Published online Aug 27, 2021. doi: 10.4240/wjgs.v13.i8.848
Peer-review started: April 13, 2021
First decision: June 23, 2021
Revised: June 28, 2021
Accepted: July 19, 2021
Article in press: July 19, 2021
Published online: August 27, 2021
Processing time: 128 Days and 11.5 Hours
Many clinicians and surgeons are unfamiliar with the sclerosing angiomatoid nodular transformation (SANT), which is gaining recognition as a benign splenic tumor. We challenge that SANT is rare and whether surgical intervention could be avoided through critical imaging review.
To evaluate the incidence of SANT among splenic tumors and the decision-making process of SANT management.
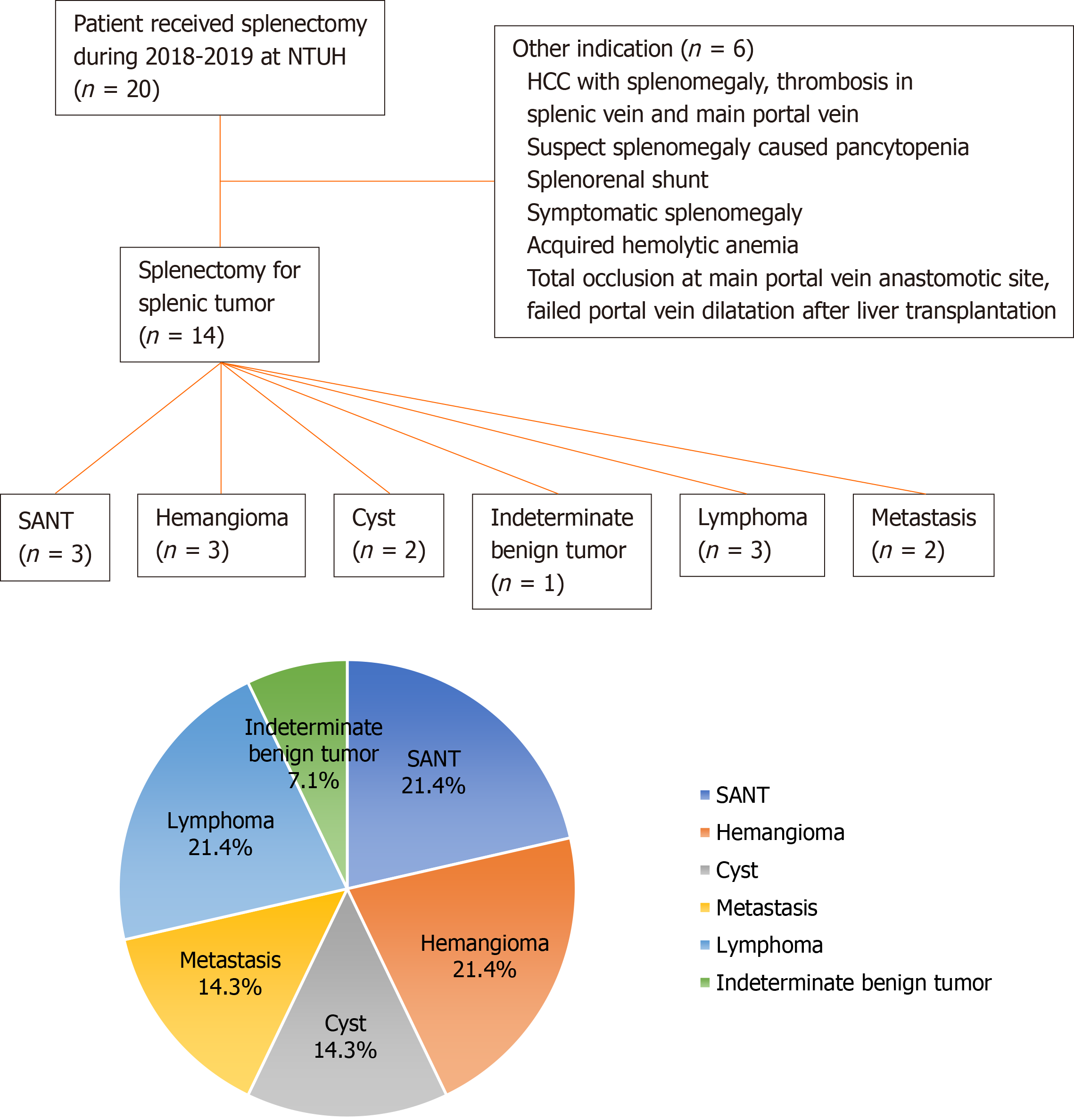
Twenty hospitalized patients who underwent splenectomy in 2018 and 2019 in a tertiary university hospital were retrospectively reviewed, and their data on imaging, diagnosis, surgical indications, and courses were recorded. All pathology results were confirmed by pathologist. Discriminative features differentiating SANT from other non-SANT splenic tumors were descriptively analyzed in this case series.
Fourteen out of 20 patients who underwent splenectomy had splenic tumors, including 3 SANTs (21% splenic tumors), 6 non-SANT benign lesions (43%), 2 metastatic tumors, and 3 lymphomas. Hypointensity on T2-weighted magnetic resonance imaging (MRI), spoke wheel enhancing pattern in contrasted computed tomography or MRI, and cold spot (low fluorodeoxyglucose uptake) in positron emission tomography (PET) scan helped establish the diagnosis of SANT. Lymphoma, presenting with a hot spot on the PET scan were differentiated from SANT. Surgical indications were reformatted for splenic tumors. Splenectomy need not be performed in patients with typical imaging features of SANT.
SANT is not a rare disease entity in clinical practice. Splenectomy should not be routinely indicated as the only management option for SANT with typical imaging features.
Core Tip: Sclerosing angiomatoid nodular transformation (SANT) used to be considered a rare but benign lesion since it was recognized. However, SANT comprised one fifth patients who received splenectomy for splenic tumors in our university hospital cohort. The unique diagnostic image features of SANT include spoke wheel enhancing pattern in contrasted computed tomography or magnetic resonance imaging (MRI) and hypo-intensity on T2-weighted images of MRI. Clinicians should recognize this disease entity to avoid unnecessary overtreatment.
- Citation: Tseng H, Ho CM, Tien YW. Reappraisal of surgical decision-making in patients with splenic sclerosing angiomatoid nodular transformation: Case series and literature review. World J Gastrointest Surg 2021; 13(8): 848-858
- URL: https://www.wjgnet.com/1948-9366/full/v13/i8/848.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i8.848
With the increasing use of imaging modalities, such as computed tomography (CT), incidental finding of splenic lesions is increasingly common. Clinicians and surgeons are frequently faced with difficult decision-making regarding the management of splenic tumors. Benign splenic masses include cysts, hemangiomas, lymphangiomas, hamartomas, sclerosing angiomatoid nodular transformation (SANT), and sarcoidosis, whereas malignant lesions include lymphoma, metastasis, and rarely angiosarcoma[1-3]. Splenic cysts and hemangiomas are well recognized, but the others are not common in daily practice, and therefore cause a diagnostic dilemma that may eventually lead to unnecessary splenectomy.
SANT, a newly defined rare benign tumor, first reported in 2004, was re-named as cord capillary hemangioma, a variant of hamartoma, or a multinodular hemangioma[4]. Reported patterns in CT images include well-circumscribed lesions that are solitary, hypodense, and present with peripheral enhancement with central progression and radiating lines. On magnetic resonance imaging (MRI), SANT appears isointense and hypointense on T1 and T2 images, with the same enhancing pattern as CT images, with the so-called spoke-wheel enhancement[2]. Splenectomy is considered the standard treatment because the diagnostic imaging features of SANT are not well-established[5]. However, in light of the benign nature of the pathogenesis[6] and the clinical course (no reported relapse or metastasis after splenectomy), the indication for splenectomy is controversial.
Splenectomy is commonly performed in the setting of hematological autoimmune disease (idiopathic thrombocytopenic purpura and autoimmune hemolytic anemia) and malignancies[7], whereas for other splenic tumors (including SANT), the indications are not well recognized. In our study, we reviewed the patients who underwent splenectomy for splenic tumors and found that many SANTs were diagnosed within a short period. We hypothesize that SANT is not rare, but an unrecognized disease entity. We aimed to evaluate the decision-making process for the management of SANT (conservative observation or surgery) by determining the typical imaging features to distinguish SANT and critically review current indications for splenectomy in the literature.
We retrospectively reviewed 20 hospitalized patients who underwent splenectomy at the National Taiwan University Hospital in 2018 and 2019. Six patients were excluded from the study because the indications for splenectomy were not the presence of tumors. Fourteen eligible patients were further divided into SANT and non-SANT groups based on the histopathological diagnosis. Demographic and clinical characteristics, including age, sex, symptoms, past history, radiographic imaging findings [CT, MRI, positron emission tomography (PET)], tentative diagnosis made by radiologists, operative methods (open or laparoscopic), and postoperative complications were reviewed. Furthermore, we compared the imaging features of SANT and non-SANT tumors largely based on literature[1-3,8], with an intent to identify the decisive factor distinguishing non-SANT from SANT. This study was approved by the Institutional Review Board of the National Taiwan University Hospital, Taipei, Taiwan (NTUH REC: 202102011RIND). Because this was a retrospective study using chart review, the institutional review board waived the need for informed consent.
Descriptive statistics were used to summarize the characteristics (frequency distribution, central tendency, and variation) of the dataset. Data are presented as mean, median, range, or percentage when appropriate. Analysis were performed using the Statistical Package for Social Sciences (SPSS)® version 21.0 (SPSS Inc., Chicago, IL, United States).
Between 2018 and 2019, 20 patients underwent splenectomy (11 open and 9 Laparoscopic approaches). Their surgical indication and final pathologic diagnosis are shown in Figure 1. Among these, 14 cases were splenic tumors, and 9 (64.3%) were benign, including 3 cases of SANT. Malignant tumors included two metastatic lesions (renal cell carcinoma and lung pleomorphic carcinoma), and three newly diagnosed lymphomas, with a median hospital stay of eight days (4-105 d). One patient with diffuse large B-cell lymphoma underwent re-operation for gastric perforation 12 d after open distal pancreatectomy and splenectomy, and was transferred to the intensive care unit after re-open surgery. A total of seven patients had minor complications (two cases of intra-abdominal infection, two cases of pneumonia, and three cases of wound infection) according to the Clavien-Dindo classification.
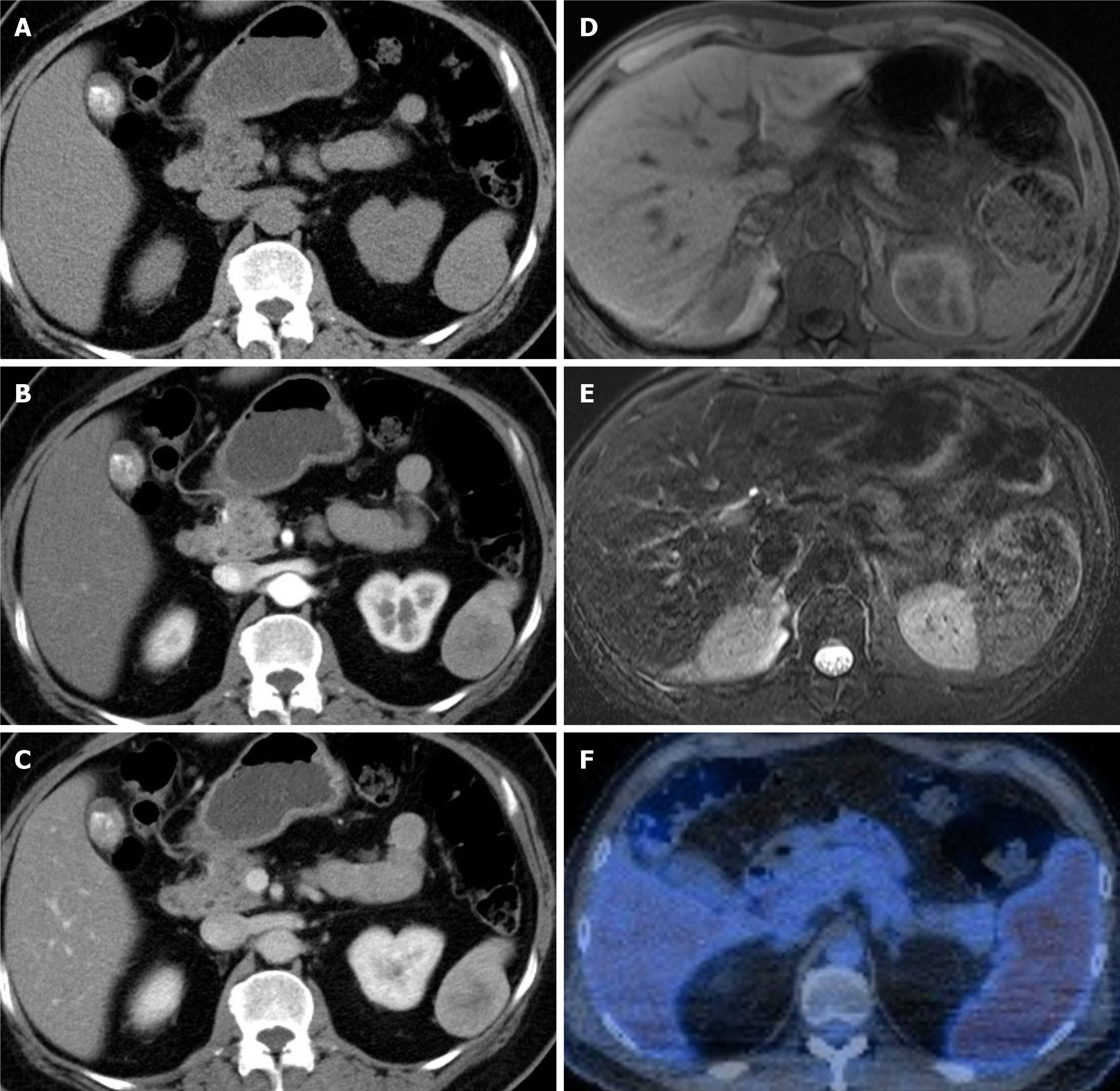
The clinical characteristics of the three patients who were finally diagnosed with SANT are summarized in Table 1. The average tumor size was 4.7 cm (3.5-5.5 cm). On CT scan, all three tumors showed non-contrast hypodense to isodense lesions with progressive peripheral enhancement, with a hypodense center in the venous phase (Figure 2). Case 1 showed a typical lobulated lesion and septate enhancement, which is referred to as centripetal enhancement or spoke wheel pattern[2]. SANTs cases 1 and 2 had hypo- to iso-intense appearance on T1-weighted and T2-weighted sequences, with peripheral or lobulated enhancement (Figure 2). Case 3 underwent a PET scan for lymphoma follow-up, and presented with diffuse mild hypermetabolism within the splenic tumor (standardized uptake value max = 4.2, Figure 2F). Splenectomy was indicated for diagnostic purpose (n = 2) or symptom relief (n = 1).
| No. | Age (yr) | Sex | Indication | Symptoms | Size | CT | MRI | PET | Follow up | Surgery | ||
| A phase | V phase | T1/T2 | Contrast | |||||||||
| 1 | 48 | M | Diagnostic splenectomy, for Atypical hemangioma MRI pattern, suspect lymphoma | 8 kg loss in 2 yr; left abdominal painFatigue | 5.5 cm | Septate enhancement | Mild progressing peripheral enhancement with centrally remained iso-hypodense | Iso-hypodense/iso-hypodense | Heterogenous gradual enhancement and diffusion restriction | Nil | 2 mo | Open |
| 2 | 61 | F | Diagnostic splenectomy, for elevated CA19-9, malignancy cannot be rule out | Nil | 4.1 cm | Mild enhancement | Mild progressing peripheral enhancement with centrally remained iso-hypodense | Iso-hypodense/hypodense | Mild peripheral enhancement | Nil | 6 mo | Laparoscopy |
| 3 | 52 | M | Diagnostic splenectomy, for suspect lymphoma splenic involvement1 | Nil | 3.5 cm | Peripheral enhancement | Nil | Nil | Diffuse mild hypermetabolism at spleen (2SUVmax = 4.2) | 4 mo | Laparoscopy | |
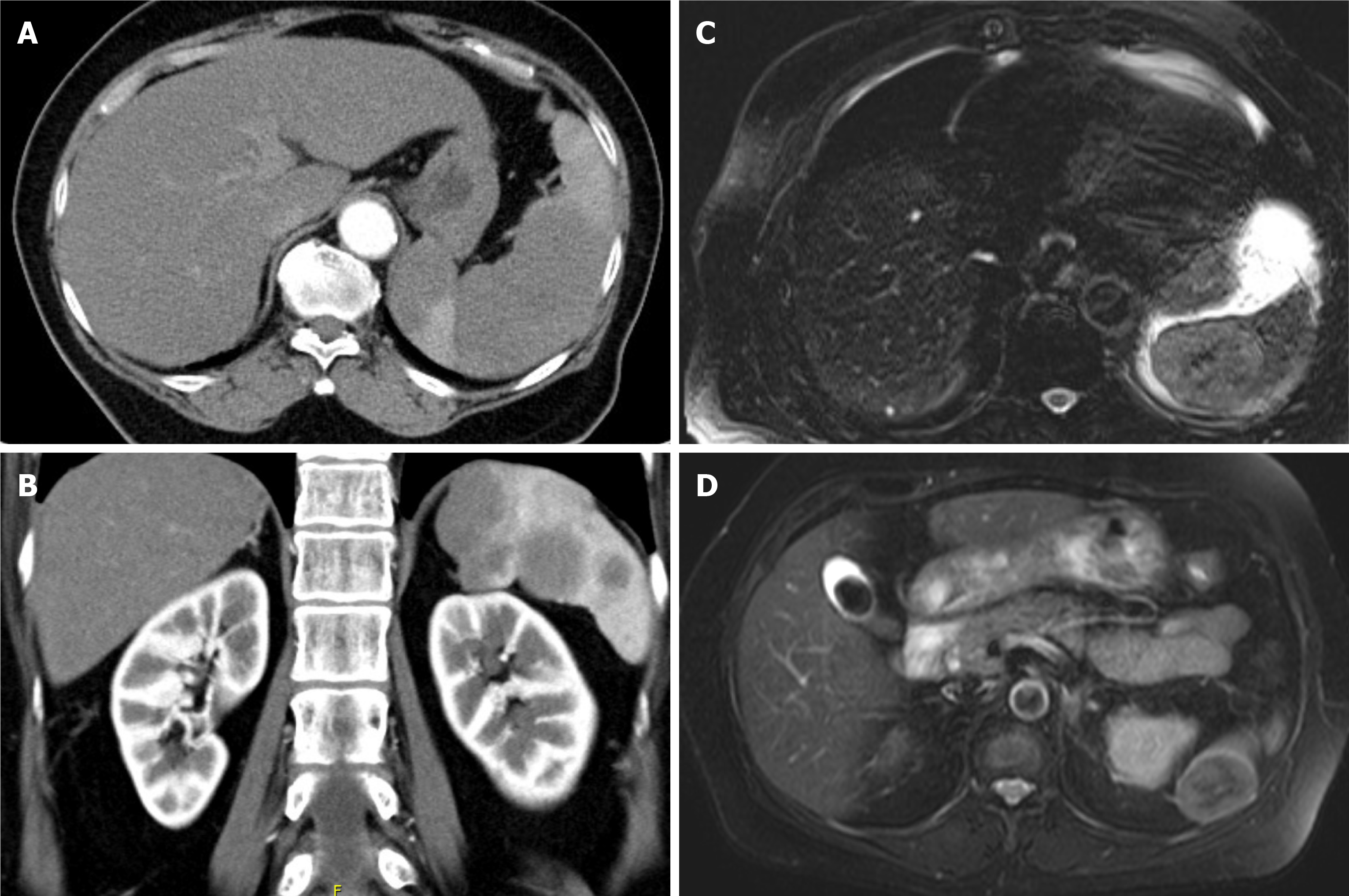
Splenectomies performed for other benign or malignant splenic tumors (n = 11) were included in this study to compare imaging features of SANT and five types of non-SANT tumors (Table 2). All 20 splenic tumors were hypodense on non-contrast CT. Some features, such as enhancement patterns (e.g., peripheral for hemangioma and poor for lymphoma) may be suggestive of SANT, but are difficult for radiologists to definitely diagnose. We reasoned that MRI outperformed CT in that hyperintensity on T2-weighted images (Figure 3) could suggest hemangioma or non-lymphoma metastasis (Figure 3B and D)[1,2], and the latter is rarely found in patients without an underlying malignancy[9]. In contrast, both SANT and lymphoma showed hypo intensity on T2 and a poor enhancement pattern (Figure 3A and C). Homogeneous splenomegaly with a focal infarct (Figure 4A) or multifocal splenic tumors (Figure 4B) could indicate a systemic lymphoma; however, when isolated lymphoma presents as a solitary splenic tumor (Figure 4C), distinguishing it from SANT becomes difficult because the spoke-wheel pattern can also be seen in lymphoma (Figure 4C and D). Additional imaging, such as PET (Figure 2F), or biopsy are necessary if splenectomy is not considered.
| Tumor | Tumor shape, features, and characteristics | MRI T1 | MRI T2 | CT/MRI enhancing pattern | Other image characteristics probably aid diagnosis | |
| Benign | SANT | Well-circumscribed, commonly lobulated, solitary mass | Iso-hypointense | Hypointense | Spoke-Wheel pattern; Poor enhancement | PET/CT: Low FDG accumulation |
| Hemangioma | Single nodule; multiple nodules; diffuse masses enlarging the spleen | Hypointense | Hyperintense | Homogenous or peripheral enhancement | Mostly small, asymptomatic, and slow growth | |
| Cyst | Cystic lesion | Hypointense | Hyperintense | No enhancement | Ultrasound: Thin wall, anechoic, peripheral brightly echogenicity, and distal shadows due to wall calcification | |
| Malignant | Systemic lymphoma | Homogenous splenomegaly; Infiltrative miliary lesions (1-5 mm); Multifocal lesions (2-10 cm); Uncommon solitary lesions (7-14 cm) | Isointense | Hypointense | Poor enhancing | Splenomegaly; Splenic infarct; PET: Intense heterogenous FDG activity; Extra-splenic findings: Hepatomegaly, lymphadenopathy |
| Primary splenic lymphoma | Solitary mass > uncommonly Splenomegaly | Isointense | Hypointense | Poor enhancing | Central necrosis | |
| Metastasis | Variously | Iso-hypointense | Hyperintensity | Similar to the primary tumor | PET/CT: FDG-avid tumor; Poorly marginated; Heterogenous |
No cases of SANT were diagnosed preoperatively. Indications of splenectomy for two cases of SANT and five malignant splenic tumors were oncological concerns. Interestingly, although one MRI reported a splenic tumor resembling lymphoma or SANT, splenectomy was still performed for the increasing tumor size, and the final diagnosis given was lymphoma. Notably, none of the three lymphomas in our series had abnormal blood parameters.
The indications for splenectomy in the two metastatic cases were a rapidly enlarging cystic lesion (8 cm in 6 mo) during follow-up and a newly suspected metastasis showing hyperintensity on T2-weighted images with central necrosis (Figure 3D).
Benign neoplasms in our series other than SANT were resected due to symptoms or an increase in size during follow-up, and the final pathologic reports were compatible with the preoperative diagnoses.
In our small cohort including patients from the 2-year study period, SANT was not uncommon, accounting for 3 out of the 14 splenic tumors. Since the first case series published in 2004[4], less than 200 cases have been reported, and almost all reports defined this novel lesion as a rare benign tumor. A disease is defined as rare when the approximate case number ranges between 1/1500 and 1/2000[10,11]. We argue that SANT is unidentified rather than rare in common clinical practice. Milosavljević et al[12] reported that SANT represented 3.3% of all the benign lesions that underwent splenectomy and in our series, 21.4% of all splenectomies in the 2 years. As more SANT cases are reported in the literature, the term “rare” may be inappropriate and deviates from reality.
Differential diagnosis of splenic tumors based on imaging features is generally difficult. However, T2 weighted imaging on MRI may provide additional diagnostic value for SANT (hypointensity) by excluding hemangioma and metastasis (hyperintensity). The presence of primary malignancy also suggests a diagnosis of metastatic splenic tumor[13], although rare. Solitary splenic metastasis is more uncommon, with only 5% of all metastases involving the spleen[9]. The chance of spleen as a metastatic focus is probably considered only in patients with an underlying known malignancy. Although rare, solitary lymphoma[8] could appear very similar to SANT on CT or MRI. Clinical clues, such as lymphadenopathy, hepatomegaly, symptoms of systemic lymphoma[1] tumor central necrosis, cytopenia, and size-increasing primary splenic lymphoma may help distinguish lymphoma from SANT[1,14]. However, SANT may coexist with extrasplenic lymphoma, as seen in case 3 in Table 1. High fluorodeoxyglucose uptake in the PET scan may be a promising diagnostic feature of lymphoma, which warrants further investigation[15].
Management of splenic tumors involves conservative follow-up or surgical resection. Surgical resection (total or partial splenectomy) is universally recommended for the diagnosis and treatment of SANT in the literature[16]; however, since a diagnosis can be made without pathology, the indication for surgery is questionable. Recently, Jin et al[16] reported a large series of 37 patients who were diagnosed with SANT over a 10-year-period, estimating an average of 4 cases per year. This finding was consistent with our statement that SANT should not be considered a rare tumor. A further review of 37 patients revealed that progressive enhancement was present in 9 cases on dynamic contrast-enhanced MRI studies that also looked more like malignant disease. Splenectomy could have been avoided in at least 28 cases, if SANT was identified by the clinicians[16]. We propose a list of indications for splenectomy for splenic tumors (Table 3). For those with typical imaging features of benign tumors, such as cysts, hemangiomas, and SANTs, symptoms were the most reasonable indication for splenectomy, although malignant splenic tumors tend to be more symptomatic than benign tumors[17]. For suspected benign splenic tumors, surgical intervention is appropriate for increasing size, splenic rupture, patient decision, and diagnostic purpose. In addition to surgical complications, splenectomy may be associated with an increased risk of infection, thromboembolism, and possibly cancer development[18]. When diagnosed, malignant splenic tumors do not always require splenectomy. Accumulating evidence has shown that splenic lymphoma can be effectively treated with immunochemotherapy, resulting in a decreasing need for therapeutic splenectomy[19,20]. Splenectomy for splenic lymphoma is reserved for patients who experience abdominal fullness due to large spleens and cytopenia due to spleen sequestration[19,20]. Diagnosis of tissue proof to determine the nature of lymphoma could be another important indication of splenectomy for highly suspected systemic lymphoma with absence of a definite diagnosis from another site. Splenectomy for splenic metastasis can be beneficial in metastatic carcinoma, either to achieve tumor-free status or to reduce tumor load in debulking surgery (such as ovarian cancer)[9]. Splenectomy could be avoided in disseminated metastatic disease because the benefit of survival or diagnosis remains low.
| Imaging features | Rationale for splenectomy |
| Benign tumor: Cyst; Hemangioma; SANT; other | Symptom-relief |
| Probable splenic rupture | |
| Increasing tumor size or numbers | |
| Rule out malignancy with atypical image features | |
| Patient desire | |
| Lymphoma | Symptom-relief |
| Treatment for cytopenia without heavy bone marrow infiltration | |
| Diagnosis for tissue proof to determine the nature of lymphoma | |
| Angiosarcoma | Treatment |
| Metastasis | Total tumor free |
| Debulking after chemotherapy | |
| Diagnosis and total tumor free for isolated splenic metastasis |
If surgery is not performed based on the radiological findings, the best strategy for the follow up of these patients and how the patients are counselled require additional consideration. In patients with underlying malignancies, the follow-up interval could be in line with the current schedule. As for incidental cases, 6-mo or 12-mo follow-up imaging is recommended[13]. The changing nature of tumor in images or clinical presentation should initiate a surgical re-evaluation. However, if patients do not feel reassured after counselling, an individualized decision of a short-interval follow-up recommendation or a direct referral to a surgeon is also justified.
Our study is limited by the small number of patients and its retrospective nature, precluding an extensive analysis of the specificity and sensitivity of imaging features. Patients who underwent conservative management of splenic tumors were not included. However, we aim to raise the awareness of this emerging diagnosis of SANT, which is currently categorized as a rare disease, but hopefully will be more commonly recognized with time, among clinicians and surgeons. We hope that this would assist the decision-making in the future management of splenic tumors, particularly SANT.
SANT should be considered uncommon. The surgical indications for SANT should be reconsidered. Further studies are needed to confirm the diagnostic features of SANT in imaging, such as hypo-intensity on T2-weighted images of MRI and spoke wheel enhancing pattern.
Clinicians are not familiar with the sclerosing angiomatoid nodular transformation (SANT), which is gaining recognition as a benign splenic tumor.
We challenge that SANT is rare and whether critical imaging review could help avoid unnecessary splenectomy.
This study aimed to evaluate the incidence of SANT among splenic tumors and the decision-making process of SANT management.
Twenty hospitalized patients who underwent splenectomy in 2018 and 2019 in a tertiary university hospital were retrospectively reviewed. Discriminative features differentiating SANT from other non-SANT splenic tumors were descriptively analyzed.
Fourteen splenectomies were indicated for splenic tumors, including 3 SANTs (21%). Hypointensity on T2-weighted magnetic resonance imaging, spoke wheel enhancing pattern, and cold spot in positron emission tomography scan helped establish the diagnosis of SANT. Splenectomy need not be performed in patients with typical imaging features of SANT.
SANT is not rare. Splenectomy should not be routinely indicated as the only management option for SANT with typical imaging features.
Further studies are needed to confirm the diagnostic imaging features of SANT in the future.
We thank Dr. Chen CY (Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital) for providing critical comments.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali SM S-Editor: Gao CC L-Editor: A P-Editor: Li JH
| 1. | Ricci ZJ, Kaul B, Stein MW, Chernyak V, Rozenblit AM, Oh SK, Flusberg M, Mazzariol FS. Improving diagnosis of atraumatic splenic lesions, Part III: malignant lesions. Clin Imaging. 2016;40:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Ricci ZJ, Mazzariol FS, Flusberg M, Chernyak V, Oh SK, Kaul B, Stein MW, Rozenblit AM. Improving diagnosis of atraumatic splenic lesions, part II: benign neoplasms/nonneoplastic mass-like lesions. Clin Imaging. 2016;40:691-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Ricci ZJ, Oh SK, Chernyak V, Flusberg M, Rozenblit AM, Kaul B, Stein MW, Mazzariol FS. Improving diagnosis of atraumatic splenic lesions, part I: nonneoplastic lesions. Clin Imaging. 2016;40:769-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Martel M, Cheuk W, Lombardi L, Lifschitz-Mercer B, Chan JK, Rosai J. Sclerosing angiomatoid nodular transformation (SANT): report of 25 cases of a distinctive benign splenic lesion. Am J Surg Pathol. 2004;28:1268-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Cipolla C, Florena AM, Ferrara G, Di Gregorio R, Unti E, Giannone AG, Lazzaro LA, Graceffa G, Pantuso G. Sclerosing Angiomatoid Nodular Transformation: Laparoscopic Splenectomy as Therapeutic and Diagnostic Approach at the Same Time. Case Rep Surg. 2018;2018:7020538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Awamleh AA, Perez-Ordoñez B. Sclerosing angiomatoid nodular transformation of the spleen. Arch Pathol Lab Med. 2007;131:974-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Browning MG, Bullen N, Nokes T, Tucker K, Coleman M. The evolving indications for splenectomy. Br J Haematol. 2017;177:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Lee HJ, Kim JW, Hong JH, Kim GS, Shin SS, Heo SH, Lim HS, Hur YH, Seon HJ, Jeong YY. Cross-sectional Imaging of Splenic Lesions: RadioGraphics Fundamentals | Online Presentation. Radiographics. 2018;38:435-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Sauer J, Sobolewski K, Dommisch K. Splenic metastases--not a frequent problem, but an underestimate location of metastases: epidemiology and course. J Cancer Res Clin Oncol. 2009;135:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Griggs RC, Batshaw M, Dunkle M, Gopal-Srivastava R, Kaye E, Krischer J, Nguyen T, Paulus K, Merkel PA; Rare Diseases Clinical Research Network. Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab. 2009;96:20-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 11. | Baldovino S, Moliner AM, Taruscio D, Daina E, Roccatello D. Rare Diseases in Europe: from a Wide to a Local Perspective. Isr Med Assoc J. 2016;18:359-363. [PubMed] |
| 12. | Milosavljević V, Tadić B, Grubor N, Erić D, Matić S. Laparoscopic Vs. Open Surgery in Management of Benign Neoplasms of Spleen—Single Institution Experience. Indian J Surg. 2020;82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Heller MT, Harisinghani M, Neitlich JD, Yeghiayan P, Berland LL. Managing incidental findings on abdominal and pelvic CT and MRI, part 3: white paper of the ACR Incidental Findings Committee II on splenic and nodal findings. J Am Coll Radiol. 2013;10:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Li M, Zhang L, Wu N, Huang W, Lv N. Imaging findings of primary splenic lymphoma: a review of 17 cases in which diagnosis was made at splenectomy. PLoS One. 2013;8:e80264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Karunanithi S, Sharma P, Roy SG, Vettiyil B, Sharma A, Thulkar S, Bal C, Kumar R. Use of 18F-FDG PET/CT imaging for evaluation of patients with primary splenic lymphoma. Clin Nucl Med. 2014;39:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Jin Y, Hu H, Regmi P, Li F, Cheng N. Treatment options for sclerosing angiomatoid nodular transformation of spleen. HPB (Oxford). 2020;22:1577-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Siewert B, Millo NZ, Sahi K, Sheiman RG, Brook OR, Sun MRM, Kane RA. The Incidental Splenic Mass at CT: Does It Need Further Work-up? Radiology. 2018;287:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Kristinsson SY, Gridley G, Hoover RN, Check D, Landgren O. Long-term risks after splenectomy among 8,149 cancer-free American veterans: a cohort study with up to 27 years follow-up. Haematologica. 2014;99:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 19. | Arcaini L, Rossi D, Paulli M. Splenic marginal zone lymphoma: from genetics to management. Blood. 2016;127:2072-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Fallah J, Olszewski AJ. Diagnostic and therapeutic splenectomy for splenic lymphomas: analysis of the National Cancer Data Base. Hematology. 2019;24:378-386. [PubMed] |