Published online Aug 27, 2021. doi: 10.4240/wjgs.v13.i8.822
Peer-review started: February 12, 2021
First decision: May 4, 2021
Revised: May 12, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: August 27, 2021
Processing time: 188 Days and 22.1 Hours
Hirschsprung’s disease (HD) is a congenital disorder, characterized by aganglionosis in the distal part of the gastrointestinal tract. Despite complete surgical resection of the aganglionic segment, both constipation and fecal incontinence persist in a considerable number of patients with limited treatment options. There is growing evidence for structural abnormalities in the ganglionic bowel proximal to the aganglionosis in both humans and animals with HD, which may play a role in persistent bowel dysfunction. These abnormalities include: (1) Histopathological abnormalities of enteric neural cells; (2) Imbalanced expression of neurotransmitters and neuroproteins; (3) Abnormal expression of enteric pacemaker cells; (4) Abnormalities of smooth muscle cells; and (5) Abnormalities within the extracellular matrix. Hence, a better understanding of these previously unrecognized neuropathological abnormalities may improve follow-up and treatment in patients with HD suffering from persistent bowel dysfunction following surgical correction. In the long term, further combination of clinical and neuropathological data will hopefully enable a translational step towards more individual treatment for HD.
Core Tip: Hirschsprung’s disease (HD) is a congenital disorder, characterized by aganglionosis in the distal part of the gastrointestinal tract. Despite surgical resection of the aganglionic bowel segment, bowel dysfunction persists in a considerable number of patients with limited treatment options. There is growing evidence regarding structural abnormalities in the proximal, ganglionic colon of both humans and animals with HD, which may play a role in persistent bowel dysfunction. Hence, understanding these previously unrecognized neuropathological abnormalities in the proximal, ganglionic bowel of patients with HD may improve current follow-up and treatment of persistent postoperative bowel dysfunction in certain patients.
- Citation: Verkuijl SJ, Friedmacher F, Harter PN, Rolle U, Broens PM. Persistent bowel dysfunction after surgery for Hirschsprung’s disease: A neuropathological perspective. World J Gastrointest Surg 2021; 13(8): 822-833
- URL: https://www.wjgnet.com/1948-9366/full/v13/i8/822.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i8.822
Hirschsprung’s disease (HD) is one of the most common congenital disorders of the lower gastrointestinal tract, with an incidence of approximately 1:5000 live births[1,2]. Children with HD usually present within the first six months of their life with symptoms related to bowel obstruction, such as delayed first passage of meconium, abdominal distension, and bilious vomiting[2,3]. However, later presentations are also recognized, where intractable constipation and Hirschsprung-associated enterocolitis may be more prominent[1,3]. The underlying congenital defect of HD is a complete absence of ganglion cells in the most distal part of the gastrointestinal tract. Due to this aganglionosis, there is a lack of peristaltic movement in the affected bowel with loss of smooth muscle relaxation, resulting in functional constriction. In around 80% of patients, the aganglionic segment is located in the rectosigmoid area, but it may also cover a longer segment, up to the total colon or even the small bowel in rare cases[2-4]. The current method of establishing aganglionosis is by contrast enema, anorectal manometry, and rectal biopsies[2,4-6]. Of these options, rectal biopsies are considered to have the highest diagnostic specificity[4,6,7].
Surgical resection of the aganglionic segment remains the gold-standard treatment of HD[2,8]. The aim of surgery is to resect the aganglionic bowel and pull-through the bowel segment that contains a normal enteric nervous system. Therefore, it is important to check intraoperatively in which part ganglion cells are present. This is usually performed by neuropathological review of a frozen biopsy of the whole bowel circumference during open surgery or multiple biopsies during laparoscopic surgery[8]. The most widely applied techniques to identify ganglionic and aganglionic bowel segments are hematoxylin and eosin (HE) and acetylcholinesterase (AChE) histochemistry[4,5]. Using HE staining, no ganglion cells are identified in the affected aganglionic bowel. The AChE enzyme histochemistry usually shows abundant hypertrophic extrinsic nerve fibers in the aganglionic segment[4,5]. However, both techniques have their limitations in the visualization of ganglion cells and extrinsic nerve fibers. For that reason, more and more laboratories additionally use enzyme histochemistry for the detection of ganglion cells, such as lactate dehydrogenase (LDH), nicotinamide adenine dinucleotide (NADH) tetrazolium reductase reactions, and/or succinate dehydrogenase (SDH)[9]. Most ganglion cells are stained using any of these three enzymatic techniques. Therefore, negative LDH, NADH and/or SDH staining is an additional indication of aganglionosis[10,11]. There is growing evidence that immunohistochemistry against calretinin may also be supportive in detecting aganglionic segments[12,13]. In general, it is argued that the dissection level should be at least five centimeters proximal to the biopsy location were ganglion cells were confirmed to be sure that ganglionic bowel is pulled through[4,8]. However, the transition zone may extend even longer in patients with a longer aganglionic segment, which requires a longer resection margin[14].
Since the first description of surgical treatment of HD, which consisted of resection of the aganglionic bowel and pulling through the ganglionic bowel by Swenson[15], various surgical techniques have been proposed. In recent decades, the main advances in surgery for HD have been the performance of primary repairs instead of two-staged or three-staged procedures as well as the utilization of laparoscopic and transanal techniques[16,17]. Today, the Duhamel pull-through and the endorectal pull-through are the most commonly performed procedures, without obvious preference for one of these techniques in terms of complications and outcomes[4]. One of the long-term complications after successful surgery for HD is Hirschsprung-associated enterocolitis[18], but that is beyond the scope of this review.
Despite surgical removal of the aganglionic segment, postoperative bowel function is not always favorable. Bowel dysfunction after surgery for HD can be clinically divided into constipation and fecal incontinence-related symptoms. Both are known to have a considerable impact on the physical and mental well-being of children, adolescents, and adults with HD[19-21].
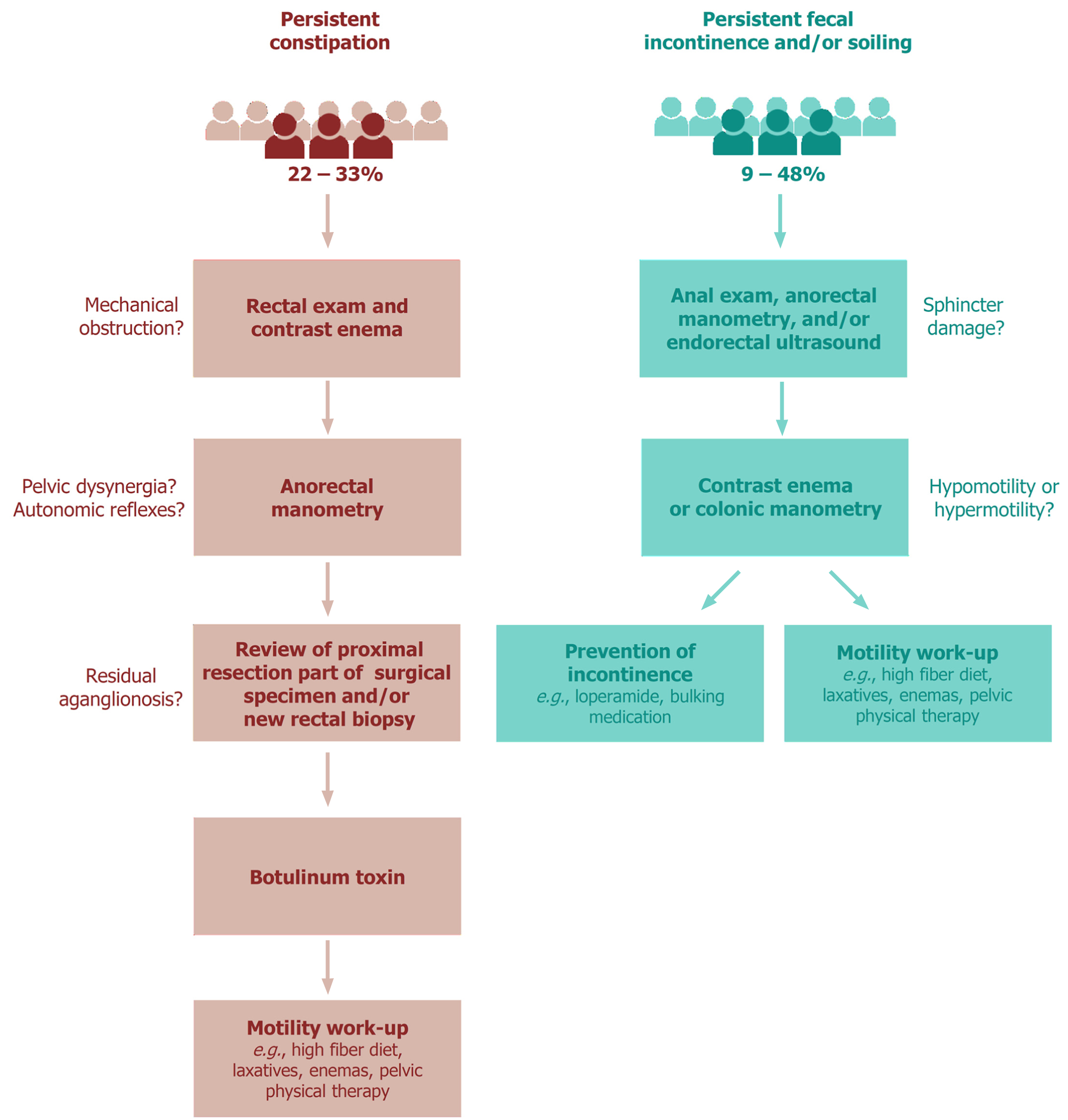
Constipation was reported in 8% to 71% of patients who underwent pull-through surgery for HD[1]. This widely varying prevalence of constipation may be explained by differences in follow-up duration and definitions of constipation. Nevertheless, a notably high rate of 22% to 33% of patients still suffer from constipation in adulthood[20,22-24], and there seems to be no clear improvement with ageing[20,25,26].
Although the causes of persistent obstructive symptoms following pull-through surgery for HD are numerous, the first differentiation is between anatomical and functional etiologies[27]. Various reasons for the obstructive complaints have been shown, including mechanical obstruction (e.g., twisting or adhesions), persistent aganglionosis or hypoganglionosis, internal sphincter achalasia, motility disorder, and functional megacolon[28]. Furthermore, it is known that patients with HD lack the rectoanal inhibitory reflex (RAIR), which cannot be restored by surgery and thus makes them prone to postoperative constipation[7,29]. Based on these observations, the following work-up for the diagnosis and treatment of postoperative obstruction can be recommended (Figure 1). First, following a thorough medical and surgical history, a rectal exam and contrast enema should be performed to exclude mechanical obstruction requiring revision surgery. Second, anorectal manometry may be helpful to test the presence of different autonomic reflexes. Anorectal manometry may also show functional causes for the obstructive complaints, such as pelvic dyssynergia[28,30]. Third, the proximal resection part of the HD specimen may be reviewed again by an experienced neuropathologist and/or a new rectal biopsy at the anastomotic site may be obtained to look for persisting aganglionosis or a transition zone, which might require a redo pull-through in specific cases. When neither of these two examinations show irregularities, a botulinum toxin injection may be given, which can be repeated if it works[28,31]. If botulinum toxin has no effect, the child should take part in a motility work-up program[28]. This includes a high-fiber diet, laxatives, prokinetic agents, enemas, psychological support, biofeedback training and/or pelvic physical therapy[28]. For intractable constipation, bowel management using transanal or (antegrade) colonic irrigation may be advised[4]. Unfortunately, the impact of a structured work-up on persistent constipation has not yet been evaluated in a randomized setting.
Postoperative fecal incontinence is described in between 1% to 50% of HD patients[1]. In contrast to constipation, there is a clear decreasing prevalence with increasing age[20,25,26,32]. Nevertheless, 9% to 19% of adult patients still suffer from fecal incontinence[20,22-24,33], and 16% to 48% from soiling[20,22-24,34].
Fecal continence requires anal sensation and control of the anal sphincter muscles. Therefore, in the case of fecal incontinence after a pull-through procedure, the integrity of the anal canal and sphincters should be checked[31,35]. This can be performed during physical examination with or without sedation, performing anorectal manometry or endorectal ultrasound (Figure 1)[31,35,36]. In addition to sphincter damage, it is important to determine if there is either colonic hypomotility leading to overflow incontinence, or colonic hypermotility with loss of rectal reservoir function[4,31,35,36]. A contrast enema or colonic manometry can be used to disclose the presence of either hypomotility or hypermotility[31,35]. Both conditions require a completely different treatment. In the case of hypomotility, the child is in fact pseudo-incontinent and the management should be directed towards prevention of constipation[31,35]. In contrast, when the child suffers from hypermotility, the goal of treatment is to slow down colonic transit and thicken stool consistency, including a constipating diet, loperamide, bulking medication and/or retrograde enemas[4,31,35,36]. If social continence is not achieved, colonic irrigation or stoma formation may be considered as the final treatment option[4,35].
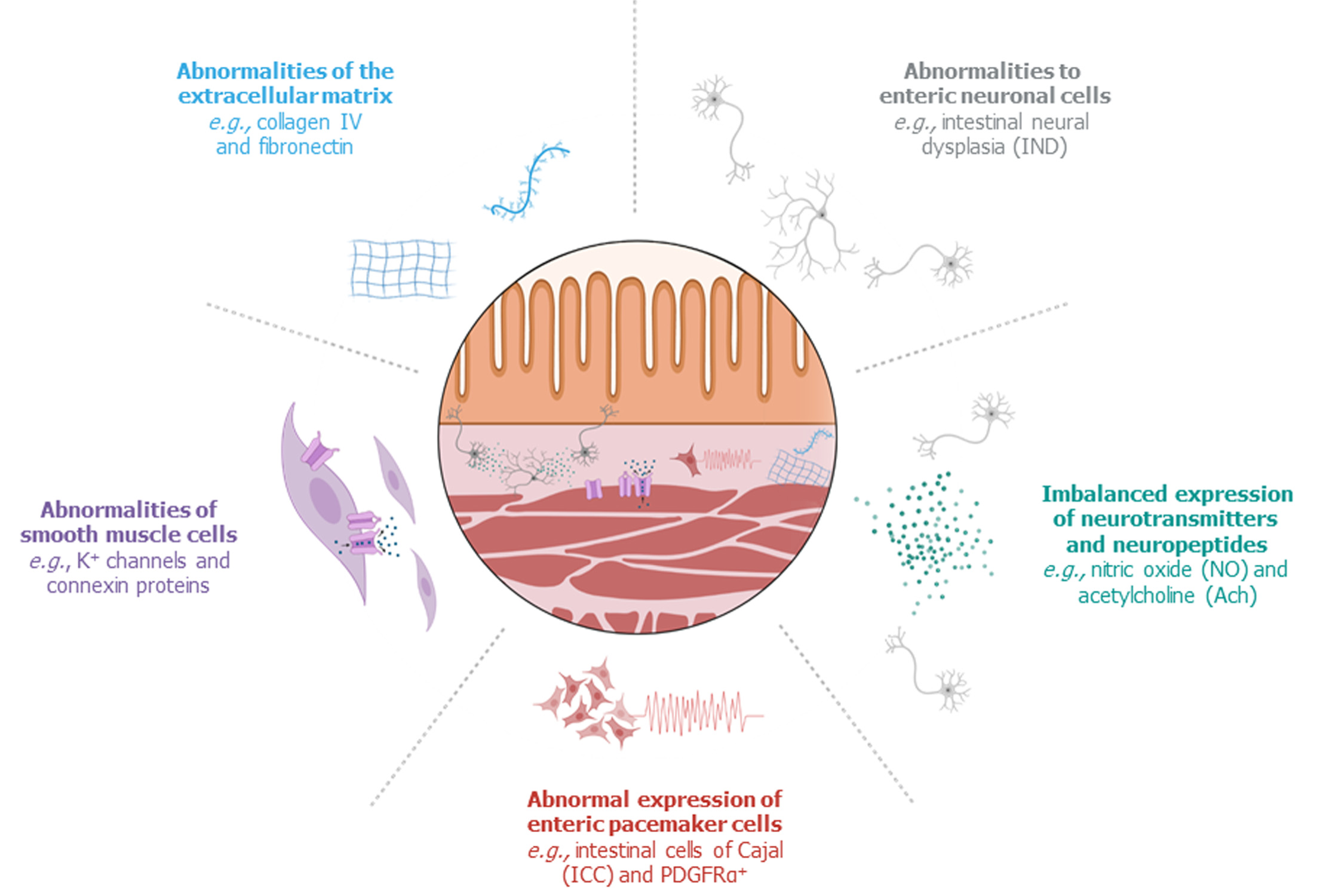
Persistent bowel dysfunction following successful surgery raises the issue that the presence of ganglion cells does not necessarily indicate that the proximal, ganglionic colon of HD patients is completely “healthy” and thus functional. Recent studies have confirmed structural abnormalities in the proximal, ganglionic colon of humans and animals with HD, which can be divided into: (1) Histopathological abnormalities of enteric neural cells; (2) Imbalanced expression of neurotransmitters and neuroproteins; (3) Abnormal expression of enteric pacemaker cells; (4) Abnormalities of smooth muscle cells; and (5) Abnormalities within the extracellular matrix (Figure 2).
The neural cells that are present in the proximal, remaining colon following surgery for HD may exhibit abnormalities in their appearance and/or distribution. One of the most frequently described pathological findings in the proximal, ganglionic bowel is intestinal neural dysplasia (IND). IND is inconsistently defined in the literature, but the main feature is giant submucosal ganglia[8,37]. IND has been observed in the proximal colon segment of 20 to 70% of surgically treated patients with HD[8]. Several studies have associated IND or graded features of IND in the proximal, ganglionic colon segment of surgically treated HD patients with persistent postoperative bowel dysfunction[38-41], but others have contested this statement[42]. Other histopathological alterations in the proximal colon segment of patients with HD include myenteric hypoganglionosis and submucosal nerve hypertrophy[8,43,44]. Additionally, several studies reported an increased amount of immature neural cells in the proximal colon of HD patients[45-47], although the clinical consequences of neural immaturity are not clear yet.
Enteric neural cells communicate using neurotransmitters and neuropeptides. Peristaltic movements require both contraction and relaxation, which is mediated by a balance between excitatory and inhibitory neurotransmitters and neuropeptides. Disproportionate expression of a specific neurotransmitter or neuropeptide may therefore lead to colonic dysmotility.
The primary inhibitory neurotransmitter of the enteric nervous system is nitric oxide (NO), which is produced by nitrergic neurons that contain NO synthase (NOS)[48]. Alterations in the expression of NOS-containing neurons in the proximal, ganglionic bowel have been observed in mouse models of HD[49,50]. In addition, recent studies in humans have shown a relative overabundance of nitrergic neurons in ganglionic bowel of HD patients in comparison to healthy controls[48,50,51]. Two of these studies correlated their findings with functional outcomes of the patients, but were unable to show a significant relation with postoperative bowel function[48,50]. Nevertheless, patients with worse bowel function tended to have a greater proportion of NOS-containing neurons, a finding which requires future research with larger study populations. It has been hypothesized that the overabundance of nitrergic neurons may be an additional expression of neuronal immaturity, as these neurons are the first subtypes to appear during embryonic development[50]. However, it remains unclear if this is a primary or secondary phenomenon.
Neuropeptides that act predominantly as inhibitory neurotransmitters are vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), galanin, and neuropeptide Y (NPY), all inducing smooth muscle relaxation. In line with the increased presence of inhibitory nitrergic neurons, VIP-immunoreactive neurons were also more frequently found in ganglionic bowel in a mouse model of HD, compared to healthy control mice[49]. However, a more recent human study found that VIP-immunoreactive neurons were as frequently found in the proximal, ganglionic bowel of HD patients as in healthy controls[51]. With regard to PACAP, galanin and NPY, a decreased number of immunoreactive neurons was found in the ganglionic colon in a mouse model of HD compared to healthy control mice[52].
One of the most widely studied excitatory neurotransmitters of the enteric nervous system is acetylcholine, which is produced by cholinergic neurons containing choline acetyltransferase (ChAT)[48,51]. Although it seems clear that the excessive expression of acetylcholine plays a crucial role in the permanent constriction of the affected, aganglionic segment[51], a slightly decreased expression in the proximal, ganglionic segment of both mice[49] and humans with HD has been shown[51]. Therefore, an inverse relationship in the expression of NOS and ChAT has been suggested[49,51]; however, future studies are needed as previous investigations consisted of small study populations and a short follow-up time.
Substance P (SP) is a well-studied excitatory neuropeptide. Its presence was significantly reduced in the proximal colon of a mouse model of HD in comparison to healthy control mice[52]. However, other authors did not find this difference in both mice[49] and humans[51]. Similarly, no obviously different expression of the neuropeptide calcitonin gene-related peptide (CGRP) was found in the proximal colon of a mouse model of HD in comparison to healthy control mice[52].
Coordinated propulsive contractions of smooth muscle cells are dependent on electrical pacemaker activity, which is provided by an intramural network of interstitial cells of Cajal (ICCs)[53]. Two other major functions of ICCs are facilitation of muscle innervation and mediation of sensory transmission[53]. The traditional method of visualizing ICCs is by immunohistochemistry of its c-Kit membrane receptor tyrosine kinase[53,54]. By using this technique, a total reduction of ICCs in the proximal, ganglionic colon of HD patients has been observed in comparison with healthy controls[54]. This observation has been contested by others, who did not find an overall difference in the distribution of c-Kit positive ICCs[55-58]. However, a marked variability of ICC values in patients with HD has been noted, which may be a reflection of the heterogeneous character of the disease[55]. Furthermore, two studies linked poor clinical outcomes in patients to very low numbers of ICCs and a low ratio of ICCs to neural innervation[55,59]. More recently, the use of c-Kit has been replaced by a more specific ICC marker: anoctamin-1[53,54,60]. Use of this marker showed a moderate reduction of ICC fibers in ganglionic HD colon, compared to the colon of non-HD controls[60]. These contradicting results are likely to arise from small study populations, but may have also been biased by the fact that the distribution of ICCs can vary with age and location in the gastrointestinal tract[53,54,60]. Therefore, the role of structural ICC abnormalities in persistent bowel dysfunction in patients with HD is still to be elucidated.
Adjacent to ICCs, platelet-derived growth factor receptor alpha positive (PDGFRα+) cells form a second network of pacemaker cells, which works closely together with ICCs and smooth muscle cells to regulate bowel motility[61]. A striking decrease in PDGFRα+ protein expression has been found in ganglionic colon, compared to non-HD controls[62], which requires future research.
Coordinated colonic motility requires more than just an intact nerve network, balanced neurotransmitters and neuropeptides, and stimuli from ICCs and PDGFRα+ cells. Smooth muscle cells are the effectors of colonic motility. The chemical and electrical stimuli provoke activity of smooth muscles, leading to peristaltic movements. The response of smooth muscle cells to these stimuli is regulated by a wide variety of ion channels and receptors. Furthermore, smooth muscle cells also need an intact cytoskeleton for maintenance of muscle structural and functional integrity.
With regard to cytoskeletal proteins, there are contradictory reports. On the one hand, a lack of desmin, dystrophin, vinculin[63], and ε-sarcoglycan[64] has been found in aganglionic bowel, whilst on the other hand, comparable amounts of some of these substances in ganglionic and aganglionic bowel were reported[65]. Unfortunately, little is known about differences in the cytoskeleton between ganglionic HD colon vs healthy colon. The exception to that is a recent study by Zhu et al[47], which showed that HD patients with an accumulation of neurofilaments in neural cells of their proximal, ganglionic colon had a significantly worse postoperative bowel function one to six years after surgery.
In contrast, reductions of many ion channels and receptors have been reported in the ganglionic bowel of patients with HD in comparison to healthy controls: small-conductance Ca2+ activated K+ (SK3) channels[66,67], different members of the two-pore domain K+ (K2P or KCNK) channels[68,69], voltage-dependent K+ channels[70,71], hyperpolarization-activated nucleotide-gated (HCN) 3 channels[72], voltage-gated sodium channel type 1β (SCN1B), chloride channel subunit FXYD1[73], and ryanodine receptors (Ryr)[74]. Another factor which is important for the connection between smooth muscles cells and chemical and electric stimuli are gap junctions. The gap junction channels are formed by connexin proteins, of which reductions have been observed in the ganglionic colon of HD patients relative to healthy control tissue[75]. Hence, there is mounting evidence today regarding ion channel and receptor deficiencies in the ganglionic colon of patients with HD. However, if and how all these abnormalities translate into differences in postoperative bowel function is a question that remains to be elucidated.
Around the smooth muscle cells lies a three-dimensional structure called the extracellular matrix (ECM). The composition of the ECM varies from tissue to tissue, but mainly consists of two main classes of macromolecules: collagens and glycoproteins[76]. These ECM components serve as a structural skeleton, but also influence migration, proliferation, survival, and/or differentiation of cells[76]. Therefore, it is not surprising that the ECM was found to have a critical role in the development of the enteric nervous system[77]. Various studies have determined imbalances in the composition of ECM in the proximal, ganglionic colon of HD patients.
Collagens are the most abundant proteins in the ECM[76,77]. Among them is collagen IV, a major component of basement membranes surrounding smooth muscle cells in human colon[65,78,79]. Increased amounts of collagen IV have been observed in the aganglionic segment of patients with HD[78], and in the proximal, ganglionic segment in almost half of these patients in comparison to healthy controls[79]. Similarly, the presence of collagen VI was two to three times greater in the proximal, ganglionic bowel segment of HD patients compared to healthy controls[80].
Laminins are a large family of glycoproteins, composed of α, β, and γ chains[65,76,77]. An overall increase in laminin concentration was found in the proximal, ganglionic bowel segment of patients with HD in comparison to controls[79,81]. More detailed analysis of the different laminin chains revealed an increased accumulation of the laminin α5 chain in the proximal, ganglionic bowel of almost half the studied HD patients[65]. It has been postulated that the overabundance of laminin may be a reflection of the immature state of HD colon[81].
Additionally, fibronectin - another family of glycoproteins[76] - showed a marked increase in aganglionic tissue, in comparison to the proximal, ganglionic segment[82-84]. A comparison with healthy controls is lacking to assess the overall presence of fibronectin, but an up-regulated expression of the fibronectin 1 gene has been described in the proximal, ganglionic colon of patients with HD compared to healthy colon[85]. For two other glycoproteins, tenascin and nidogen, the intensity of the immunoreactivity in the proximal, ganglionic colon segment was similar to the colon of non-HD controls[83,86]. Aberrant expression of the above ECM components in the proximal ganglionic bowel, may have an influence on postoperative bowel dysfunction in HD, but this has not yet been proven.
Despite complete surgical resection of the aganglionic colon in patients with HD, bowel dysfunction frequently seems to persist even into adulthood with limited treatment options. At present, there is a growing body of evidence regarding neuropathological abnormalities in the proximal, ganglionic colon of surgically treated HD patients, which may have important future clinical implications. However, most studies are limited by solely reporting laboratory findings, retrospective reporting of functional outcomes based on medical reports, short follow-up periods, and small study populations. Therefore, the current knowledge is too limited to alter surgical management or to extend the standard neuropathological analyses performed.
In the short-term, it will be important to further establish the association between the structural abnormalities of the proximal, ganglionic colon and the postoperative functional outcome. Brooks et al[48] and Zhu et al[47] were the first to associate neuropathological findings to prospectively obtained patient-reported bowel function measures, which is a first step in the right direction. The optimum would be the combination of longitudinal patient-reported functional outcomes after surgery for HD combined with intraoperative and postoperative detailed neuropathological analysis of the proximal, ganglionic colon segment. Thus, new, innovative ways of studying the complex intramural network of different colonic cells should be explored, for example three-dimensional imaging of the enteric nervous system, which may lead to new insights[87]. In this way, it may be possible to predict which patients might benefit from intensified follow-up and bowel management to prevent bowel obstruction and other types of postoperative dysfunction. Eventually, these studies may lead to a different evaluation and treatment of certain HD patients with persistent postoperative constipation and/or fecal incontinence.
In the long-term, it may become clearer whether certain HD patients might benefit from additional surgical resection. This would require further study on the extent of the investigated neuropathological abnormalities as well as the permanent or transient character of the structural deviations. Finally, these findings may represent a target for future therapies, but it is premature to advocate a certain direction of these therapies yet.
An understanding of previously unrecognized neuropathological abnormalities in the proximal, ganglionic bowel of HD patients may improve follow-up and treatment for patients suffering from persistent bowel dysfunction following pull-through surgery. In future, the combination of longitudinal assessment of postoperative functional outcomes and in-depth studies of the underlying colonic neuropathology will enable a translational step towards innovative surgical techniques, structured follow-up programs, and new targeted treatment options for HD.
The figures of this review were created with Biorender.com.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lourencao P S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Zhang YL
| 1. | Wester T, Granström AL. Hirschsprung disease-Bowel function beyond childhood. Semin Pediatr Surg. 2017;26:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Heuckeroth RO. Hirschsprung disease - integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol. 2018;15:152-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 3. | Langer JC. Hirschsprung disease. Curr Opin Pediatr. 2013;25:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Kyrklund K, Sloots CEJ, de Blaauw I, Bjørnland K, Rolle U, Cavalieri D, Francalanci P, Fusaro F, Lemli A, Schwarzer N, Fascetti-Leon F, Thapar N, Johansen LS, Berrebi D, Hugot JP, Crétolle C, Brooks AS, Hofstra RM, Wester T, Pakarinen MP. ERNICA guidelines for the management of rectosigmoid Hirschsprung's disease. Orphanet J Rare Dis. 2020;15:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 5. | Ambartsumyan L, Smith C, Kapur RP. Diagnosis of Hirschsprung Disease. Pediatr Dev Pathol. 2020;23:8-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 6. | de Lorijn F, Kremer LC, Reitsma JB, Benninga MA. Diagnostic tests in Hirschsprung disease: a systematic review. J Pediatr Gastroenterol Nutr. 2006;42:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Meinds RJ, Trzpis M, Broens PMA. Anorectal Manometry May Reduce the Number of Rectal Suction Biopsy Procedures Needed to Diagnose Hirschsprung Disease. J Pediatr Gastroenterol Nutr. 2018;67:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Smith C, Ambartsumyan L, Kapur RP. Surgery, Surgical Pathology, and Postoperative Management of Patients With Hirschsprung Disease. Pediatr Dev Pathol. 2020;23:23-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Bruder E, Knecht Y, Kasper M, Chaffard R, Ipsen S, Terracciano L, Meier-Ruge WA. [Enzyme histochemical diagnosis of gastrointestinal motility disorders. A laboratory guide]. Pathologe. 2007;28:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Meier-Ruge WA, Bruder E. Pathology of chronic constipation in pediatric and adult coloproctology. Pathobiology. 2005;72:1-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Braczynski AK, Gfroerer S, Beschorner R, Harter PN, Baumgarten P, Rolle U, Mittelbronn M. Cholinergic innervation and ganglion cell distribution in Hirschsprung's disease. BMC Pediatr. 2020;20:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Veras LV, Arnold M, Avansino JR, Bove K, Cowles RA, Durham MM, Goldstein AM, Krishnan C, Langer JC, Levitt M, Monforte-Munoz H, Rabah R, Reyes-Mugica M, Rollins MD 2nd, Kapur RP, Gosain A; American Pediatric Surgical Association Hirschsprung Disease Interest Group. Guidelines for synoptic reporting of surgery and pathology in Hirschsprung disease. J Pediatr Surg. 2019;54:2017-2023. [PubMed] [DOI] [Full Text] |
| 13. | Takawira C, D'Agostini S, Shenouda S, Persad R, Sergi C. Laboratory procedures update on Hirschsprung disease. J Pediatr Gastroenterol Nutr. 2015;60:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Coyle D, O'Donnell AM, Tomuschat C, Gillick J, Puri P. The Extent of the Transition Zone in Hirschsprung Disease. J Pediatr Surg. 2019;54:2318-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Swenson O, Rheinlander HF, Diamond I. Hirschsprung's disease; a new concept of the etiology; operative results in 34 patients. N Engl J Med. 1949;241:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 131] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Georgeson KE, Fuenfer MM, Hardin WD. Primary laparoscopic pull-through for Hirschsprung's disease in infants and children. J Pediatr Surg. 1995;30:1017-21; discussion 1021. [PubMed] [DOI] [Full Text] |
| 17. | De la Torre-Mondragón L, Ortega-Salgado JA. Transanal endorectal pull-through for Hirschsprung's disease. J Pediatr Surg. 1998;33:1283-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 233] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Demehri FR, Halaweish IF, Coran AG, Teitelbaum DH. Hirschsprung-associated enterocolitis: pathogenesis, treatment and prevention. Pediatr Surg Int. 2013;29:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Sood S, Lim R, Collins L, Trajanovska M, Hutson JM, Teague WJ, King SK. The long-term quality of life outcomes in adolescents with Hirschsprung disease. J Pediatr Surg. 2018;53:2430-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Meinds RJ, van der Steeg AFW, Sloots CEJ, Witvliet MJ, de Blaauw I, van Gemert WG, Trzpis M, Broens PMA. Long-term functional outcomes and quality of life in patients with Hirschsprung's disease. Br J Surg. 2019;106:499-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 21. | Collins L, Collis B, Trajanovska M, Khanal R, Hutson JM, Teague WJ, King SK. Quality of life outcomes in children with Hirschsprung disease. J Pediatr Surg. 2017;52:2006-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Ieiri S, Nakatsuji T, Akiyoshi J, Higashi M, Hashizume M, Suita S, Taguchi T. Long-term outcomes and the quality of life of Hirschsprung disease in adolescents who have reached 18 years or older--a 47-year single-institute experience. J Pediatr Surg. 2010;45:2398-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Jarvi K, Laitakari EM, Koivusalo A, Rintala RJ, Pakarinen MP. Bowel function and gastrointestinal quality of life among adults operated for Hirschsprung disease during childhood: a population-based study. Ann Surg. 2010;252:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Onishi S, Nakame K, Kaji T, Kawano M, Moriguchi T, Sugita K, Yano K, Nomura M, Yamada K, Yamada W, Masuya R, Kawano T, Machigashira S, Mukai M, Ieiri S. The bowel function and quality of life of Hirschsprung disease patients who have reached 18 years of age or older - the long-term outcomes after undergoing the transabdominal soave procedure. J Pediatr Surg. 2017;52:2001-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Aworanti OM, McDowell DT, Martin IM, Quinn F. Does Functional Outcome Improve with Time Postsurgery for Hirschsprung Disease? Eur J Pediatr Surg. 2016;26:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Mills JL, Konkin DE, Milner R, Penner JG, Langer M, Webber EM. Long-term bowel function and quality of life in children with Hirschsprung's disease. J Pediatr Surg. 2008;43:899-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Kapur RP, Ambartsumyan L, Smith C. Are We Underdiagnosing Hirschsprung Disease? Pediatr Dev Pathol. 2020;23:60-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Langer JC, Rollins MD, Levitt M, Gosain A, Torre L, Kapur RP, Cowles RA, Horton J, Rothstein DH, Goldstein AM; American Pediatric Surgical Association Hirschsprung Disease Interest Group. Guidelines for the management of postoperative obstructive symptoms in children with Hirschsprung disease. Pediatr Surg Int. 2017;33:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Sintusek P, Rybak A, Mutalib M, Thapar N, Borrelli O, Lindley KJ. Preservation of the colo-anal reflex in colonic transection and post-operative Hirschsprung's disease: Potential extrinsic neural pathway. Neurogastroenterol Motil. 2019;31:e13472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Meinds RJ, Eggink MC, Heineman E, Broens PM. Dyssynergic defecation may play an important role in postoperative Hirschsprung's disease patients with severe persistent constipation: analysis of a case series. J Pediatr Surg. 2014;49:1488-1492. [PubMed] [DOI] [Full Text] |
| 31. | Levitt MA, Dickie B, Peña A. The Hirschsprungs patient who is soiling after what was considered a "successful" pull-through. Semin Pediatr Surg. 2012;21:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Bjørnland K, Pakarinen MP, Stenstrøm P, Stensrud KJ, Neuvonen M, Granström AL, Graneli C, Pripp AH, Arnbjörnsson E, Emblem R, Wester T, Rintala RJ; Nordic Pediatric Surgery Study Consortium. A Nordic multicenter survey of long-term bowel function after transanal endorectal pull-through in 200 patients with rectosigmoid Hirschsprung disease. J Pediatr Surg. 2017;52:1458-1464. [PubMed] [DOI] [Full Text] |
| 33. | Heikkinen M, Rintala RJ, Louhimo I. Bowel function and quality of life in adult patients with operated Hirschsprung's disease. Pediatr Surg Int. 1995;10:342-344. [DOI] [Full Text] |
| 34. | Gustafson E, Larsson T, Danielson J. Controlled outcome of Hirschsprung's disease beyond adolescence: a single center experience. Pediatr Surg Int. 2019;35:181-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Saadai P, Trappey AF, Goldstein AM, Cowles RA, De La Torre L, Durham MM, Huang EY, Levitt MA, Rialon K, Rollins M, Rothstein DH, Langer JC; American Pediatric Surgical Association Hirschsprung Disease Interest Group. Guidelines for the management of postoperative soiling in children with Hirschsprung disease. Pediatr Surg Int. 2019;35:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Levitt MA, Dickie B, Peña A. Evaluation and treatment of the patient with Hirschsprung disease who is not doing well after a pull-through procedure. Semin Pediatr Surg. 2010;19:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Friedmacher F, Puri P. Classification and diagnostic criteria of variants of Hirschsprung's disease. Pediatr Surg Int. 2013;29:855-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Kobayashi H, Hirakawa H, Surana R, O'Briain DS, Puri P. Intestinal neuronal dysplasia is a possible cause of persistent bowel symptoms after pull-through operation for Hirschsprung's disease. J Pediatr Surg. 1995;30:253-7; discussion 257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Moore SW, Laing D, Kaschula RO, Cywes S. A histological grading system for the evaluation of co-existing NID with Hirschsprung's disease. Eur J Pediatr Surg. 1994;4:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Schulten D, Holschneider AM, Meier-Ruge W. Proximal segment histology of resected bowel in Hirschsprung's disease predicts postoperative bowel function. Eur J Pediatr Surg. 2000;10:378-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Schmittenbecher PP, Sacher P, Cholewa D, Haberlik A, Menardi G, Moczulski J, Rumlova E, Schuppert W, Ure B. Hirschsprung's disease and intestinal neuronal dysplasia--a frequent association with implications for the postoperative course. Pediatr Surg Int. 1999;15:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Swaminathan M, Oron AP, Chatterjee S, Piper H, Cope-Yokoyama S, Chakravarti A, Kapur RP. Intestinal Neuronal Dysplasia-Like Submucosal Ganglion Cell Hyperplasia at the Proximal Margins of Hirschsprung Disease Resections. Pediatr Dev Pathol. 2015;18:466-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Moore SW. Genetic impact on the treatment & management of Hirschsprung disease. J Pediatr Surg. 2017;52:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Kapur RP, Kennedy AJ. Histopathologic delineation of the transition zone in short-segment Hirschsprung disease. Pediatr Dev Pathol. 2013;16:252-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Gonzales J, Le Berre-Scoul C, Dariel A, Bréhéret P, Neunlist M, Boudin H. Semaphorin 3A controls enteric neuron connectivity and is inversely associated with synapsin 1 expression in Hirschsprung disease. Sci Rep. 2020;10:15119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Miyahara K, Kato Y, Seki T, Arakawa A, Lane GJ, Yamataka A. Neuronal immaturity in normoganglionic colon from cases of Hirschsprung disease, anorectal malformation, and idiopathic constipation. J Pediatr Surg. 2009;44:2364-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Zhu J, Zhang Y, Wang Y, Yu S, Chen Y, Guo Z, Zhao Y. Dysmorphic Neurofilament-Positive Ganglion Cells in the Myenteric Plexus at the Proximal Resection Margin Indicate Worse Postoperative Prognosis in Hirschsprung's Disease. Pediatr Dev Pathol. 2020;23:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Brooks LA, Fowler KL, Veras LV, Fu M, Gosain A. Resection margin histology may predict intermediate-term outcomes in children with rectosigmoid Hirschsprung disease. Pediatr Surg Int. 2020;36:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Zaitoun I, Erickson CS, Barlow AJ, Klein TR, Heneghan AF, Pierre JF, Epstein ML, Gosain A. Altered neuronal density and neurotransmitter expression in the ganglionated region of Ednrb null mice: implications for Hirschsprung's disease. Neurogastroenterol Motil. 2013;25:e233-e244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Cheng LS, Schwartz DM, Hotta R, Graham HK, Goldstein AM. Bowel dysfunction following pullthrough surgery is associated with an overabundance of nitrergic neurons in Hirschsprung disease. J Pediatr Surg. 2016;51:1834-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Coyle D, O'Donnell AM, Gillick J, Puri P. Altered neurotransmitter expression profile in the ganglionic bowel in Hirschsprung's disease. J Pediatr Surg. 2016;51:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Sandgren K, Larsson LT, Ekblad E. Widespread changes in neurotransmitter expression and number of enteric neurons and interstitial cells of Cajal in lethal spotted mice: an explanation for persisting dysmotility after operation for Hirschsprung's disease? Dig Dis Sci. 2002;47:1049-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Huizinga JD, Chen JH. Interstitial cells of Cajal: update on basic and clinical science. Curr Gastroenterol Rep. 2014;16:363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Gfroerer S, Rolle U. Interstitial cells of Cajal in the normal human gut and in Hirschsprung disease. Pediatr Surg Int. 2013;29:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Bettolli M, De Carli C, Jolin-Dahel K, Bailey K, Khan HF, Sweeney B, Krantis A, Staines WA, Rubin S. Colonic dysmotility in postsurgical patients with Hirschsprung's disease. Potential significance of abnormalities in the interstitial cells of Cajal and the enteric nervous system. J Pediatr Surg. 2008;43:1433-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Newman CJ, Laurini RN, Lesbros Y, Reinberg O, Meyrat BJ. Interstitial cells of Cajal are normally distributed in both ganglionated and aganglionic bowel in Hirschsprung's disease. Pediatr Surg Int. 2003;19:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Taguchi T, Suita S, Masumoto K, Nada O. Universal distribution of c-kit-positive cells in different types of Hirschsprung's disease. Pediatr Surg Int. 2003;19:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Vanderwinden JM, Rumessen JJ, Liu H, Descamps D, De Laet MH, Vanderhaeghen JJ. Interstitial cells of Cajal in human colon and in Hirschsprung's disease. Gastroenterology. 1996;111:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 207] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 59. | Taguchi T, Suita S, Masumoto K, Nagasaki A. An abnormal distribution of C-kit positive cells in the normoganglionic segment can predict a poor clinical outcome in patients with Hirschsprung's disease. Eur J Pediatr Surg. 2005;15:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Coyle D, Kelly DA, O'Donnell AM, Gillick J, Puri P. Use of anoctamin 1 (ANO1) to evaluate interstitial cells of Cajal in Hirschsprung's disease. Pediatr Surg Int. 2016;32:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Kurahashi M, Zheng H, Dwyer L, Ward SM, Koh SD, Sanders KM. A functional role for the 'fibroblast-like cells' in gastrointestinal smooth muscles. J Physiol. 2011;589:697-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 62. | O'Donnell AM, Coyle D, Puri P. Deficiency of platelet-derived growth factor receptor-α-positive cells in Hirschsprung's disease colon. World J Gastroenterol. 2016;22:3335-3340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Nemeth L, Rolle U, Puri P. Altered cytoskeleton in smooth muscle of aganglionic bowel. Arch Pathol Lab Med. 2002;126:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Arena S, Cutroneo G, Favaloro A, Sinatra MT, Trimarchi F, Scarvaglieri S, Mallamace A, Arena F, Anastasi G, Di Benedetto V. Abnormal distribution of sarcoglycan subcomplex in colonic smooth muscle cells of aganglionic bowel. Int J Mol Med. 2010;25:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 65. | Alpy F, Ritié L, Jaubert F, Becmeur F, Méchine-Neuville A, Lefebvre O, Arnold C, Sorokin L, Kedinger M, Simon-Assmann P. The expression pattern of laminin isoforms in Hirschsprung disease reveals a distal peripheral nerve differentiation. Hum Pathol. 2005;36:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Coyle D, O'Donnell AM, Puri P. Altered distribution of small-conductance calcium-activated potassium channel SK3 in Hirschsprung's disease. J Pediatr Surg. 2015;50:1659-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Gunadi, Sunardi M, Budi NYP, Kalim AS, Iskandar K, Dwihantoro A. The impact of down-regulated SK3 expressions on Hirschsprung disease. BMC Med Genet. 2018;19:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Tomuschat C, O'Donnell AM, Coyle D, Dreher N, Kelly D, Puri P. Altered expression of a two-pore domain (K2P) mechano-gated potassium channel TREK-1 in Hirschsprung's disease. Pediatr Res. 2016;80:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | O'Donnell AM, Nakamura H, Parekh B, Puri P. Decreased expression of TRAAK channels in Hirschsprung's disease: a possible cause of postoperative dysmotility. Pediatr Surg Int. 2019;35:1431-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | O'Donnell AM, Coyle D, Puri P. Decreased expression of Kv7 channels in Hirchsprung's disease. J Pediatr Surg. 2017;52:1177-1181. [PubMed] [DOI] [Full Text] |
| 71. | O'Donnell AM, Nakamura H, Tomuschat C, Marayati NF, Puri P. Altered expression of KCNG3 and KCNG4 in Hirschsprung's disease. Pediatr Surg Int. 2019;35:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | O'Donnell AM, Coyle D, Puri P. Decreased expression of hyperpolarisation-activated cyclic nucleotide-gated channel 3 in Hirschsprung's disease. World J Gastroenterol. 2015;21:5635-5640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | O'Donnell AM, Nakamura H, Tomuschat C, Marayati NF, Puri P. Abnormal Scn1b and Fxyd1 gene expression in the pulled-through ganglionic colon may influence functional outcome in patients with Hirschsprung's disease. Pediatr Surg Int. 2019;35:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | O' Donnell AM, Nakamura H, Puri P. Altered ryanodine receptor gene expression in Hirschsprung's disease. Pediatr Surg Int. 2019;35:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Coyle D, Doyle B, Murphy JM, O'Donnell AM, Gillick J, Puri P. Expression of connexin 26 and connexin 43 is reduced in Hirschsprung's disease. J Surg Res. 2016;206:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15:771-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1041] [Cited by in RCA: 996] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 77. | Rauch U, Schäfer KH. The extracellular matrix and its role in cell migration and development of the enteric nervous system. Eur J Pediatr Surg. 2003;13:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Gao N, Wang J, Zhang Q, Zhou T, Mu W, Hou P, Wang D, Lv X, Li A. Aberrant Distributions of Collagen I, III, and IV in Hirschsprung Disease. J Pediatr Gastroenterol Nutr. 2020;70:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | Parikh DH, Tam PK, Van Velzen D, Edgar D. Abnormalities in the distribution of laminin and collagen type IV in Hirschsprung's disease. Gastroenterology. 1992;102:1236-1241. [PubMed] |
| 80. | Soret R, Mennetrey M, Bergeron KF, Dariel A, Neunlist M, Grunder F, Faure C, Silversides DW, Pilon N; Ente-Hirsch Study Group. A collagen VI-dependent pathogenic mechanism for Hirschsprung's disease. J Clin Invest. 2015;125:4483-4496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 81. | Parikh DH, Tam PK, Lloyd DA, Van Velzen D, Edgar DH. Quantitative and qualitative analysis of the extracellular matrix protein, laminin, in Hirschsprung's disease. J Pediatr Surg. 1992;27:991-5; discussion 995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Gao N, Hou P, Wang J, Zhou T, Wang D, Zhang Q, Mu W, Lv X, Li A. Increased Fibronectin Impairs the Function of Excitatory/Inhibitory Synapses in Hirschsprung Disease. Cell Mol Neurobiol. 2020;40:617-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Parikh DH, Tam PK, Van Velzen D, Edgar D. The extracellular matrix components, tenascin and fibronectin, in Hirschsprung's disease: an immunohistochemical study. J Pediatr Surg. 1994;29:1302-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Zheng Y, Lv X, Wang D, Gao N, Zhang Q, Li A. Down-regulation of fibronectin and the correlated expression of neuroligin in hirschsprung disease. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Gunadi, Budi NYP, Kalim AS, Santiko W, Musthofa FD, Iskandar K, Makhmudi A. Aberrant expressions of miRNA-206 target, FN1, in multifactorial Hirschsprung disease. Orphanet J Rare Dis. 2019;14:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Parikh DH, Leibl M, Tam PK, Edgar D. Abnormal expression and distribution of nidogen in Hirschsprung's disease. J Pediatr Surg. 1995;30:1687-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 87. | Graham KD, López SH, Sengupta R, Shenoy A, Schneider S, Wright CM, Feldman M, Furth E, Valdivieso F, Lemke A, Wilkins BJ, Naji A, Doolin EJ, Howard MJ, Heuckeroth RO. Robust, 3-Dimensional Visualization of Human Colon Enteric Nervous System Without Tissue Sectioning. Gastroenterology. 2020;158:2221-2235.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |