improved QOL score, improvement in gut-related anxiety (VSI scale) |
| Majeed et al[107], 2016 | D | 36 | Bacillus coagulans MTCC 5856 | Decrase in bloating, diarhea, vomiting, abdominal pain, improvement in Bristol stool score |
| Mezzasalma et al[108],2019 | C | 150 | Lactobacillus acidophilus, L. reuteri, L.s plantarum, L.s rhamnosus, Bifidobacterium animalis subps. lactis | QOL improved in probiotic group, incresead healthier characteristic in stool samples |
| Hod et al[109],2017 | D | 107 | Lactobacillus rhamnosus LR5, L. casei LC5, L. paracasei LPC5, L. plantarum LP3, L. acidophilus LA1, Bifidobacterium bifidum, BF3, B. longum BG7, B. breve BR3, B. infantis BT1, Streptococcus thermophilus ST3, L. bulgaricus LG1, Lactococcus lactis | No difference between groups overall, no CRP and fecal calprotectin levels difference |
| Ishaque et al[110],2018 | D | 360 | Bio-Kult® | Reduced overal IBS-SSS score by 145 point in 30 d, reduced number of bowel movements, symptom free patients (33.7% vs 12.8%) |
| Khodadoostan et al[111],2018 | D | 67 | Lactobacillus casei, L. acidophilus, L. rhamnosus, L. bulgaricus, Bifidobacterium breve, B. longum, and Streptococcus thermophilus with prebiotic of ructooligosaccharides | Improvement in stool consistency adn defecation rate after 3 mo, decrease in abdominal pain after 6 mo |
| Sun et al[112], 2018 | D | 200 | Clostridium butyricum | Reduction in IBS-SSS score (-62.12 vs -40.74), no difference in abdominal pain and bloating, improvement in QOL, no change in Bristol stool scale. |
The symptoms of this form of the disease are similar to those in other subtypes, with more frequent bowel movements and increased peristalsis, which results in softer stools or diarrhoea. It is also characterised by the urgency for defecation. According to the Bristol stool scale, patients define this form of the disease as the presence of fluffy or watery stools (≤ 25% of all stools) and hard or lumpy stools (< 25% of all stools). When using a mixture of L. plantarum (5 × 107 CFU/mL) and 3.6 g of fibre, the results showed that the presence of gas/wind was significantly lower, intensity of abdominal pain was reduced and overall function of the gastrointestinal system was much better after one year of using symbiotics[113]. These effects can be explained by slowing down the passage through the intestine, facilitating the flow, electrolyte reabsorption and consequently, reducing diarrhoea. The combination of the probiotics L. acidophilus, L. plantarum, L. rhamnosus, B. breve, B. lactis, B. longum and S. thermophilus in a dose of 1.0 × 1010 CFU also produced promising results. The application lasted for eight weeks, and the effect manifested as alleviation of overall symptoms and improvement in stool consistency, although no specific effect on individual symptoms was observed[106]. However, regarding the primary symptom of this subgroup, diarrhoea, probiotics did not prove effective in reducing the number of diarrhoeal stools. Several studies have been conducted, testing different probiotics, but the results have not been successful[46,78,114]. Moreover, in one study, an even more significant deterioration was reported[115]. In contrast, in this subtype of disease, Bacillus coagulans MTCC 5856, at a dose of 2 × 109 CFU/d for 90 d of use, proved to be quite successful. All symptoms in patients belonging to the D-IBS group were significantly alleviated, including diarrhoea[107].
The aim of one study was to evaluate the change in the frequency and intensity of abdominal pain in patients with a predominantly diarrhoeal form of the disease. A combination of strains were evaluated in the study: Bacillus subtilis PXN 21, B. bifidum PXN 23, B. breve PXN 25, B. infantis PXN 27, B. longum PXN 30, L. acidophilus PXN 35, L. delbrueckii spp. bulgaricus PXN39, L. casei PXN 37, L. plantarum PXN 47, L. rhamnosus PXN 54, L. helveticus PXN 45, L. salivarius PXN 57), Lactococcus lactis PXN 63, and S. thermophilus PXN 66 at 2 million colonies per capsule, twice daily for 16 wk. After this treatment, patients reported a reduction in the intensity of abdominal pain, as well as other symptoms comprising the IBS-SSS (Irritable Bowel Syndrome Severity Scoring System), including the intensity of abdominal pain, number of days of abdominal pain during the last 10 d, severity of abdominal distension, discomfort during urination, and reduced quality of life. The participants were examined every month for five months, and during these controls, the results showed an improvement in all examined elements of the disease, compared to the initial condition and results of the group of patients receiving placebo. This study included a large number of subjects (360 patients) that were relatively homogeneous with a certain subtype of the disease, resulting in a more relevant study compared to a large number of other processed analyses[110]. In Figure 3, exhibited is the effect of probiotics on different IBS type symptoms.
Unlike probiotics, prebiotics are not metabolised in the intestines of the host, and their ultimate purpose is to positively impact the microenvironment of the digestive system. The best known prebiotics are oligofructose, inulin, galactooligosaccharides, lactulose, and oligosaccharides from breast milk. In fact, these compounds are an integral part of the food we eat every day. Some of the positive effects include an increase in the number of bifidobacteria, calcium absorption, and fecal mass, shortening of the retention time of fecal mass in the intestines, and a possible decrease in blood lipids[32]. Several studies have investigated the effect of prebiotics[47], revealing the importance of choice of prebiotic, as well as the dose, since doses that were too small could be useless, and larger ones can stimulate gas production, which worsens symptoms[116-118].
Most of the studies highlighted in this article did not report side effects or listed them as “unimportant”. In fact, it is an interesting that probiotics, prebiotics and symbiotics used in the treatment of IBS can sometimes cause, or even worsen, some symptoms. This phenomenon is most commonly observed in D-IBS, in which the use of prebiotics and fibre could lead to worsening of symptoms. These side effects include gas production, bloating, softer stools and abdominal pain; all of which are mostly temporary[119].
There exist several variables that affect changes in the microbiota (i.e., differences in sample storage, DNA extraction, and analytical methods), as well as the diet of individuals, which were not strictly regulated in any studies. Many foods serve as prebiotics and may also contain probiotics. It has been proven that the application of multiple strains of probiotic bacteria, or even multiple species, is much more effective than the application of only one probiotic strain. It is difficult to predict which strain or species most contributed to the welfare of IBS patients. Several groups investigated the effect of a particular strain on a specific symptom, which could be considered a good research direction and a way to prove the effectiveness of a particular probiotic on the symptom that causes the most discomfort to an individual patient. A major drawback of these studies, however, is the design of clinical studies in which the types of IBS are not clearly defined, or the analysis of IBS types is performed after the study is completed. In most of the clinical studies, a small number of patients were grouped into IBS subtypes, which made it difficult to draw conclusions. In addition, it is unlikely that the same probiotic or multispecies probiotic preparation will influence all four types of IBS. The biggest unknown remains as the mixed and unclassified types of IBS, which are present in small numbers in the conducted studies. The way to design a suitable study for mixed and unclassified types of IBS is questionable, as we do not currently have a probiotic or symbiotic that would affect the modification of the various symptoms that occur in these types of IBS. The solution may be to group patients with specific subtype and gather critical mass. Furthermore, numerous studies have shown the impact of probiotics on certain areas of the brain and their activity. Studies of this type certainly have their limitations, mostly related to the complicated interrelationships of the intestinal brain axis, such as those present in patients with IBS, which are not easy to transfer to an animal model and then map to human subjects. The need to understand the connection between the intestinal microbiota and functional diseases of the gastrointestinal system is central to the research in this field of medicine. The cognition that controlling the intake of dietary supplements can affect bowel functions, as well as the psychological manifestations of the disease, is the basis for setting new therapeutic options in the treatment of IBS and other similar disorders of the gastrointestinal system.
We would like to thank Darko Rodi for helping with the illustrations.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Frascio M, Sahin Y S-Editor: Gao CC L-Editor: A P-Editor: Li JH
| 1. | Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43-II47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 830] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 2. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3413] [Cited by in RCA: 3381] [Article Influence: 177.9] [Reference Citation Analysis (1)] |
| 3. | Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759-6773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 318] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (14)] |
| 4. | Lacy BE, Mearin F, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterol. 2016;150:1393-1407. |
| 5. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1895] [Article Influence: 210.6] [Reference Citation Analysis (3)] |
| 6. | Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 371] [Article Influence: 41.2] [Reference Citation Analysis (1)] |
| 7. | Parkes GC, Brostoff J, Whelan K, Sanderson JD. Gastrointestinal microbiota in irritable bowel syndrome: their role in its pathogenesis and treatment. Am J Gastroenterol. 2008;103:1557-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Bhattarai Y, Muniz Pedrogo DA, Kashyap PC. Irritable bowel syndrome: a gut microbiota-related disorder? Am J Physiol Gastrointest Liver Physiol. 2017;312:G52-G62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 9. | Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 613] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Distrutti E, Salvioli B, Azpiroz F, Malagelada JR. Rectal function and bowel habit in irritable bowel syndrome. Am J Gastroenterol. 2004;99:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3642] [Article Influence: 280.2] [Reference Citation Analysis (0)] |
| 12. | Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 540] [Cited by in RCA: 624] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 13. | Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. 2014;11:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 261] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 14. | Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The Microbiome and Irritable Bowel Syndrome - A Review on the Pathophysiology, Current Research and Future Therapy. Front Microbiol. 2019;10:1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 15. | Bellini M, Gambaccini D, Stasi C, Urbano MT, Marchi S, Usai-Satta P. Irritable bowel syndrome: a disease still searching for pathogenesis, diagnosis and therapy. World J Gastroenterol. 2014;20:8807-8820. [PubMed] |
| 16. | Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, Störsrud S, Le Nevé B, Öhman L, Simrén M. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology. 2017;152:111-123.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 443] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 17. | Pozuelo M, Panda S, Santiago A, Mendez S, Accarino A, Santos J, Guarner F, Azpiroz F, Manichanh C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci Rep. 2015;5:12693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 18. | Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512-519, e114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 773] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 20. | Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 21. | Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 482] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 22. | Jalanka-Tuovinen J, Salojärvi J, Salonen A, Immonen O, Garsed K, Kelly FM, Zaitoun A, Palva A, Spiller RC, de Vos WM. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 23. | Villarreal AA, Aberger FJ, Benrud R, Gundrum JD. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. WMJ. 2012;111:17-20. [PubMed] |
| 24. | Rajilić-Stojanović M, Jonkers DM, Salonen A, Hanevik K, Raes J, Jalanka J, de Vos WM, Manichanh C, Golic N, Enck P, Philippou E, Iraqi FA, Clarke G, Spiller RC, Penders J. Intestinal microbiota and diet in IBS: causes, consequences, or epiphenomena? Am J Gastroenterol. 2015;110:278-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 268] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 25. | Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34:15490-15496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 632] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 26. | Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, Mayer EA. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394-1401, 1401.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 758] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 27. | Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050-16055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2500] [Cited by in RCA: 2633] [Article Influence: 188.1] [Reference Citation Analysis (0)] |
| 28. | Kelly JR, Borre Y, O' Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 1138] [Article Influence: 126.4] [Reference Citation Analysis (0)] |
| 29. | Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (1)] |
| 30. | Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 31. | Jeffery IB, O'Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 635] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 32. | Hauser G, Benjak Horvat I, Zelić M, Prusac M, Škopić OV. Probiotici i prebiotici – koncept. Medicus. 2020;29:95-114. |
| 33. | Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin FP, Cominetti O, Welsh C, Rieder A, Traynor J, Gregory C, De Palma G, Pigrau M, Ford AC, Macri J, Berger B, Bergonzelli G, Surette MG, Collins SM, Moayyedi P, Bercik P. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology. 2017;153:448-459.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 562] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 34. | Medani M, Collins D, Docherty NG, Baird AW, O'Connell PR, Winter DC. Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm Bowel Dis. 2011;17:1620-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Schemann M, Grundy D. Role of hydrogen sulfide in visceral nociception. Gut. 2009;58:744-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Distrutti E. Hydrogen sulphide and pain. Inflamm Allergy Drug Targets. 2011;10:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Distrutti E, Cipriani S, Renga B, Mencarelli A, Migliorati M, Cianetti S, Fiorucci S. Hydrogen sulphide induces micro opioid receptor-dependent analgesia in a rodent model of visceral pain. Mol Pain. 2010;6:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, Roviezzo F, Morelli A, Cirino G, Wallace JL, Fiorucci S. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006;316:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 39. | Oleskin AV, Shenderov BA. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb Ecol Health Dis. 2016;27:30971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Perez-Burgos A, Wang L, McVey Neufeld KA, Mao YK, Ahmadzai M, Janssen LJ, Stanisz AM, Bienenstock J, Kunze WA. The TRPV1 channel in rodents is a major target for antinociceptive effect of the probiotic Lactobacillus reuteri DSM 17938. J Physiol. 2015;593:3943-3957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Kamiya T, Wang L, Forsythe P, Goettsche G, Mao Y, Wang Y, Tougas G, Bienenstock J. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 42. | Zhou L, Foster JA. Psychobiotics and the gut-brain axis: in the pursuit of happiness. Neuropsychiatr Dis Treat. 2015;11:715-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1494] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 44. | Bär F, Von Koschitzky H, Roblick U, Bruch HP, Schulze L, Sonnenborn U, Böttner M, Wedel T. Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: evidence from an in vitro organ bath study. Neurogastroenterol Motil. 2009;21:559-566, e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Guarino MP, Altomare A, Stasi E, Marignani M, Severi C, Alloni R, Dicuonzo G, Morelli L, Coppola R, Cicala M. Effect of acute mucosal exposure to Lactobacillus rhamnosus GG on human colonic smooth muscle cells. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 2:S185-S190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 47. | O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O'Sullivan GC, Kiely B, Collins JK, Shanahan F, Quigley EM. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 959] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 48. | Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 352] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 49. | Spaiser SJ, Culpepper T, Nieves C Jr, Ukhanova M, Mai V, Percival SS, Christman MC, Langkamp-Henken B. Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2 Ingestion Induces a Less Inflammatory Cytokine Profile and a Potentially Beneficial Shift in Gut Microbiota in Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. J Am Coll Nutr. 2015;34:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Abbas Z, Yakoob J, Jafri W, Ahmad Z, Azam Z, Usman MW, Shamim S, Islam M. Cytokine and clinical response to Saccharomyces boulardii therapy in diarrhea-dominant irritable bowel syndrome: a randomized trial. Eur J Gastroenterol Hepatol. 2014;26:630-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 52. | Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4055] [Cited by in RCA: 5548] [Article Influence: 504.4] [Reference Citation Analysis (2)] |
| 53. | Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547-61; quiz 1546, 1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 489] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 54. | Moraes-Filho JP, Quigley EM. THE INTESTINAL MICROBIOTA AND THE ROLE OF PROBIOTICS IN IRRITABLE BOWEL SYNDROME: a review. Arq Gastroenterol. 2015;52:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 541] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 56. | Axelsson L. Lactid acid bacteria: Classification and physiology. In: Salminen S, von Wright A, Ouwehand A. Lactic acid bacteria: Microbiological and funcional aspects. 3rd ed. New York: Marcel Dekker Inc, 2004: 3-66. |
| 57. | Šušković J, Brkić B, Matošić S. Mehanizam probiotičkog djelovanja bakterija mliječne Kiseline. Mljekarstvo. 1997;47:57-73. |
| 58. | Dai C, Zheng CQ, Meng FJ, Zhou Z, Sang LX, Jiang M. VSL#3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-κB pathway in rat model of DSS-induced colitis. Mol Cell Biochem. 2013;374:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 59. | Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One. 2013;8:e63893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 60. | Do EJ, Hwang SW, Kim SY, Ryu YM, Cho EA, Chung EJ, Park S, Lee HJ, Byeon JS, Ye BD, Yang DH, Park SH, Yang SK, Kim JH, Myung SJ. Suppression of colitis-associated carcinogenesis through modulation of IL-6/STAT3 pathway by balsalazide and VSL#3. J Gastroenterol Hepatol. 2016;31:1453-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Dolpady J, Sorini C, Di Pietro C, Cosorich I, Ferrarese R, Saita D, Clementi M, Canducci F, Falcone M. Oral Probiotic VSL#3 Prevents Autoimmune Diabetes by Modulating Microbiota and Promoting Indoleamine 2,3-Dioxygenase-Enriched Tolerogenic Intestinal Environment. J Diabetes Res. 2016;2016:7569431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 62. | Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 742] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 63. | Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, Thomforde G, Zinsmeister AR. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 249] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 64. | Roager HM, Hansen LB, Bahl MI, Frandsen HL, Carvalho V, Gøbel RJ, Dalgaard MD, Plichta DR, Sparholt MH, Vestergaard H, Hansen T, Sicheritz-Pontén T, Nielsen HB, Pedersen O, Lauritzen L, Kristensen M, Gupta R, Licht TR. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol. 2016;1:16093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 308] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 65. | Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65:57-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 705] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 66. | Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 67. | Aragon G, Graham DB, Borum M, Doman DB. Probiotic therapy for irritable bowel syndrome. Gastroenterol Hepatol (N Y). 2010;6:39-44. [PubMed] |
| 68. | Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, Zinsmeister AR. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 305] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 69. | Guandalini S, Magazzù G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, Sibal A, Romano C, Canani RB, Lionetti P, Setty M. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 70. | Staudacher HM, Lomer MCE, Farquharson FM, Louis P, Fava F, Franciosi E, Scholz M, Tuohy KM, Lindsay JO, Irving PM, Whelan K. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology. 2017;153:936-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 302] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 71. | Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, Chang DK, Kim YH, Kim JJ, Rhee JC, Rhee PL. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53:2714-2718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 72. | Hong KS, Kang HW, Im JP, Ji GE, Kim SG, Jung HC, Song IS, Kim JS. Effect of probiotics on symptoms in korean adults with irritable bowel syndrome. Gut Liver. 2009;3:101-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 74. | Cui S, Hu Y. Multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Int J Clin Exp Med. 2012;5:238-244. [PubMed] |
| 75. | Dapoigny M, Piche T, Ducrotte P, Lunaud B, Cardot JM, Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized, double-blind study. World J Gastroenterol. 2012;18:2067-2075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 76. | Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012-4018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 175] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (6)] |
| 77. | Amirimani B, Nikfam S, Albaji M, Vahedi S, Nasseri-Moghaddam S, Sharafkhah M, Ansari R, Vahedi H. Probiotic vs. Placebo in Irritable Bowel Syndrome:A Randomized Controlled Trial. Middle East J Dig Dis. 2013;5:98-102. [PubMed] |
| 78. | Begtrup LM, de Muckadell OB, Kjeldsen J, Christensen RD, Jarbøl DE. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome--a randomised, double-blind, placebo controlled trial. Scand J Gastroenterol. 2013;48:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 79. | Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FD. A randomised controlled trial of a probiotic 'functional food' in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Jafari E, Vahedi H, Merat S, Momtahen S, Riahi A. Therapeutic effects, tolerability and safety of a multi-strain probiotic in Iranian adults with irritable bowel syndrome and bloating. Arch Iran Med. 2014;17:466-470. [PubMed] |
| 81. | Ludidi S, Jonkers DM, Koning CJ, Kruimel JW, Mulder L, van der Vaart IB, Conchillo JM, Masclee AA. Randomized clinical trial on the effect of a multispecies probiotic on visceroperception in hypersensitive IBS patients. Neurogastroenterol Motil. 2014;26:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Pedersen N, Andersen NN, Végh Z, Jensen L, Ankersen DV, Felding M, Simonsen MH, Burisch J, Munkholm P. Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol. 2014;20:16215-16226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 83. | Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome--a 12 wk double-blind study. Aliment Pharmacol Ther. 2014;40:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 84. | Yoon JS, Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, Lee HL, Yoon BC, Choi HS, Chung WS, Seo JG. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 85. | Pineton de Chambrun G, Neut C, Chau A, Cazaubiel M, Pelerin F, Justen P, Desreumaux P. A randomized clinical trial of Saccharomyces cerevisiae vs placebo in the irritable bowel syndrome. Dig Liver Dis. 2015;47:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 86. | Yoon H, Park YS, Lee DH, Seo JG, Shin CM, Kim N. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Biochem Nutr. 2015;57:129-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 87. | Lyra A, Hillilä M, Huttunen T, Männikkö S, Taalikka M, Tennilä J, Tarpila A, Lahtinen S, Ouwehand AC, Veijola L. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22:10631-10642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 88. | Spiller R, Pélerin F, Cayzeele Decherf A, Maudet C, Housez B, Cazaubiel M, Jüsten P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: improvement in abdominal pain and bloating in those with predominant constipation. United European Gastroenterol J. 2016;4:353-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 89. | Preston K, Krumian R, Hattner J, de Montigny D, Stewart M, Gaddam S. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R and Lactobacillus rhamnosus CLR2 improve quality-of-life and IBS symptoms: a double-blind, randomised, placebo-controlled study. Benef Microbes. 2018;9:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 90. | Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: probiotics for the treatment of irritable bowel syndrome--focus on lactic acid bacteria. Aliment Pharmacol Ther. 2012;35:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 91. | Martoni CJ, Evans M, Chow CT, Chan LS, Leyer G. Impact of a probiotic product on bowel habits and microbial profile in participants with functional constipation: A randomized controlled trial. J Dig Dis. 2019;20:435-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 92. | Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol. 2014;7:473-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 93. | Lorenzo-Zúñiga V, Llop E, Suárez C, Alvarez B, Abreu L, Espadaler J, Serra J. I.31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J Gastroenterol. 2014;20:8709-8716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 94. | Liang D, Longgui N, Guoqiang X. Efficacy of different probiotic protocols in irritable bowel syndrome: A network meta-analysis. Medicine (Baltimore). 2019;98:e16068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 95. | Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 2017;66:1517-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 96. | Szajewska H, Gyrczuk E, Horvath A. Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2013;162:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 97. | Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 98. | Urbańska M, Gieruszczak-Białek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 99. | Ouwehand AC. A review of dose-responses of probiotics in human studies. Benef Microbes. 2017;8:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 100. | Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003;48:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 101. | Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102:837-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 102. | Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, Schlumberger A, Jakob S, Whorwell PJ. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 103. | Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 104. | Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, Matuchansky C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 105. | Michail S, Kenche H. Gut microbiota is not modified by Randomized, Double-blind, Placebo-controlled Trial of VSL#3 in Diarrhea-predominant Irritable Bowel Syndrome. Probiotics Antimicrob Proteins. 2011;3:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 106. | Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, Hyuk J, Kyung Chang S, Kim K, Chung WS, Seo JG. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 107. | Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Ali F, Pande A, Majeed S, Karri SK. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant Irritable Bowel Syndrome: a double blind randomized placebo controlled pilot clinical study. Nutr J. 2016;15:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 108. | Mezzasalma V, Manfrini E, Ferri E, Sandionigi A, Ferla B, Schiano I, Michelotti A, Nobile V, Labra M, Di Gennaro P. Corrigendum to "A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation". Biomed Res Int. 2019;2019:9042956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 109. | Hod K, Sperber AD, Ron Y, Boaz M, Dickman R, Berliner S, Halpern Z, Maharshak N, Dekel R. A double-blind, placebo-controlled study to assess the effect of a probiotic mixture on symptoms and inflammatory markers in women with diarrhea-predominant IBS. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 110. | Ishaque SM, Khosruzzaman SM, Ahmed DS, Sah MP. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2018;18:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 111. | Khodadoostan M, Shavakhi A, Sherafat Z. Effect of Probiotic Administration Immediately and 1 Month after Colonoscopy in Diarrhea-predominant Irritable Bowel Syndrome Patients. Adv Biomed Res. 2018;7:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | Sun YY, Li M, Li YY, Li LX, Zhai WZ, Wang P, Yang XX, Gu X, Song LJ, Li Z, Zuo XL, Li YQ. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Sci Rep. 2018;8:2964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 113. | Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 376] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 114. | Ringel-Kulka T, Palsson OS, Maier D, Carroll I, Galanko JA, Leyer G, Ringel Y. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 vs placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011;45:518-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 115. | Ligaarden SC, Axelsson L, Naterstad K, Lydersen S, Farup PG. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial. BMC Gastroenterol. 2010;10:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 116. | Hasler WL. Lactulose breath testing, bacterial overgrowth, and IBS: just a lot of hot air? Gastroenterology. 2003;125:1898-900; discussion 1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 117. | Snook J, Shepherd HA. Bran supplementation in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8:511-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 118. | Badiali D, Corazziari E, Habib FI, Tomei E, Bausano G, Magrini P, Anzini F, Torsoli A. Effect of wheat bran in treatment of chronic nonorganic constipation. A double-blind controlled trial. Dig Dis Sci. 1995;40:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 119. | Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (1)] |