Published online Sep 27, 2021. doi: 10.4240/wjgs.v13.i9.1102
Peer-review started: March 20, 2021
First decision: June 5, 2021
Revised: June 5, 2021
Accepted: July 20, 2021
Article in press: July 20, 2021
Published online: September 27, 2021
Processing time: 182 Days and 2.9 Hours
Although acute graft-vs-host disease (aGvHD) is a rare complication of liver transplantation, it is poorly understood and has an extremely high mortality rate. No standardized diagnostic criteria or treatment regimens currently exist.
The present study investigated the etiology, diagnosis, and treatment of aGvHD following liver transplantation. Presentation, diagnosis, disease course, histology, and treatment of an aGvHD case are reported, and associated literature is reviewed. A 64-year-old female required LTx due to primary biliary cirrhosis. The donor was a 12-year-old male. Three weeks following liver transplantation, the recipient developed pyrexia, diarrhea, rashes, and antibiotic-unresponsive pancytopenia. Clinical symptoms together with laboratory investigations suggested a diagnosis of aGvHD, which was confirmed via peripheral blood fluorescent in situ hybridization. Donor XY chromosome fluorescent in situ hybridization indicating early chimerism achieved 93% sensitivity in the detection of GvHD. Existing immunosuppressants were discontinued, and high-dose intravenous methylprednisolone was initiated along with antibiotics. While diarrhea resolved, the patient’s general condition continued to deteriorate until demise due to multi-system organ failure at 37 d post-liver transplantation. This case illustrates the life-threatening nature of aGvHD.
Herein, we have summarized a post-LTx aGvHD case and reviewed associated literature in order to increase awareness and provide potentially risk-mitigating recommendations.
Core Tip: At present, the risk factors, pathogenesis, optimal treatment, and prognosis associated with acute graft-vs-host disease following liver transplantation are unclear. Currently, the most reliable diagnostic method is specific immunostaining for donor-specific antigens. If the donor is male and the recipient is female, fluorescent in situ hybridization-based detection of the Y chromosome is a diagnostic option. In the present case, acute graft-vs-host disease was confirmed via fluorescent in situ hybridization, demonstrating the presence of male donor DNA.
- Citation: Xiao JJ, Ma JY, Liao J, Wu D, Lv C, Li HY, Zuo S, Zhu HT, Gu HJ. Fluorescence in situ hybridization-based confirmation of acute graft-vs-host disease diagnosis following liver transplantation: A case report. World J Gastrointest Surg 2021; 13(9): 1102-1109
- URL: https://www.wjgnet.com/1948-9366/full/v13/i9/1102.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i9.1102
Acute graft-vs-host disease (aGvHD) is one of the most dangerous complications following liver transplantation (LTx)[1]. It involves overactivation of donor helper T lymphocytes by recipient antigen-presenting cells, leading to a local inflammatory reaction against recipient tissue. Although the rate of aGvHD incidence after LTx is low (1%-2%), the mortality rate is extremely high (85%-90%)[2]. Skin rash and pyrexia are the most frequently noted early signs, followed by leukopenia. Although aGvHD was first proposed as a clinical entity in 1988, its mechanisms and optimal treatment strategies remain controversial[3]. Modification of the post-transplant treatment plan, including incorporation of more effective immunosuppressants, has a limited effect on the course of aGvHD[4,5]. In most cases, death results from overwhelming sepsis or gastrointestinal hemorrhage as a consequence of bone marrow involvement[6]. Due to the low incidence (but high mortality) of aGvHD following LTx, analysis of the present case with respect to existing literature is worthwhile in order to raise awareness regarding the condition, which may assist in the early diagnosis of suspected cases. It will also help improve diagnostic criteria and establish standardized evidence-based treatment regimens. Moreover, we wish to draw atten
The patient was a 64-year-old female with primary biliary cirrhosis, esophageal-fundal variceal hemo
A 64-year-old female received a liver from an ABO-matched (A-positive) 12-year-old male cadaveric donor. The donor and recipient details are shown in Table 1. The donor was a 12-year-old male. Three weeks following liver transplantation, the recipient developed pyrexia, diarrhea, rashes, and antibiotic-unresponsive pancytopenia.
| Recipient | Donor | |
| Age | 64 | 12 |
| Sex | Female | Male |
| Primary complaint | PBC | Hypoxic-ischemic encephalopathy |
| Special history | Low-dose glucocorticoids | NA |
| Blood group | A | A |
| HLA | NA | NA |
A 64-year-old female with primary biliary cirrhosis, esophageal-fundal variceal hemorrhages, and decompensated hepatocirrhosis.
The patient grew up in her locality, denies any contact with contaminated water or radiation exposure, and denies smoking and alcohol consumption.
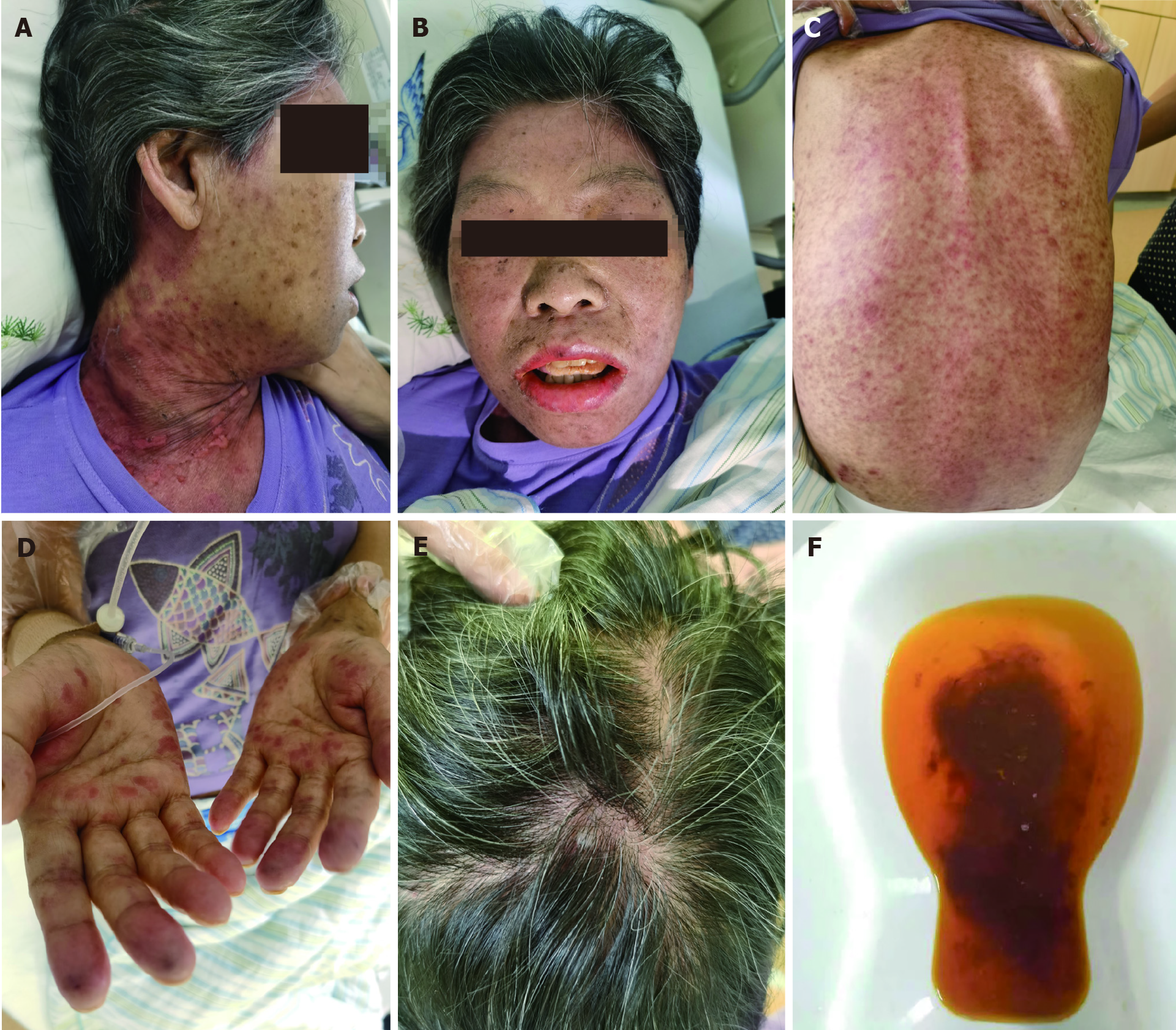
On physical examination, we found her poor nutritional status, the abdomen was moderately distended with mild tenderness, and there was moderately yellow staining of the skin and mucous membranes. The rest of the physical examination revealed no abnormal findings.
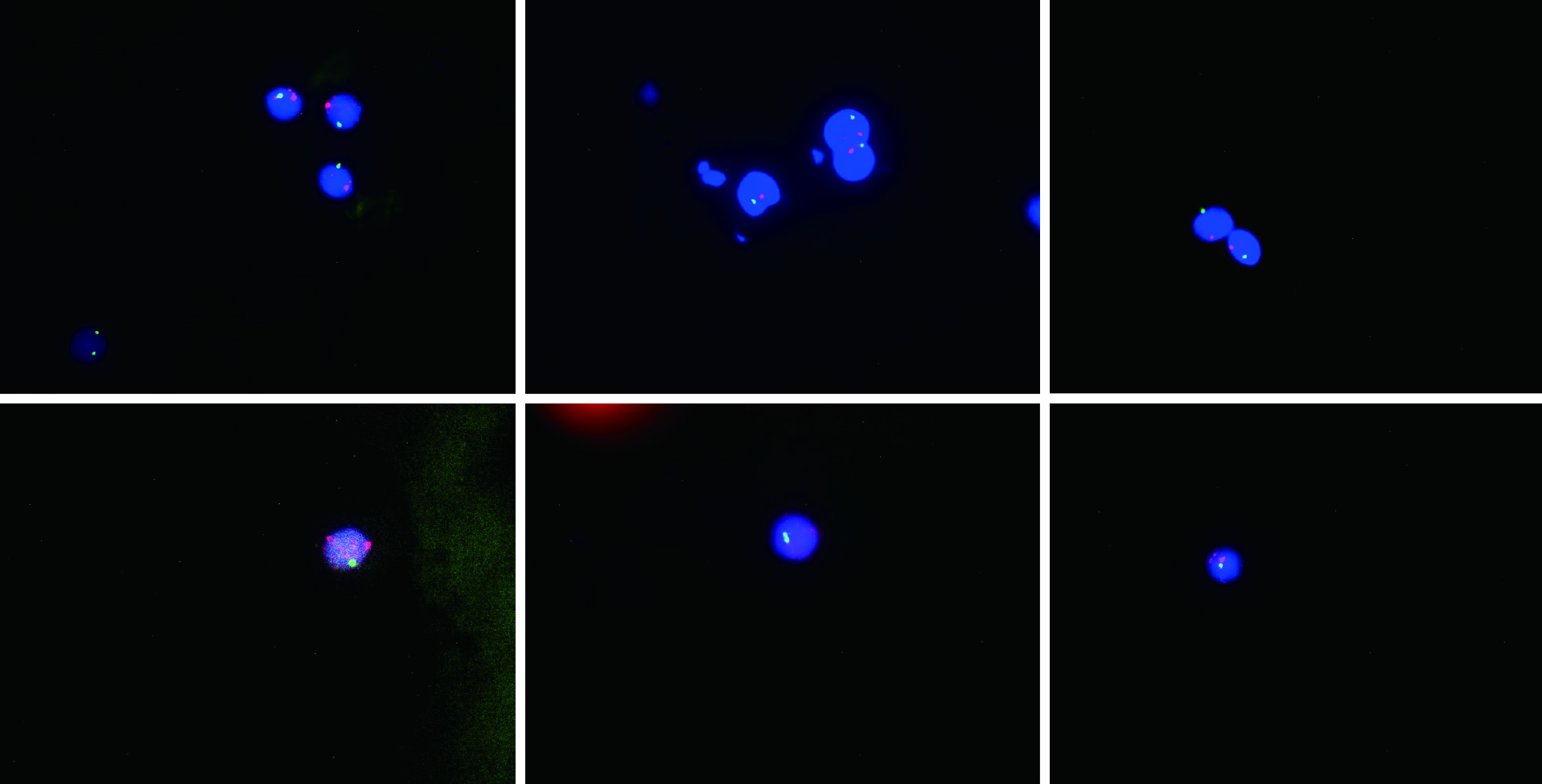
The following timeline of events refers to post-operative days. On day 22, the patient developed pyrexia of unknown origin, fluctuating between 38.2 °C and 39.3 °C. On day 26, sex chromosome FISH was performed on peripheral venous blood samples. No gastrointestinal tract lesions were apparent, and no evidence of aGvHD was noted on gastrointestinal endoscopic biopsy (histologically normal esophagus, stomach, and ileum). On day 31, the presumptive diagnosis of GvHD was made based on the following clinical ground observations: Generalized maculopapular eruption (largely involving the back, neck, and face), pyrexia, pancytopenia, low blood pressure, and watery diarrhea (Figure 1 and Table 2). FISH revealed chimerism (presence of the fluorescently stained donor XY chromosome) consistent with aGvHD (Figure 2).
| Manifestations | Drugs | ||||||||
| PO day | Temperature (°C) | Skin rash | Diarrhea | Myelosuppression | Tacrolimus (mg/d) | MMF (g/d) | MP | IgG (g/d) | Antibiotics |
| 22 | 38.3 | Palm | 2 | NA | 3 | 0.25 | 500 | 10 | Yes |
| 24 | 38.6 | Neck | 3 | NA | 2 | 0 | 500 | 10 | Yes |
| 26 | 38.5 | Face | 6 | Yes | 2 | 0 | 120 | NA | Yes |
| 28 | 38.2 | Trunk | 7 | Yes | 2 | 0 | 40 | NA | Yes |
| 30 | 39 | > 35% | 6 | Yes | 2 | 0 | 20 | NA | Yes |
| 32 | 38.6 | > 50% | 5 | Yes | 1.5 | 0 | 20 | 10 | Yes |
| 34 | 38.7 | > 55% | 4 | Yes | 1.5 | 0 | 20 | 10 | NA |
| 36 | Demise | ||||||||
Two days following the development of thrombocytopenia, a bone marrow biopsy revealed marked hypocellularity. No skin rash was yet apparent. The findings of detai
| Post-operative laboratory investigation timeline | |||||||||||
| Value/PO day | 0 | 4 | 8 | 12 | 16 | 20 | 24 | 26 | 30 | 34 | 36 |
| AST (U/L) | 643 | 47.6 | 56 | 48 | 64 | 75 | 63 | 56 | 44 | 52 | 74 |
| ALT (U/L) | 772 | 88.1 | 96 | 86 | 107.3 | 62 | 59 | 64 | 66 | 71 | 83 |
| Total bilirubin (mg/dL) | 231.4 | 123.5 | 119.5 | 76.9 | 65.5 | 23.7 | 25.8 | 24.2 | 35.1 | 45.6 | 48.7 |
| Direct bilirubin (mg/dL) | 146.1 | 63.2 | 59.3 | 43.2 | 38.1 | 13.3 | 15.6 | 16.5 | 24.7 | 28.5 | 31.2 |
| Leukocyte count × 109/L | 17.5 | 8.7 | 12.4 | 17.3 | 7.2 | 6.7 | 1.3 | 0.39 | 0.24 | 0.12 | 0.08 |
| Neutrophil % | 93 | 79 | 86 | 92 | 81 | 80 | 63 | 17.9 | 0 | 0 | 0 |
| Hemoglobin (g/L) | 89 | 92 | 176 | 113 | 92 | 85 | 75 | 63 | 58 | 53 | 47 |
| Hematocrit % | 42 | 46 | 50 | 32 | 26.5 | 23.3 | 22 | 17.6 | 16.5 | 15.6 | 14.8 |
| Platelets × 109/L | 21 | 26 | 44 | 58 | 77 | 73 | 71 | 56 | 47 | 46 | 41 |
| Prothrombin time (s) | 17.9 | 19.9 | 16.5 | 22.4 | 13.5 | 13.1 | 12.7 | 13.1 | 12.8 | 13.2 | 13.6 |
| INR | 1.82 | 1.7 | 1.34 | 1.98 | 1.05 | 1.01 | 0.97 | 0.99 | 0.98 | 1.02 | 1.07 |
| Sodium (mmol/L) | 147 | 145 | 142 | 139 | 136 | 134 | 143 | 141 | 138 | 139 | 143 |
| Potassium (mmol/L) | 3.8 | 4.5 | 3.1 | 3.4 | 3.6 | 3.8 | 3.9 | 4.2 | 4.1 | 3.9 | 3.7 |
| Urea (mmol/L) | 32.52 | 29.8 | 16.42 | 4.55 | 4.77 | 4.46 | 4.13 | 3.8 | 4.17 | 3.74 | 4.02 |
| Creatinine (μmol/L) | 89.73 | 85.64 | 64.59 | 43.78 | 58.44 | 53.76 | 49.19 | 46.52 | 27.26 | 24.54 | 30.45 |
| PCT (ng/mL) | 5.73 | 3.86 | 11.5 | 5.1 | 1.86 | 2.65 | 2.58 | 2.45 | 2.18 | 3.65 | 4.53 |
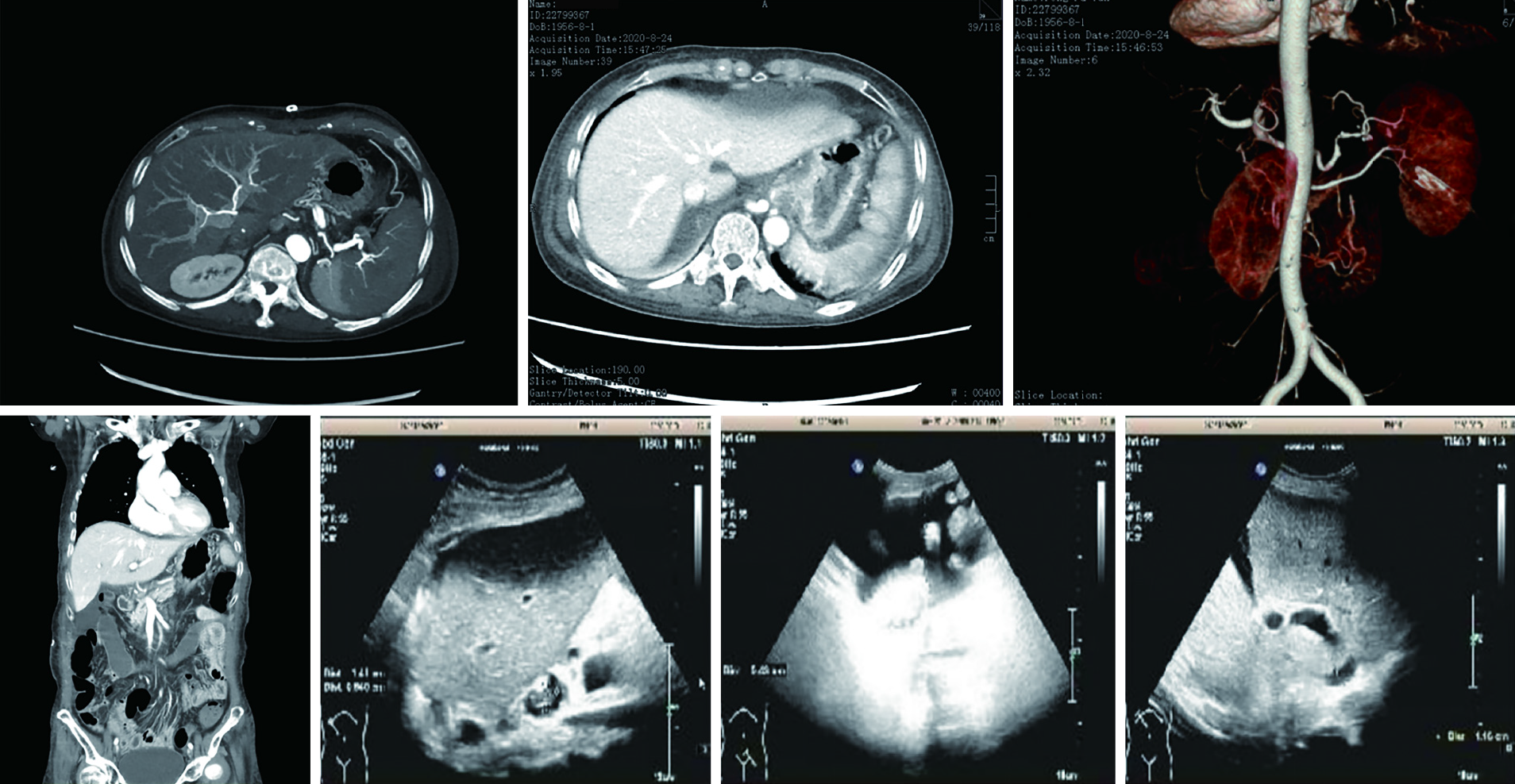
Abdominal computed tomography and color ultrasound findings suggested laminar portal vein, inferior vena cava, hepatic artery, and hepatic venous flow (Figure 3).
aGvHD, primary biliary cirrhosis, esophageal-fundal variceal hemorrhages, and decom
Initial treatment involved tapering the dosage of immunosuppressants to allow the recipient immune system to reject donor lymphocytes. Due to the inefficacy of this approach, the following treatment was administered subsequently: High-dose (500 mg/d) intravenous methylprednisolone, antibiotics, and immunoglobulin G (Table 2).
Severe inflammation induced multi-system organ failure, which led to the patient’s demise on post-operative day 37.
At present, the risk factors, pathogenesis, optimal treatment, and prognosis associated with aGvHD following LTx are unclear. Current (incomplete) understanding of aGvHD pathogenesis may be summarized as follows. The conditioning regimen induces initial recipient tissue damage, followed by auto- and alloantigen denudation in the recipient concomitant with antigen-presenting cell activation and massive inflammatory cytokine release (a “cytokine storm”). If a sufficient number of donor lymphocytes, especially T lymphocytes, of the correct specificity are present, direct recognition of and activation by antigen-presenting cell (either locally or within secondary lymphoid tissues) results in T lymphocyte interleukin (IL)-2 and IL-2R expression. Activated T-cells then stimulate donor monocytes to produce significant levels of myeloid cytokines (e.g., IL-1 and tumor necrosis factor) and also trigger a cascade of cytotoxic signal transduction pathways, such as the perforin/granzyme B or Fas/FasL pathways (although direct cytokine-mediated injury is also possible). Finally, inflammatory infiltration in the digestive tract, skin, and bone marrow leads to severe clinical presentations[7]. In the present case, abnormally high numbers of CD8+ T lymphocytes were present during the acute phase of GvHD, while the CD4+ T lymphocyte:CD8+ T lymphocyte ratio was less than 0.1. This indicates that perhaps cytotoxic T lymphocytes (with a minor contribution by helper T lymphocytes) are the cells primarily involved in GvHD pathogenesis. In summary, the necessary conditions for the occurrence of aGvHD[8-10] include the presence of donor immunoreactive cells within graft tissue, presence of recipient tissue antigens not present in donor organ tissue, and inability of the recipient immune system to eliminate effectively donor leukocytes.
Triulzi et al[9] have described the diagnostic criteria for aGvHD following LTx in the following three requirements: (1) Characteristic clinical symptoms affecting related organ systems (e.g., skin, gastrointestinal tract, and bone marrow), including rash, diarrhea, and pancytopenia, among others; (2) Abnormal skin or digestive tract histology; and (3) HLA or DNA evidence of donor immunoreactive lymphocytes in involved organs or peripheral blood of the recipient. In addition to the above criteria, T lymphocyte counts and cytokine quantitation provide clear diagnostic support. Currently, the most reliable diagnostic method is specific immunostaining for donor-specific antigens. If the donor is male and the recipient is female, FISH-based detection of the Y chromosome is a diagnostic option[9,11,12]. At present, no false negatives have been reported for this method. In the present case, aGvHD was confirmed via FISH, demonstrating the presence of male donor DNA.
Due to inter-individual differences in post-operative GvHD pathogenesis and presentation, no unified treatment plan exists. Each hospital follows a unique treat
In order to lessen mortality resulting from aGvHD, early detection and optimal standardized treatment are paramount. Additionally, an improved understanding of pathogenesis may assist in the prevention and treatment of this disorder. Based on our experience and the literature review, we make the following recommendations: Baseline (presurgical) donor and recipient blood samples should be obtained and cryopreserved. High-risk patients should routinely undergo HLA typing as a preli
In the present case, aGvHD was confirmed via FISH, demonstrating the presence of male donor DNA. If the donor is male and the recipient is female, FISH-based dete
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferreira GSA S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu RR
| 1. | Perri R, Assi M, Talwalkar J, Heimbach J, Hogan W, Moore SB, Rosen CB. Graft vs. host disease after liver transplantation: a new approach is needed. Liver Transpl. 2007;13:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Taylor AL, Gibbs P, Bradley JA. Acute graft vs host disease following liver transplantation: the enemy within. Am J Transplant. 2004;4:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Burdick JF, Vogelsang GB, Smith WJ, Farmer ER, Bias WB, Kaufmann SH, Horn J, Colombani PM, Pitt HA, Perler BA. Severe graft-versus-host disease in a liver-transplant recipient. N Engl J Med. 1988;318:689-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Lee SJ, Onstad L, Chow EJ, Shaw BE, Jim HSL, Syrjala KL, Baker KS, Buckley S, Flowers ME. Patient-reported outcomes and health status associated with chronic graft-versus-host disease. Haematologica. 2018;103:1535-1541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Qian L, Dima D, Berce C, Liu Y, Rus I, Raduly LZ, Petrushev B, Berindan-Neagoe I, Irimie A, Tanase A, Jurj A, Shen J, Tomuleasa C. Protein dysregulation in graft vs host disease. Oncotarget. 2018;9:1483-1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Taylor AL, Gibbs P, Sudhindran S, Key T, Goodman RS, Morgan CH, Watson CJ, Delriviere L, Alexander GJ, Jamieson NV, Bradley JA, Taylor CJ. Monitoring systemic donor lymphocyte macrochimerism to aid the diagnosis of graft-versus-host disease after liver transplantation. Transplantation. 2004;77:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Schrager JJ, Vnencak-Jones CL, Graber SE, Neff AT, Chari RS, Wright KJ Jr, Pinson CW, Stewart JH, Gorden DL. Use of short tandem repeats for DNA fingerprinting to rapidly diagnose graft-versus-host disease in solid organ transplant patients. Transplantation. 2006;81:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Jacobs MT, Olson M, Ferreira BP, Jin R, Hachem R, Byers D, Witt C, Ghobadi A, DiPersio JF, Pusic I. The use of ruxolitinib for acute graft-versus-host disease developing after solid organ transplantation. Am J Transplant. 2020;20:589-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Triulzi D, Duquesnoy R, Nichols L, Clark K, Jukic D, Zeevi A, Meisner D. Fatal transfusion-associated graft-versus-host disease in an immunocompetent recipient of a volunteer unit of red cells. Transfusion. 2006;46:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Kanehira K, Riegert-Johnson DL, Chen D, Gibson LE, Grinnell SD, Velgaleti GV. FISH diagnosis of acute graft-versus-host disease following living-related liver transplant. J Mol Diagn. 2009;11:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Gonultas F, Akbulut S, Barut B, Kutluturk K, Yilmaz S. Graft-versus-host disease after living donor liver transplantation: an unpredictable troublesome complication for liver transplant centers. Eur J Gastroenterol Hepatol. 2020;32:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Di Ianni M, Del Papa B, Baldoni S, Di Tommaso A, Fabi B, Rosati E, Natale A, Santarone S, Olioso P, Papalinetti G, Giancola R, Accorsi P, Di Bartolomeo P, Sportoletti P, Falzetti F. NOTCH and Graft-Versus-Host Disease. Front Immunol. 2018;9:1825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Triulzi DJ, Nalesnik MA. Microchimerism, GVHD, and tolerance in solid organ transplantation. Transfusion. 2001;41:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Perkins JL, Neglia JP, Ramsay NK, Davies SM. Successful bone marrow transplantation for severe aplastic anemia following orthotopic liver transplantation: long-term follow-up and outcome. Bone Marrow Transplant. 2001;28:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Ramachandran V, Kolli SS, Strowd LC. Review of Graft-Versus-Host Disease. Dermatol Clin. 2019;37:569-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 16. | Hill L, Alousi A, Kebriaei P, Mehta R, Rezvani K, Shpall E. New and emerging therapies for acute and chronic graft versus host disease. Ther Adv Hematol. 2018;9:21-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 17. | Schroeder T, Haas R, Kobbe G. Treatment of graft-versus-host disease with monoclonal antibodies and related fusion proteins. Expert Rev Hematol. 2010;3:633-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. |
Aladağ E, Kelkitli E, Göker H.
Acute Graft-Versus-Host Disease: A Brief Review |
| 19. | Murray J, Stringer J, Hutt D. Graft-Versus-Host Disease (GvHD). 2017 Nov 22. In: Kenyon M, Babic A, editors. The European Blood and Marrow Transplantation Textbook for Nurses: Under the Auspices of EBMT [Internet]. Cham (CH): Springer; 2018. Chapter 11. [PubMed] [DOI] [Full Text] |
| 20. | Whalen JG, Jukic DM, English JC 3rd. Rash and pancytopenia as initial manifestations of acute graft-versus-host disease after liver transplantation. J Am Acad Dermatol. 2005;52:908-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |