Published online Aug 27, 2021. doi: 10.4240/wjgs.v13.i8.814
Peer-review started: February 9, 2021
First decision: May 13, 2021
Revised: May 24, 2021
Accepted: July 13, 2021
Article in press: July 13, 2021
Published online: August 27, 2021
Processing time: 191 Days and 19.1 Hours
Colorectal cancer (CRC) is a common malignancy of the digestive system. Colorectal liver cancer metastasis (CRLM) occurs in approximately 50% of the patients and is the main cause of CRC mortality. Surgical resection is currently the most effective treatment for CRLM. However, given that the remnant liver volume after resection should be adequate, only a few patients are suitable for radical resection. Since Dr. Hans Schlitt first performed the associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) for CRLM in 2012, ALPPS has received considerable attention and has continually evolved in recent years. This review explains the technical origin of the ALPPS procedure for CRLM and evaluates its efficacy, pointing to its favorable postoperative outcomes. We also discuss the patient screening strategies and optimization of ALPPS to ensure long-term survival of patients with CRLM in whom surgery cannot be performed. Finally, further directions in both basic and clinical research regarding ALPPS have been proposed. Although ALPPS surgery is a difficult and high-risk technique, it is still worth exploration by experienced surgeons.
Core Tip: Several previous reviews have discussed the associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) technique and its applications. However, this is the first review on ALPPS in colorectal liver cancer metastasis, in which the technical evolution of the procedure is described, its safety and efficacy are evaluated, patient selection process and technique improvement are discussed, and further directions are proposed.
- Citation: Wen XD, Xiao L. Associating liver partition and portal vein ligation for staged hepatectomy in the treatment of colorectal cancer liver metastases. World J Gastrointest Surg 2021; 13(8): 814-821
- URL: https://www.wjgnet.com/1948-9366/full/v13/i8/814.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i8.814
Colorectal cancer (CRC) is a common malignancy of the digestive system and is the third and second most common cancer in males and females, respectively. CRC forms metastases in the liver in 50% of patients, and approximately 15% to 25% of patients have concurrent colorectal liver cancer metastases (CRLM) at initial diagnosis, while nearly 25% of the patients have metachronous CRLM after colectomy. The median survival time in untreated patients is less than 6.9 mo[1,2]. Surgical resection is currently recognized as the most effective treatment for CRLM. Complete resection of the liver metastases improves patient outcomes, with the median survival time increasing to 35 mo and the 5-year survival rate ranging from 30% to 57%[3]. Therefore, a comprehensive evaluation of the technique by a multidisciplinary team followed by an increase in the rate of surgical resection could effectively improve the survival rate of patients suffering from CRLM[4].
Patients with multiple liver metastases located on one side of the liver usually require extensive hepatectomy; however, a sufficient volume of the remnant liver must be preserved in these patients, making radical resection virtually impossible in patients with CRLM[5,6]. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) was first reported in 2012 in the context of resection of advanced tumors. The procedure is considered a revolutionary breakthrough in hepatobiliary surgery because of its remarkable curative effect by inducing hypertrophy of the liver in a short time[7]. The application of ALPPS in patients with CRC, who were considered unsuitable for radical surgery in the past, has shown therapeutic promise. Although ALPPS has been used in patients with cholangiocarcinoma, hepatocellular carcinoma, and neuroendocrine tumors with liver metastasis, ALPPS is most commonly performed in patients with CRLM.
As a new surgical technique, and despite its encouraging efficacy, ALPPS has been the subject of debate. In the early years of the ALPPS procedure, the surgical complications and mortality rates ranged from 40% to 64% and 22% to 29%, respectively. These high rates were considered to be primarily due to the evolving surgical technique and improper case selection. More recently, questions about whether improvement in the quality of life and survival benefits of the procedure balance the risks of complications and death have been raised[8]. However, with the development of the ALPPS procedure, an increasing number of patients with CRLM have undergone radical resection and survived for a long time afterwards; this has provided new hope to patients considered unsuitable for surgery. Thus, it is necessary to further standardize the technical methodology of ALPPS, summarize the technical points and preventive measures for complications, and optimize patient selection strategies. In this study, we will evaluate and review the current therapeutic value of ALPPS for CRLM.
Portal vein embolization or ligation (PVE/PVL) is used to increase the volume of the future remnant lobe of the liver to a safe range before two-stage hepatectomy (TSH). This increases the liver volume so that more patients can undergo radical hepatectomy. Following the PVE procedure, it takes approximately 6–8 wk for the liver to regenerate by 20%–46%. However, due to the spread or progression of the tumor during the waiting period, one-third of patients become ineligible for second-stage radical resection[7]. As a result, most patients have to undergo interventional therapy, radiofrequency ablation, or other non-surgical methods.
In 2007, Dr. Hans Schlitt from Regensburg, Germany, planned to perform right hepatectomy for a patient with hilar cholangiocarcinoma[9]. During the surgery, he found that the future remnant liver volume would be inadequate and temporarily decided to perform selective hepaticojejunostomy on the left biliary system. To completely expose the left hepatic duct to facilitate hepaticojejunostomy, he had to split the liver parenchyma between the left medial lobe and the left lateral lobe along the falciform ligament and ligate the right portal vein to induce hypertrophy of the left lobe of the liver. On the 8th d after surgery, computed tomography indicated that the left lobe had significantly enlarged; thus, the right lobe could be resected. Based on this finding, Dr. Hans Schlitt attempted to implement this new strategy in a CRLM patient with inadequate liver volume for radical tumor resection. This was the earliest record of the application of ALPPS to treat CRLM. In 2012, Schnitzbauer et al[10] reported 25 cases of patients who underwent this procedure, which attracted widespread interest and attention. This technique was later abbreviated as ALPPS.
ALPPS is mainly performed in patients with CRLM, primary liver cancer, hilar cholangiocarcinoma, breast cancer liver metastasis, and neuroendocrine cancer liver metastasis; however, it has been mainly used for CRLM due to its favorable oncological characteristics. The initial reported incidence of postoperative complications and mortality was 40%–64% and 22%–29%, respectively[8]. Analyses of postoperative complications revealed that the liver section is prone to cause bile leakage and that secondary infection is the main cause of mortality after surgery[11,12]. Hence, further technical improvements mainly focused on reducing cross-sectional bile leakage and infection, simplifying the surgery, and facilitating second-stage surgery. Currently, at the initial stage, the liver is wrapped with sterile plastic bags to collect bile and prevent bile peritonitis[13]. Moreover, some surgeons use radiofrequency and microwave ablation to complete liver parenchymal isolation in the first step of the surgery, which promotes hypertrophy of the future remnant liver, increasing its size by 62.3%[14]. Petrowsky et al[15] reported that ligation of at least 50% of the liver parenchyma could result in considerable liver proliferation. Favorable liver proliferation can also be achieved by intraoperative ultrasound confirmation that the blood flow between the segments is completely cut off and by placing a circumferential hepatic belt around the liver to block the blood vessels between the two segments[16].
The application of the anterior approach method to cut off the liver or to use the “hybrid ALPPS” method for postsurgical PVE when the portal vein is difficult to separate is more in line with the “No Touch” principle, thereby reducing the probability of tumor spread[17,18]. To adapt the procedure for tumor removal on the left side or even other parts of the liver, traditional hepatectomy has been modified to left-sided ALPPS and monosegment future liver remnant ALPPS. Many scholars believe that patients can benefit from laparoscopic ALPPS, stage 1 laparoscopy ALPPS, stage 2 open surgery, and full laparoscopic ALPPS[19,20]. However, currently, ALPPS is mainly performed by open surgery. Due to differences between various surgical facilities, the current development of ALPPS technology is mainly focused on achieving portal vein branch blockade and liver isolation and on simplifying the surgery. Thus, the surgical method is selected according to the tumor growth characteristics and expertise of the performing surgeon (Table 1).
| ALPPS strategies | Technical points |
| Classical ALPPS | Right portal vein ligation and right trisectionectomy |
| Rescue ALPPS | Failure of PVE with subsequent ALPPS |
| Laparoscopic ALPPS | Laparoscopy for stage 1 or both stages 1 and 2 |
| PVE ALPPS | The intentional use of PVE as part of the first stage is stated by using PVE-ALPPS |
| Partial ALPPS | Transection at least 50% of the future transection plane at stage 1 |
| Left ALPPS | Left portal vein ligation, left trisectionectomy |
| Tourniquet ALPPS | Tourniquet in the umbilical fissure and portal vein occlusion |
| Radiofrequency ALPPS | Radio-frequency-assisted liver partition |
| Microwave ALPPS | Microwave transection of the liver |
| Monosegment ALPPS | Extending hepatectomy, only sparing a single or adjacent segment |
In recent years, continuous technical optimization of ALPPS has significantly reduced the incidence of associated postoperative complications and mortality. Hernandez-Alejandro et al[21] reported 14 patients with CRLM who underwent the first stage of ALPPS, and all patients successfully underwent the second stage of surgery. The incidence of postoperative complications above Clavien–Dindo grade IIIB was 14%, and no perioperative deaths were reported. In 2014, Schadde et al[22] analyzed the data of 202 patients who underwent ALPPS and who were registered in the international ALPPS.net database (including 141 patients with CRLM). The results revealed that the median interval between the two steps of the surgery was 7 d, median growth of the remnant liver volume was 80%, incidence of serious complications (Clavien–Dindo grade ≥ IIIB) was 27%, and mortality rate was 9%. All 141 cases with CRLM had good oncological results, which demonstrated that CRLM patients under 60 years of age were most likely to benefit from ALPPS. A study on the postoperative quality of life found that patients had an excellent quality of life after ALPPS, equivalent to that of healthy people[23].
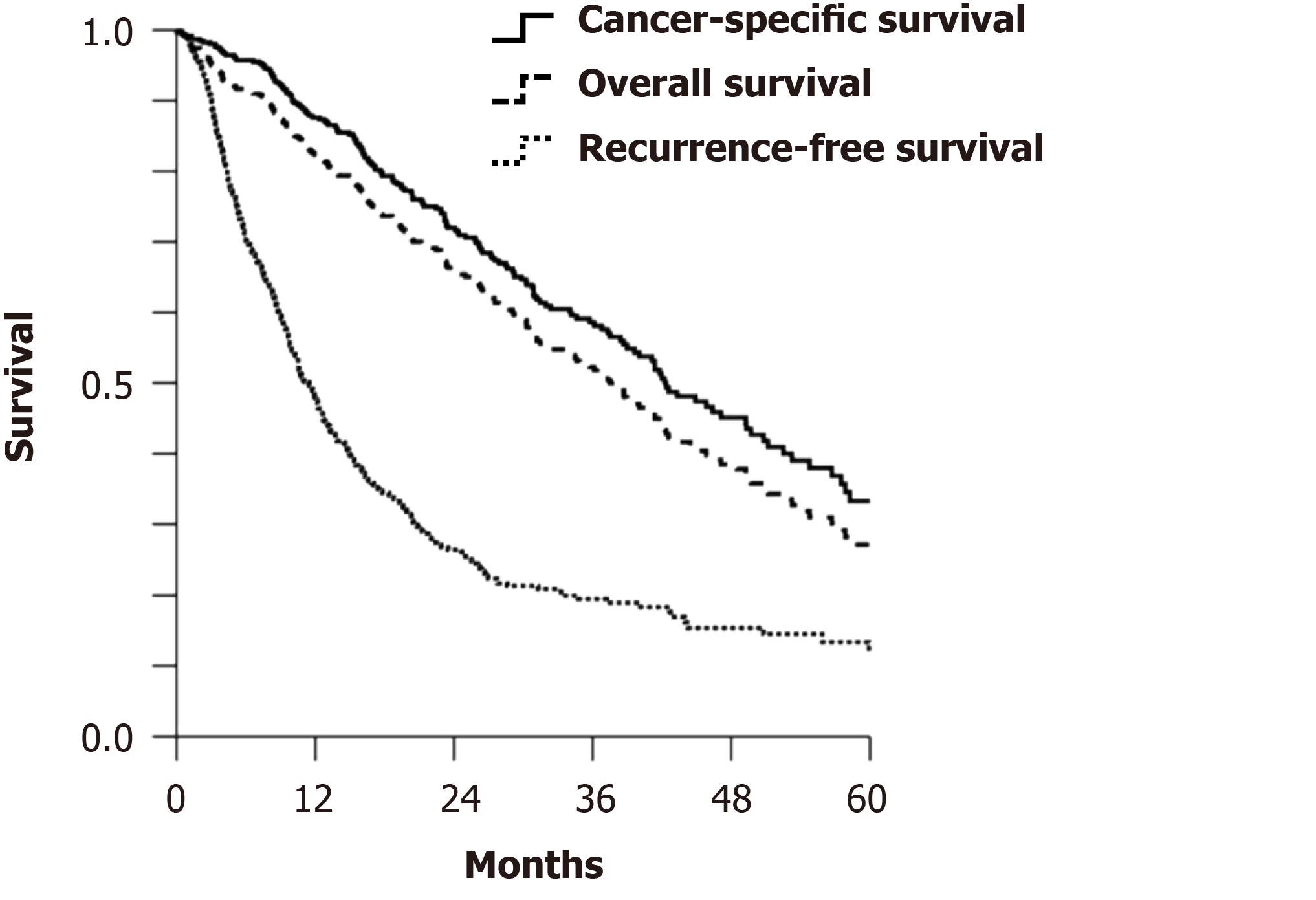
The comparative results between ALPPS and traditional TSH for the treatment of CRLM are remarkable. A study compared 48 patients who underwent ALPPS and 83 who underwent TSH for initially unresectable advanced CRLM. The proportion of patients who underwent second-stage surgical resection was significantly higher in the ALPPS group than in the TSH group, and there was no increase in the recurrence of early-stage tumors[22]. The LIGRO Trial, a high-quality randomized controlled trial, further proved that ALPPS could achieve higher resectability than TSH in patients with advanced CRLM, with a comparable perioperative complication rate[24]. In 2020, Petrowsky et al[25] retrospectively analyzed the efficacy of ALPPS in 510 patients with CRLM in 22 centers over the past 10 years. The median cancer-specific survival (CSS) time, overall survival time, and recurrence-free survival time were 42, 37, and 11 mo, respectively (Figure 1). This large cohort provided the first convincing evidence that patients with unresectable CRLM who underwent ALPPS not only had low perioperative mortality but also achieved satisfactory long-term oncological outcomes. This was especially seen in patients with good tumor biobehavior and response to chemotherapy (Table 2). Moreover, on multivariate analysis, tumor characteristics (primary T4, right colon), biological characteristics (K/N-RAS status), and chemotherapy response (solid tumor response evaluation criteria) were found to be independent predictors of CSS, while the occurrence of serious surgical complications indicated a poor prognosis. These findings stress the importance of proper patient screening and the selection of suitable surgical methods. However, the comparability of data is limited as there are considerable differences in indications and patient selection methods between TSH and ALPPS in various therapeutic centers. Whether ALPPS should be an auxiliary tool for patients with unsuccessful TSH or a more optimal choice for patients with TSH requires long-term and systematic studies and evaluation.
| Variable | Number of patients | Data completion | Value |
| Interstage interval, d | 468 | 92% | 13 (9–21) |
| Stage 2 performed, n (%) | 510 | 100% | 492 (96) |
| R0 resection at stage 2, n (%) | 302 | 59% | 220 (73) |
| 90-d mortality, n (%) | 510 | 100% | 25 (5) |
| Complications ≥ 3B stage 1, n (%) | 501 | 98% | 37 (7) |
| Complications ≥ 3B stage 2, n (%) | 485 | 95% | 100 (21) |
Strict and uniform patient selection and optimization of surgical methods could effectively reduce the complication and mortality rates of ALPPS in the treatment of CRLM. In light of the high 90-d mortality rate of simultaneous ALPPS and colorectal resection (15%), Wanis et al[26] recommended against simultaneous ALPPS and colorectal resection. However, a study on a large cohort of patients found that resection of primary colorectal lesions during primary ALPPS surgery did not increase the perioperative mortality rate[25]. Thus, ALPPS can be considered in simultaneous or heterogeneous CRLM with the likelihood of inadequate future remnant liver volume. Current clinical trials have not conclusively shown whether preoperative chemotherapy or direct surgery is beneficial for the survival of CRLM patients; nevertheless, preoperative chemotherapy remains an option for patients with poor tumor biology and surgical tolerance.
We believe that empirical surgical indications for ALPPS in the treatment of CRLM should be limited to the following situations: patients with good overall condition, with good liver function, and ability to tolerate large-volume liver resection; patients with extensive left/right liver metastases that require large hemihepatic or hepatic trilobe resection with the future remnant liver volume likely to be less than 30% of that before surgery; patients with no evidence of metastasis to organs other than the liver, in whom surgery could technically achieve an R0 resection; cases in which the tumor does not invade the main portal vein of the liver and there is no tumor thrombus; and patients who are fully or partially sensitive to systemic chemotherapy and have stopped chemotherapy for more than 4 wk. Before the second ALPPS stage, careful attention should be paid to the functional evaluation of the hyperplastic liver and the overall condition of the patient after first-stage ALPPS, including recovery from serious complications and the patient’s nutritional status. A model for end-stage liver disease score of less than 10 is the deciding indication for stage 2 surgery. The majority of CRLM patients reportedly have a history of chemotherapy prior to undergoing ALPPS. Existing evidence suggests that chemotherapy has no significant inhibitory effect on the hypertrophy of the future remnant liver. However, it is important to rule out sinusoidal obstruction syndrome and decreased liver reserve ability caused by chemotherapeutic drugs such as oxaliplatin.
For patients with CRLM in whom ALPPS is being considered, the first step is to determine whether they have technically resectable tumors. The oncological characteristics of the tumor are used to determine whether tumors suitable for resection should be treated with direct surgery or neoadjuvant chemotherapy, while conversion therapy can be used for unresectable tumors. It should be noted that resectability is a partially subjective judgment made by hepatobiliary surgeons; therefore, differences in opinion as well as significant differences in methods and efficacy are seen across surgical centers worldwide. Individualized technical judgments should be made based on the technical ability of the surgeon and following a joint discussion on oncological characteristics of a given case by a multidisciplinary surgical team.
In a comparison of liver proliferation in clinical and pre-clinical trials, the liver growth rate following ALPPS was 11 times higher than that after pulmonary vein obstruction (PVO)[22], and the hepatocyte proliferation rate was 10 times higher than that after portal vein ligation (PLV)[27]. Thus, the combination of portal vein branch ligation and liver isolation appears to trigger rapid growth of the remnant liver by an unknown mechanism. It has been hypothesized that this rapid growth could be due to the following factors: hemodynamic changes after PVO[28,29], inflammatory stimulation as a result of local trauma caused by liver separation[27,30], and activation of specific signaling pathways that lead to significant liver regeneration after ALPPS[31-33]. The results of early research have partially confirmed the importance of the abovementioned factors, which are likely to jointly contribute to hepatic growth. Results of a breakthrough research on the mechanism of liver regeneration and CRLM metastasis could simplify the surgery, reduce the rapid increase in the size of the remnant liver induced by surgery, or inhibit malignant progression during the process of liver regeneration. Treatment of CRLM using ALPPS has created an excellent research platform, which provides the possibility for an in-depth study of tumor growth and metastasis patterns during liver regeneration.
The favorable outcomes of ALPPS in the treatment of CRLM encourage surgeons to attempt technical optimization strategies. However, given the small number of ALPPS cases performed in a single center and differences in technical preferences of surgeons, standardized surgical procedures require long-term exploration and optimization. A large-scale comparative study of different ALPPS methods and a more detailed meta-analysis of the data of registered cases in the ALPPS.net database might facilitate the ongoing process of ALPPS evaluation. ALPPS remains a complex and challenging surgery and most surgeons consider it an auxiliary tool in the treatment of CRLM rather than as the first choice. Selecting CRLM patients who can achieve maximum benefit from this approach is challenging, but important. Currently, the ALPPS risk score can be used to predict the risk of related complications. Based on the oncological outcomes of ALPPS treatment for CRLM, the standardization of other evaluation indicators or grading systems can help avoid the unnecessary use of ALPPS.
CRLM with the likelihood of a low future remnant liver volume is considered a good indication for ALPPS. Existing research on ALPPS has proven the technique to be safe, effective, and associated with favorable therapeutic outcomes. It is expected that with meticulous patient screening and optimization of surgical methods, ALPPS will be considered appropriate for a large number of patients with CRLM who are considered ineligible for surgery. Although ALPPS is a difficult and high-risk technique, it is still worth exploration by experienced surgeons.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S S-Editor: Zhang H L-Editor: Filipodia P-Editor: Xing YX
| 1. | Shimada H, Tanaka K, Endou I, Ichikawa Y. Treatment for colorectal liver metastases: a review. Langenbecks Arch Surg. 2009;394:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Gallinger S, Biagi JJ, Fletcher GG, Nhan C, Ruo L, McLeod RS. Liver resection for colorectal cancer metastases. Curr Oncol. 2013;20:e255-e265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 586] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 4. | Margonis GA, Sergentanis TN, Ntanasis-Stathopoulos I, Andreatos N, Tzanninis IG, Sasaki K, Psaltopoulou T, Wang J, Buettner S, Papalois ΑE, He J, Wolfgang CL, Pawlik TM, Weiss MJ. Impact of Surgical Margin Width on Recurrence and Overall Survival Following R0 Hepatic Resection of Colorectal Metastases: A Systematic Review and Meta-analysis. Ann Surg. 2018;267:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 6. | Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 1005] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 7. | Baumgart J, Lang S, Lang H. A new method for induction of liver hypertrophy prior to right trisectionectomy: A report of three cases. HPB. 2011;13:71-72. |
| 8. | Hasselgren K, Sandström P, Björnsson B. Role of associating liver partition and portal vein ligation for staged hepatectomy in colorectal liver metastases: a review. World J Gastroenterol. 2015;21:4491-4498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the "ALPPS" approach. Ann Surg. 2012;255:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 280] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 10. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 928] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 11. | Alvarez FA, Ardiles V, Sanchez Claria R, Pekolj J, de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg. 2013;17:814-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Andriani OC. Long-term results with associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Ann Surg. 2012;256:e5; author reply e16-e5; author reply e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Torres OJ, Moraes-Junior JM, Lima e Lima NC, Moraes AM. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): a new approach in liver resections. Arq Bras Cir Dig. 2012;25:290-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Gall TM, Sodergren MH, Frampton AE, Fan R, Spalding DR, Habib NA, Pai M, Jackson JE, Tait P, Jiao LR. Radio-frequency-assisted Liver Partition with Portal vein ligation (RALPP) for liver regeneration. Ann Surg. 2015;261:e45-e46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Petrowsky H, Györi G, de Oliveira M, Lesurtel M, Clavien PA. Is partial-ALPPS safer than ALPPS? Ann Surg. 2015;261:e90-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Robles Campos R, Parrilla Paricio P, López Conesa A, Brusadín R, López López V, Jimeno Griñó P, Fuster Quiñonero M, García López JA, de la Peña Moral J. [A new surgical technique for extended right hepatectomy: tourniquet in the umbilical fissure and right portal vein occlusion (ALTPS). Clinical case]. Cir Esp. 2013;91:633-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Vennarecci G, Levi Sandri GB, Ettorre GM. Performing the ALPPS Procedure by Anterior Approach and Liver Hanging Maneuver. Ann Surg. 2016;263:e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Li J, Kantas A, Ittrich H, Koops A, Achilles EG, Fischer L, Nashan B. Avoid "All-Touch" by Hybrid ALPPS to Achieve Oncological Efficacy. Ann Surg. 2016;263:e6-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Brustia R, Scatton O, Perdigao F, El-Mouhadi S, Cauchy F, Soubrane O. Vessel identifications tags for open or laparoscopic associating liver partition and portal vein ligation for staged hepatectomy. J Am Coll Surg. 2013;217:e51-e55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Xiao L, Li JW, Zheng SG. Totally laparoscopic ALPPS in the treatment of cirrhotic hepatocellular carcinoma. Surg Endosc. 2015;29:2800-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, Croome KP. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery. 2015;157:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, Amacker N, Baumgart J, Croome K, Hernandez-Alejandro R, Lang H, de Santibaňes E, Clavien PA. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014;38:1510-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 23. | Wanis KN, Ardiles V, Alvarez FA, Tun-Abraham ME, Linehan D, de Santibañes E, Hernandez-Alejandro R. Intermediate-term survival and quality of life outcomes in patients with advanced colorectal liver metastases undergoing associating liver partition and portal vein ligation for staged hepatectomy. Surgery. 2018;163:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Sandström P, Røsok BI, Sparrelid E, Larsen PN, Larsson AL, Lindell G, Schultz NA, Bjørnbeth BA, Isaksson B, Rizell M, Björnsson B. ALPPS Improves Resectability Compared With Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg. 2018;267:833-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 25. | Petrowsky H, Linecker M, Raptis DA, Kuemmerli C, Fritsch R, Kirimker OE, Balci D, Ratti F, Aldrighetti L, Voskanyan S, Tomassini F, Troisi RI, Bednarsch J, Lurje G, Fard-Aghaie MH, Reese T, Oldhafer KJ, Ghamarnejad O, Mehrabi A, Abraham MET, Truant S, Pruvot FR, Hoti E, Kambakamba P, Capobianco I, Nadalin S, Fernandes ESM, Kron P, Lodge P, Olthof PB, van Gulik T, Castro-Benitez C, Adam R, Machado MA, Teutsch M, Li J, Scherer MN, Schlitt HJ, Ardiles V, de Santibañes E, Brusadin R, Lopez-Lopez V, Robles-Campos R, Malagó M, Hernandez-Alejandro R, Clavien PA. First Long-term Oncologic Results of the ALPPS Procedure in a Large Cohort of Patients With Colorectal Liver Metastases. Ann Surg. 2020;272:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Wanis KN, Buac S, Linecker M, Ardiles V, Tun-Abraham ME, Robles-Campos R, Malago M, de Santibañes E, Clavien PA, Hernandez-Alejandro R. Patient Survival After Simultaneous ALPPS and Colorectal Resection. World J Surg. 2017;41:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Schlegel A, Lesurtel M, Melloul E, Limani P, Tschuor C, Graf R, Humar B, Clavien PA. ALPPS: from human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann Surg. 2014;260:839-46; discussion 846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Colak D, Al-Harazi O, Mustafa OM, Meng F, Assiri AM, Dhar DK, Broering DC. RNA-Seq transcriptome profiling in three liver regeneration models in rats: comparative analysis of partial hepatectomy, ALLPS, and PVL. Sci Rep. 2020;10:5213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Dhar DK, Mohammad GH, Vyas S, Broering DC, Malago M. A novel rat model of liver regeneration: possible role of cytokine induced neutrophil chemoattractant-1 in augmented liver regeneration. Ann Surg Innov Res. 2015;9:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Yao L, Li C, Ge X, Wang H, Xu K, Zhang A, Dong J. Establishment of a rat model of portal vein ligation combined with in situ splitting. PLoS One. 2014;9:e105511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Langiewicz M, Graf R, Humar B, Clavien PA. JNK1 induces hedgehog signaling from stellate cells to accelerate liver regeneration in mice. J Hepatol. 2018;69:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Otsuka N, Yoshioka M, Abe Y, Nakagawa Y, Uchinami H, Yamamoto Y. Reg3α and Reg3β Expressions Followed by JAK2/STAT3 Activation Play a Pivotal Role in the Acceleration of Liver Hypertrophy in a Rat ALPPS Model. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Uribe M, Uribe-Echevarría S, Mandiola C, Zapata MI, Riquelme F, Romanque P. Insight on ALPPS - Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy - mechanisms: activation of mTOR pathway. HPB (Oxford). 2018;20:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |