Published online Aug 27, 2021. doi: 10.4240/wjgs.v13.i8.772
Peer-review started: February 9, 2021
First decision: May 13, 2021
Revised: May 24, 2021
Accepted: July 16, 2021
Article in press: July 16, 2021
Published online: August 27, 2021
Processing time: 191 Days and 22 Hours
Endoscopic submucosal dissection (ESD) is a globally accepted minimally invasive therapy for early-stage gastrointestinal tract tumors. Although numerous electrosurgical knives have been developed for ESD, technical difficulties and high complication rates (bleeding and perforation) have limited their use worldwide. The grasping-type scissors forceps [clutch cutter (CC)] is the first forceps-type resection device developed with reference to hemostatic forceps. The aim was to allow easy and safe ESD throughout the gastro
Core Tip: The grasping-type scissors forceps [clutch cutter (CC)] is the first forceps-type resection device developed with reference to hemostatic forceps. It was designed for easy and safe endoscopic submucosal dissection (ESD) throughout the gastrointestinal tract, as a biopsy technique, using one device. Unlike knives, the CC is a thin pliers-like forceps that can grasp the target tissue accurately and perform sufficient hemostasis and incision. Reported clinical outcomes of ESD using the CC (ESD-CC) are good. This review describes the structural features of the CC, how it is used, the effectiveness of ESD-CC, and other proprietary endoscopic treatments performed with the CC.
- Citation: Akahoshi K, Komori K, Akahoshi K, Tamura S, Osada S, Shiratsuchi Y, Kubokawa M. Advances in endoscopic therapy using grasping-type scissors forceps (with video). World J Gastrointest Surg 2021; 13(8): 772-787
- URL: https://www.wjgnet.com/1948-9366/full/v13/i8/772.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i8.772
Endoscopic submucosal dissection (ESD) is recognized as an effective treatment method for early gastrointestinal tumors, and this technique is becoming widespread all over the world[1-12]. However, unsolved problems in ESD using conventional knives remain, namely technical difficulties and severe complications, such as perforation and bleeding. The main cause of these problems is mechanical factors related to conventional knives. These knives have poor targeting accuracy, leading to unintentional incision, and insufficient hemostatic capability, leading to bleeding[12]. Grasping-type scissors forceps (GSF) designed for safer ESD were developed in 2007 as high-frequency forceps for use instead of knives[6]. The GSF can be used to perform electrosurgical cuts and coagulation under direct vision while grasping, fixing, compressing, and pulling the target tissue as for a biopsy technique[6,13-15]. The basic approach in ESD using the GSF involves first grasping the tissue (release and regrasp is available), pulling the tissue, followed by cutting or coagulating. Theoretically, unintentional perforation should never happen with GSF compared with conventional knives, which may induce perforation through unintentional movement[6,12]. Furthermore, the GSF has sufficient hemostatic capacity by a mechanism similar to hemostatic forceps because the GSF can grasp (compress) target blood vessels, pull them out of the muscle layer, and coagulate using electrosurgical currents. Therefore, ESD using the GSF requires no other electrosurgical hemostatic forceps[6,8]. The GSF is currently available as the clutch cutter (CC) from Fujifilm Co. (Tokyo, Japan) in Japan and other Asian countries, and in the European Union, United States, and South America[12]. In this paper, we describe the structural features of the CC, how to use the device, efficacies of CC for ESD, and unique indications for other treatments.
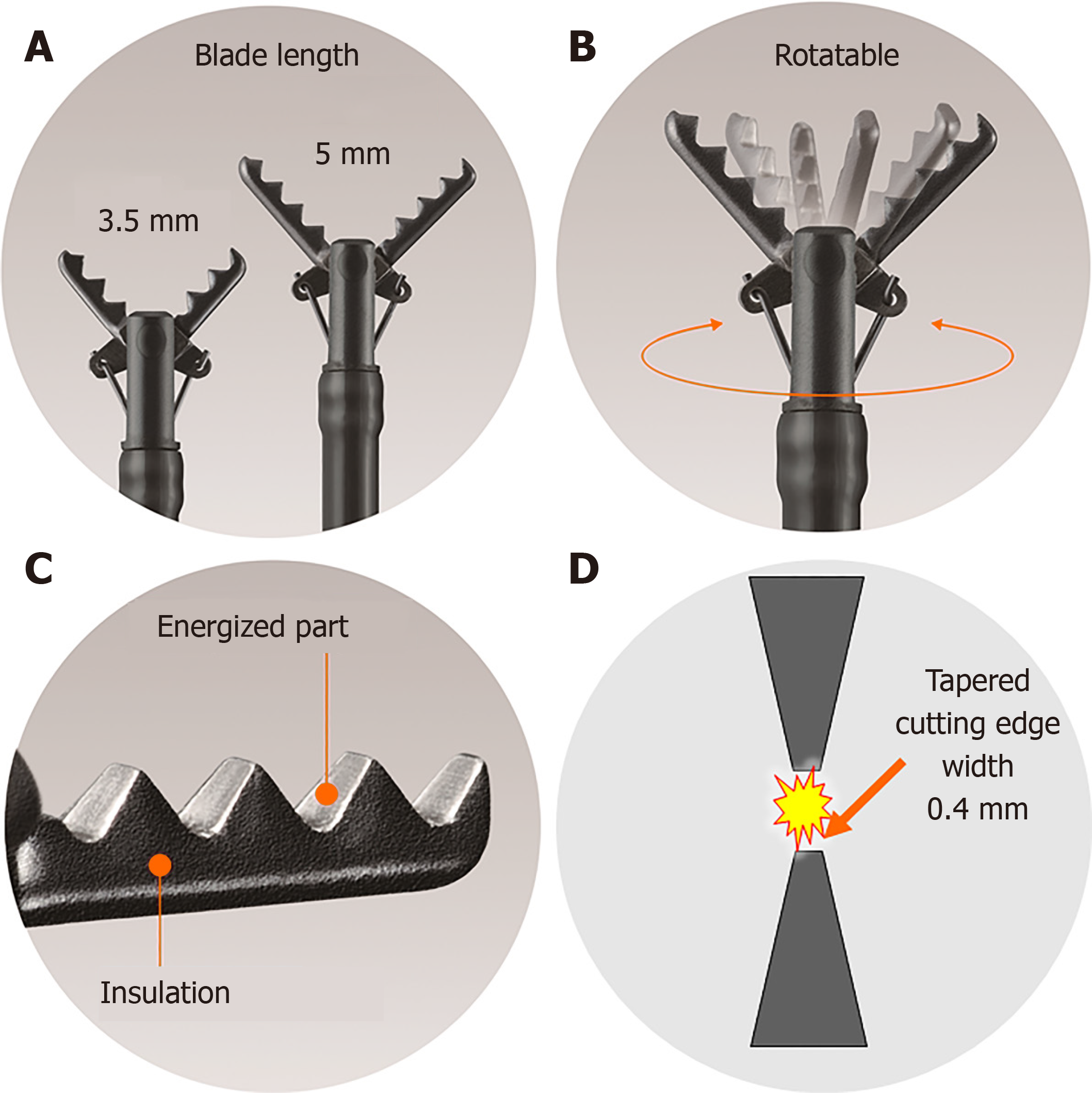
The diameter of the CC is 2.7 mm, and the device can be used with standard endoscopes with a working channel width of ≥ 2.8 mm. This device is disposable and cannot be reused. The length of the CC is 1800 mm, and it can be used with up to medium-length colonoscopes and with all gastroscopes. Furthermore, the CC is available with a 1550-mm-long therapeutic double-balloon endoscope (EI-580BT, Fujifilm). The CC is basically a device that grips target tissue, and is not a knife. The CC has five unique and characteristic mechanisms as detailed below (Figure 1). The CC can grasp the target tissue accurately and pull it up and away from the underlying muscle layer prior to being energized for safe and effective incision and hemostasis during ESD.
The CC grasps and pulls the target tissue and accurately coagulates and cuts using an electrosurgical current; the cutting edge is serrated over the entire length. Therefore, theoretically, it is possible to perform energization coagulation/incision from pinpoint to 5-mm lengths after accurately grasping and fixing tissue without slipping, under endoscopic observation (Figure 1A).
There are two blade lengths, a long blade (5 mm) and a short blade (3.5 mm). The long blade is used for long incisions and is designed for gastrointestinal areas with thick walls (stomach). The short blade is used for short incisions and is designed for gastrointestinal areas with thin walls (esophagus, duodenum, small intestine, large intestine) (Figure 1A).
The CC has a function to rotate the scissors, and the grasping direction can be changed freely; therefore, accurate cutting and coagulation are available (Figure 1B).
The CC is a monopolar energizing forceps. However, because all parts other than the scissors blade are insulated so that only the grasped tissue is energized, localized energization is possible (Figure 1C).
The width of the cutting edge is as narrow as 0.4 mm, which is almost the same as the diameter of the polypectomy snare, and sharp incision and pinpoint coagulation can be performed without impairing the endoscopic view during ESD (Figure 1D).
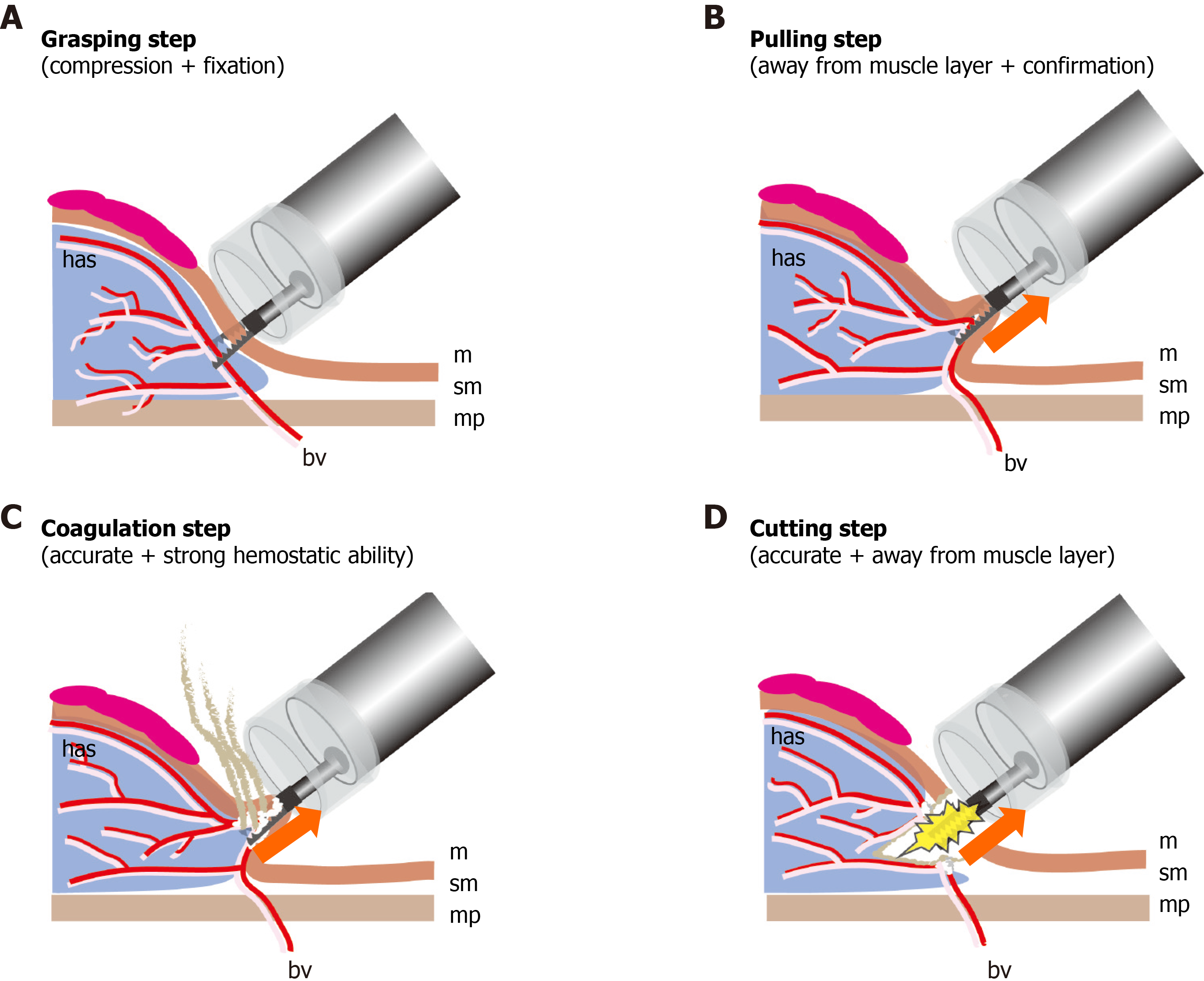
Because the CC has a serrated blade to the tip, unlike a knife, the CC can grasp, fix, and compress the target tissue, pull it away from the muscle layer, and allow energization excision and coagulation. The CC has the following four safe and effective procedure steps for ESD.
Grasping step (lock-on-like accurate targeting): Because the target tissue can be grasped, compressed, fixed, and re-grasped, the CC permits accurate treatment without slippage and does not cause unexpected incision or coagulation (Figure 2 and Video 1).
Pulling step (reduces electrical damage to the muscle layer): Because the target tissue is grasped and pulled up prior to incision and coagulation using an electrosurgical current, there is minimal electrical damage to the muscle layer.
Coagulation step (accurate and effective hemostatic ability): Because the CC coagulates the vessel using an electrosurgical current during grasping and compression, the CC has accurate and effective hemostatic ability. Intraoperative bleeding can be reduced, and even if spurting bleeding occurs, bleeding can be sufficiently stopped; therefore, hemostatic forceps are not required. If there are no visible vessels near the target tissue, this step can be skipped.
Cutting step (accurate mucosal incision and submucosal dissection): After confir
ESD-CC can be completed simply by repeating the above four steps without changing the CC device[6,8,16].
Local injection liquid: A 0.4%-sodium hyaluronate solution is mixed with 0.1 mg of epinephrine and 0.3 mL of indigo carmine.
High-frequency generator: In our institute, we use the VIO3 or VIO300D (Erbe, Tubingen, Germany). Because the CC grasps and pulls the tissue and performs energization and coagulation away from the muscle layer, it is not necessary to change the power setting to address differences in wall thickness in different parts of the gastrointestinal tract. Therefore, the output settings are common to all areas of the gastrointestinal tract, as shown in Table 1[8,16]. There are only three settings, and there is little risk of mistakes when changing settings.
Endoscope: A therapeutic endoscope with a water jet system is recommended to facilitate the ESD-CC procedure.
Hood [8,16]: In ESD-CC, a long transparent hood is always attached to the tip of the endoscope for safe and reliable treatment. The purposes of using a long hood are: (1) to maintain the same distance between the tip of the endoscope and the lesion; (2) to secure the field of view without air inflation; (3) to facilitate obtaining a frontal view of the lesion; (4) to obtain counter traction up and down; and (5) to secure a safe energizing space in the hood. For lesions without fibrosis, we use a long hood (F-01, TOP Corp., Tokyo, Japan) that allows both long and short CCs to open, close, and rotate in the hood, during ESD-CC. For lesions in a narrow submucosal space (esophagus, duodenum, colorectum, and lesions with fibrosis), a tapered hood (ST hood Long type, DH-40GR; Fujifilm) is recommended, which allows the short CC to open, close, and rotate in the hood, and facilitates inserting the hood into narrow submucosal spaces (Figure 3).
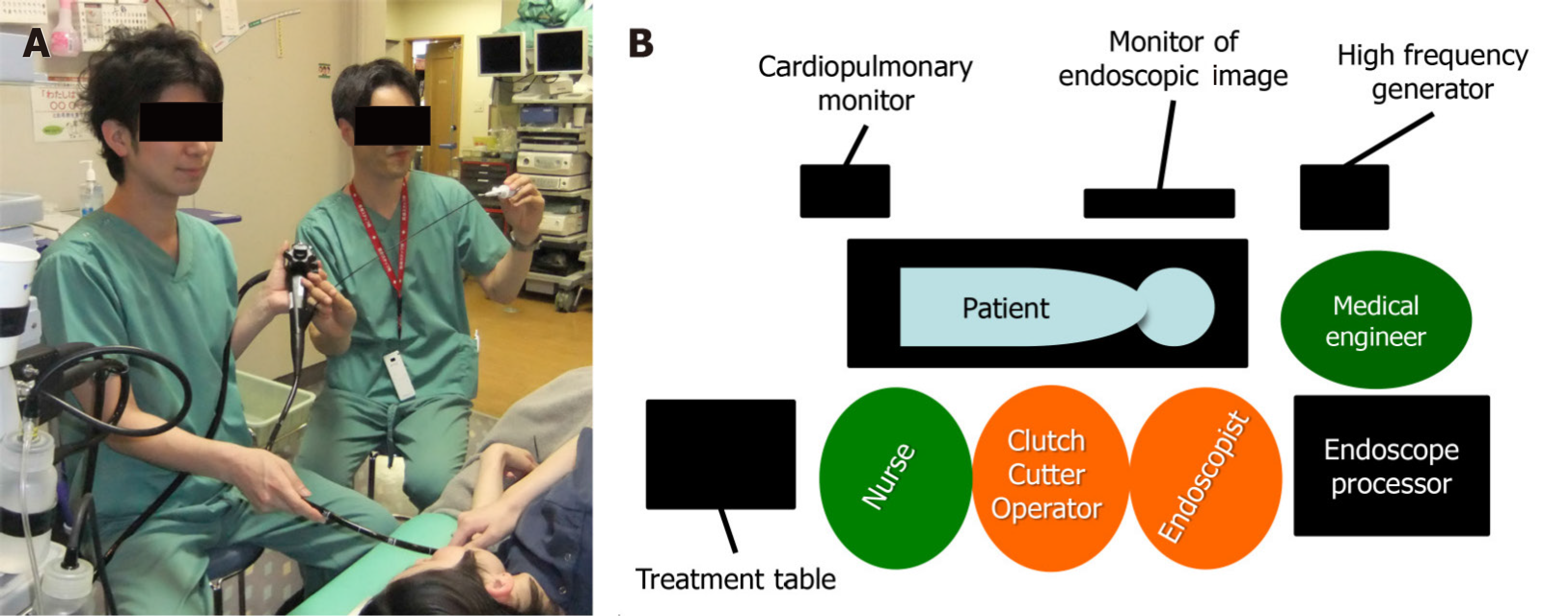
Figure 4 shows the placement of equipment and personnel for ESD-CC in our endoscopy room. The CC must adjust the incision direction by rotating the scissors and the cutting depth by the opening/closing angle and the pressing force, which is handled by the CC operator. For effective ESD, the endoscopist and the CC operator must work side by side looking at the same monitor for good cooperative operation. To perform this method quickly, it is indispensable to have a CC operator who is skilled in rotating the CC, adjusting the opening/closing angle, grasping, and pulling. It is desirable that a dedicated endoscopist, nurse, or clinical engineer performs the role of the CC operator.
Light-contact energization method (marking): This method is used to mark points and the mucosal incision introduction holes. With the scissors completely closed, lightly touch the tip to the area to be marked and energize for a moment (0.1 s) to create the mark (Video 2-a). With the scissors fully open, lightly press the tip against the planned mucosal incision area and energize for a moment to create an introduction hole for the mucosal incision (Video 2-b).
Pull energization method: Angle operation of the endoscope when energizing is not required, and it is possible to safely perform incision and hemostasis while pulling the grasped tissue as for a biopsy. This is especially useful when the treated area can be approached only vertical to the muscle layer, or when angle operation cannot be adequately performed. Grasp the tissue and pull the scissors lightly toward a safe space inside the hood to perform energization incision and coagulation (Video 2-c).
Scooping-up energization method: This method is used in situations where angled operation is achievable. Grasp the tissue and push the CC slightly to scoop (lift up) the tissue by angled operation away from the muscle layer to perform energization incision and hemostasis (Video 2-d).
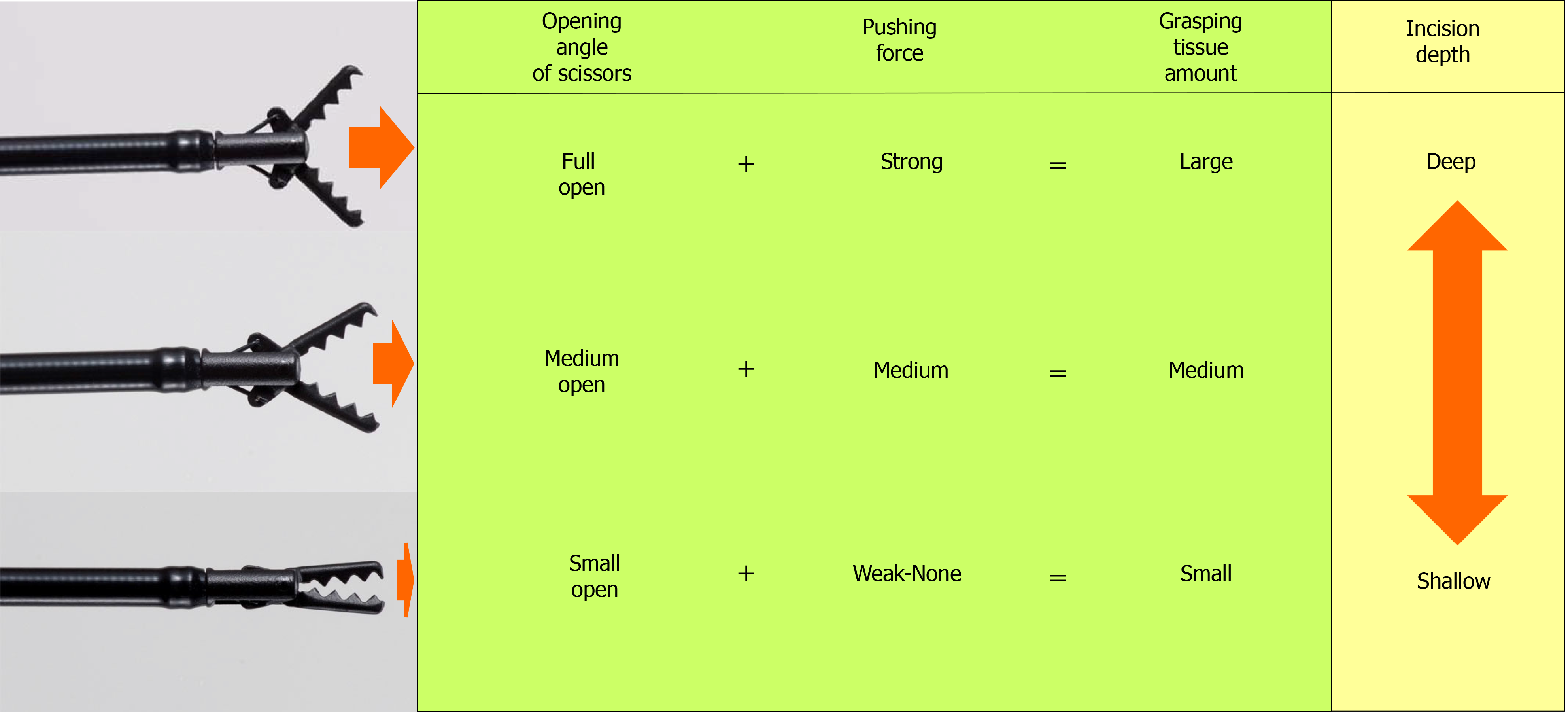
The amount of tissue grasped by the CC is determined by the opening/closing angle of the scissors and the force that presses the cutting edge against the tissue. When the scissors are fully opened and pressed strongly against the tissue and the scissors are closed, a large amount of tissue is grasped, and when energized after traction, a deep incision depth is obtained. Conversely, when the scissors are opened slightly and pressed lightly against the tissue to close the scissors, a small amount of tissue is grasped, and when energized after traction, a shallow incision depth is obtained. In this way, the CC can freely change the incision depth from approximately 0 mm to 5 mm (short blade, 3.5 mm). The depth of coagulation depends on the energization time; the coagulation depth is shallow when energized for a short time, and the coagulation depth is deeper when energized for a long time (Figure 5).
Marking: With the scissors completely closed, lightly press the tip of the scissors against the mucosal surface using as vertical an approach as possible to energize and mark (Video 2A). If determining a tumor margin using a therapeutic endoscope is difficult, replacing the scope with a diagnostic endoscope equipped with narrow-band imaging or blue laser imaging and precise marking with a hook knife is necessary (Video 3 and Figure 6).
Submucosal injection: One to 2 milliliters of the sodium hyaluronate solution is locally injected into the submucosal layer at several points at the planned site of incision or dissection.
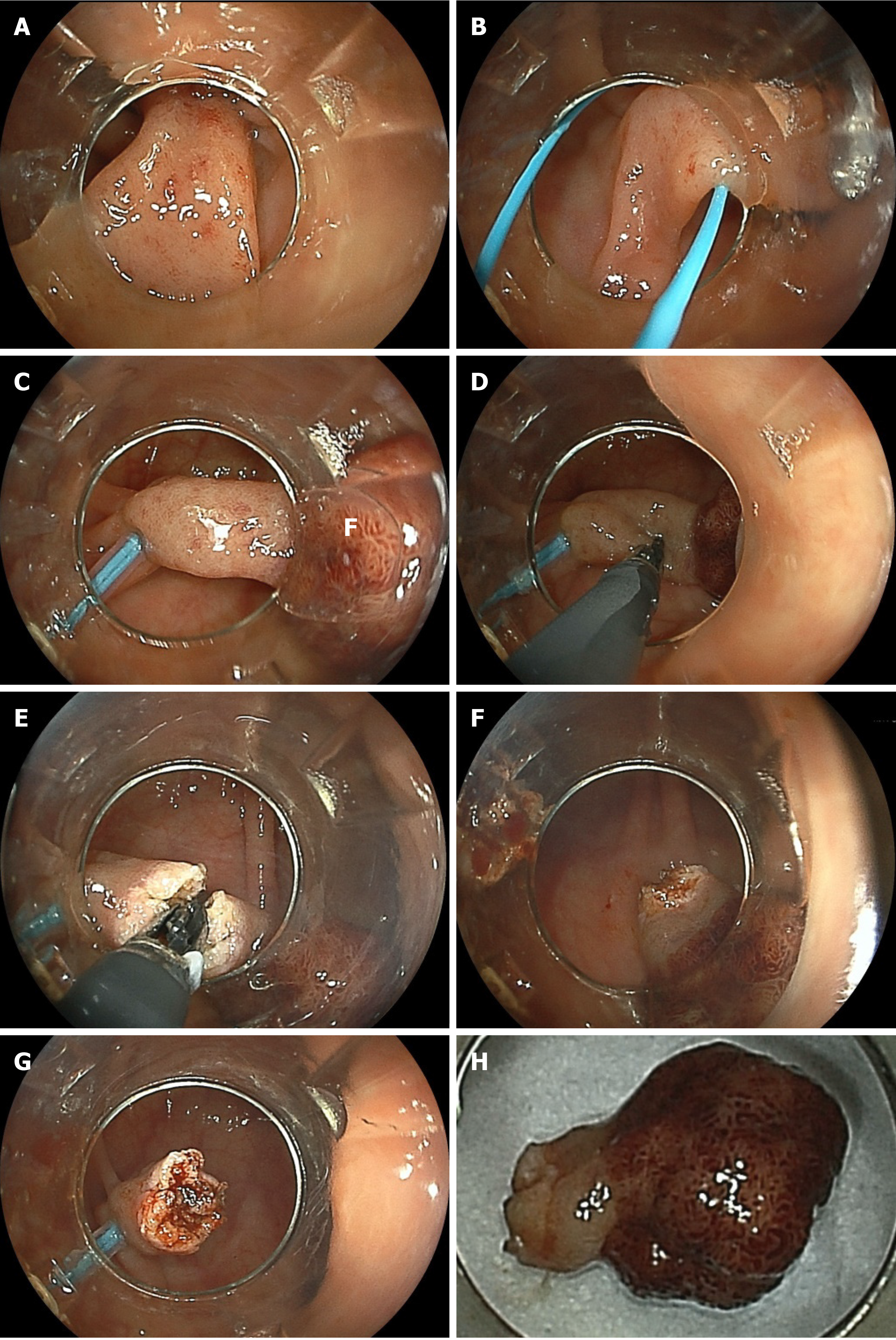
Mucosal incision and submucosal dissection: If the cutting edge slips, and it is difficult to grasp the mucosa, open the cutting edge fully and lightly press the edge against the mucosal surface and energize for a moment to create an introduction hole (Video Video 2-b). Unexpected bleeding occurs because the blood vessels under the mucosa cannot be seen endoscopically during mucosal incision. To prevent bleeding during mucosal incision, the mucosa and superficial submucosa are grasped and raised with the CC, and soft coagulation is energized for prophylactic hemostasis, after which energization is performed with ENDO CUT mode for the incision; thus, mucosal incision is achieved without bleeding. For every 2–3 cm of mucosal incision, additional deep and wide submucosal dissection is needed for the initial incised area. During exfoliation, the tissue in the lower third of the submucosal layer is grasped, compressed, and pulled up, which lifts the tissue away from the muscle layer; the tissue is then energized. If no blood vessels are seen through the submucosa during dissection, exfoliate with only ENDO CUT mode energization. If small blood vessels (≤ 1 mm) are seen, preventive coagulation is performed using soft coagulation, and then cutting is performed using the ENDO CUT mode, for bleeding-free dissection. When a thick blood vessel measuring ≥ 1 mm is seen, the surrounding tissue is first peeled off to expose the blood vessel (Video Video 4-a). Next, the muscular layer side of the blood vessel is preventively coagulated using soft coagulation, and the mucosal side of the blood vessel is also coagulated for blood flow blockage. Next, the blood vessel between both coagulation sites is grasped and pulled and coagulated using soft coagulation and cut using ENDO CUT mode. There is no bleeding during this cutting.
Hemostasis for intraoperative bleeding: The bleeding blood vessel is pinpointedly grasped and compressed, and after confirming that the bleeding has temporarily stopped, the vessel is slightly pulled up and coagulated using soft coagulation, for permanent hemostasis (Video 4-b). If the blood vessel cannot be seen because of severe bleeding, inject 1 cc of hypertonic saline–epinephrine solution into 3–5 points near the site where bleeding is suspected (Video 4-c). Once the bleeding momentum has subsided and the blood vessel becomes visible, accurately grasp the bleeding blood vessel, pull up it gently, and coagulate. Aggressive pulling should be avoided because this leads to severe bleeding owing to blood vessel rupture. Furthermore, blind grasping coagulation carries the risk of perforation and should also be avoided.
Preventive vascular coagulation to prevent post-ESD hemorrhage: Visible blood vessels at the bottom of the post-ESD ulcer are pinpointedly grasped by the CC, pulled up, and coagulated using soft coagulation to prevent post-ESD bleeding (Video Video 4-d). Coagulation by pressing the CC toward the muscle layer may cause perforation and should be avoided.
Table 2 shows the reported clinical outcomes of ESD-CC. Reported performance ranges for the use of CC in early esophageal tumor resections were as follows: surgical time: 56.4–78.3 min; en-bloc resection rate: 88.9%–100%; perforation rate: 0%; and delayed bleeding rate: 0%–1.4%[18-20]. The ranges in early gastric tumor resection were: surgical time: 44–97.2 min; en-bloc resection rate: 93%–100%; perforation rate: 0%–3.6%; and delayed bleeding rate: 0%–3.6%[6,21-23]. The ranges in early duodenal tumor resection were: surgical time: 36 min; en-bloc resection rate: 100%; perforation rate: 2.1%; and delayed bleeding rate: 4.2%[24]. The ranges in early colorectal tumor resection were surgical time: 56–88.3 min; en-bloc resection rate: 98%–99.3%; perforation rate: 2%–2.3%; and delayed bleeding rate: 0%–2.3%[8,12]. Thus, high en bloc resection rates and low complication rates were achieved by ESD-CC in every intestinal section.
| Ref. | Organ | Numberof patients | Histology | Procedure time, min | En bloc (R0) resection rate % | Perforation rate % (n) | Delayed bleeding rate % (n) | Surgical1 intervention rate % (n) |
| Sawas et al[18], 2018 | Esophagus | 14 | ADCA, DYS | ND | 100 (41.6) | 0 (0) | 0 (0) | 0 (0) |
| Esaki et al[19], 2020 | Esophagus | 36 | SCC | 56.4 | 88.9 (80.6) | 0 (0) | 0 (0) | 0 (0) |
| Hanada et al[20], 2020 | Esophagus | 96 | ADCA, DYS, SCC | 78.3 | 94.3 (ND) | 0 (0) | 1.4 (2) | 0 (0) |
| Akahoshi et al[6], 2015 | Stomach | 325 | ADCA | 97.2 | 99.7 (95.3) | 0.3 (1) | 3.4 (11) | 0 (0) |
| Nagai et al[21], 2016 | Stomach | 56 | ADCA, ADM | 66 | 93 (ND) | 3.6 (2) | 3.6 (2) | 0 (0) |
| Hayashi et al[22], 2018 | Stomach | 50 | ADCA | 49 | 100 (100) | 0 (0) | 2.3 (1) | 0 (0) |
| Dohi et al[23], 2019 | Stomach | 61 | ADCA | 44 | 100 (93.4) | 0 (0) | 0 (0) | 0 (0) |
| Dohi et al[24], 2020 | Duodenum | 47 | ADCA, ADM | 36 | 100 (97.9) | 2.1 (1) | 4.2 (2) | 0 (0) |
| Akahoshi et al[8], 2019 | Colorectum | 437 | ADCA, ADM | 88.3 | 99.3 (87.0) | 2.3 (10) | 2.3 (10) | 0 (0) |
| Yoshida et al[12], 2019 | Colorectum | 51 | ND | 56 | 98 (ND) | 2.0 (ND) | 0 (0) | ND |
Five comparison studies between ESD-CC and ESD-conventional knives have been reported (Table 3)[19,21-24]. In the esophagus, Esaki et al[19] reported that the ESD-CC group showed no significant difference for several parameter comparisons, and only the median procedure time was significantly shorter with ESD-CC vs with ESD-conventional knives (44.0 min vs 66.5 min, respectively; P = 0.020). In the stomach, two of the three papers reported that ESD-CC had a significantly shorter resection time than ESD-conventional knives[21-23]. In the duodenum, Dohi et al[24] reported that ESD-CC had a significantly shorter resection time, higher R0 resection rate, and lower intraoperative perforation rate compared with ESD-conventional knives.
| Organ | Ref. | ESD using the clutch cutter | ESD using the conventional knives | P value | |
| Esophagus | Esaki et al[19], 2020 | Type of knife | - | IT-KN, DK, FK, SMK | - |
| Number of patients1 | 36 | 36 | - | ||
| Median procedure time (min) | 44 | 66.5 | 0.02 | ||
| En-bloc resection rate (%) | 100 | 97.2 | 1 | ||
| R0 resection rate (%) | 88.9 | 86.1 | 1 | ||
| Perforation rate (%) | 0 | 5.6 | 0.20 | ||
| Delayed bleeding rate (%) | 0 | 0 | - | ||
| Stomach | Nagai et al[21], 2016 | Type of knife | - | IT-2K | - |
| Number of patients | 56 | 61 | - | ||
| Median procedure time (min) | 66 | 41 | 0.04 | ||
| En-bloc resection rate (%) | 93 | 98 | 0.195 | ||
| R0 resection rate (%) | ND | ND | - | ||
| Perforation rate (%) | 3.6 | 1.6 | - | ||
| Delayed bleeding rate (%) | 3.6 | 1.6 | - | ||
| Stomach | Hayashi et al[22], 2018 | Type of knife | - | IT-2K,SMK | - |
| Number of patients1 | 44 | 44 | - | ||
| Median procedure time (min) | 49 | 88.5 | <0.001 | ||
| En-bloc resection rate (%) | 100 | 100 | - | ||
| R0 resection rate (%) | 100 | 100 | - | ||
| Perforation rate (%) | 0 | 0 | - | ||
| Delayed bleeding rate (%) | 2.3 | 6.8 | 0.620 | ||
| Stomach | Dohi et al[23], 2019 | Type of knife | - | IT-2K | - |
| Number of patients1 | 61 | 61 | - | ||
| Median procedure time (min) | 44 | 56 | 0.005 | ||
| En-bloc resection rate (%) | 100 | 100 | 1 | ||
| R0 resection rate (%) | 93.4 | 100 | 0.06 | ||
| Perforation rate (%) | 0 | 4.9 | 0.12 | ||
| Delayed bleeding rate (%) | 0 | 6.6 | 0.06 | ||
| Duodenum | Dohi et al[24], 2020 | Type of knife | - | FK | - |
| Number of patients | 47 | 37 | - | ||
| Median procedure time (min) | 36 | 54 | 0.045 | ||
| En-bloc resection rate (%) | 100 | 94.6 | 0.191 | ||
| R0 resection rate (%) | 97.9 | 83.8 | 0.04 | ||
| Intra-ESD perforation rate (%) | 0 | 4.9 | 0.014 | ||
| Delayed perforation rate (%) | 2.1 | 8.1 | 0.316 | ||
| Delayed bleeding rate (%) | 4.2 | 0 | 0.501 |
Shiga et al[25] reported the learning curve of colorectal ESD using conventional knives. Dividing a total of 180 cases into three learning phases, the en-bloc resection rate was gradually improved to 88.3%, 93.3%, and 98.3% in the early, middle, and late phases, respectively. In contrast, the rate of perforation decreased from 10.0% in the early phase to 5.0% in the middle phase and 3.3% in the late phase. Research from France evaluating the learning curve of rectal ESD using conventional knives showed similar results in that from the start phase to the late phase, the en-bloc resection rate increased from 52% to 82%, and the perforation rate decreased significantly from 34% to 0%[26].
Research evaluating the learning curve for colorectal ESD-CC showed a flat learning curve from the start phase to the late phase for both the R0 resection rate (82% to 80%, respectively) and perforation rate (0% to 3.4%, respectively)[8]; however, there was statistically significant proficiency regarding operating time (127 min to 82 min, respectively). Thus, the CC is considered a safe and effective device for facilities that are introducing ESD for the first time.
Dohi et al[23] reported that the rate of self-completion of gastric ESD by non-experts was significantly better with the CC than with the IT knife 2 (61.7% vs 24.5%, respectively; P < 0.001). The authors also reported additional data for gastric ESD performed by non-experts in which the number of intraoperative bleeding points and the number of points requiring hemostatic forceps were significantly lower in the CC group than in the IT knife 2 group. The CC has unique mechanical features, such as accurate incision and hemostasis by grasping without slippage, opening and re-grasping functions, and high hemostasis ability. These advantages of the CC allow for safe, effective, and easy ESD training by experts[6,8,12,27]. However, the ESD-CC procedure requires the technical skills of the assistant to rotate the device at an appropriate position and grasp the target tissue. ESD using conventional type knife does not need it. Esaki et al[28] reported that procedure time of the ESD-CC assisted by an expert was significantly shorter than that of the ESD-CC assisted by a non-expert in an ex vivo porcine stomach model. They also showed that assistant skill was significantly associated with the difficulty of ESD. Assistance by an expert is needed to obtain a favorable learning curve of ESD-CC.
The CC (¥43000) is a little more expensive than conventional knives (Insulation-tipped diathermic knife (KD-611L; Olympus, ¥38000), Flush knife BT-S (DK2620J; Fujifilm, ¥28000), et al. However, in ESD using conventional knives, it is necessary to use the other types of devices properly depending on the aspect of ESD[6]. For example: (1) ESD using insulation-tipped diathermic knife needs a needle knife (KD-lL-1; Olympus, ¥27500) to make a start hole for mucosal incision; and (2) If the submucosal dissection step needs a vertical approach to the proper muscle in ESD using the Flush knife-BT-S, pull energization method available devices such as the hook knife (KD-620QR; Olympus, ¥30000) or the CC (¥43000) are needed to reduce the risk of perforation. Furthermore, conventional knives have an insufficient hemostatic ability and often require hemostatic forceps [Coagrasper, (FD-411QR; Olympus, ¥15000), et al] for bleeding during ESD. Therefore, conventional knives need additional costs for combined devices. On the other hand, since CC can perform incision and coagulation while grasping and pulling the target tissue, it can handle all aspects during ESD even if a vertical approach of submucosal excision to the proper muscle is unavoidable. Furthermore, CC has high hemostatic ability, so hemostat forceps are not required for intraoperative bleeding. In ESD-CC, additional use of other types of knives are unnecessary (single device ESD is possible). Therefore, CC reduces the total clinical cost during ESD.
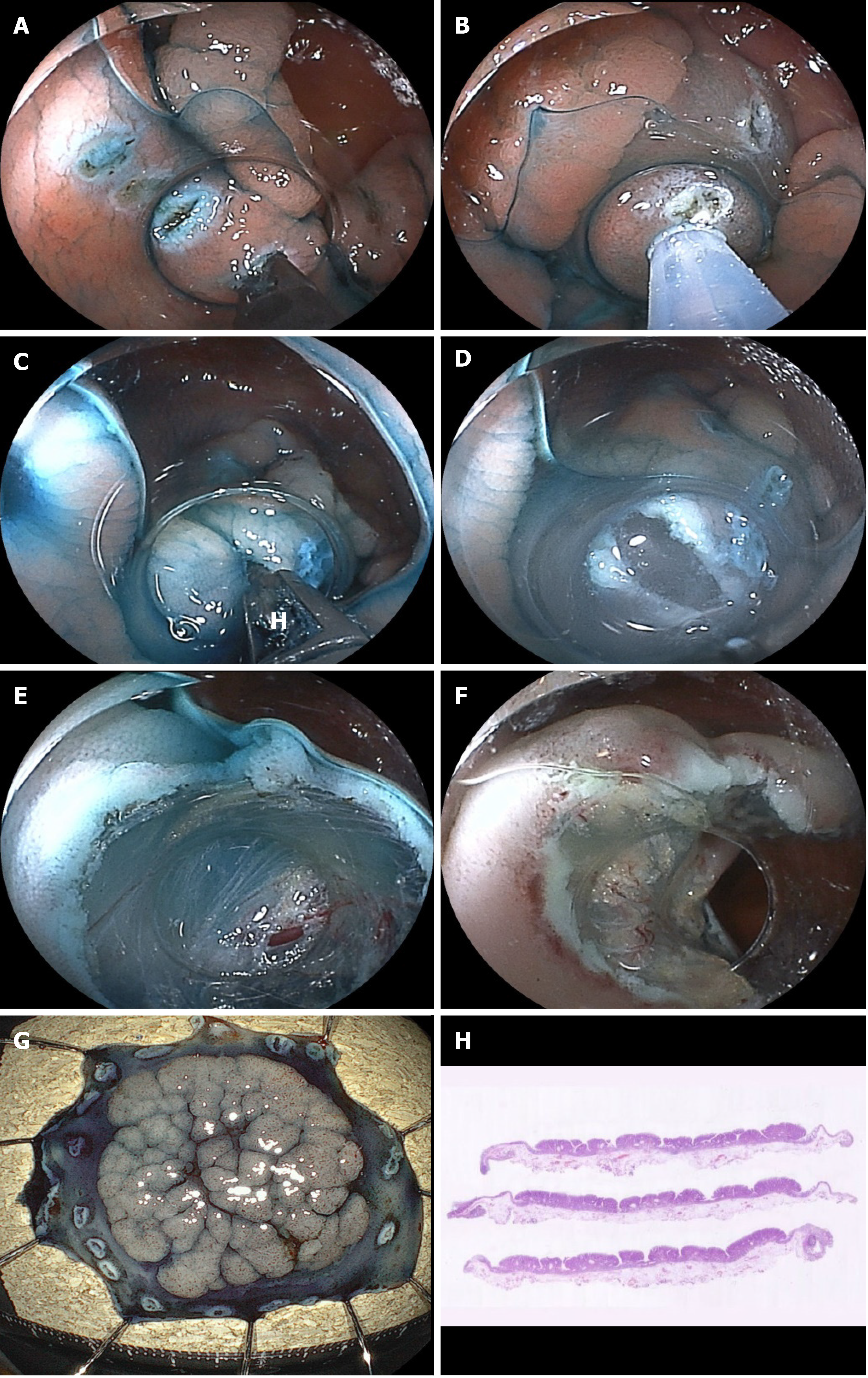
When an endoscopist performs polypectomy for a large pedunculated polyp, the snare on the back side of the thick stalk cannot be seen, and it is difficult to check for adequate positioning of the distal side of the snare using an endoscopic view and to determine whether the distal part of the snare is on the stalk of the polyp or on the normal mucosa on the distal side. No visibility of the distal part of the snare during the procedure leads to piecemeal polypectomy or severe adverse events, such as perforation and bleeding[29,30]. If the CC is used to remove a large pedunculated polyp, the large stalk is also grasped and pulled by the CC and cut little by little, so that the distal side of the stalk can be accurately incised under good endoscopic view[30-32]. Like the snare, the CC is energized while grasping and compressing; therefore, it provides strong hemostasis, less intraoperative bleeding, and even if there is bleeding, this can be stopped immediately by the CC. Furthermore, a thick artery and perivascular fibrosis of the stalk portion of a large pedunculated polyp sometimes leads to the electrosurgical snare becoming stuck in the stalk during polypectomy. When this occurs, cutting the stalk using an electrosurgical current and releasing the snare are both impossible. However, if the CC is used, releasing the blades by opening the CC is easy [30]. Previous reports of polypectomy using the CC for large pedunculated polyps indicated 100% R0 resection without complications[30-32]. This appears to be a promising future indication for the CC (Figure 7 and Video 5).
Zenker’s diverticulum is an acquired extruded diverticulum consisting of the mucosa and submucosa through Killian’s triangle dorsally at the pharyngoesophageal junction[33,34]. Currently, endoscopic myotomy of the cricopharyngeal muscle is performed as less invasive therapy in symptomatic patients[34-36]. However, if the incision is too short, recurrence occurs owing to incomplete cricopharyngeal myotomy. In contrast, cutting too deeply leads to mediastinal perforation; another complication is bleeding. Accurate cutting and sufficient coagulation are the keys to the success of this procedure. A pilot study using the CC showed 100% complete diverticulotomy without complications because the CC has a grasping function (accurate targeting) and high hemostatic capability[34,36]. However, further studies are needed.
Aso et al[37] reported using the CC for endoscopic necrosectomy. In this case, the areas of walled-off pancreatic necrosis were so large that the authors could not remove the dense and massive necrotic tissue satisfactorily, even though the procedure was repeated over five sessions using conventional devices. In the sixth session using the CC, the dense and massive necrotic tissues were easily dissected and fragmented with the CC. In addition, endoscopic hemostasis was also achieved for intraoperative bleeding, and the authors grasped and removed the fragmented necrotic tissue from the cavity and successfully accomplished this procedure.
Another indication is endoscopic management of buried bumper syndrome. Experience with a case has been reported[38]. In this report, overlying mucosa and granulomatous-fibrotic tissue on the buried bumper were completely incised and opened by the CC without complications, and the buried bumper was then successfully released into the gastric lumen.
The CC is a unique endoscopic forceps-type resection device that enables safe and easy endoscopic surgery with a single device, according to the movements of the device itself, and the protection system. Endoscopic surgery can be completed by repeating the same simple procedure as for biopsy. Previous studies showed that even non-expert endoscopists can use the CC with confidence. Further clinical studies using the CC for endoscopic treatment are needed to clarify the efficacy and safety of CC and to expand its use to new indications.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zou BC S-Editor: Zhang H L-Editor: A P-Editor: Xing YX
| 1. | Nishizawa T, Suzuki H. Long-Term Outcomes of Endoscopic Submucosal Dissection for Superficial Esophageal Squamous Cell Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Ma MX, Bourke MJ. Endoscopic submucosal dissection in the West: Current status and future directions. Dig Endosc. 2018;30:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Nishizawa T, Yahagi N. Endoscopic mucosal resection and endoscopic submucosal dissection: technique and new directions. Curr Opin Gastroenterol. 2017;33:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 5. | Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Naruke A, Kim M, Koizumi W. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer. 2014;17:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Akahoshi K, Motomura Y, Kubokawa M, Gibo J, Kinoshita N, Osada S, Tokumaru K, Hosokawa T, Tomoeda N, Otsuka Y, Matsuo M, Oya M, Koga H, Nakamura K. Endoscopic Submucosal Dissection for Early Gastric Cancer using the Clutch Cutter: a large single-center experience. Endosc Int Open. 2015;3:E432-E438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Esaki M, Ihara E, Gotoda T. Endoscopic instruments and techniques in endoscopic submucosal dissection for early gastric cancer. Expert Rev Gastroenterol Hepatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Akahoshi K, Shiratsuchi Y, Oya M, Koga H, Kubokawa M, Nakama N, Akahoshi K, Ihara E. Endoscopic submucosal dissection with a grasping-type scissors for early colorectal epithelial neoplasms: a large single-center experience. VideoGIE. 2019;4:486-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Kotzev AI, Yang D, Draganov PV. How to master endoscopic submucosal dissection in the USA. Dig Endosc. 2019;31:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakaya N, Nakamura T, Shimosegawa T. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: "eCura system". Am J Gastroenterol. 2017;112:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 11. | Yamamoto K, Michida T, Nishida T, Hayashi S, Naito M, Ito T. Colorectal endoscopic submucosal dissection: Recent technical advances for safe and successful procedures. World J Gastrointest Endosc. 2015;7:1114-1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Yoshida N, Dohi O, Inoue K, Yasuda R, Ishida T, Hirose R, Naito Y, Ogiso K, Murakami T, Morinaga Y, Kishimoto M, Inada Y, Itoh Y, Gotoda T. Efficacy of scissor-type knives for endoscopic mucosal dissection of superficial gastrointestinal neoplasms. Dig Endosc. 2020;32:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Dhaliwal L, Codipilly DC, Rowan DJ, Wong Kee Song LM, Iyer PG. Water-pocket endoscopic submucosal dissection of an early esophageal adenocarcinoma in a patient with portal hypertension and varices. VideoGIE. 2020;5:646-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Abiko S, Yoshikawa A, Harada K, Kawagishi N, Sano I, Oda H, Miyagishima T. Usefulness of a clutch cutter combined with an S-O clip in improving stability when opening the pocket in the pocket-creation method. Endoscopy. 2020;52:E128-E129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Tashima T, Nonaka K, Ryozawa S, Fujino T. Duodenal endoscopic submucosal dissection for a large protruded lesion located just behind the pyloric ring with a scissor-type knife. VideoGIE. 2019;4:447-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Akahoshi K, Kubokawa M, Tamura S. Clutch Cutter (Long type & Short type). In: Yahagi N. Electrosurgical units for therapeutic endoscopy-Settings and Its effective usage. 3rd ed. Tokyo: Japan Medical Center, 2020: 177-184. |
| 17. | Akahoshi K, Motomura Y, Kubokawa M, Kinoshita N. Characteristics and effective use of the Clutch Cutter. Endoscopia Digestiva. 2014;26: 1399-1406. |
| 18. | Sawas T, Visrodia KH, Zakko L, Lutzke LS, Leggett CL, Wang KK. Clutch cutter is a safe device for performing endoscopic submucosal dissection of superficial esophageal neoplasms: a western experience. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Esaki M, Hayashi Y, Ikehara H, Ihara E, Horii T, Tamura Y, Ichijima R, Yamakawa S, Irie A, Shibuya H, Suzuki S, Kusano C, Minoda Y, Akiho H, Ogawa Y, Gotoda T. The effect of scissor-type versus non-scissor-type knives on the technical outcomes in endoscopic submucosal dissection for superficial esophageal cancer: a multi-center retrospective study. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Hanada Y, Wang KK. Safety and feasibility of same-day discharge after esophageal endoscopic submucosal dissection. Gastrointest Endosc. 2021;93:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Nagai K, Uedo N, Yamashina T, Matsui F, Matsuura N, Ito T, Yamamoto S, Hanaoka N, Takeuchi Y, Higashino K, Ishihara R, Iishi H. A comparative study of grasping-type scissors forceps and insulated-tip knife for endoscopic submucosal dissection of early gastric cancer: a randomized controlled trial. Endosc Int Open. 2016;4:E654-E660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Hayashi Y, Esaki M, Suzuki S, Ihara E, Yokoyama A, Sakisaka S, Hosokawa T, Tanaka Y, Mizutani T, Tsuruta S, Iwao A, Yamakawa S, Irie A, Minoda Y, Hata Y, Ogino H, Akiho H, Ogawa Y. Clutch Cutter knife efficacy in endoscopic submucosal dissection for early gastric neoplasms. World J Gastrointest Oncol. 2018;10:487-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Dohi O, Yoshida N, Terasaki K, Azuma Y, Ishida T, Kitae H, Matsumura S, Ogita K, Takayama S, Mizuno N, Nakano T, Hirose R, Inoue K, Kamada K, Uchiyama K, Ishikawa T, Takagi T, Kishimoto M, Konishi H, Naito Y, Itoh Y. Efficacy of Clutch Cutter for Standardizing Endoscopic Submucosal Dissection for Early Gastric Cancer: A Propensity Score-Matched Analysis. Digestion. 2019;100:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Dohi O, Yoshida N, Naito Y, Yoshida T, Ishida T, Azuma Y, Kitae H, Matsumura S, Takayama S, Ogita K, Mizuno N, Nakano T, Majima A, Hirose R, Inoue K, Kamada K, Uchiyama K, Takagi T, Ishikawa T, Konishi H, Morinaga Y, Kishimoto M, Itoh Y. Efficacy and safety of endoscopic submucosal dissection using a scissors-type knife with prophylactic over-the-scope clip closure for superficial non-ampullary duodenal epithelial tumors. Dig Endosc. 2020;32:904-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Shiga H, Ohba R, Matsuhashi T, Jin M, Kuroha M, Endo K, Moroi R, Kayaba S, Iijima K. Feasibility of colorectal endoscopic submucosal dissection (ESD) carried out by endoscopists with no or little experience in gastric ESD. Dig Endosc. 2017;29 Suppl 2:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Rönnow CF, Uedo N, Toth E, Thorlacius H. Endoscopic submucosal dissection of 301 large colorectal neoplasias: outcome and learning curve from a specialized center in Europe. Endosc Int Open. 2018;6:E1340-E1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Ichijima R, Esaki M, Yamakawa S, Minoda Y, Suzuki S, Kusano C, Ikehara H, Gotoda T. Ex vivo porcine model study on the treatment outcomes of scissor-type knife versus needle-type knife in endoscopic submucosal dissection performed by trainees. BMC Surg. 2020;20:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Esaki M, Horii T, Ichijima R, Wada M, Sakisaka S, Abe S, Tomoeda N, Kitagawa Y, Nishioka K, Minoda Y, Tsuruta S, Suzuki S, Akiho H, Ihara E, Ogawa Y, Gotoda T. Assistant skill in gastric endoscopic submucosal dissection using a clutch cutter. World J Gastrointest Surg. 2021;13:116-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Yang CW, Yen HH, Chen YY, Soon MS. Use of dual knife for large pedunculated colorectal polyps. Surg Laparosc Endosc Percutan Tech. 2014;24:444-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Akahoshi K, Kubokawa M, Gibo J, Osada S, Tokumaru K, Shiratsuchi Y, Oya M, Ihara E, Nakamura K. Endoscopic resection using the Clutch Cutter and a detachable snare for large pedunculated colonic polyps. Endoscopy. 2017;49:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Zimmer V. Rapid upfront stalk transection for endoscopic resection of pedunculated colorectal lesions using a grasping-type scissor forceps. Endoscopy. 2021;53:E253-E254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Jawaid S, Draganov PV, Yang D. Endoscopic resection of large pedunculated colon polyps using only a scissor-type knife: a case series. VideoGIE. 2020;5:264-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Ferreira LE, Simmons DT, Baron TH. Zenker's diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus. 2008;21:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Rath T, Siebler J, Neurath MF, Nägel A. Treatment of Zenker's diverticulum using a novel grasping-type scissors forceps allows fast, safe, and effective endoscopic diverticulotomy. Endosc Int Open. 2018;6:E659-E663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Ishaq S, Sultan H, Siau K, Kuwai T, Mulder CJ, Neumann H. New and emerging techniques for endoscopic treatment of Zenker's diverticulum: State-of-the-art review. Dig Endosc. 2018;30:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 36. | Neumann H, Löffler S, Rieger S, Kretschmer C, Nägel A. Endoscopic therapy of Zenker's diverticulum using a novel endoscopic scissor - the Clutch Cutter device. Endoscopy. 2015;47 Suppl 1 UCTN:E430-E431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Aso A, Igarashi H, Matsui N, Ihara E, Takaoka T, Osoegawa T, Niina Y, Oono T, Akahoshi K, Nakamura K, Ito T, Takayanagi R. Large area of walled-off pancreatic necrosis successfully treated by endoscopic necrosectomy using a grasping-type scissors forceps. Dig Endosc. 2014;26:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Rath T, Vitali F, Nägel A, Neurath MF. Endoscopic management of buried bumper syndrome using a novel grasping-type scissor forceps. Endoscopy. 2021;53:E21-E22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |