Published online Sep 27, 2021. doi: 10.4240/wjgs.v13.i9.1025
Peer-review started: March 1, 2021
First decision: June 3, 2021
Revised: June 18, 2021
Accepted: August 19, 2021
Article in press: August 19, 2021
Published online: September 27, 2021
Processing time: 201 Days and 4.6 Hours
Distal cholangiocarcinoma (DCC) presents as one of the relatively rare malignant tumors in the digestive system and has a poor long-term prognosis. Curative resection is currently the most appropriate therapy for patients with DCC because of the lack of effective adjuvant therapies. Therefore, it is important to accurately predict the prognosis for formulating a reasonable treatment plan and avoiding unnecessary surgical trauma.
To minimize the interference of obstructive jaundice on carbohydrate antigen 19-9 (CA19-9) level by adapting CA19-9 to γ-glutamyltransferase (GGT) as an indicator, to determine the strong associations between CA19-9/GGT and postoperative neoplasm recurrence and long-term outcome of DCC.
We enrolled 186 patients who were diagnosed with DCC between January 2010 and December 2019 and performed radical excision with strict criteria as follows in our hospital. Receiver operating characteristic curves were drawn according to preoperative CA19-9/GGT and 1-year survival. Based on this, patients were divided into two groups (group 1, low-ratio, n = 81; group 2, high-ratio, n = 105). Afterwards, by the way of univariate and multivariate analysis, the risk factors influencing postoperative tumor recrudesce and long-term prognosis of patients with DCC were screened out.
Optimum cut-off value of CA19-9/GGT was 0.12. Patients in group 2 represented higher CA19-9 and lymphatic metastasis rate accompanied by lower GGT, when compared with group 1 (P < 0.05). The 1-, 3- and 5-year overall survival rates of patients in groups 1 and 2 were 88.3%, 59.2% and 48.1%, and 61.0%, 13.6% and 13.6%, respectively (P = 0.000). Multivariate analysis indicated that CA19-9/GGT, lymphatic metastasis and tumor differentiation were independent risk factors for tumor recurrence and long-term prognosis of DCC.
Elevation of CA19-9/GGT performed better as a biomarker of aggressive carcinoma and predictor of poor clinical outcomes by reducing the effect of obstruction of biliary tract on CA19-9 concentration in patients with DCC.
Core Tip: Distal cholangiocarcinoma (DCC) is a rare malignant tumor in the digestive system and has a poor long-term prognosis. Curative resection is currently the best treatment for patients with DCC because of the lack of effective adjuvant therapies. Therefore, it is important to accurately predict the prognosis for formulating a reasonable treatment plan and avoiding unnecessary surgical trauma. Carbohydrate antigen 19-9 to serum γ-glutamyltransferase (CA19-9/GGT) ratio was adapted as an indicator to minimize the interference of obstructive jaundice CA19-9 level, to determine the strong associations between CA19-9/GGT and postoperative neoplasm recurrence and long-term outcome of DCC.
- Citation: Jiang T, Lyu SC, Zhou L, Wang J, Li H, He Q, Lang R. Carbohydrate antigen 19-9 as a novel prognostic biomarker in distal cholangiocarcinoma. World J Gastrointest Surg 2021; 13(9): 1025-1038
- URL: https://www.wjgnet.com/1948-9366/full/v13/i9/1025.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i9.1025
Cholangiocarcinoma is a primary biliary system malignant tumor that originates from bile duct epithelial cells and is one of the rare malignant tumors in the digestive system and has a poor long-term prognosis. The incidence of cholangiocarcinoma appears low, accounting for about 3% of malignant tumors of the digestive system[1]. Cholangiocarcinomas are usually classified as intrahepatic, hilar or distal, depending on their anatomical location. Treatment and long-term prognosis of cholangiocarcinoma differ according to location. Distal cholangiocarcinoma (DCC) refers to extrahepatic cholangiocarcinoma located outside the perihilar region, that is, the primary tumor originates from the bile duct malignant tumor in the middle and lower segments of the common bile duct. It accounts for about 20%–40% of cholangiocarcinoma and is relatively rare clinically[2,3]. Radical surgery remains the optimum therapy for curing DCC because of the lack of effective adjuvant therapies. However, the 5-year survival rate for postoperative patients remains poor at about 20%[4]. It is important to accurately predict the prognosis for formulating a reasonable treatment plan and avoiding unnecessary surgical trauma. At present, the differentiation of tumor, lymphatic metastasis and other related risk factors can only be obtained after surgery, and the information acquisition is delayed[5,6].
There is a strong association between carbohydrate antigen 19-9 (CA19-9) and the diagnosis, recurrence and prognosis of malignant tumors[7]. CA19-9 is not restricted to tumor cells; epithelial cells in the pancreas, bile duct, stomach and colon are also able to synthesize CA19-9[8]. Under the circumstance of biliary obstruction, CA19-9 originating from bile duct epithelial cells cannot be excreted into the intestinal tract normally, and CA19-9 from pancreatic epithelial cells may flow back into the biliary tract abnormally. Local inflammation secondary to biliary obstruction leads to the proliferation of bile duct epithelial cells. All of these will induce an abnormal increase in serum CA19-9[9]. In the absence of specific symptoms, most patients with DCC do not seek treatment until they have jaundice symptoms. At that time, biliary obstruction has already occurred; therefore, the concentration of CA19-9 would be inconsistent with the increase of tumor invasiveness, resulting in a decline in its predictive function for the prognosis of DCC.
γ-Glutamyltransferase (GGT) is widely distributed in the human body and located on the surface of cell membranes, and is a key enzyme involved in glutathione (GSH) metabolism. GGT participates in oxidative stress and plays a proinflammatory role, leading to the occurrence of various chronic metabolic diseases, and is closely related to the occurrence and development of tumors[10,11]. Serum GGT is mainly secreted from the hepatobiliary system and is excreted by bile[12]. After biliary obstruction leads to bile drainage obstruction, GGT produced by bile duct epithelial cells and hepatocytes increases, and, due to bile excretion obstruction, GGT enters the blood in reverse flow, and may result in an atypical increase in GGT. However, GGT is commonly used clinically as a diagnostic test; mainly as a biomarker of hepatobiliary disease and alcohol intake[13]. Although GGT is released in a variety of tumor types, its role in malignant tumor behavior and prognosis remains unclear.
In view of the above considerations, we adjusted CA19-9 by CA19-9/GGT, thereby eliminating or reducing the impact of biliary obstruction on the concentration of CA19-9. The aim of our study was to establish the role of CA19-9/GGT in DCC and its influence as a prognostic biomarker.
This study was approved by the Ethical Committee of Beijing Chao-Yang Hospital (No. 2020-D.-301) and in accordance with the Declaration of Helsinki of the World Medical Association. Since this was a retrospective study design, participants’ informed consent was not required.
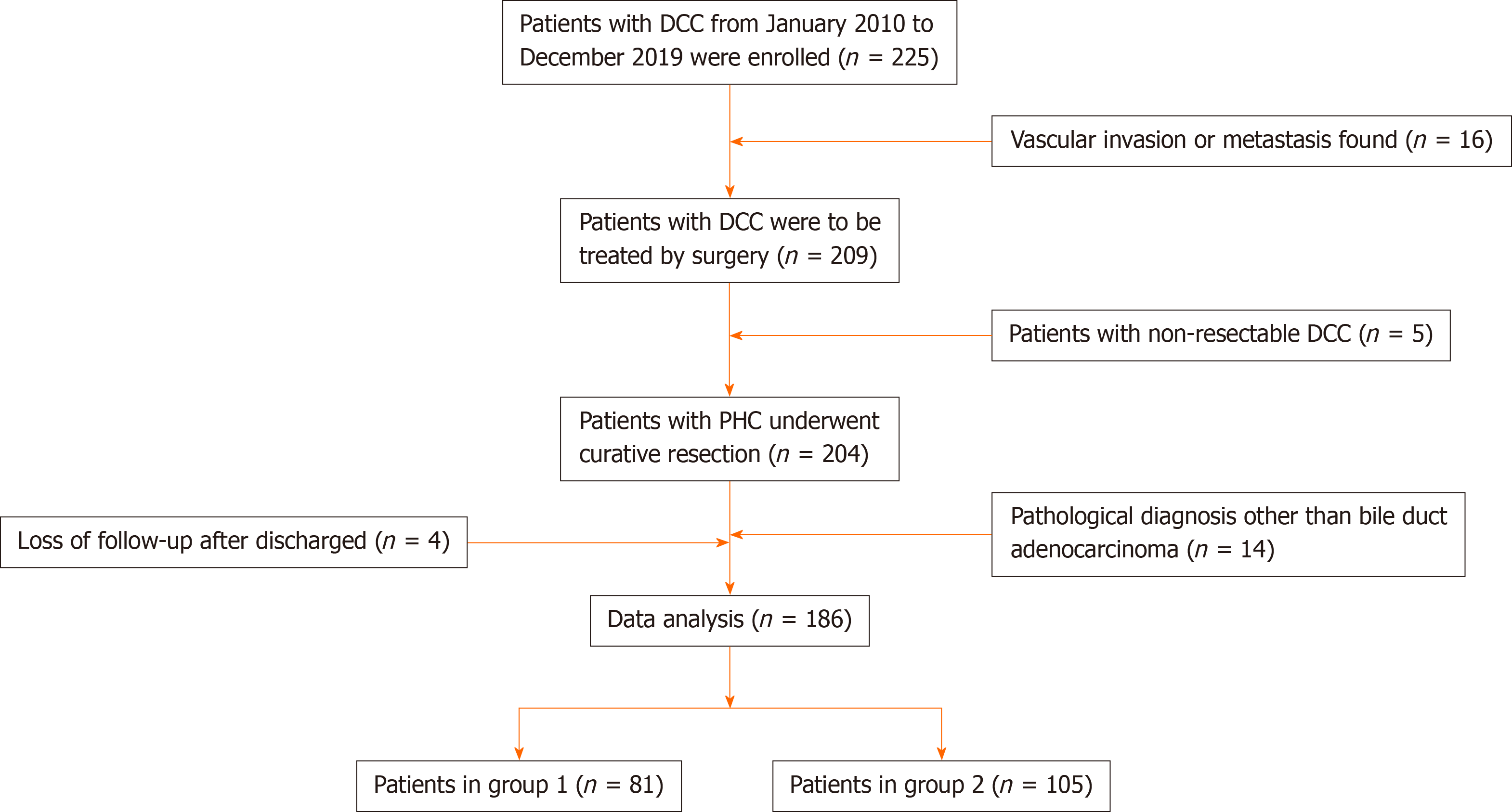
The data of patients who underwent pancreaticoduodenectomy (pancreaticoduodenectomy, PD) for DCC between January 2010 and December 2019 at our hospital were collected and analyzed. We screened 186 patients with DCC who met the criteria (Figure 1). Inclusion criteria: (1) DCC patients who underwent PD from January 2010 to December 2019; (2) Age 20–85 years; (3) Preoperative imaging showed no invasion of celiac vessels; (4) Tumor was completely removed during the operation; (5) Postoperative pathology confirmed bile duct adenocarcinoma; and (6) Informed consent of the patients and their families was obtained for the surgical methods and treatment strategies. Exclusion criteria: (1) Tumor was not removed for various reasons during the operation; (2) Patients with complicated cancers of other systems; (3) Pathological diagnosis was nonconventional ductal adenocarcinoma; and (4) Incomplete follow-up data or loss to follow-up.
Of 186 patients who were screened out, there were 73 women, with a male: female ratio of 1.5:1, mean age 64.9 ± 8.6 years. The primary symptoms mainly included jaundice (n = 156) and epigastric pain (n = 17) and the other 10 patients were identified during physical examination. Among the included patients, 62 (33.3%) had a history of smoking and 53 had diabetes (28.5%). Ninety of 158 patients who had jaundice received preoperative biliary drainage (PBD), which included 23 cases of endoscopic retrograde cholangiopancreatography and 67 of percutaneous transhepatic biliary drainage.
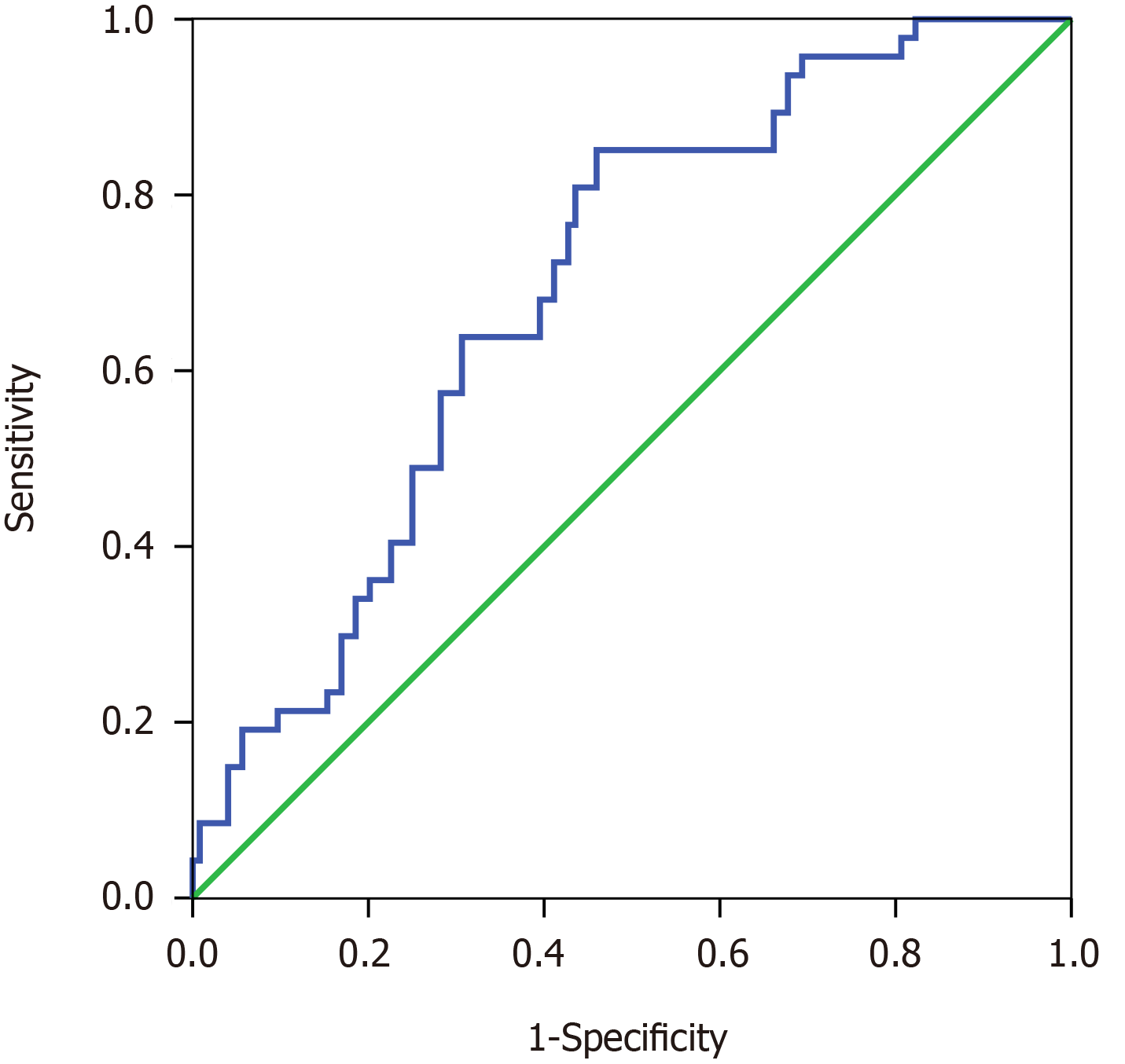
ROC curves were drawn based on preoperative CA19-9/GGT and 1-year survival. The best cut-off value of CA19-9/GGT was 0.12 [area under the curve, 0.695, 95%confidence interval (CI): 0.613–0.777] (Figure 2), and the patients were divided into two different groups (group 1, low-ratio, n = 81; group 2, high-ratio, n = 105). The CA19-9 and GGT assays were used to obtain the results from the last blood sample before surgery. For the patients who underwent PBD, our center rechecked the CA19-9 and GGT index the day before the surgery.
The clinicopathological data during the perioperative period were extracted from the medical records. After surgery, routine laboratory tests were performed once every 3 mo within 2 years and once every 6 mo thereafter, as were imaging examinations including abdominal enhanced computed tomography (CT), pulmonary CT, electroconvulsive therapy, etc. and subsequent treatment regimens, tumor recurrence and survival were compared in different groups. The end points of follow-up were usually defined as tumor recurrence and death.
All data analysis was carried out by SPSS version 22.0 software, and each index was expressed as mean ± SD. Survival rates, including overall survival (OS) and disease-free survival (DFS), were calculated using the Kaplan–Meier method and evaluated with the log-rank test. The Cox proportional model was used to analyze multivariate survival, and the independent risk factors affecting the survival time. Qualitative variables were compared using χ2 tests. Statistical significance was defined as P < 0.05.
During the perioperative period, bleeding volume was 500 (400–600) mL, and 66 patients (35.5%) received blood transfusions. The duration of the operation was 9.8 ± 1.9 h. Pathology showed the degree of tumor differentiation was as follows: poor in 52 cases (28.0%), moderate in 109 (58.6%) and high in 25 (13.4%). Tumor size was 2.2 ± 1.0 cm, and positive lymph nodes was detected in 75 patients (40.3%). Radical resection (R0) was performed in 178 cases (95.7%).
Fifty-four patients (29.0%) had postoperative complications. Among them, 19 were accompanied with biochemical fistula (10.2%), six with grade B pancreatic fistula (3.2%), seven with grade C pancreatic fistula (3.8%), 16 with intra-abdominal infection (8.6%), 11 with hemorrhage (5.9%), eight with disturbance of gastric emptying (4.3%), two each with biliary fistula, gastrointestinal bleeding or myocardial infarction (1.1%). There was one case each with intracranial hemorrhage or pulmonary embolism (0.5%). Among them, perioperative mortality was 3.8% in seven cases. Four patients died of grade C pancreatic fistula with abdominal hemorrhage, and one each with myocardial infarction, pulmonary embolism or intracranial hemorrhage.
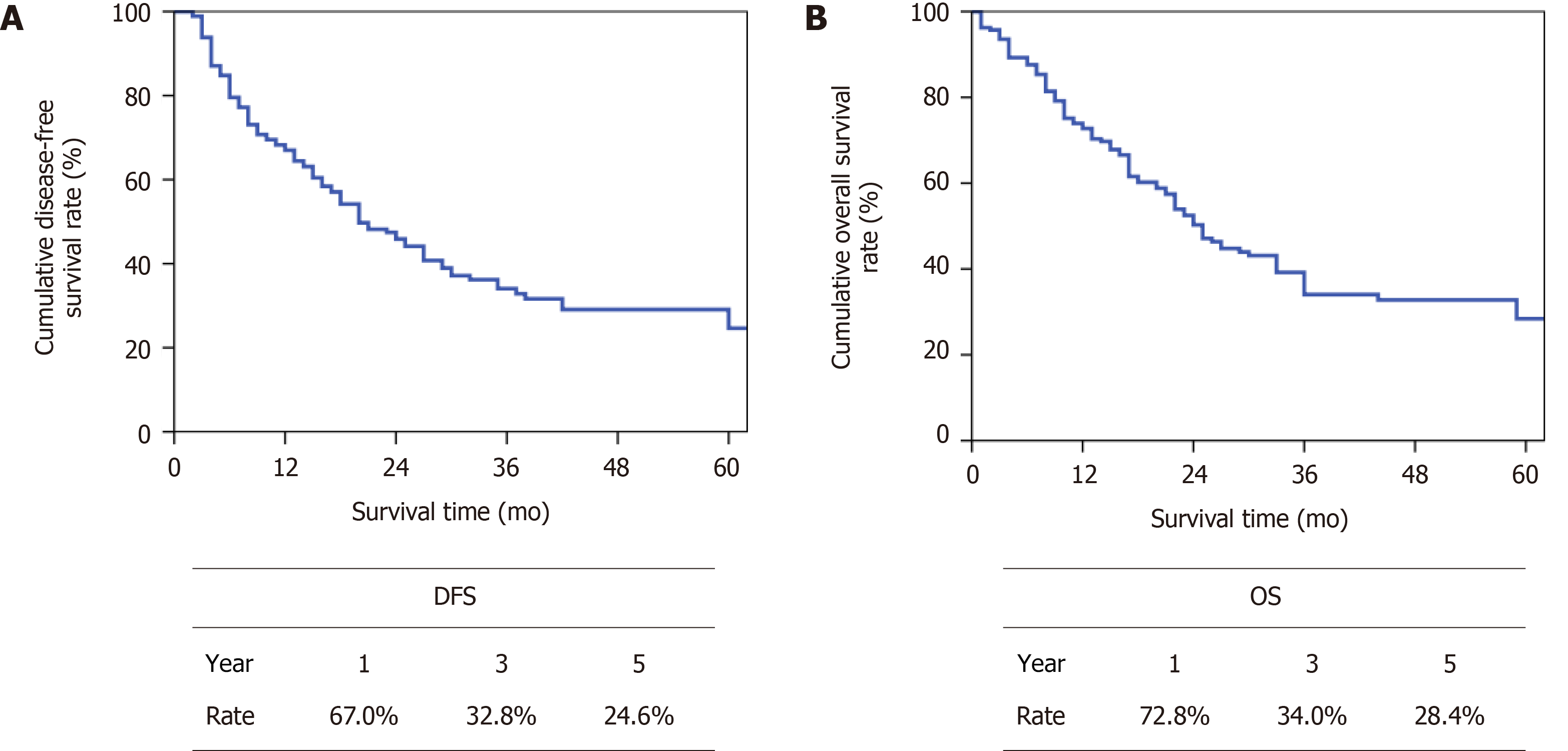
The median follow-up period was 38 mo until March 2020. The median overall DFS was 20 mo (Figure 3A) and the median OS was 25 mo (Figure 3B).
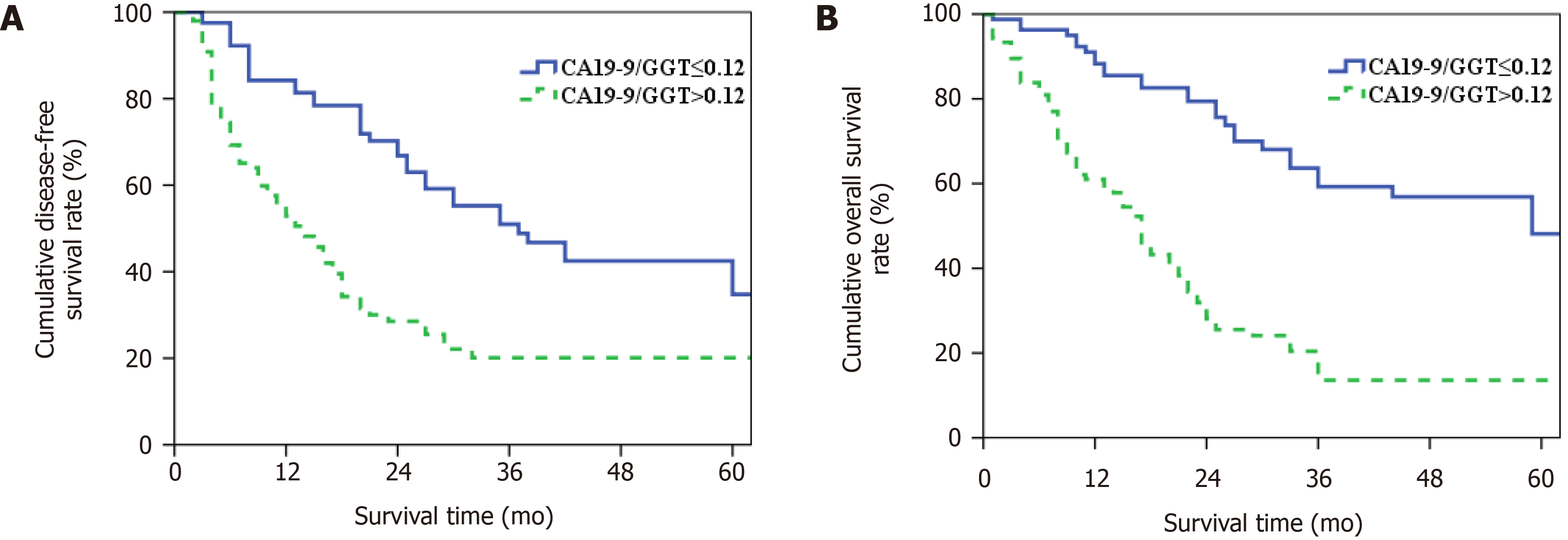
Patients in group 2 had higher CA19-9 and lymph node metastasis, accompanied by lower GGT, when compared with group 1 (P < 0.05) (Table 1). Postoperative morbidity between the groups was compared (Table 2), and there was no significant difference in postoperative mortality and morbidity rate (P > 0.05, Table 3). Patients had a median DFS of 37 mo in group 1 and 14 mo in group 2. The 1-, 3- and 5-year DFS rates were 84.2%, 51.0% and 34.8% and 52.9%, 20.1% and 20.1% (P = 0.000, Figure 4A). The median OS of patients in groups 1 and 2 was 59 and 17 mo, respectively, and the 1-, 3- and 5-year OS rates were 88.3%, 59.2% and 48.1% and 61.0%, 13.6% and 13.6% (P = 0.000, Figure 4B).
| Variables | Group 1 (n = 81) | Group 2 (n = 105) | P |
| Gender (M/F) | 50/31 | 63/42 | 0.811 |
| Age, mean ± SD, yr | 63.7 ± 9.1 | 65.8 ± 8.1 | 0.093 |
| Smoking (Y/N) | 24/57 | 38/67 | 0.347 |
| Diabetes (Y/N) | 18/63 | 35/70 | 0.096 |
| PBD (Y/N) | 35/46 | 55/50 | 0.215 |
| TB (μmol/L) | 76.7 (35.4–211.3) | 110.0 (24.1–203.4) | 0.78 |
| CA19-9 (U/mL) | 27.3 (11.7–45.6) | 139.8 (42.7–316.2) | 0 |
| γ-GGT (U/L) | 706 (395–1194) | 207 (80–446) | 0 |
| Tumor size, mean ± SD, cm | 2.1 ± 0.9 | 2.2 ± 1.1 | 0.82 |
| Tumor differentiation (poor/moderate & high) | 20/61 | 32/73 | 0.412 |
| Nerve invasion (Y/N) | 68/13 | Nov-94 | 0.261 |
| Intraoperative blood loss (mL) | 500 (400–600) | 500 (400–800) | 0.222 |
| Blood transfusion (Y/N) | 30/51 | 36/69 | 0.697 |
| OP time, mean ± SEM, h | 9.5 ± 1.4 | 10.0 ± 2.1 | 0.079 |
| LN metastasis (+/–) | 18/63 | 57/48 | 0 |
| Resection margin (R0/R1) | Jan-80 | Jul-98 | 0.141 |
| Postoperative chemotherapy (Y/N) | 20/61 | 19/86 | 0.273 |
| Variable | n | 1-yr OS (%) | 3-yr OS (%) | χ2 | P |
| Gender | 2.434 | 0.119 | |||
| Male | 113 | 69.6 | 42.9 | ||
| Female | 73 | 63 | 23.3 | ||
| Age, yr | 1.155 | 0.283 | |||
| ≤ 60 | 53 | 67.7 | 39.7 | ||
| > 60 | 133 | 66.8 | 31.8 | ||
| Smoking | 0.883 | 0.347 | |||
| Yes | 62 | 69.3 | 32 | ||
| No | 124 | 65.9 | 35.5 | ||
| Diabetes | 0.734 | 0.391 | |||
| Yes | 53 | 61.6 | 32.7 | ||
| No | 133 | 69.1 | 34.9 | ||
| PBD | 0.519 | 0.471 | |||
| Yes | 90 | 67 | 31.3 | ||
| No | 96 | 67.1 | 36.8 | ||
| TB (μmol/L) | 2.556 | 0.11 | |||
| ≤ 21 | 38 | 82.4 | 34.3 | ||
| > 21 | 148 | 63.3 | 32.4 | ||
| CA19-9 (U/mL) | 5.688 | 0.017 | |||
| ≤ 37 | 69 | 83.5 | 43.7 | ||
| > 37 | 117 | 56.5 | 28 | ||
| γ-GGT (U/L) | 0.06 | 0.806 | |||
| ≤ 45 | 11 | 51.1 | 51.1 | ||
| > 45 | 175 | 68 | 34 | ||
| CA19-9/GGT | 26.824 | 0 | |||
| ≤ 0.12 | 81 | 84.2 | 51 | ||
| > 0.12 | 105 | 52.9 | 20.1 | ||
| OP time, h | 0.299 | 0.585 | |||
| ≤ 9 | 83 | 65.4 | 36.3 | ||
| > 9 | 103 | 68.2 | 31.5 | ||
| Intraoperative blood loss (mL) | 1.282 | 0.258 | |||
| ≤ 500 | 117 | 69.3 | 35.5 | ||
| > 500 | 69 | 62.6 | 30.7 | ||
| Blood transfusion | 7.235 | 0.007 | |||
| Yes | 66 | 56.3 | 24.7 | ||
| No | 120 | 72.7 | 37.9 | ||
| Degree of differentiation | 20.848 | 0 | |||
| Poor | 52 | 34.2 | 22.2 | ||
| Moderate & high | 134 | 79.9 | 39 | ||
| Tumor size, cm | 3.313 | 0.069 | |||
| ≤ 2 | 114 | 72.7 | 39.6 | ||
| > 2 | 72 | 57.7 | 24.5 | ||
| LN metastasis | 32.491 | 0 | |||
| Yes | 75 | 45.6 | 15.4 | ||
| No | 111 | 81.1 | 47.8 | ||
| Nerve invasion | 4.963 | 0.026 | |||
| Yes | 162 | 65.4 | 31.8 | ||
| No | 24 | 80 | 60 | ||
| Resection margin | 0.943 | 0.332 | |||
| R0 | 178 | 67 | 34.8 | ||
| R1 | 8 | 66.7 | 16.7 | ||
| Postoperative complication | 0.197 | 0.657 | |||
| Yes | 54 | 75.2 | 30.1 | ||
| No | 132 | 64.3 | 34.9 | ||
| Postoperative chemotherapy | 0.011 | 0.917 | |||
| Yes | 39 | 65.7 | 36.5 | ||
| No | 147 | 67.4 | 33.5 |
| Variables | Group 1 (n = 81) | Group 2 (n = 105) | P |
| Postoperative hospital stay (d) | 21 (16–24) | 20 (16–29) | 0.368 |
| In-hospital death | 1 | 6 | 0.229 |
| Complications | 20 | 34 | 0.252 |
| Biochemical fistula | 12 | 7 | 0.069 |
| Pancreatic fistula | |||
| Grade B | 4 | 2 | 0.458 |
| Grade C | 3 | 4 | 0.726 |
| Delayed gastric emptying | 2 | 6 | 0.473 |
| Intra-abdominal infection | 7 | 9 | 0.986 |
| Abdominal hemorrhage | 4 | 7 | 0.856 |
Postoperative tumor recurrence was taken as a dependent variable and preoperative data [gender, age, smoking history, diabetes, PBD, total bilirubin (TB), GGT, CA19-9, CA19-9/GGT], intraoperative data, pathological data, postoperative complications and chemotherapy as independent variables for univariate and multivariate analysis (Tables 3 and 4). CA19-9/GGT [relative risk (RR) = 2.134, 95% CI: 1.319–3.451), carcinoma differentiation (RR = 1.695, 95% CI: 1.115–2.576) and lymphatic node metastasis (RR = 2.145, 95% CI: 1.404–3.277) were independent risk factors for tumor recurrence in DCC. Patients with the smaller CA19-9/GGT, higher degree of tumor differentiation and the absence of lymphatic metastasis, the lower the risk of tumor recurrence.
| Variable | RR | 95%CI | P |
| CA19-9 | 0.921 | 0.578–1.468 | 0.728 |
| CA19-9/GGT | 2.134 | 1.319–3.451 | 0.002 |
| Blood transfusion | 0.74 | 0.497–1.103 | 0.139 |
| Degree of differentiation | 1.695 | 1.115–2.576 | 0.013 |
| LN metastasis | 2.145 | 1.404–3.277 | 0 |
| Nerve invasion | 1.238 | 0.520–2.951 | 0.63 |
The long-term outcome of DCC after surgery was considered as the dependent variable and intraoperative, preoperative, pathological and postoperative data were used as independent variables for univariate and multivariate analysis (Tables 5 and 6). CA19-9/GGT (RR = 2.837, 95% CI: 1.727–4.660), carcinoma differentiation (RR = 1.725, 95% CI: 1.140–2.690) and lymphatic metastasis (RR = 2.050, 95% CI: 1.336–3.144) were independent risk factors for long-term outcome in DCC.
| Variable | n | 1-yr OS (%) | 3-yr OS (%) | χ2 | P |
| Gender | 1.351 | 0.245 | |||
| Male | 113 | 76.1 | 40.8 | ||
| Female | 73 | 67.9 | 25.9 | ||
| Age, yr | 2.381 | 0.123 | |||
| ≤ 60 | 53 | 80.9 | 36.4 | ||
| > 60 | 133 | 69.5 | 32.3 | ||
| Smoking | 0.822 | 0.364 | |||
| Yes | 62 | 78.2 | 27.3 | ||
| No | 124 | 70.2 | 36.1 | ||
| Diabetes | 0.014 | 0.906 | |||
| Yes | 53 | 70.8 | 32.6 | ||
| No | 133 | 73.5 | 34.6 | ||
| PBD | 1.217 | 0.27 | |||
| Yes | 90 | 70.2 | 27.7 | ||
| No | 96 | 75.1 | 39.9 | ||
| TB (μmol/L) | 0.623 | 0.43 | |||
| ≤ 21 | 38 | 78.9 | 44.4 | ||
| > 21 | 148 | 70.9 | 32.3 | ||
| CA19-9 (U/ml) | 8.239 | 0.004 | |||
| ≤ 37 | 69 | 85 | 49 | ||
| > 37 | 117 | 65.5 | 25.5 | ||
| γ-GGT (U/L) | 0.169 | 0.681 | |||
| ≤ 45 | 11 | 71.6 | 43 | ||
| > 45 | 175 | 72.9 | 34.1 | ||
| CA19-9/GGT | 38.091 | 0 | |||
| ≤ 0.12 | 81 | 88.3 | 59.2 | ||
| > 0.12 | 105 | 61 | 13.6 | ||
| OP time, h | 0.008 | 0.929 | |||
| ≤ 9 | 83 | 68.8 | 38.4 | ||
| > 9 | 103 | 76 | 31.6 | ||
| Intraoperative blood loss (mL) | 2.693 | 0.101 | |||
| ≤ 500 | 117 | 72.8 | 39.1 | ||
| > 500 | 69 | 72.5 | 26.2 | ||
| Blood transfusion | 8.307 | 0.004 | |||
| Yes | 66 | 65.1 | 26.1 | ||
| No | 120 | 76.9 | 37.3 | ||
| Degree of differentiation | 21.212 | 0 | |||
| Poor | 52 | 51.5 | 19.4 | ||
| Moderate & high | 134 | 80.9 | 40 | ||
| Tumor size, cm | 1.544 | 0.214 | |||
| ≤ 2 | 114 | 78.4 | 35.7 | ||
| > 2 | 72 | 63.6 | 31.3 | ||
| LN metastasis | 30.845 | 0 | |||
| Yes | 75 | 59.8 | 15.8 | ||
| No | 111 | 81.4 | 48.4 | ||
| Nerve invasion | 1.861 | 0.173 | |||
| Yes | 162 | 73.7 | 30.3 | ||
| No | 24 | 66.2 | 66.2 | ||
| Resection margin | 3.343 | 0.067 | |||
| R0 | 178 | 73.2 | 35.2 | ||
| R1 | 8 | 62.5 | 12.5 | ||
| Postoperative complication | 2.357 | 0.125 | |||
| Yes | 54 | 67.8 | 28.8 | ||
| No | 132 | 74.9 | 36.3 | ||
| Postoperative chemotherapy | 0.073 | 0.788 | |||
| Yes | 39 | 70.4 | 36.8 | ||
| No | 147 | 73.4 | 33.5 |
| Variable | RR | 95%CI | P |
| CA19-9 | 0.974 | 0.607–1.561 | 0.911 |
| CA19-9/GGT | 2.837 | 1.727–4.660 | 0 |
| Blood transfusion | 0.763 | 0.513–1.135 | 0.182 |
| Degree of differentiation | 1.725 | 1.140–2.609 | 0.01 |
| LN metastasis | 2.05 | 1.336–3.144 | 0.001 |
DCC is mainly managed by surgical resection to achieve DFS; however, the long-term outcome of patients remained unsatisfactory. The data of 1490 patients who were diagnosed with DCC and received PD in the USA were retrospectively analyzed by Andrianello et al[14]. They included patients with median OS of 31 mo and at 1-, 3- and 5-year postoperative survival of 89%, 40% and 18%, respectively. Further analysis indicated the independent risk factors for long-term prognosis in patients with DCC, including lymph node metastasis and tumor differentiation. However, these predictive factors had their own limitations in optimizing treatment decisions preoperatively in clinical practice since most of them were not available before surgery and were influenced by human factors. Therefore, developing noninvasive blood-based biomarkers that can make accurate prognostic prediction of DCC preoperatively will be of importance clinically.
CA19-9 is a glycolipid tumor-associated antigen on the cell membrane. As a serological marker, CA19-9 is important in clinical diagnosis of cholangiocarcinoma[15]. It has also been proved to correlate with the long-term outcome of patients. Eighty-nine patients diagnosed with cholangiocarcinoma were reviewed by Coelho et al[16], from which they identified CA19-9 as an independent risk factor for long-term prognosis. Nevertheless, patients with DCC were not specifically distinguished. Tella et al[17] retrospectively analyzed the data from the National Cancer Database; 2100 patients with extrahepatic cholangiocarcinoma were included and 1474 (70.2%) had elevated CA19-9. They observed a particularly lower median survival time in patients with increasing level of CA19-9 compared to those with normal level of CA19-9 (8.5 vs 16.0 mo) and they confirmed CA19-9 as an independent risk factor for long-term prognosis in patients with extrahepatic cholangiocarcinoma. Nevertheless, some researchers have indicated that the efficacy of CA19-9 in the diagnosis and prognosis of biliary tract carcinoma is greatly reduced in the presence of biliary obstruction. Lin et al[18] showed that CA19-9 alone is not enough to distinguish malignant or benign biliary obstructive diseases, based on a group of patients with biliary obstruction. In their research 39 patients with benign biliary diseases were included whose level of CA19-9 was 401.9 U/mL on average, and the CA19-9 value of 10 patients was > 1000 U/mL. In a study conducted by Tan et al[19], clinical data of 84 patients diagnosed with DCC were reviewed. A lower level of CA19-9 indicated better long-term prognosis, but multivariate analysis revealed that CA19-9 was not an independent risk factor for poor outcome. Bolm et al[20] also demonstrated that CA19-9 could not be a prognostic indicator for patients with DCC, which is in urgent need of confirmation. In our study, 62.9% of patients with DCC were accompanied by elevated CA19-9 (> 37 U/mL) and had worse long-term prognosis than those patients with normal level of CA19-9 (≤ 37 U/mL). Nevertheless, CA19-9 has been proved not to be an independent risk factor for poor long-term prognosis in multivariate analysis. We attribute it to the high proportion of patients (79.6%) who had combined biliary obstruction in this cohort. Due to bile excretion disorders resulting from biliary obstruction, these patients tended to have a higher overall level of CA19-9, making CA19-9 a less accurate indicator in evaluating the prognosis of DCC patients.
γ-GGT is a membrane-bound glycoprotein and a mitochondrial enzyme containing a sulfhydryl group. γ-GGT plays a key role in the metabolism of GSH and is mostly distributed in the liver, kidney, pancreas and other substantial organs[21]. GGT can be used in the diagnosis and prognosis of malignant tumors, kidney and cardiovascular diseases, and metabolic syndrome[22-25]. According to underlying biological mechanisms illustrating the relationship between GGT expression and cancer, GGT may facilitate the progression, invasion and drug resistance of tumor by modulating a series of vital redox-sensitive functions, including antioxidant/antitoxic defenses and the cellular proliferative/apoptotic balance[26-28]. GGT mainly originates from hepatic Kupffer cells and endothelial cells of the bile duct, and has a significantly higher expression level in hepatocellular carcinoma tissues and fetal liver[29]. However, no related research has been done to reveal the clinical value of GGT in patients diagnosed with DCC. Most patients with DCC are accompanied with various degrees of biliary obstruction, resulting in an abnormal increase in serum GGT, which accounts for up to 94.1% of the data in this group. Therefore, GGT can reflect the degree of biliary obstruction to some extent and is more sensitive than bilirubin. By aligning the ratio of CA19-9 to GGT, we corrected CA19-9 to minimize the effect of biliary obstruction on the level of serum CA19-9. As far as we know, no similar retrospective studies uncovering the relationship between CA19-9/GGT and DCC have been done. Moreover, CA19-9/GGT is identified as an independent risk factor for long-term outcome in patients with DCC according to our results, and its predictive value even exceeds that of differentiation degree and lymph node metastasis due to the highest RR. Patients with smaller CA19-9/TB have a lower rate of postoperative tumor recurrence and better prognosis in the long term.
Our results also revealed an association between CA19-9/GGT and lymphatic metastasis. A smaller CA19-9/GGT indicated a higher rate of lymphatic metastasis; nevertheless, no significant relation between CA19-9/GGT and tumor size and differentiation was observed in our research. Bergquist et al[30] asserted that, among patients with DCC, 28.7% whose CA19-9 was ≤ 37 U/mL presented with lymph node metastasis, which was significantly lower than 43.8% in patients whose CA19-9 was > 37 U/mL. In addition, by mediating extracellular GSH cleavage and intracellular GSH synthesis, overexpression of GGT may increase the metastatic activity in melanoma, and intertissue flow of GSH may have a growth-promoting effect on GGT-positive tumors[31] . This emphasizes the significant correlation between higher GGT level and lymph node involvement. The exact mechanism for the relation between GGT level and lymph node metastasis remains unknown and requires further study, to elucidate the role that GGT plays in tumor invasion. Nevertheless, most patients with DCC are accompanied with various degrees of biliary obstruction, resulting in abnormal increase of serum GGT. It has also been confirmed that lymphatic metastasis is an independent risk factor for long-term prognosis of patients with DCC, and it is considered to be an important factor in judging the degree of malignancy and local spread of malignant tumor. Therefore, it is reasonable to assume that CA19-9/GGT can function as a better biomarker in reflecting the malignancy and aggressiveness of DCC.
Our study had some limitations. First, as a single-center retrospective study, a certain degree of bias was inevitable. Second, the proportion relationship between CA19-9 and bilirubin remained unclear, as did whether CA19-9 decreased in proportion to bilirubin after relief of biliary obstruction. Therefore, for patients with different degrees of biliary obstruction, however, the effect of GGT correction may be biased to a certain extent of DCC after curative resection.
Elevation of CA19-9/GGT performed better as a biomarker of aggressiveness of DCC, as well as a predictor of poor clinical outcomes by reducing the effect of biliary tract obstruction of CA19-9 concentration. CA19-9/GGT might be a significant indicator for identifying DCC patients at high risk of early recurrence and unfavorable prognosis.
Distal cholangiocarcinoma (DCC) is a rare malignant tumor in the digestive system and has a poor long-term prognosis. Curative excision is currently the most appropriate therapy for patients with DCC because of the lack of effective adjuvant therapies. Therefore, it is important to determine the long-term prognosis for formulating a reasonable treatment plan and avoiding unnecessary surgical trauma.
At present, tumor differentiation, lymphatic metastasis and other pathological risk factors for DCC can only be obtained after surgery, and the information acquisition is delayed.
We aimed to minimize the interference effect of obstructive jaundice on the concentration of carbohydrate antigen 19-9 (CA19-9), so as to determine the strong association between CA19-9/γ-glutamyltransferase (GGT) and postoperative tumor recurrence and long-term outcome of DCC.
We enrolled 186 patients. Receiver operating characteristic curves were drawn according to preoperative CA19-9/GGT and 1-year survival, and the patients were divided into two groups (group 1, low-ratio, n = 81; group 2, high-ratio, n = 105). By univariate and multivariate analyses, the risk factors influencing tumor recurrence and long-term outcome of patients with DCC were screened out.
The optimum value of CA19-9/GGT was 0.12. Patients in group 2 had higher CA19-9 and lymphatic metastasis rate accompanied by lower GGT, when compared with group 1 (P < 0.05). The 1-, 3- and 5-year overall survival rates of patients in group 1 and group 2 were 88.3%, 59.2% and 48.1% and 61.0%, 13.6% and 13.6%, respectively (P = 0.000). Multivariate analysis indicated that CA19-9/GGT, lymphatic metastasis and tumor differentiation were independent risk factors for tumor recurrence and long-term prognosis of DCC.
Elevation of CA19-9/GGT performed better as an indicator of aggressive tumor behavior, as well as a predictor of poor clinical outcomes by reducing the effect of biliary obstruction on CA19-9 concentration in patients with DCC. CA19-9/GGT might be a significant indicator for identifying DCC patients at high risk of early recurrence and unfavorable prognosis.
CA19-9/GGT is more valuable in judging DCC patients at high risk of early recurrence and unfavorable outcomes.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Han IW S-Editor: Zhang H L-Editor: Kerr C P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1378] [Article Influence: 125.3] [Reference Citation Analysis (1)] |
| 3. | Lad N, Kooby DA. Distal cholangiocarcinoma. Surg Oncol Clin N Am. 2014;23:265-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Lee RM, Maithel SK. Approaches and Outcomes to Distal Cholangiocarcinoma. Surg Oncol Clin N Am. 2019;28:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Strijker M, Belkouz A, van der Geest LG, van Gulik TM, van Hooft JE, de Meijer VE, Haj Mohammad N, de Reuver PR, Verheij J, de Vos-Geelen J, Wilmink JW, Groot Koerkamp B, Klümpen HJ, Besselink MG; Dutch Pancreatic Cancer Group. Treatment and survival of resected and unresected distal cholangiocarcinoma: a nationwide study. Acta Oncol. 2019;58:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Wellner UF, Shen Y, Keck T, Jin W, Xu Z. The survival outcome and prognostic factors for distal cholangiocarcinoma following surgical resection: a meta-analysis for the 5-year survival. Surg Today. 2017;47:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Bergquist JR, Puig CA, Shubert CR, Groeschl RT, Habermann EB, Kendrick ML, Nagorney DM, Smoot RL, Farnell MB, Truty MJ. Carbohydrate Antigen 19-9 Elevation in Anatomically Resectable, Early Stage Pancreatic Cancer Is Independently Associated with Decreased Overall Survival and an Indication for Neoadjuvant Therapy: A National Cancer Database Study. J Am Coll Surg. 2016;223:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Lee SP, Sung IK, Kim JH, Lee SY, Park HS, Shim CS. Usefulness of Carbohydrate Antigen 19-9 Test in Healthy People and Necessity of Medical Follow-up in Individuals with Elevated Carbohydrate Antigen 19-9 Level. Korean J Fam Med. 2019;40:314-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | La Greca G, Sofia M, Lombardo R, Latteri S, Ricotta A, Puleo S, Russello D. Adjusting CA19-9 values to predict malignancy in obstructive jaundice: influence of bilirubin and C-reactive protein. World J Gastroenterol. 2012;18:4150-4155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Xiao Y, Yang H, Lu J, Li D, Xu C, Risch HA. Serum gamma-glutamyltransferase and the overall survival of metastatic pancreatic cancer. BMC Cancer. 2019;19:1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 807] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 12. | Mei Y, Chen L, Zeng PF, Peng CJ, Wang J, Li WP, Du C, Xiong K, Leng K, Feng CL, Jia JH. Combination of serum gamma-glutamyltransferase and alkaline phosphatase in predicting the diagnosis of asymptomatic choledocholithiasis secondary to cholecystolithiasis. World J Clin Cases. 2019;7:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (2)] |
| 13. | Griffith OW, Bridges RJ, Meister A. Transport of gamma-glutamyl amino acids: role of glutathione and gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1979;76:6319-6322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Andrianello S, Paiella S, Allegrini V, Ramera M, Pulvirenti A, Malleo G, Salvia R, Bassi C. Pancreaticoduodenectomy for distal cholangiocarcinoma: surgical results, prognostic factors, and long-term follow-up. Langenbecks Arch Surg. 2015;400:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Grunnet M, Mau-Sørensen M. Serum tumor markers in bile duct cancer--a review. Biomarkers. 2014;19:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Coelho R, Silva M, Rodrigues-Pinto E, Cardoso H, Lopes S, Pereira P, Vilas-Boas F, Santos-Antunes J, Costa-Maia J, Macedo G. CA 19-9 as a Marker of Survival and a Predictor of Metastization in Cholangiocarcinoma. GE Port J Gastroenterol. 2017;24:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Tella SH, Kommalapati A, Yadav S, Bergquist JR, Goyal G, Durgin L, Borad M, Cleary SP, Truty MJ, Mahipal A. Novel staging system using carbohydrate antigen (CA) 19-9 in extra-hepatic cholangiocarcinoma and its implications on overall survival. Eur J Surg Oncol. 2020;46:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Lin MS, Huang JX, Yu H. Elevated serum level of carbohydrate antigen 19-9 in benign biliary stricture diseases can reduce its value as a tumor marker. Int J Clin Exp Med. 2014;7:744-750. [PubMed] |
| 19. | Tan X, Xiao K, Liu W, Chang S, Zhang T, Tang H. Prognostic factors of distal cholangiocarcinoma after curative surgery: a series of 84 cases. Hepatogastroenterology. 2013;60:1892-1895. [PubMed] |
| 20. | Bolm L, Petrova E, Weitz J, Rückert F, Wittel UA, Makowiec F, Lapshyn H, Bronsert P, Rau BM, Khatkov IE, Bausch D, Keck T, Wellner UF, Distler M. Prognostic relevance of preoperative bilirubin-adjusted serum carbohydrate antigen 19-9 in a multicenter subset analysis of 179 patients with distal cholangiocarcinoma. HPB (Oxford). 2019;21:1513-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Hanigan MH. Gamma-glutamyl transpeptidase: redox regulation and drug resistance. Adv Cancer Res. 2014;122:103-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 22. | Fu SJ, Zhao Q, Ji F, Chen MG, Wu LW, Ren QQ, Guo ZY, He XS. Elevated Preoperative Serum Gamma-glutamyltranspeptidase Predicts Poor Prognosis for Hepatocellular Carcinoma after Liver Transplantation. Sci Rep. 2016;6:28835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Kunutsor SK, Laukkanen JA. Gamma-glutamyltransferase and risk of chronic kidney disease: A prospective cohort study. Clin Chim Acta. 2017;473:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Dalos D, Binder C, Duca F, Aschauer S, Kammerlander A, Hengstenberg C, Mascherbauer J, Reiberger T, Bonderman D. Serum levels of gamma-glutamyltransferase predict outcome in heart failure with preserved ejection fraction. Sci Rep. 2019;9:18541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Coku V, Shkembi X. Serum Gamma-glutamyltransferase and Obesity: is there a Link? Med Arch. 2018;72:112-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol. 2006;71:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | Franzini M, Corti A, Lorenzini E, Paolicchi A, Pompella A, De Cesare M, Perego P, Gatti L, Leone R, Apostoli P, Zunino F. Modulation of cell growth and cisplatin sensitivity by membrane gamma-glutamyltransferase in melanoma cells. Eur J Cancer. 2006;42:2623-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Dominici S, Valentini M, Maellaro E, Del Bello B, Paolicchi A, Lorenzini E, Tongiani R, Comporti M, Pompella A. Redox modulation of cell surface protein thiols in U937 lymphoma cells: the role of gamma-glutamyl transpeptidase-dependent H2O2 production and S-thiolation. Free Radic Biol Med. 1999;27:623-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Yao DF, Dong ZZ. Hepatoma-related gamma-glutamyl transferase in laboratory or clinical diagnosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2007;6:9-11. [PubMed] |
| 30. | Bergquist JR, Ivanics T, Storlie CB, Groeschl RT, Tee MC, Habermann EB, Smoot RL, Kendrick ML, Farnell MB, Roberts LR, Gores GJ, Nagorney DM, Truty MJ. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: A national cohort analysis. J Surg Oncol. 2016;114:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Obrador E, Carretero J, Ortega A, Medina I, Rodilla V, Pellicer JA, Estrela JM. gamma-Glutamyl transpeptidase overexpression increases metastatic growth of B16 melanoma cells in the mouse liver. Hepatology. 2002;35:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |