Published online Sep 27, 2021. doi: 10.4240/wjgs.v13.i9.885
Peer-review started: February 12, 2021
First decision: May 13, 2021
Revised: May 28, 2021
Accepted: July 20, 2021
Article in press: July 20, 2021
Published online: September 27, 2021
Processing time: 218 Days and 2.1 Hours
Patients affected by pancreatic ductal adenocarcinoma (PDAC) frequently present with advanced disease at the time of diagnosis, limiting an upfront surgical approach. Neoadjuvant treatment (NAT) has become the standard of care to downstage non-metastatic locally advanced PDAC. However, this treatment increases the risk of a nutritional status decline, which in turn, may impact therapeutic tolerance, postoperative outcomes, or even prevent the possibility of surgery. Literature on prehabilitation programs on surgical PDAC patients show a reduction of postoperative complications, length of hospital stay, and read
Core Tip: Among pancreatic ductal adenocarcinoma patients with resectable or borderline resectable disease, and those with locally advanced disease with a feasibility of surgical resection of up to 30%, neoadjuvant treatment (NAT) has become the standard of care. NAT may impair functional reserve and lead to nutritional depletion, which may affect therapeutic tolerance, postoperative outcomes or even prevent the possibility of surgery. This review suggests that NAT timeframe may provide a valuable opportunity for nutritional prehabilitation program to minimize the NAT-related nutritional derangements, increase patient’s capacity to complete planned therapy, promote tissue healing, and enhance patient’s recovery, thus potentially improve outcomes.
- Citation: Trestini I, Cintoni M, Rinninella E, Grassi F, Paiella S, Salvia R, Bria E, Pozzo C, Alfieri S, Gasbarrini A, Tortora G, Milella M, Mele MC. Neoadjuvant treatment: A window of opportunity for nutritional prehabilitation in patients with pancreatic ductal adenocarcinoma. World J Gastrointest Surg 2021; 13(9): 885-903
- URL: https://www.wjgnet.com/1948-9366/full/v13/i9/885.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i9.885
Pancreatic ductal adenocarcinoma (PDAC), with 458918 new cases in 2018, represents the 14th neoplasia in incidence, and with a 95% overall mortality rate, is the 7th leading cause of cancer-related death[1].
Surgical resection is the only potentially curative treatment for PDAC[2]. Nevertheless, an upfront surgical approach is often unfeasible, because most patients are diagnosed with an advanced PDAC stage, due to a lack of early symptoms and to the fast tumor progression[2]. In this context, neoadjuvant treatment (NAT) consisting of chemotherapy and/or chemoradiation has become the standard of care to downstage non-metastatic locally advanced PDAC patients[3]. Moreover, NAT is gaining popularity in both borderline and fully resectable patients to allow a more accurate and complete cytoreduction (R0)[4-6]. Resection rates range from 26% to 60% in patients showing a good NAT response, rising to 67.8% in patients with anatomically borderline resectable disease[7], with a high percentage of R0 resections and a more than doubled median overall survival (OS)[8,9]. Adjuvant treatment after surgery, using a combination of chemotherapy and radiotherapy, is used to increase the local control of disease[3].
Common presentation hallmarks of PDAC are unintentional weight loss (WL) and malnutrition, defined as “a state resulting from lack of intake or uptake of nutrition that leads to altered body composition and body cell mass leading to diminished physical and mental function and impaired clinical outcome from disease”[10], and sustained by cancer-induced metabolic changes and by a reduced nutrient intake[11]. Moreover, the loss of parenchyma and/or obstruction of the main pancreatic duct may affect both the production of enzymes and their transportation into the duodenum, resulting in nutrients maldigestion and/or malabsorption[12].
Over 80% of PDAC patients present with WL at diagnosis, and more than one-third reports a WL > 10% of their usual body weight[13]; moreover, NAT may worsen nutritional status, impairing postoperative outcomes and even delaying or preventing surgical intervention[14,15]. Malnutrition in PDAC patients may reach high rates (up to 80%) at diagnosis, and it is associated with a worse performance status and a worse OS[16].
Sarcopenia, defined as “a progressive and generalized skeletal muscle (SKM) disorder that is associated with increased likelihood of adverse outcomes”[17], is another frequent condition reported in more than 50% of PDAC patients[18]. It is associated with poorer surgical outcomes and a higher length of hospital stay (LOS)[19], and has been identified as a relevant prognostic factor for OS in patients treated with both gemcitabine (GEM) based and FOLFIRINOX-like (leucovorin, fluorouracil, irinotecan, and oxaliplatin) chemotherapies[20-22].
Improvement of patient nutritional status during NAT and prior to surgery may lead to better surgical outcomes and be an important part of oncological management[23,24]. The best nutritional strategy to manage PDAC patients is still under debate, even if an appropriate nutritional support represents an important therapeutic strategy in the preoperative period[23]. While cancer-related malnutrition is still an underrecognized and undertreated burden in clinical practice[25,26], emerging data show that early closing of the nutritional gap during anticancer treatment can stabilize WL, improve treatment tolerability, reduce the performance status deterioration, and ameliorate survival rate[27,28].
The aim of this review is to explore if the NAT period may represent an exploitable therapeutic window to perform a nutritional prehabilitation program improving clinical and survival outcomes.
This review was conducted on Medline, from inception to January 2021, aiming to identify published studies exploring the role of nutritional status and preoperative nutritional prehabilitation on the outcomes in pancreatic cancer (PC) patients. The inclusion criteria for the studies were as follows: observational, prospective and retrospective studies, case-control studies, cohort studies, narrative reviews, systematic reviews, and meta-analyses; studies including information about nutritional status and/or nutritional prehabilitation on PC patients; exclusive PC studies; and studies written in English. All studies that did not fall into the previous criteria were excluded from the review process.
Approximately one-third of PDAC patients are diagnosed with locally advanced disease, which prevents an immediate surgical approach[29]. In this setting, NAT has become the standard of care as either exclusive treatment or to achieve resectability[9,30,31], while its use in "borderline" and "resectable" disease is still under debate, even if it has become more popular[32].
This therapeutic implementation represents an additional nutritional concern; in fact, NAT cause several side effects, such as oral ulceration, xerostomia, dysgeusia, indigestion, nausea, vomiting, diarrhea, and alteration of intestinal motility, leading to a reduced food intake, with significant consequences on body composition, and in particular on SKM mass[33]. This in turn, may impact NAT completion, postoperative outcomes or even impede the possibility of surgery[10,34].
A prospective study including patients with upper gastrointestinal cancers found that NAT-treated patients experienced greater losses in the SKM area measured at L3 vertebra by computed tomography (CT) scan, compared with patients receiving palliative chemotherapy (-6.6 cm2, 95% confidence interval [CI] -10.2 to -3.1; P < 0.001 and -1.2 kg, 95%CI -1.8 to -0.5; P < 0.001, respectively)[35].
Naumann et al[35] analyzed 100 consecutive locally advanced PDAC patients, treated with 4 wk of GEM-based NAT and found that body weight (mean weight from 69.0 kg to 66.4 kg; P < 0.0001), body mass index (BMI) (mean BMI from 24.3 kg/m2 to 23.4 kg/m2; P < 0.0001), and CT-derived subcutaneous adipose tissue (SAT) area (mean SAT from 167.1 cm2 to 139.5 cm2; P < 0.0001) significantly decreased after NAT. Interestingly, there was no significant correlation between increasing extent of WL and survival (WL < 2.5%: median survival of 10.8 mo (range 3.2–46.8); 2.5% ≤ WL < 5.0%: 10.9 mo (range 5.0–27.6); 5.0% ≤ WL < 7.5%: 10.0 mo (range 3.1–26.5); 7.5% ≤ WL < 10.0%: 8.4 mo (range 3.1–16.3); WL ≥ 10.0%: 7.3 mo (range 6.1–10.2)[36]. A retrospective analysis of 89 patients with potentially resectable PDAC, who received a 12-wk regimen of neoadjuvant GEM/cisplatin followed by short-course radiotherapy with concurrent GEM as part of a phase II study, reported a significant depletion of SKM, (median SKM area/height2from 47.5 cm2/m2 to 46.3 cm2/m2; P = 0.01), visceral adipose tissue (VAT) (median VAT area/height2 from 45.1 cm2/m2 to 41.2 cm2/m2; P = 0.01), and SAT (median SAT area/height2 from 53.0 cm2/m2 to 48.7 cm2/m2; P = 0.02). Progressive SKM during NAT was related to a shorter disease-free survival (DFS) (hazard ratio [HR] 0.89, 95%CI 0.80-1.00; P = 0.04), while VAT loss was associated with both shorter progression-free survival (HR 0.98, 95%CI 0.96-0.99; P = 0.01) and OS (HR 0.97, 95%CI 0.95-0.99; P = 0.001)[37]. Another retrospective study evaluated 127 PDAC patients who achieved resectability following approximately 5 mo of NAT, using an array of different chemotherapy regimens (mostly GEM- or fluorouracil-based regimens), found minimal changes in SKM (−0.5 ± 7.8%; P > 0.05), VAT (−1.8 ± 62.6%; P < 0.001), and SAT (−4.8 ± 27.7%; P < 0.001)[38]. A more recent retrospective analysis of 147 locally advanced PDAC patients, treated with NAT, showed a mean WL of 3.7 kg (P < 0.0001), a mean SKM area reduction of 4.2 cm2 (P < 0.0001), while a WL > 5% and a SKM loss were associated with a worse OS (14.5 mo vs 20.3 mo; P = 0.04 and 15.1 mo vs 22.2 mo; P = 0.007, respectively)[39]. Similarly, Naumann et al[39] observed a significant decrease in weight (mean relative WL of 5.3%; P < 0.001), as well as in SAT (from 142.1 cm2 to 115.2 cm2; P < 0.0001), VAT (from 114.7 cm2 to 95.0 cm2; P < 0.0001) and SKM (from 126.0 cm2 to 121.5 cm2; P < 0.0001) during NAT among 141 PDAC patients. Moreover, WL > 5% (HR 2.8, 95%CI 1.28-5.91; P = 0.009) and a reduction in SKM > 5% (HR 5.54, 95%CI 2.56-12.45; P < 0.001) were independently associated with survival[40].
A large multicenter study by Sandini et al[40] including 193 PDAC patients who received NAT (64.2% of patients receiving FOLFIRINOX and 44.6% also undergoing chemoradiotherapy) observed a significant loss of adipose tissue during treatment (median total adipose tissue area from 284 cm2 to 250 cm2; P < 0.001), with no wasting of lean body mass (median SKM from 122.1 cm2 to 123 cm2; P = 0.001). Furthermore, the authors found that an SKM increase was associated with a higher resectability rate (OR 3.7; P = 0.006), suggesting that anabolic potential was preserved in this subset of patients and that the ability to enhance muscle tissue may be related to the treatment response[41]. Conversely, a more recent prospective analysis of 67 PDAC patients reported a deterioration in SKM (median SKM from 128.4 cm2 to 120 cm2; P < 0.001), and adipose tissue (intra-muscular adipose tissue, VAT, and SAT) during NAT, using different chemotherapy regimens (FOLFIRINOX: 44% of patients; GEM-based chemotherapy: 47%) (P < 0.0001). In addition, loss of lean tissue (mean fat-free mass loss 2.6 kg, HR 1.1, P = 0.003, mean SKM loss 1.5 kg, HR 1.21; P = 0.001) and loss of fat mass (mean loss 2.8 kg HR 1.09, P = 0.004) during treatment were related to a higher mortality risk. In multivariable analysis, the preservation of muscle during NAT was predictive of better survival (HR 1.21, 95%CI 1.08-1.35; P = 0.025)[42]. An overview of these studies is reported in Table 1.
| Ref. | Number of patients enrolled | Neoadjuvant treatment | Body composition changes (P value) | Body parameters and clinical outcomes (HR; P value) |
| Naumann et al[35], 2013, Retrospective | 100 | Gemcitabine-based chemoradiation | Weight decrease (P < 0.0001), BMI decrease (P < 0.0001), SAT decrease (P < 0.0001) | WL tended to negatively impact on OS (P > 0.05) |
| Cooper et al[36], 2015, Retrospective | 89 | Gemcitabine and Cisplatin followed by Gemcitabine-based chemoradiation | SKM area/height2 decrease (P = 0.01), VAT area/height2 decrease (P = 0.01), SAT/height2 decrease (P = 0.02) | Loss of SKM was related to a shorter DFS (HR 0.89, P = 0.04), loss of VAT was related to shorter PFS (HR 0.97, P = 0.01) and OS (HR 0.97, P = 0.001) |
| Cloyd et al[37], 2018, Retrospective | 127 | Gemcitabine, Capecitabine or 5-FU based chemoradiation | SKM stability (P > 0.05), VAT decrease (P < 0.001), SAT decrease (P = 0.02) | Body composition changes during were not associated with OS (P > 0.05) |
| Sandini et al[40], 2018, Retrospective | 193 | FOLFIRINOX-based chemoradiotherapy | TAT area decrease (P < 0.001), VAT area decrease (P < 0.0001), SKM area increase (P < 0.0001) | SKM area/height2 was higher in patients who underwent resection (P = 0.004) |
| Naumann et al[38], 2019, Retrospective | 147 | Gemcitabine-based chemoradiation | Weight decrease (P < 0.0001), SKM area decrease (P < 0.0001) | WL > 5% was associated with poor OS (HR 1.56, P = 0.028), SKM area loss > 5% was associated with poor OS (HR 1.50, P = 0.036) |
| Griffin et al[41], 2019, Retrospective | 78 | FOLFIRINOX and gemcitabine-based treatments | SKM area decrease (P < 0.0001), VAT decrease (P < 0.0001), SAT decrease (P < 0.0001) | Loss of lean mass was related to poor OS (HR 1.1, P = 0.003), loss of SKM was related to poor OS (HR 1.21, P = 0.001) |
| Naumann et al[39], 2019, Retrospective | 141 | Gemcitabine-based chemoradiation | Weight decrease (P < 0.001), BMI decrease (P < 0.0001), SAT, VAT and SKM areas decrease (P < 0.0001) | WL > 5% was associated with worse OS (HR 2.8, P = 0.009), SKM area loss > 5% was associated with poor OS (HR 5.54, P < 0.001) |
In PDAC patients treated with NAT, these data highlight that anthropometric, as well as CT-scan derived body composition parameters can be useful to identify high-risk nutritional phenotypes. In the same setting, the inability to maintain body weight and SKM is associated with poor survival outcomes, while the preservation of body composition compartments represents a positive prognostic feature. However, this topic deserves well-designed and adequately sized trials to confirm these preliminary data.
Pancreatic surgery is associated with a relatively high risk of postoperative complications (POCs), due to its technical complexity and to the anatomical location of the pancreas. Most frequent POCs are postoperative pancreatitis (incidence up to 25%-30%)[43,44], delayed gastric emptying (incidence 20%-30%)[45,46], and postoperative pancreatic fistula (10%-15%)[47,48]. Other less common POCs are represented by post pancreatectomy hemorrhage (PPH), intra-abdominal abscesses, anastomotic leakage, venous thrombosis, and biliary stenosis[49,50].
Several studies have highlighted the impact of malnutrition on the incidence of POC. In a retrospective study performed by Kanda et al[51] in 2011, a low prognostic nutritional index (PNI), based on serum albumin concentration and total lymphocyte count, was independently associated with the development of POC, particularly postoperative fistula (HR 2.52, 95%CI 1.37-4.63). La Torre et al[52] retrospectively correlated nutritional status, assessed by the Malnutrition Universal Screening Tool (MUST), with POC and found that MUST was an independent predictor of overall morbidity (HR 2.66, 95%CI 1.36-8.57; P = 0.001) in 143 PDAC patients. In a prospective study published by Darnis et al[53] the Nutritional Index Risk resulted an independent factor for the development of PPH (P = 0.048). Nanashima et al[54] performed a prospective study of 222 PDAC patients to evaluate the relationship between PNI and POC, finding a lower PNI value in patients who developed POC, without statistically significant differences. In a very recent study, Mackay et al[55] performed a nationwide analysis of 1306 PDAC patients and found an incidence of 24% of severe POC, which was identified among the independent factors for not receiving adjuvant chemotherapy (OR 0.32), in particular pancreatic fistula (OR 0.51) and PPH (OR 0.36).
Body composition, particularly the presence of sarcopenia, is also associated with POC development[56]. Amini et al[57,58] reported that sarcopenia, assessed by total psoas volume, was associated with higher risk of POC (OR 1.79). Nishida et al[59] performed a retrospective study finding a higher rate of major POC and particularly of pancreatic fistula development (OR 2.87) in sarcopenic patients (assessed by the SKM index). However, a recent meta-analysis of 42 studies with 7619 patients involved, showed that preoperative sarcopenia was not associated with overall POC development nor with pancreatic fistula[60].
Preoperative nutritional status may play a crucial role in survival rate after surgical oncologic resection. A recent meta-analysis by Liu et al[61], including 11 studies with 2123 PDAC patients, indicated that a low PNI was a significant independent predictor of a worse OS (HR 1.57, 95%CI 1.40-1.77; P < 0.001). Furthermore, preoperative PNI was found to be an independent risk factor for failure to complete planned adjuvant chemotherapy (OR 6.47; P = 0.033)[62].
Serum albumin may be associated with the nutritional status and is a prognostic factor for several cancers[63-65]. Hendifar et al[66], in a cohort of 106 patients with resected PDAC, the authors observed that a decrease in serum albumin was significantly correlated with a worse DFS (HR 2.2; P = 0.024) and preoperative albumin was correlated with a worse OS (HR 0.48; P = 0.008), while preoperative BMI and BMI changes during therapies were not associated with survival outcome, in line with previous analyses[67].
A recent systematic review of PDAC patients showed that sarcopenia was independently associated with a shorter OS in five of eight studies, many of which used measurements of total psoas area or total psoas index for comparison, without identifying an optimal cut-off, that indeed varied widely[56]. Another systematic review and meta-analysis by Bundred et al[60] reported that preoperative sarcopenia was related to lower OS in both resectable (HR 1.95; P < 0.001) and in actually resected patients (HR 1.78; P < 0.001), even if the conclusions were limited by the high heterogeneity (I2: 92%) between studies, due to the different methods of body composition assessment.
Regarding DFS, a retrospective analysis by Okumura et al[68] determined that a low preoperative SKM was a negative independent prognostic factor both for OS (HR 2.0; P < 0.001) and DFS (HR 1.6; P = 0.007), the completion rate of adjuvant chemotherapy in patients with low psoas muscle mass index was significantly lower (65.6% vs 80.1%; P < 0.001) but upon multivariate analysis, only a low PNI remained an independent prognostic factor for worse OS and DFS. In line with these findings, Sugimoto et al retrospectively showed that the measure of height-adjusted and sex-standardized amount of the SKM area was related to both OS (HR 1.36; P = 0.035) and DFS (HR 0.84; P = 0.007) in patients undergoing upfront surgical resection for PDAC[69].
Sarcopenic obesity (defined as the presence of sarcopenia in an obese patient)[70] was significantly associated with a worse OS (12.9 mo vs 20.7 mo; P = 0.04) in the study by Cooper et al[36] in patients with potentially resectable PDAC treated within a phase II trial of NAT. A meta-analysis by Mintziras et al[18] including 11 studies comprising 2297 PDAC patients, found that sarcopenia was significantly associated with a poor OS (HR 1.49; P < 0.001) and the mortality risk was even higher in sarcopenic obese patients (HR 2.01; P < 0.001). Recently, a retrospective analysis of PDAC patients that underwent pancreatic resection, confirmed that sarcopenic obese patients had a worse OS (14 vs 23 mo; P = 0.007)[19].
In the Enhanced Recovery After Surgery (ERAS) era, the “prehabilitation,” an intervention aimed at enhancing a patient’s functional capacity to enable him/her to better cope with a stressful event, has become an evolving area of interest[71]. In this context, preoperative nutritional therapy is increasingly recognized as a crucial component to optimize nutrient stores in preparation for the metabolic demands of surgical trauma, conditioning patients to become stronger for an earlier recovery[23]. Major surgery involves several metabolic and nutritional changes, through the activation of an inflammatory cascade and the release of stress hormones and cytokines, whose intensity is correlated with the degree of tissue injury[72]. Therefore, an adequate preoperative physiological reserve is required to meet the functional demands of the surgical stress and to support the stress-induced mobilization of nutritional substrates[73]. Of note, patients with low preoperative reserves, including malnourished, frail and sarcopenic ones, may exhaust their nutritional reserves rapidly and, therefore, they cannot respond to the increased requirements following surgery[23].
Pancreatic surgery is identified as one of the most challenging surgical areas, due to the magnitude of the dissection and resection, the anatomical location, the resultant global stress, and the relatively high rate of morbidity[34,74]. Several studies have reported improved postoperative outcomes and shorter LOS in patients treated according to ERAS principles, as compared to those receiving conventional care[61,75,76]. As many patients scheduled for PDAC surgery are nutritionally depleted, particular attention should be paid to the preoperative nutritional optimization in this clinical scenario[77], as recommended by evidence-based guidelines for preoperative care for pancreaticoduodenectomy by the ERAS Society, the European Society for Clinical Nutrition and Metabolism (ESPEN), and the International Association for Surgical Metabolism and Nutrition, published in 2013[78]. Preoperative care should include careful nutritional assessment, detection of body composition parameters, and thus a personalized preoperative nutritional optimization[79]. Oral feeding remains the best approach[80], while the role of oral nutritional supplements (ONSs) in malnourished patients is well established, and the ONS role in well-nourished ones is still debated.
According to ERAS Society guidelines, routine use of preoperative enteral nutrition is not recommended, but there is a low-level evidence suggesting that a preoperative nutritional support may be indicated in patients with malnutrition[78]. A recent systematic review of studies conducted on ERAS protocols for patients scheduled for pancreaticoduodenectomy since 2013 emphasized the role of preoperative oral immuno-nutrition in the prevention of incisional wound infections, as well as in the reduction of surgical stress, and suggested that preoperative enteral nutrition should be applied for 10 to 14 d before surgery in patients with severe malnutrition[81].
The recently published International Study Group on Pancreatic Surgery consensus statement regarding nutritional support for pancreatic surgery established that nutritional counselling and ONSs are recommended in patients with moderate malnutrition with no evidence of gastric obstruction, or in those who have a moderate risk of nutritional worsening in the early postoperative period. An aggressive preoperative nutritional support by enteral or parenteral feeding should be considered if at least one of the following criteria, reflecting severe malnutrition, is met[34]: WL > 15% within 6 mo; BMI < 18.5 kg/m2; subjective global assessment grade “C” or nutritional risk score ≥ 5; and serum albumin < 30 g/dL (with no evidence of hepatic or renal dysfunction).
The benefit of preoperative nutritional intervention combined with physical exercise is still a subject of debate. In this regard, a recent Asian analysis among 108 patients undergoing hepato-pancreato-biliary surgeries for malignancy showed that the implementation of prehabilitation, integrating preoperative exercise and nutritional therapy, has the potential to improve outcome, preventing serum albumin deterioration (median, 0.10 vs -0.30; P = 0.001), increasing total muscle/fat ratio (median, 1.83 vs 1.75; P < 0.001), shortening postoperative LOS (median, 23 d vs 30 d; P = 0.045), leading to a potential positive economic impact[82].
Focusing on prehabilitation in 40 patients (45% PDAC) undergoing pancreaticoduodenectomy, a recent randomized controlled trial by Ausania et al[83] estimated the effect of preoperative nutritional support, control of diabetes and exocrine pancreatic insufficiency and physical, as well as respiratory training. Although prehabilitation was not associated with a lower POC incidence, a lower rate of delayed gastric emptying (5.6% vs 40.9%; P = 0.01) and a lower clinically relevant pancreatic fistula rate (11.1% vs 27.3%; P = n.s.) were found in the prehabilitation group. However, this study had several limitations in terms of methodology and was potentially flawed by the short prehabilitation time (patients receiving only 7 d of prehabilitation were included).
In this context, Okumura et al[68] suggested that although the ideal period of preoperative nutritional and exercise therapeutic protocols is not established, at least 1 mo before surgery is required to improve nutritional status. Unfortunately, routine nutritional assessment within the ERAS programs is only partially implemented, probably due to insufficient awareness about nutritional issues among health professionals, lack of structured collaboration between surgeons and clinical nutrition specialists, and the absence of dedicated resources[79].
Studies evaluating nutritional optimization before surgery for PDAC are producing encouraging early results, but definitive clinical evidence is very limited. Further studies on this topic are eagerly warranted.
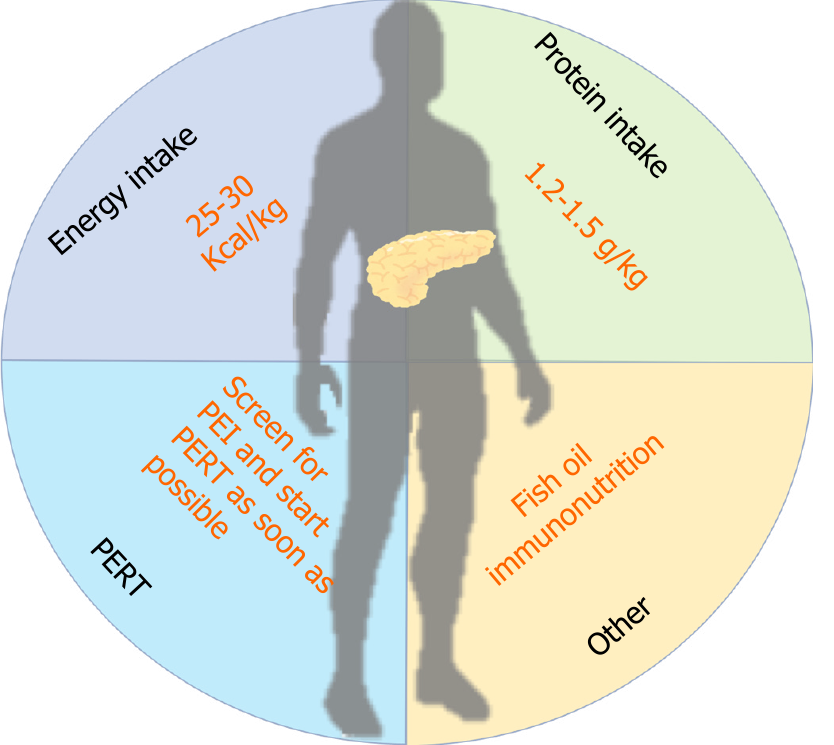
Nutritional management should be started preoperatively to optimize nutritional status in preparation for the increased metabolic requirements of surgical injury. An overview of the suggested nutritional interventions during NAT for PDAC is shown in Figure 1 and in Table 2. An accurate identification of patients at high nutritional risk or already malnourished is crucial to choose the optimal type and timing of nutritional intervention[84]. There are many nutritional risk tools that can be used in clinical practice. Of note, none of the available clinic-biological scores for nutritional assessment meets the diagnostic performance criteria to predict POC after pancreatic surgery, and the proportion of patients at high risk for deranged nutritional status varies using different scores[85].
| Nutritional recommendation during neoadjuvant therapy in patients with pancreatic cancer | |
| Energy intake | Total energy expenditure should be measured; otherwise, 25 to 30 kcal/kg/d should be guaranteed |
| Protein intake | 1.2-1.5 g/kg/d should be prescribed |
| Fish oil | Fish oil supplementation may improve the metabolic derangements |
| PERT | Tumor in the head: start PERT immediately |
| Tumor in the body/tail: Perform PEI test before prescribing PERT | |
| Immunonutrition | Immunonutrition-based supplements may improve clinical outcomes |
The inconsistency in predicting poor outcomes with different nutritional screening tools may lead to either insufficient or excessive nutritional treatments, with potentially harmful effects. In this regard, the new Global Leadership Initiative on Malnutrition (GLIM) criteria for the diagnosis and grading of malnutrition have been introduced and recently validated in a large population of patients undergoing abdominal surgery, including pancreatic resections[86]. According to GLIM criteria, a patient can be defined malnourished if, after a positive risk screening test for malnutrition, presents at least one phenotypic criterion (non-intentional WL, low BMI, or reduced muscle mass) and one etiologic criterion (reduced food intake/assi
Numerous studies have shown the prognostic impact of body composition assessment by CT-scan in oncological patients and in those undergoing cancer treatments[88-91]. Especially, in patients undergoing NAT, CT scans are usually performed several times during treatment. Therefore, CT scan-based body composition analysis could be easily implemented in routine clinical practice.
Energy balance in catabolic condition, as in PDAC patients, is deeply influenced by changes in dietary intake[92], therapy-linked factors[93], and decreased levels of physical activity[94].
Okusaka et al[95] found that a longer survival time was associated with a high energy intake in patients affected by advanced PDAC (P = 0.02). Moreover, Bye et al[96] in their work, found a correlation between PDAC-specific symptoms (e.g., pain, fatigue, nausea), WL, and poor energy intake. The caloric requirement of PDAC patients should be assessed in a personalized way and when energy expenditure is not measured individually, ESPEN guidelines suggest an intake of 25-30 kcal/kg/day[97].
In conclusion, assessment of energy intake should be guaranteed in PDAC patients with the aim to elaborate a personalized nutritional strategy in order to decrease the risk of WL and consequently malnutrition.
Recent literature data on nutritional support in oncological patients attribute high relevance to correct protein intake (PI), with the aim to promote muscle anabolism[98]. Sarcopenic patients, similar to oncological ones, have poor protein stores and this could contribute to increased POC, LOS and mortality[23]. The most recent and interesting theories affirm that the best way to prepare the patient to the surgical trauma should be a multimodal approach, which includes nutritional changes, psychological support, and physical training[99,100]. ESPEN guidelines on nutrition in cancer patients set the PI target to 1.2-1.5 g/kg per day. However, it is not clarified if there are specific amino acid mixtures that can improve clinical outcomes in this setting[97].
Several studies have highlighted the role of branched-chain amino acids (BCAAs) to decrease muscle catabolism[101]. Between the first studies conducted on this field of research, Tayek et al[102] found a higher response in terms of protein accretion and albumin synthesis using BCAA-enriched parenteral nutrition (PN) formulas with respect to standard PN. More recently, Deutz et al[103] conducted a randomized, controlled, double-blind study on 25 cancer patients, with the interventional group receiving a functional food enriched with 40 g of protein and leucine. In this group, the rate of muscle protein synthesis was higher than the control group (P = 0.02). β-hydroxy-β-methylbutyrate (HMB) is a metabolite of the essential amino acid leucine, which induces an anabolic effect in cancer patients, promoting regenerative events, suppressing protein degradation, and activating anabolic signaling pathways[104]. In many murine preclinical studies HMB showed the potential to reduce WL, tumor weight, and to attenuate protein degradation[105,106]. In the study by May et al[107], which also included advanced PDAC patients, the authors found an increase of fat-free mass (FFM) in patients treated with a mixture of HMB (3 g/d), L-arginine (14 g/d), and L-glutamine (14 g/d) vs the control group. On the other hand, a randomized, double-blind, placebo-controlled trial performed by Berk et al[108], showed no statistical differences in FFM among patients treated with the same mixture. Despite the lack of a strong evidence from the literature, it is advisable to refer to ESPEN guidelines and recommend a PI of 1.2-1.5 g/kg per day in PDAC patients.
Fish oil (FO) is an anti-inflammatory nutraceutical, which is often used to improve the imbalance between omega-3 (w-3 FA) and omega-6 fatty acids (w-6 FA) in oncologic patients among others[109]. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are the w-3 FA acids found in FO that are able to induce an anti-inflammatory response[110]. Moreover, many epidemiological studies have suggested that a high consumption of FO and therefore of w-3 FA reduces the risk of pancreatic cancer[111]. These molecules are also involved in the synthesis of cell membranes[112], hormones, receptors, prostaglandins, and leukotrienes[113]. Moreover, EPA and DHA show anabolic effects when used on sarcopenic patients like oncological ones[114]. Barber[115] analyzed the impact of FO on patients with cancer cachexia, concluding that FO had the potential to normalize the metabolic derangements of oncological patients.
For all of these reasons, FO can be useful both in surgical and medical patients affected by PDAC, through oral, enteral, or parenteral administration. Werner et al[116] performed a randomized, double-blind, and controlled trial, comparing the administration of 500 mg FO (60% of FO and 40% of medium-chain triglycerides, 6.9 g/100 g of EPA and 13.6 g/100 g DHA) vs 500 mg marine phospholipids (8.5 g/100 g EPA and 12.3 g/100 g DHA) three times per day for 6 consecutive weeks. The authors found a stabilization of body weight after 6 wk in both groups (P = 0.001 and P = 0.003, respectively). Moreover, they found a significant increase in the amount of anti-inflammatory EPA and DHA, a decrease of the pro-inflammatory arachidonic acid and an increase in high-density lipoprotein in the patient’s plasma of FO group. Arshad et al[117] described a reduction in the concentration of pro-inflammatory cytokines and growth factors using intravenous omega-3 enriched lipid emulsion with improvement in survival outcomes, in patients affected by locally advanced or metastatic PDAC eligible for GEM treatment.
Pancreatic exocrine insufficiency (PEI) is a frequent condition that can profoundly affect the nutritional status of PDAC patients. PEI is defined as the clinical condition in which the quantity of enzymes secreted by the pancreas are not sufficient to guarantee the physiological digestive processes and can be caused by lack of production by the pancreas and/or by the obstruction of ducts by external causes, such as tumors[118]. Symptoms of PEI may vary from micronutrient deficiency to abdominal pain, flatulence, WL, and steatorrhea, defined as the presence of more than 7 g fat in the feces per day[119,120]. PEI in PDAC patients ranges from 30% to 100%, according to the method used to diagnose the condition[121].
Pancreatic enzyme replacement therapy (PERT) can improve the malabsorption-related symptoms through the amelioration of protein and fat digestion processes[122,123].
In the paper by Roberts et al[124], the use of PERT was associated with an increase of survival among PDAC patients (survival time ratio: 2.62, 95%CI 2.27-3.02). Landers et al[125] performed a pilot study to determine the efficacy of PERT in metastatic PDAC patients, using 50.000 IU of pancrealipase for each meal and 25.000 IU for each snack, showing an improvement of symptoms and quality of life (QoL) assessed at 1 and 3 wk after the start of treatment. A retrospective analysis by Domínguez-Muñoz et al[126] was conducted among 160 patients with unresectable PDAC. The authors divided into two groups the study population: the first group followed the standard of care, while the second one was screened for PEI and started PERT if necessary. Survival in the second group was longer than in the first one (HR 2.117, 95%CI 1.493-3.002; P < 0.001). Moreover, also in patients with significant WL at diagnosis, PERT was associated with longer survival (HR 2.52, 95%CI 1.55-4.11; P < 0.001). PERT is, therefore, useful to treat malnutrition in PDAC patients affected by PEI, and is associated with an improvement in QoL and survival[127]. Nevertheless, the optimal dose and optimal timing of PERT administration in PDAC patients is not well defined[128]. A very recent position paper by Pezzilli et al[12] aimed to give recommendations on PERT in the PDAC setting, concluding that patients with head PDAC should be given enzymes, while a diagnostic evaluation should be performed using fecal elastase in patients affected by body or tail neoplasm prior to giving them PERT. Moreover, in the next few months, a Cochrane Systematic Review on this issue is planning to be published[129].
In conclusion, due to the underrecognition of this condition and its metabolic consequences, PEI should be investigated in all patients affected by PDAC, and PERT should be started as soon as possible, when necessary.
Immunonutrition (IN) can be defined as modulation of the activity of the immune system by specific food or nutrients, called immunonutrients; the most important are w-3 FA, glutamine, arginine, and nucleotides[130].
The role of the IN has been studied only in a few series in the surgical setting. In 2011 Klek et al[131] performed a prospective, randomized, double-blind clinical trial evaluating the impact of IN on surgical patients affected by PDAC or gastric cancer, finding differences in postoperative LOS (P = 0.006), infectious POC (P = 0.04), overall morbidity (P = 0.01), and mortality (P = 0.03). The group of Shirakawa et al[132] also found a lower rate of incisional wound infection in the IN group vs standard therapy (P = 0.012). Gade et al[133] performed a randomized controlled trial enrolling 35 surgical patients, with the aim to define the effect of 7 d of oral IN supplementation in PDAC patients. However, the author found no statistically significant improvements in the IN group. Silvestri et al[134] studied the impact of oral IN in non-malnourished PDAC patients undergoing pancreaticoduodenectomy and found a significative impact on LOS (P = 0.035) and infectious POC (P = 0.034). On the contrary, no differences in terms of mortality and overall morbidity rate were found. While IN in surgical PDAC patients reduces POC, LOS, and improves survival rate, no data were found in the recent literature on IN use during NAT.
Patients undergoing multimodal oncological care are at increased risk of progressive nutritional worsening, with deleterious effects on surgical and oncological outcomes[135,136]. In this setting, current standard of care creates a minimum timeframe of four to 6 mo for NAT completion. This time period could thus represent a valuable opportunity for prehabilitation, to minimize the nutritional/metabolic impact of NAT, but published literature is scarce on this topic[34,137]. Indeed, most studies investigating ERAS programs/prehabilitation for PDAC excluded patients who had received preoperative NAT[82,84].
Recently, a prospective randomized control study by Akita et al[138] aimed at exploring whether a nutritional intervention consisting in 560 kcal/day of EPA-enriched nutritional supplements might impact on nutritional status in PDAC patients who received GEM-based neoadjuvant chemoradiotherapy. The authors reported that the psoas major muscle area ratio was significantly higher in the nutritional intervention group (median, 0.96 vs 0.89; P = 0.001), and that patients who consumed ≥ 50% of the EPA-enriched supplement presented significantly higher SKM ratios (P = 0.042). With regards to patients following NAT for locally advanced PDAC, a recent prospective analysis evaluated the impact of the preoperative IN supplementation on surgical outcomes in subjects undergoing irreversible electroporation surgery. Patients receiving IN presented a lower decrease in nutritional risk index (-12.6 vs -16.2; P = 0.03), serum albumin levels (-1.1 vs -1.5; P < 0.01), and experienced a statistically significant decrease in POC (P = 0.05) and LOS (10.7 vs 17.4; P = 0.01)[139].
Only a preliminary prospective study has reported the feasibility of a preoperative prehabilitation program, including nutritional counselling by a dietitian, of IN for 5 d before surgery and an exercise program, in patients with borderline resectable PDAC who received NAT[82].
In other areas of surgery, multimodal prehabilitation in patients receiving NAT has recently generated growing interest and seems to have a potential clinical benefit. Recently, a retrospective study of 22 patients, planning to undergo NAT for esophageal cancer, found a trend to a lower WL (3.0% vs 4.4%; P = 0.05) and a lower percentage of patients requiring postsurgical readmission rates at 30-d and 90-d (0.0% vs 18.2%; P = 0.14 and 18.2% vs 27.3%; P = 0.6, respectively) in those submitted to a structured prehabiliation program, which included tailored nutritional counselling, psychological support and supervised physical exercise[140].
Despite the lack of high-quality clinical evidence, many PDAC patients with resectable, borderline resectable, and locally advanced disease, nowadays undergo NAT as part of an integrated, multimodal, treatment program. Since NAT may provide an interesting window of opportunity to implement nutritional prehabilitation in PDAC patients and the limited available data on this issue suggest a reduction in POC, LOS and readmission rates, well-designed, controlled, randomized clinical trials are needed to establish new recommendations in this NAT setting.
The authors gratefully want to thank Mr. Philip Egger for his kindly revision of the English language and style.
Manuscript source: Invited manuscript
Specialty type: Nutrition and dietetics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koustas E S-Editor: Zhang H L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55670] [Article Influence: 7952.9] [Reference Citation Analysis (132)] |
| 2. | Ghaneh P, Kleeff J, Halloran CM, Raraty M, Jackson R, Melling J, Jones O, Palmer DH, Cox TF, Smith CJ, O'Reilly DA, Izbicki JR, Scarfe AG, Valle JW, McDonald AC, Carter R, Tebbutt NC, Goldstein D, Padbury R, Shannon J, Dervenis C, Glimelius B, Deakin M, Anthoney A, Lerch MM, Mayerle J, Oláh A, Rawcliffe CL, Campbell F, Strobel O, Büchler MW, Neoptolemos JP; European Study Group for Pancreatic Cancer. The Impact of Positive Resection Margins on Survival and Recurrence Following Resection and Adjuvant Chemotherapy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2019;269:520-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 3. | Dobiasch S, Goerig NL, Fietkau R, Combs SE. Essential role of radiation therapy for the treatment of pancreatic cancer : Novel study concepts and established treatment recommendations. Strahlenther Onkol. 2018;194:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | NCCN clinical practice guidelines in oncology. Pancreatic adenocarcinoma. National Comprehensive Cancer Network. Version 1. 2020 [Internet]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. |
| 5. | Uzunoglu FG, Welte MN, Gavazzi F, Maggino L, Perinel J, Salvia R, Janot M, Reeh M, Perez D, Montorsi M, Zerbi A, Adham M, Uhl W, Bassi C, Izbicki JR, Malleo G, Bockhorn M. Evaluation of the MDACC clinical classification system for pancreatic cancer patients in an European multicenter cohort. Eur J Surg Oncol. 2019;45:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Maggino L, Malleo G, Marchegiani G, Viviani E, Nessi C, Ciprani D, Esposito A, Landoni L, Casetti L, Tuveri M, Paiella S, Casciani F, Sereni E, Binco A, Bonamini D, Secchettin E, Auriemma A, Merz V, Simionato F, Zecchetto C, D'Onofrio M, Melisi D, Bassi C, Salvia R. Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2019;154:932-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Janssen QP, Buettner S, Suker M, Beumer BR, Addeo P, Bachellier P, Bahary N, Bekaii-Saab T, Bali MA, Besselink MG, Boone BA, Chau I, Clarke S, Dillhoff M, El-Rayes BF, Frakes JM, Grose D, Hosein PJ, Jamieson NB, Javed AA, Khan K, Kim KP, Kim SC, Kim SS, Ko AH, Lacy J, Margonis GA, McCarter MD, McKay CJ, Mellon EA, Moorcraft SY, Okada KI, Paniccia A, Parikh PJ, Peters NA, Rabl H, Samra J, Tinchon C, van Tienhoven G, van Veldhuisen E, Wang-Gillam A, Weiss MJ, Wilmink JW, Yamaue H, Homs MYV, van Eijck CHJ, Katz MHG, Groot Koerkamp B. Neoadjuvant FOLFIRINOX in Patients With Borderline Resectable Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. J Natl Cancer Inst. 2019;111:782-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 8. | Habermehl D, Kessel K, Welzel T, Hof H, Abdollahi A, Bergmann F, Rieken S, Weitz J, Werner J, Schirmacher P, Büchler MW, Debus J, Combs SE. Neoadjuvant chemoradiation with Gemcitabine for locally advanced pancreatic cancer. Radiat Oncol. 2012;7:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, Strobel O, Jäger D, Ulrich A, Büchler MW. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg. 2016;264:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 380] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 10. | Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, de van der Schueren MA, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 1465] [Article Influence: 162.8] [Reference Citation Analysis (0)] |
| 11. | Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2908] [Cited by in RCA: 3809] [Article Influence: 272.1] [Reference Citation Analysis (0)] |
| 12. | Pezzilli R, Caccialanza R, Capurso G, Brunetti O, Milella M, Falconi M. Pancreatic Enzyme Replacement Therapy in Pancreatic Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Gilliland TM, Villafane-Ferriol N, Shah KP, Shah RM, Tran Cao HS, Massarweh NN, Silberfein EJ, Choi EA, Hsu C, McElhany AL, Barakat O, Fisher W, Van Buren G. Nutritional and Metabolic Derangements in Pancreatic Cancer and Pancreatic Resection. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 14. | Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, Sabbatino F, Santos DD, Allen JN, Blaszkowsky LS, Clark JW, Faris JE, Goyal L, Kwak EL, Murphy JE, Ting DT, Wo JY, Zhu AX, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 650] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 15. | Trestini I, Paiella S, Sandini M, Sperduti I, Elio G, Pollini T, Melisi D, Auriemma A, Soldà C, Bonaiuto C, Tregnago D, Avancini A, Secchettin E, Bonamini D, Lanza M, Pilotto S, Malleo G, Salvia R, Bovo C, Gianotti L, Bassi C, Milella M. Prognostic Impact of Preoperative Nutritional Risk in Patients Who Undergo Surgery for Pancreatic Adenocarcinoma. Ann Surg Oncol. 2020;27:5325-5334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Bicakli DH, Uslu R, Güney SC, Coker A. The Relationship Between Nutritional Status, Performance Status, and Survival Among Pancreatic Cancer Patients. Nutr Cancer. 2020;72:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 599] [Cited by in RCA: 1467] [Article Influence: 244.5] [Reference Citation Analysis (0)] |
| 18. | Mintziras I, Miligkos M, Wächter S, Manoharan J, Maurer E, Bartsch DK. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int J Surg. 2018;59:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 19. | Gruber ES, Jomrich G, Tamandl D, Gnant M, Schindl M, Sahora K. Sarcopenia and sarcopenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS One. 2019;14:e0215915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Park I, Choi SJ, Kim YS, Ahn HK, Hong J, Sym SJ, Park J, Cho EK, Lee JH, Shin YJ, Shin DB. Prognostic Factors for Risk Stratification of Patients with Recurrent or Metastatic Pancreatic Adenocarcinoma Who Were Treated with Gemcitabine-Based Chemotherapy. Cancer Res Treat. 2016;48:1264-1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5610] [Article Influence: 400.7] [Reference Citation Analysis (1)] |
| 22. | Kurita Y, Kobayashi N, Tokuhisa M, Goto A, Kubota K, Endo I, Nakajima A, Ichikawa Y. Sarcopenia is a reliable prognostic factor in patients with advanced pancreatic cancer receiving FOLFIRINOX chemotherapy. Pancreatology. 2019;19:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Gillis C, Wischmeyer PE. Pre-operative nutrition and the elective surgical patient: why, how and what? Anaesthesia. 2019;74 Suppl 1:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 24. | Weimann A. Is there a rationale for perioperative nutrition therapy in the times of ERAS? [Internet]. Vol. 4, Innovative Surgical Sciences. De Gruyter; 2019; 152–157. [cited 2020 Jun 12]. Available from: https://www.degruyter.com/view/journals/iss/4/4/article-p152.xml. |
| 25. | Paiella S, Trestini I, Milella M, Salvia R. ASO Author Reflections: Preoperative Nutritional Care: The 'Cinderella' of Surgical Management in Patients with Pancreatic Cancer. Ann Surg Oncol. 2020;27:5335-5336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Mele MC, Rinninella E, Cintoni M, Pulcini G, Di Donato A, Grassi F, Trestini I, Pozzo C, Tortora G, Gasbarrini A, Bria E. Nutritional Support in Lung Cancer Patients: The State of the Art. Clin Lung Cancer. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Laviano A, Di Lazzaro L, Koverech A. Nutrition support and clinical outcome in advanced cancer patients. Proc Nutr Soc. 2018;77:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Trestini I, Carbognin L, Sperduti I, Bonaiuto C, Auriemma A, Melisi D, Salvatore L, Bria E, Tortora G. Prognostic impact of early nutritional support in patients affected by locally advanced and metastatic pancreatic ductal adenocarcinoma undergoing chemotherapy. Eur J Clin Nutr. 2018;72:772-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15272] [Article Influence: 3054.4] [Reference Citation Analysis (4)] |
| 30. | Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, Carter R, Tebbutt NC, Dervenis C, Smith D, Glimelius B, Charnley RM, Lacaine F, Scarfe AG, Middleton MR, Anthoney A, Ghaneh P, Halloran CM, Lerch MM, Oláh A, Rawcliffe CL, Verbeke CS, Campbell F, Büchler MW; European Study Group for Pancreatic Cancer. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 459] [Article Influence: 35.3] [Reference Citation Analysis (1)] |
| 31. | Heinrich S, Pestalozzi B, Lesurtel M, Berrevoet F, Laurent S, Delpero JR, Raoul JL, Bachellier P, Dufour P, Moehler M, Weber A, Lang H, Rogiers X, Clavien PA. Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: a randomized multicenter phase III study (NEOPAC study). BMC Cancer. 2011;11:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Salvia R, Malleo G, Maggino L, Milella M, Bassi C. Pancreatic ductal adenocarcinoma: time for a neoadjuvant revolution? Updates Surg. 2020;72:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Gianotti L, Besselink MG, Sandini M, Hackert T, Conlon K, Gerritsen A, Griffin O, Fingerhut A, Probst P, Abu Hilal M, Marchegiani G, Nappo G, Zerbi A, Amodio A, Perinel J, Adham M, Raimondo M, Asbun HJ, Sato A, Takaori K, Shrikhande SV, Del Chiaro M, Bockhorn M, Izbicki JR, Dervenis C, Charnley RM, Martignoni ME, Friess H, de Pretis N, Radenkovic D, Montorsi M, Sarr MG, Vollmer CM, Frulloni L, Büchler MW, Bassi C. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2018;164:1035-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 34. | Nitta H, Baba H, Sugimori K, Furuse J, Ohkawa S, Yamamoto K, Minami H, Shimokawa M, Wakabayashi GO, Aiba K; CINV Study Group of Japan. Chemotherapy-induced Nausea and Vomiting in Patients with Hepatobiliary and Pancreatic Cancer Treated with Chemotherapy: A Prospective Observational Study by the CINV Study Group of Japan. Anticancer Res. 2016;36:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 35. | Naumann P, Habermehl D, Welzel T, Debus J, Combs SE. Outcome after neoadjuvant chemoradiation and correlation with nutritional status in patients with locally advanced pancreatic cancer. Strahlenther Onkol. 2013;189:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Cooper AB, Slack R, Fogelman D, Holmes HM, Petzel M, Parker N, Balachandran A, Garg N, Ngo-Huang A, Varadhachary G, Evans DB, Lee JE, Aloia T, Conrad C, Vauthey JN, Fleming JB, Katz MH. Characterization of Anthropometric Changes that Occur During Neoadjuvant Therapy for Potentially Resectable Pancreatic Cancer. Ann Surg Oncol. 2015;22:2416-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 37. | Cloyd JM, Nogueras-González GM, Prakash LR, Petzel MQB, Parker NH, Ngo-Huang AT, Fogelman D, Denbo JW, Garg N, Kim MP, Lee JE, Tzeng CD, Fleming JB, Katz MHG. Anthropometric Changes in Patients with Pancreatic Cancer Undergoing Preoperative Therapy and Pancreatoduodenectomy. J Gastrointest Surg. 2018;22:703-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Naumann P, Eberlein J, Farnia B, Hackert T, Debus J, Combs SE. Continued Weight Loss and Sarcopenia Predict Poor Outcomes in Locally Advanced Pancreatic Cancer Treated with Chemoradiation. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Naumann P, Eberlein J, Farnia B, Liermann J, Hackert T, Debus J, Combs SE. Cachectic Body Composition and Inflammatory Markers Portend a Poor Prognosis in Patients with Locally Advanced Pancreatic Cancer Treated with Chemoradiation. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Sandini M, Patino M, Ferrone CR, Alvarez-Pérez CA, Honselmann KC, Paiella S, Catania M, Riva L, Tedesco G, Casolino R, Auriemma A, Salandini MC, Carrara G, Cristel G, Damascelli A, Ippolito D, D'Onofrio M, Lillemoe KD, Bassi C, Braga M, Gianotti L, Sahani D, Fernández-Del Castillo C. Association Between Changes in Body Composition and Neoadjuvant Treatment for Pancreatic Cancer. JAMA Surg. 2018;153:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 41. | Griffin OM, Duggan SN, Ryan R, McDermott R, Geoghegan J, Conlon KC. Characterising the impact of body composition change during neoadjuvant chemotherapy for pancreatic cancer. Pancreatology. 2019;19:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 42. | Connor S. Defining post-operative pancreatitis as a new pancreatic specific complication following pancreatic resection. HPB (Oxford). 2016;18:642-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 43. | Lermite E, Sommacale D, Piardi T, Arnaud JP, Sauvanet A, Dejong CH, Pessaux P. Complications after pancreatic resection: diagnosis, prevention and management. Clin Res Hepatol Gastroenterol. 2013;37:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Hüttner FJ, Fitzmaurice C, Schwarzer G, Seiler CM, Antes G, Büchler MW, Diener MK. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev. 2016;2:CD006053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 45. | McEvoy SH, Lavelle LP, Hoare SM, O'Neill AC, Awan FN, Malone DE, Ryan ER, McCann JW, Heffernan EJ. Pancreaticoduodenectomy: expected post-operative anatomy and complications. Br J Radiol. 2014;87:20140050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Kawaida H, Kono H, Hosomura N, Amemiya H, Itakura J, Fujii H, Ichikawa D. Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery. World J Gastroenterol. 2019;25:3722-3737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (3)] |
| 47. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3504] [Article Influence: 175.2] [Reference Citation Analysis (34)] |
| 48. | Bhosale P, Fleming J, Balachandran A, Charnsangavej C, Tamm EP. Complications of Whipple surgery: imaging analysis. Abdom Imaging. 2013;38:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Hafezi-Nejad N, Fishman EK, Zaheer A. Imaging of post-operative pancreas and complications after pancreatic adenocarcinoma resection. Abdom Radiol (NY). 2018;43:476-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85:1001-1005. [PubMed] |
| 51. | Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 465] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 52. | La Torre M, Ziparo V, Nigri G, Cavallini M, Balducci G, Ramacciato G. Malnutrition and pancreatic surgery: prevalence and outcomes. J Surg Oncol. 2013;107:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 53. | Darnis B, Lebeau R, Chopin-Laly X, Adham M. Postpancreatectomy hemorrhage (PPH): predictors and management from a prospective database. Langenbecks Arch Surg. 2013;398:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Nanashima A, Hiyoshi M, Imamura N, Yano K, Hamada T, Hamada R, Nagatomo K, Ikenoue M, Tobinaga S, Nagayasu T. Clinical significance of preoperative nutritional parameter and patient outcomes after pancreatectomy: A retrospective study at two academic institute. Ann Hepatobiliary Pancreat Surg. 2019;23:168-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Mackay TM, Smits FJ, Roos D, Bonsing BA, Bosscha K, Busch OR, Creemers GJ, van Dam RM, van Eijck CHJ, Gerhards MF, de Groot JWB, Groot Koerkamp B, Haj Mohammad N, van der Harst E, de Hingh IHJT, Homs MYV, Kazemier G, Liem MSL, de Meijer VE, Molenaar IQ, Nieuwenhuijs VB, van Santvoort HC, van der Schelling GP, Stommel MWJ, Ten Tije AJ, de Vos-Geelen J, Wit F, Wilmink JW, van Laarhoven HWM, Besselink MG; Dutch Pancreatic Cancer Group. The risk of not receiving adjuvant chemotherapy after resection of pancreatic ductal adenocarcinoma: a nationwide analysis. HPB (Oxford). 2020;22:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 56. | Chan MY, Chok KSH. Sarcopenia in pancreatic cancer - effects on surgical outcomes and chemotherapy. World J Gastrointest Oncol. 2019;11:527-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 57. | Amini N, Spolverato G, Gupta R, Margonis GA, Kim Y, Wagner D, Rezaee N, Weiss MJ, Wolfgang CL, Makary MM, Kamel IR, Pawlik TM. Impact Total Psoas Volume on Short- and Long-Term Outcomes in Patients Undergoing Curative Resection for Pancreatic Adenocarcinoma: a New Tool to Assess Sarcopenia. J Gastrointest Surg. 2015;19:1593-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 58. | Amini N, Spolverato G, Gupta R, Margonis GA, Kim Y, Wagner D, Rezaee N, Weiss MJ, Wolfgang CL, Makary MM, Kamel IR, Pawlik TM. Erratum to: Impact Total Psoas Volume on Short- and Long-Term Outcomes in Patients Undergoing Curative Resection for Pancreatic Adenocarcinoma: a New Tool to Assess Sarcopenia. J Gastrointest Surg. 2016;20:1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Nishida Y, Kato Y, Kudo M, Aizawa H, Okubo S, Takahashi D, Nakayama Y, Kitaguchi K, Gotohda N, Takahashi S, Konishi M. Preoperative Sarcopenia Strongly Influences the Risk of Postoperative Pancreatic Fistula Formation After Pancreaticoduodenectomy. J Gastrointest Surg. 2016;20:1586-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 60. | Bundred J, Kamarajah SK, Roberts KJ. Body composition assessment and sarcopenia in patients with pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford). 2019;21:1603-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 61. | Liu J, Jiang S, Yang X, Li X, Wang N. The Significant Value of Preoperative Prognostic Nutritional Index for Survival in Pancreatic Cancers: A Meta-analysis. Pancreas. 2018;47:793-799.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Akahori T, Sho M, Tanaka T, Kinoshita S, Nagai M, Nishiwada S, Nishiofuku H, Ohbayashi C, Kichikawa K, Nakajima Y. Factors associated with failure to complete adjuvant chemotherapy in pancreatic cancer. Am J Surg. 2016;211:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 575] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 64. | Carr BI, Guerra V. Serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int J Biol Markers. 2017;32:e391-e396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 65. | Danan D, Shonka DC Jr, Selman Y, Chow Z, Smolkin ME, Jameson MJ. Prognostic value of albumin in patients with head and neck cancer. Laryngoscope. 2016;126:1567-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 66. | Hendifar A, Osipovl A, Khanujal J, Nissen N, Naziri J, Yang W, Li Q, Tuli R. Influence of Body Mass Index and Albumin on Perioperative Morbidity and Clinical Outcomes in Resected Pancreatic Adenocarcinoma. PLoS One. 2016;11:e0152172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | Vos T, GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5206] [Cited by in RCA: 4834] [Article Influence: 537.1] [Reference Citation Analysis (0)] |
| 68. | Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Masui T, Mizumoto M, Hammad A, Mori A, Takaori K, Uemoto S. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 69. | Sugimoto M, Farnell MB, Nagorney DM, Kendrick ML, Truty MJ, Smoot RL, Chari ST, Moynagh MR, Petersen GM, Carter RE, Takahashi N. Decreased Skeletal Muscle Volume Is a Predictive Factor for Poorer Survival in Patients Undergoing Surgical Resection for Pancreatic Ductal Adenocarcinoma. J Gastrointest Surg. 2018;22:831-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Kamarajah SK, Bundred J. Comments on: Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int J Surg. 2019;66:99-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017;152:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 2207] [Article Influence: 275.9] [Reference Citation Analysis (0)] |
| 72. | Pillinger NL, Robson JL, Kam P. Nutritional prehabilitation: physiological basis and clinical evidence. Anaesth Intensive Care. 2018;46:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Gillis C, Carli F. Promoting Perioperative Metabolic and Nutritional Care. Anesthesiology. 2015;123:1455-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 74. | Ji HB, Zhu WT, Wei Q, Wang XX, Wang HB, Chen QP. Impact of enhanced recovery after surgery programs on pancreatic surgery: A meta-analysis. World J Gastroenterol. 2018;24:1666-1678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 75. | Rinninella E, Persiani R, D'Ugo D, Pennestrì F, Cicchetti A, Di Brino E, Cintoni M, Miggiano GAD, Gasbarrini A, Mele MC. NutriCatt protocol in the Enhanced Recovery After Surgery (ERAS) program for colorectal surgery: The nutritional support improves clinical and cost-effectiveness outcomes. Nutrition. 2018;50:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 76. | Ardito F, Lai Q, Rinninella E, Mimmo A, Vellone M, Panettieri E, Adducci E, Cintoni M, Mele MC, Gasbarrini A, Giuliante F. The impact of personalized nutritional support on postoperative outcome within the enhanced recovery after surgery (ERAS) program for liver resections: results from the NutriCatt protocol. Updates Surg. 2020;72:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Goonetilleke KS, Siriwardena AK. Systematic review of peri-operative nutritional supplementation in patients undergoing pancreaticoduodenectomy. JOP. 2006;7:5-13. [PubMed] |
| 78. | Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, Parks RW, Fearon KC, Lobo DN, Demartines N, Braga M, Ljungqvist O, Dejong CH; Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg. 2013;37:240-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 79. | Sandrucci S, Beets G, Braga M, Dejong K, Demartines N. Perioperative nutrition and enhanced recovery after surgery in gastrointestinal cancer patients. A position paper by the ESSO task force in collaboration with the ERAS society (ERAS coalition). Eur J Surg Oncol. 2018;44:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 80. | West MA, Wischmeyer PE, Grocott MPW. Prehabilitation and Nutritional Support to Improve Perioperative Outcomes. Curr Anesthesiol Rep. 2017;7:340-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 81. | Xu X, Zheng C, Zhao Y, Chen W, Huang Y. Enhanced recovery after surgery for pancreaticoduodenectomy: Review of current evidence and trends. Int J Surg. 2018;50:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Nakajima H, Yokoyama Y, Inoue T, Nagaya M, Mizuno Y, Kadono I, Nishiwaki K, Nishida Y, Nagino M. Clinical Benefit of Preoperative Exercise and Nutritional Therapy for Patients Undergoing Hepato-Pancreato-Biliary Surgeries for Malignancy. Ann Surg Oncol. 2019;26:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 83. | Ausania F, Senra P, Meléndez R, Caballeiro R, Ouviña R, Casal-Núñez E. Prehabilitation in patients undergoing pancreaticoduodenectomy: a randomized controlled trial. Rev Esp Enferm Dig. 2019;111:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 84. | Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, Laviano A, Ljungqvist O, Lobo DN, Martindale R, Waitzberg DL, Bischoff SC, Singer P. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr. 2017;36:623-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 1055] [Article Influence: 131.9] [Reference Citation Analysis (0)] |
| 85. | Probst P, Haller S, Bruckner T, Ulrich A, Strobel O, Hackert T, Diener MK, Büchler MW, Knebel P. Prospective trial to evaluate the prognostic value of different nutritional assessment scores in pancreatic surgery (NURIMAS Pancreas). Br J Surg. 2017;104:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 86. | Skeie E, Tangvik RJ, Nymo LS, Harthug S, Lassen K, Viste A. Weight loss and BMI criteria in GLIM's definition of malnutrition is associated with postoperative complications following abdominal resections - Results from a National Quality Registry. Clin Nutr. 2020;39:1593-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 87. | Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R, Blaauw R, Coats A, Crivelli A, Evans DC, Gramlich L, Fuchs-Tarlovsky V, Keller H, Llido L, Malone A, Mogensen KM, Morley JE, Muscaritoli M, Nyulasi I, Pirlich M, Pisprasert V, de van der Schueren MAE, Siltharm S, Singer P, Tappenden K, Velasco N, Waitzberg D, Yamwong P, Yu J, Van Gossum A, Compher C; GLIM Core Leadership Committee; GLIM Working Group. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1623] [Article Influence: 231.9] [Reference Citation Analysis (0)] |
| 88. | Malietzis G, Aziz O, Bagnall NM, Johns N, Fearon KC, Jenkins JT. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. Eur J Surg Oncol. 2015;41:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 89. | Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 768] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 90. | Rinninella E, Cintoni M, Raoul P, Pozzo C, Strippoli A, Bria E, Tortora G, Gasbarrini A, Mele MC. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: A systematic review and meta-analysis. Clin Nutr. 2020;39:2045-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 91. | Rinninella E, Fagotti A, Cintoni M, Raoul P, Scaletta G, Scambia G, Gasbarrini A, Mele MC. Skeletal muscle mass as a prognostic indicator of outcomes in ovarian cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. 2020;30:654-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 1010] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 93. | Purcell SA, Elliott SA, Baracos VE, Chu QS, Prado CM. Key determinants of energy expenditure in cancer and implications for clinical practice. Eur J Clin Nutr. 2016;70:1230-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 94. | Ngo-Huang A, Holmes HM, des Bordes JKA, Parker NH, Fogelman D, Petzel MQB, Song J, Bruera E, Katz MHG. Association between frailty syndrome and survival in patients with pancreatic adenocarcinoma. Cancer Med. 2019;8:2867-2876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 95. | Okusaka T, Okada S, Ishii H, Ikeda M, Kosakamoto H, Yoshimori M. Prognosis of advanced pancreatic cancer patients with reference to calorie intake. Nutr Cancer. 1998;32:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 96. | Bye A, Jordhøy MS, Skjegstad G, Ledsaak O, Iversen PO, Hjermstad MJ. Symptoms in advanced pancreatic cancer are of importance for energy intake. Support Care Cancer. 2013;21:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1762] [Article Influence: 195.8] [Reference Citation Analysis (1)] |
| 98. | Baracos VE. Skeletal muscle anabolism in patients with advanced cancer. Lancet Oncol. 2015;16:13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Carli F, Gillis C, Scheede-Bergdahl C. Promoting a culture of prehabilitation for the surgical cancer patient. Acta Oncol. 2017;56:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 100. | Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M, Stein B, Charlebois P, Ghitulescu G, Morin N, Jagoe T, Scheede-Bergdahl C, Minnella EM, Fiore JF Jr. Effect of Multimodal Prehabilitation vs Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer: A Randomized Clinical Trial. JAMA Surg. 2020;155:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 327] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 101. | Holeček M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr Metab (Lond). 2018;15:33. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 466] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 102. | Tayek JA, Bistrian BR, Hehir DJ, Martin R, Moldawer LL, Blackburn GL. Improved protein kinetics and albumin synthesis by branched chain amino acid-enriched total parenteral nutrition in cancer cachexia. A prospective randomized crossover trial. Cancer. 1986;58:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 103. | Deutz NE, Safar A, Schutzler S, Memelink R, Ferrando A, Spencer H, van Helvoort A, Wolfe RR. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011;30:759-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 104. | Kim JS, Khamoui AV, Jo E, Park BS, Lee WJ. β-Hydroxy-β-methylbutyrate as a countermeasure for cancer cachexia: a cellular and molecular rationale. Anticancer Agents Med Chem. 2013;13:1188-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 105. | Eley HL, Russell ST, Tisdale MJ. Mechanism of attenuation of muscle protein degradation induced by tumor necrosis factor-alpha and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab. 2008;295:E1417-E1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 106. | Aversa Z, Bonetto A, Costelli P, Minero VG, Penna F, Baccino FM, Lucia S, Rossi Fanelli F, Muscaritoli M. β-hydroxy-β-methylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int J Oncol. 2011;38:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 107. | May PE, Barber A, D'Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 108. | Berk L, James J, Schwartz A, Hug E, Mahadevan A, Samuels M, Kachnic L; RTOG. A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support Care Cancer. 2008;16:1179-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 109. | Bosaeus I. Nutritional support in multimodal therapy for cancer cachexia. Support Care Cancer. 2008;16:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 110. | Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45:1105-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 530] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 111. | Park M, Kim H. Anti-cancer Mechanism of Docosahexaenoic Acid in Pancreatic Carcinogenesis: A Mini-review. J Cancer Prev. 2017;22:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 112. | Cholewski M, Tomczykowa M, Tomczyk M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 113. | Nabavi SF, Bilotto S, Russo GL, Orhan IE, Habtemariam S, Daglia M, Devi KP, Loizzo MR, Tundis R, Nabavi SM. Omega-3 polyunsaturated fatty acids and cancer: lessons learned from clinical trials. Cancer Metastasis Rev. 2015;34:359-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 114. | Di Girolamo FG, Situlin R, Mazzucco S, Valentini R, Toigo G, Biolo G. Omega-3 fatty acids and protein metabolism: enhancement of anabolic interventions for sarcopenia. Curr Opin Clin Nutr Metab Care. 2014;17:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 115. | Barber MD. Cancer cachexia and its treatment with fish-oil-enriched nutritional supplementation. Nutrition. 2001;17:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 116. | Werner K, Küllenberg de Gaudry D, Taylor LA, Keck T, Unger C, Hopt UT, Massing U. Dietary supplementation with n-3-fatty acids in patients with pancreatic cancer and cachexia: marine phospholipids versus fish oil - a randomized controlled double-blind trial. Lipids Health Dis. 2017;16:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 117. | Arshad A, Chung WY, Steward W, Metcalfe MS, Dennison AR. Reduction in circulating pro-angiogenic and pro-inflammatory factors is related to improved outcomes in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega-3 fish oil. HPB (Oxford). 2013;15:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 118. | Nikfarjam M, Wilson JS, Smith RC; Australasian Pancreatic Club Pancreatic Enzyme Replacement Therapy Guidelines Working Group. Diagnosis and management of pancreatic exocrine insufficiency. Med J Aust. 2017;207:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 119. | Löhr JM, Oliver MR, Frulloni L. Synopsis of recent guidelines on pancreatic exocrine insufficiency. United European Gastroenterol J. 2013;1:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Sabater L, Ausania F, Bakker OJ, Boadas J, Domínguez-Muñoz JE, Falconi M, Fernández-Cruz L, Frulloni L, González-Sánchez V, Lariño-Noia J, Lindkvist B, Lluís F, Morera-Ocón F, Martín-Pérez E, Marra-López C, Moya-Herraiz Á, Neoptolemos JP, Pascual I, Pérez-Aisa Á, Pezzilli R, Ramia JM, Sánchez B, Molero X, Ruiz-Montesinos I, Vaquero EC, de-Madaria E. Evidence-based Guidelines for the Management of Exocrine Pancreatic Insufficiency After Pancreatic Surgery. Ann Surg. 2016;264:949-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 121. | Vujasinovic M, Valente R, Del Chiaro M, Permert J, Löhr JM. Pancreatic Exocrine Insufficiency in Pancreatic Cancer. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 122. | Dominguez-Muñoz JE. Management of pancreatic exocrine insufficiency. Curr Opin Gastroenterol. 2019;35:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 123. | Forsmark CE. Diagnosis and Management of Exocrine Pancreatic Insufficiency. Curr Treat Options Gastroenterol. 2018;16:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 124. | Roberts KJ, Bannister CA, Schrem H. Enzyme replacement improves survival among patients with pancreatic cancer: Results of a population based study. Pancreatology. 2019;19:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 125. | Landers A, Brown H, Strother M. The effectiveness of pancreatic enzyme replacement therapy for malabsorption in advanced pancreatic cancer, a pilot study. Palliat Care. 2019;12:1178224218825270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 126. | Domínguez-Muñoz JE, Nieto-Garcia L, López-Díaz J, Lariño-Noia J, Abdulkader I, Iglesias-Garcia J. Impact of the treatment of pancreatic exocrine insufficiency on survival of patients with unresectable pancreatic cancer: a retrospective analysis. BMC Cancer. 2018;18:534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 127. | Layer P, Kashirskaya N, Gubergrits N. Contribution of pancreatic enzyme replacement therapy to survival and quality of life in patients with pancreatic exocrine insufficiency. World J Gastroenterol. 2019;25:2430-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 128. | Barkin JA, Westermann A, Hoos W, Moravek C, Matrisian L, Wang H, Shemanski L, Barkin JS, Rahib L. Frequency of Appropriate Use of Pancreatic Enzyme Replacement Therapy and Symptomatic Response in Pancreatic Cancer Patients. Pancreas. 2019;48:780-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 129. | Nofal YH, Abu Dail Y, Assaf Y, Abo Samra H, Abbas F, Hamzeh A, Alhaj Hasan N. Pancreatic enzyme replacement therapy for steatorrhoea in pancreatic cancer. Database Syst Rev. 2018;2:CD012952. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 130. | Grimble RF. Basics in clinical nutrition: Immunonutrition-Nutrients which influence immunity: Effect and mechanism of action. Educational Paper. 2009;4:E10-13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 131. | Klek S, Sierzega M, Szybinski P, Szczepanek K, Scislo L, Walewska E, Kulig J. The immunomodulating enteral nutrition in malnourished surgical patients - a prospective, randomized, double-blind clinical trial. Clin Nutr. 2011;30:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 132. | Shirakawa H, Kinoshita T, Gotohda N, Takahashi S, Nakagohri T, Konishi M. Compliance with and effects of preoperative immunonutrition in patients undergoing pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2012;19:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 133. | Gade J, Levring T, Hillingsø J, Hansen CP, Andersen JR. The Effect of Preoperative Oral Immunonutrition on Complications and Length of Hospital Stay After Elective Surgery for Pancreatic Cancer--A Randomized Controlled Trial. Nutr Cancer. 2016;68:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 134. | Silvestri S, Franchello A, Deiro G, Galletti R, Cassine D, Campra D, Bonfanti D, De Carli L, Fop F, Fronda GR. Preoperative oral immunonutrition versus standard preoperative oral diet in well nourished patients undergoing pancreaticoduodenectomy. Int J Surg. 2016;31:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 135. | Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Eijck CH, van Tienhoven G; Dutch Pancreatic Cancer Group. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020;38:1763-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 716] [Article Influence: 143.2] [Reference Citation Analysis (0)] |
| 136. | Versteijne E, van Eijck CH, Punt CJ, Suker M, Zwinderman AH, Dohmen MA, Groothuis KB, Busch OR, Besselink MG, de Hingh IH, Ten Tije AJ, Patijn GA, Bonsing BA, de Vos-Geelen J, Klaase JM, Festen S, Boerma D, Erdmann JI, Molenaar IQ, van der Harst E, van der Kolk MB, Rasch CR, van Tienhoven G; Dutch Pancreatic Cancer Group (DPCG). Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials. 2016;17:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 137. | Ngo-Huang A, Parker N, Martinez VA, Petzel MQ, Fogelman D, Holmes HM, Dhah SS, Katz M. Poster 68 Feasibility of a Prehabilitation Program for Patients with Potentially Resectable Pancreatic Cancer: Pilot Study. PM R. 2016;8:S183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 138. | Akita H, Takahashi H, Asukai K, Tomokuni A, Wada H, Marukawa S, Yamasaki T, Yanagimoto Y, Takahashi Y, Sugimura K, Yamamoto K, Nishimura J, Yasui M, Omori T, Miyata H, Ochi A, Kagawa A, Soh Y, Taniguchi Y, Ohue M, Yano M, Sakon M. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: Prospective randomized control study. Clin Nutr ESPEN. 2019;33:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 139. | Martin RC 2nd, Agle S, Schlegel M, Hayat T, Scoggins CR, McMasters KM, Philips P. Efficacy of preoperative immunonutrition in locally advanced pancreatic cancer undergoing irreversible electroporation (IRE). Eur J Surg Oncol. 2017;43:772-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 140. | Dewberry LC, Wingrove LJ, Marsh MD, Glode AE, Schefter TE, Leong S, Purcell WT, McCarter MD. Pilot Prehabilitation Program for Patients With Esophageal Cancer During Neoadjuvant Therapy and Surgery. J Surg Res. 2019;235:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |