Published online Aug 27, 2021. doi: 10.4240/wjgs.v13.i8.764
Peer-review started: January 28, 2021
First decision: March 8, 2021
Revised: March 17, 2021
Accepted: April 29, 2021
Article in press: April 29, 2021
Published online: August 27, 2021
Processing time: 203 Days and 18.7 Hours
Rectovaginal fistula after low anterior resection for rectal malignancy is one of the most challenging postoperative complications because it is difficult to treat and may complicate plans of adjuvant therapy. This problematic complication could lead to multiple operations, stoma formation, sexual dysfunction, fecal incontinence and psychosocial ramifications. This review comprehensively covers an overview of its incidence, risk factors, presentation and evaluation, management (ranging from conservative measures, endoscopic treatment and local tissue repair to radical resection and redo anastomosis) and treatment outcomes of rectovaginal fistula after low anterior resection. Notably, these therapeutic options and outcomes are influenced by several factors, including the size and location of the fistula, tumor clearance, cancer staging, quality of colorectal anastomosis and surrounding tissue, presence of diverting stoma, previous attempted repair, and the surgeon’s experience. Also, strategies to prevent rectovaginal fistula after low anterior resection are presented with illustrations. Finally, a decision-making algorithm for managing this complication is proposed.
Core Tip: The current article provides a comprehensive overview of the incidence, risk factors, presentation, evaluation, management and outcomes of patients with rectovaginal fistula resulting from low anterior resection. Notably, the therapeutic options and results are influenced by several factors, including size and location of the fistula, tumor clearance, cancer staging, quality of colorectal anastomosis, surrounding tissue, presence of diverting stoma, previously attempted repair, and the surgeon’s experience. Strategies to prevent rectovaginal fistula formation after rectal cancer surgery are also discussed. A decision-making algorithm for managing this complication is proposed at the end of article.
- Citation: Lohsiriwat V, Jitmungngan R. Rectovaginal fistula after low anterior resection: Prevention and management. World J Gastrointest Surg 2021; 13(8): 764-771
- URL: https://www.wjgnet.com/1948-9366/full/v13/i8/764.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i8.764
Rectovaginal fistula (RVF) is an abnormal connection between the vagina and the rectum. Patients with RVF may present with the passage of flatus or stool through the vagina, causing vaginitis, foul-smelling vaginal discharge or fecal incontinence. The common etiologies of RVF include Crohn’s disease, pelvic irradiation, obstetric injury and direct invasion of rectal or gynecologic malignancy[1,2]. Meanwhile, radical rectal surgery, especially with colorectal anastomosis, was another cause of RVF that accounted for 10%-20% of overall RVF in some studies[1,2].
RVF after low anterior resection for rectal malignancy is one of the most challenging postoperative complications because it is difficult to treat and may complicate plans of adjuvant therapy. In fact, RVF following rectal cancer surgery has been reported in the literature since the 1980s[3]. It is perceived to be associated with difficult pelvic dissections and creation of a colorectal anastomosis. This complication could occur after sphincter-preserving operations for proximal and distal rectal cancer with either stapled or hand-sewn anastomosis[4]. This problematic complication could lead to multiple operations, stoma formation, sexual dysfunction, fecal incontinence, and psychosocial ramifications. This article presents a comprehensive overview of the incidence, risk factors, management and outcome of RVF after low anterior resection. Preventive strategies and a management algorithm are also discussed.
The incidence of RVF after low anterior resection for rectal cancer is reported as 1.6%-5.1%[4-10]. Depending on fistula size and presence of diverting stoma, time to occurrence of RVF varied from days to months after surgery, with a median time of 20-25 d in two large-scale studies[5,7].
It was well evident that low colorectal anastomosis, especially within 5 cm from the anal verge, is an important risk factor for RVF after sphincter-preserving operations[5,7-9]. The increased likelihood of RVF following low anterior resection is explained by the fact that ‘safe’ low colorectal anastomosis requires complete dissection between the rectum and the vagina (sometimes until the pelvic floor muscle is reached) before creation of an anastomosis, but deep pelvic dissection can be difficult, even in a female pelvis.
Apart from low colorectal anastomosis, tumor characteristics have been reported to be associated with an increased risk of RVF, including tumors larger than 5 cm[6], locally advanced tumors requiring neoadjuvant therapy[6,11], and stage IV rectal cancer[5]. A large retrospective study from China suggested that concomitant hysterectomy and/or bilateral oophorectomy may increase the risk of RVF formation by 3-6 times[8]. Other risk factors reported for developing RVF after rectal cancer surgery are preoperative malnutrition[6], anemia[8], lateral pelvic lymph node dissection and intraoperative blood loss of more than 200 mL[6].
The diagnosis of RVF after low anterior resection is not difficult because it is well characterized by postoperative passage of flatus or stool through the vagina, in most cases. To a lesser extent, patients with such a fistula may present with recurrent vaginitis and foul-smelling vaginal discharge[12]. Because RVF is caused by inclusion of the posterior vaginal wall in a colorectal anastomosis, rectal and vaginal examination including bimanual palpation of the rectovaginal septum may reveal the fistula or a dimple at the site of colorectal anastomosis.
Endoscopic examination together with rectal or vaginal contrast study may confirm and give detailed information of the fistula, especially when digital, anoscopic and speculum examination cannot display the lesion. In some cases, a tampon is placed in the vagina while a small amount of dye solution (methylene blue or India ink) is administered via enema. The presence of dye color on the tampon indicates a fistula.
The integrity and diameter of colorectal anastomosis should also be noted because RVF might be secondary to colorectal anastomotic leakage[5] in which a pelvic abscess is drained through the injured vagina, or anastomotic stricture may complicate the management of RVF. However, if a patient presents with a late-onset RVF, recurrent rectal cancer and radiation injury to the pelvic organs should be ruled out. It is worth noting that occurrence of enterovaginal fistula could mimic or present with RVF, especially in those receiving pelvic irradiation[1].
RVF after low anterior resection can be classified based on the location of the fistula as low (close to the posterior fourchette), middle or high (close to the cervix). However, some investigators have classified the fistula based on radiologic and/or endoscopic studies into four types, namely RVF alone, RVF with dead space, RVF with anastomotic stricture and RVF with dead space, and anastomotic stricture[13]. This newly proposed classification of RVF after rectal cancer surgery may indicate different approaches, e.g., RVF with dead space may require drainage prior to surgical correction, or RVF with anastomotic stricture is more likely to have re-resection and redo coloanal anastomosis.
Various approaches to RVF after low anterior resection for rectal malignancy have been reported, ranging from conservative management, endoscopic treatment and local tissue repair to radical resection of the fistula and redo colorectal anastomosis. These therapeutic options are influenced by several factors, including the patient’s age and comorbidities, the size and location of the fistula, tumor clearance, cancer staging, needs of adjuvant therapy, quality of colorectal anastomosis and surrounding tissue, presence of diverting stoma, previous attempted repair, and the surgeon’s experience. Because most published data on the management of RVF after rectal cancer surgery were from small retrospective studies or case series, it is difficult to compare these results and find the treatment of choice for this condition. Although most patients need surgical correction, conservative or endoscopic approaches can be used in selected cases. The viable options of treating RVF after low anterior resection are discussed. Major studies utilizing various treatment modalities and the reported outcome in these studies are shown in Table 1.
| Ref. | Number of cases | Treatment modalities and their outcomes |
| Rex and Khubchandani[4], 1992 | 57 | Conservative treatment: 14 (success: 70%) |
| Diversion only: 17 (success: 35%) | ||
| Diversion with staged endoanal repair: 8 (success: 63%) | ||
| Diversion with re-anastomosis: 3 (success: 100%) | ||
| Endoanal repair: 3 (success: 67%) | ||
| Re-anastomosis: 3 (success: 100%) | ||
| Pull-through operation: 2 (success: 100%) | ||
| Abdominoperineal resection: 3 (success: 100%) | ||
| Lamazza et al[20], 2016 | 15 | Endoscopic stent: 13 (success: 92%) |
| Endoscopic stent with subsequent repair: 2 (success: 100%) | ||
| Zheng et al[7], 2017 | 24 | Diversion only: 22 (success: 64%) |
| Diversion with staged endoanal repair: 1 (success 100%) | ||
| Endoanal repair: 1 (success: 100%) | ||
| Woo et al[9], 2019 | 18 | Conservative treatment: 3 (success: 0%) |
| Diversion only: 1 (success: 100%) | ||
| Diversion with staged endoanal repair: 2 (success: 100%) | ||
| Diversion with re-anastomosis: 3 (success: 100%) | ||
| Endoanal repair: 6 (success: 100%) | ||
| Re-anastomosis: 3 (success: 100%) |
It is known that RVF after low anterior resection is refractory to conservative treatment[5,7,9], but such an approach may be effective in those having small RVF with minimal symptoms (e.g., only passage of flatus but not feces per vagina) and no pelvic irradiation. This approach includes bowel rest and total parenteral nutrition. However, it could take up to 2 mo before the fistula heals spontaneously[14-16]. Notably, the diameter of the RVF with spontaneous healing reported in the literature was not larger than 1 cm. Some investigators have suggested giving concentrated coagulation factor XIII intravenously for 5 d during nonoperative treatment to promote fistula healing because this coagulation factor is decreased significantly in the early postoperative period and may interfere with wound healing[14,17]. A large survey of active members of the American Society of Colon and Rectal Surgeons in the 1990s identified a total of 57 RVF after low anterior resection, and 14 of them were managed conservatively. Spontaneous closure was reported in 10 of the cases treated nonoperatively, thus accounting for a 70% success rate[4].
It is worth noting that there is no study evaluating the benefit of hyperbaric oxygen therapy for RVF after low anterior resection in which the fistulas could display severe reactive tissue inflammation and ischemia, especially in individuals with pelvic sepsis and pelvic irradiation. So far, only a case series from Dohgomori et al[18] demons
Many patients with low colorectal anastomosis had prophylactic diverting stoma so that RVF in such patients could be detected (clinically, radiologically or endoscopically) with a later onset after surgery than that in individuals without protective stoma. In the latter group, fecal diversion alone has resulted in the closure of fistula in 25%-100% of reported cases[4,7,9,20]. Half of the RVF were reported to heal within 6 mo after the creation of a diverting stoma[10]. Large fistula and the presence of pelvis sepsis are two important predictors for the failure of fecal diversion alone[10]. Therefore, after fecal diversion is performed, concomitant or subsequent repair of the fistula is suggested in patients with these two risk factors.
Endoluminal treatment has been proposed as a minimally invasive approach to RVF after low anterior resection with or without diverting stoma. Lamazza et al[21] showed a satisfactory result of covered self-expandable metallic stent placement over the fistula in 10 patients with neoadjuvant radiotherapy (3 with diverting stoma). At an average follow-up of 2 years, the RVF healed in 8 cases and the others had a decrease fistula, which allowed a successful flap transposition. It appeared that diverting stoma plus endoscopic placement of self-expandable metal stents had a better rate of fistula closure than fecal diversion alone[20]. However, some of the patients receiving the endoscopic placement of self-expandable metal stents experienced stent dislodgement or severe tenesmus requiring stent removal. Other endoluminal interventions for treating RVF after low anterior resection reported in the literature include fibrin glue application[22], fistula coverage with polyglycolic acid sheet[17], endoluminal clipping[23,24], and transanal endoscopic suturing[25].
A perineal procedure is appropriate for low to middle RVF via transanal, transvaginal, transperineal or combined approaches after the resolution of pelvic inflammation or infection. Patients usually undergo staged operations, starting from diverting stoma and followed by local tissue repair. Endorectal advancement flap (partial or full thickness) appears to be a simple method for treating RVF after low anterior resection, especially small and low-lying fistula. Many investigators have reported a 30%-60% success rate of fistula closure after primary repair with mucosal advancement flap[4,9], but the rate of healing has declined with the number of previous local repairs[26]. Well-vascularized tissue interposition via a perineal approach may be beneficial to patients with poor tissue quality surrounding the fistula or those with recurrent RVF. Viable options of tissue interposition via this approach include Martius labial fat pad, levator ani muscle, pedicled muscle flap (gracilis or gluteal muscles), and biologic mesh.
Abdominal procedure via an open or laparoscopic approach is suitable for high RVF and RVF with colorectal anastomotic stricture. If the quality of tissues surrounding the fistula is acceptable (i.e., minimal fibrosis, good blood supply, free from infection and no evidence of cancer recurrence), the fistula can be excised. Then, a tension-free multilayered closure of the rectum and the vagina can be performed with an interposition of well-vascularized omental tissue.
With the possibility of multiple adhesions and difficult pelvic dissection, a resection of colorectal anastomosis including the RVF and redo colorectal (or coloanal) anastomosis is reserved for recurrent or refractory RVF, radiation-related RVF and RVF associated with the stricture of colorectal anastomosis. Although re-resection and redo anastomosis might be more aggressive than other abdominal or perineal procedures, it appears to have the highest success rate of fistula healing (approximately 90%), especially in the presence of diverting stoma[9]. Functional outcomes were reportedly satisfactory (no or minimal anterior resection syndrome) in about 80% of successful cases[27].
Transanal colonic pull-through with delayed coloanal anastomosis is an alternative procedure, or salvage operation, for standard re-resection and redo anastomosis. This two-step operation, also known as the Turnbull-Cutait abdominoanal procedure[28], was proposed for managing the ‘most difficult or complex’ RVF after rectal cancer surgery[2]. In the first stage, completion proctectomy including previous colorectal anastomosis and the fistula were performed. Then, the descending colon, the splenic flexure and the transverse colon were fully mobilized until the colon was pulled through the anal canal leaving a 5 cm colonic stump externally. Anal mucosa above the dentate line may be removed. On postoperative day 5-7, the second stage was performed by resecting distal colonic stump and performing hand-sewn coloanal anastomosis at the dentate line. This procedure could ensure that the newly forming coloanal anastomosis lies below the fistula site with a minimal chance of anastomotic failure. Recently, Maggiori et al[27] reported a 79% success rate of transanal colonic pull-through with delayed coloanal anastomosis for patients with chronic anastomosis leakage after rectal resection including RVF formation.
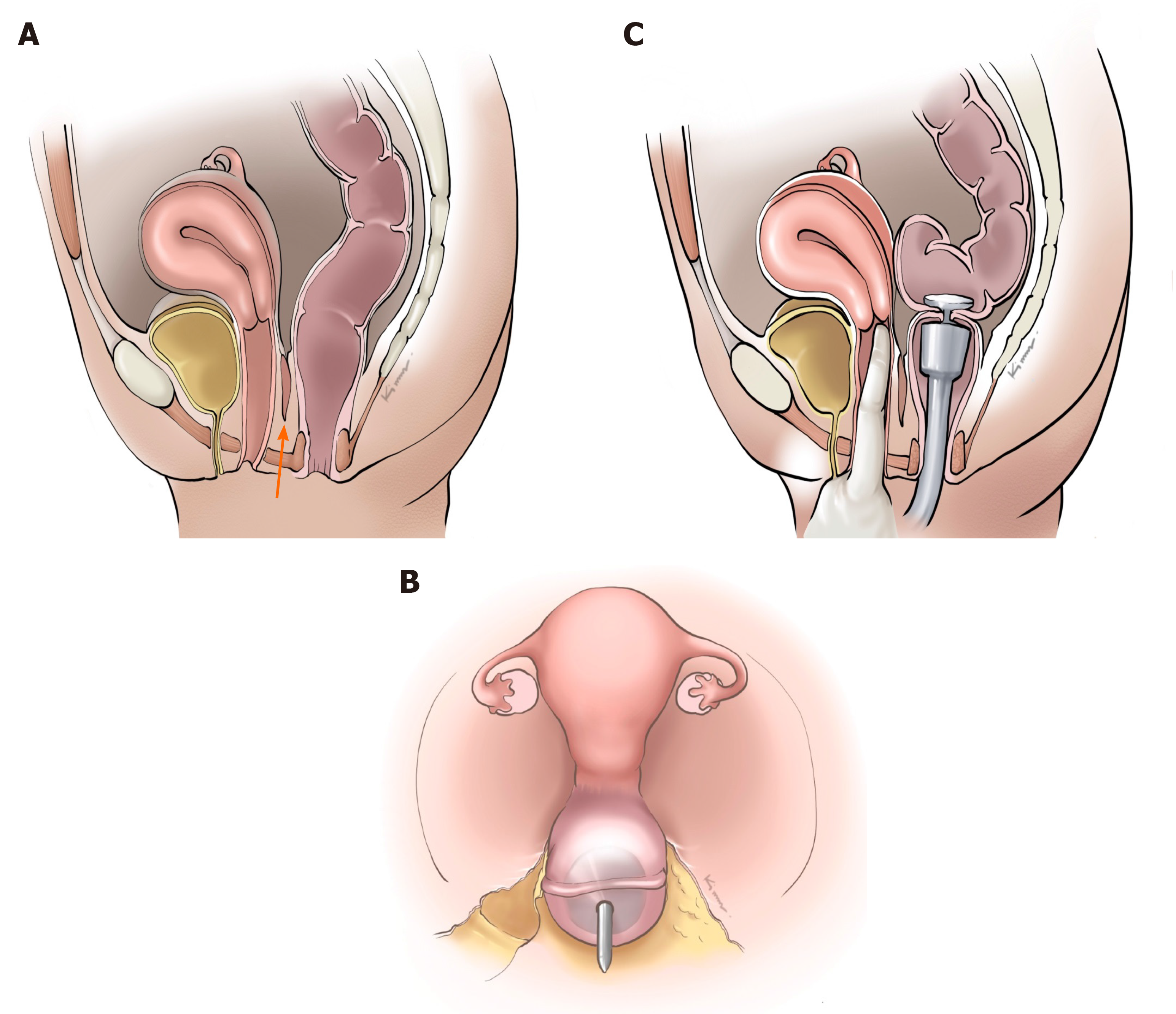
Because RVF after low anterior resection is usually caused by inadvertent incorporation of the posterior wall of the vagina into a colorectal anastomosis, the adequate dissection of the rectum from the vagina and direct vision of the dissected posterior vaginal wall from a rectal transection line and a colorectal anastomosis as well as digital examination of the vagina before firing a circular staple are essential steps before the creation of the anastomosis (Figure 1). Notably, in a stapled colorectal anastomosis, it is advisable that a stapler pin should pass through the rectal stump posterior to the rectal transection line, thereby allowing more space between the vagina and the colorectal anastomosis. To a lesser extent, RVF after rectal cancer surgery low anterior resection is secondary to colorectal anastomotic leakage[5] in which a pelvic collection is drained through vaginal wall injuries missed in the index operation or through an unsecured vaginal stump during concomitant hysterectomy. In this case, an intraoperative air leak test through the vagina may help to detect the missed injury to the vagina or unsecured vaginal stump.
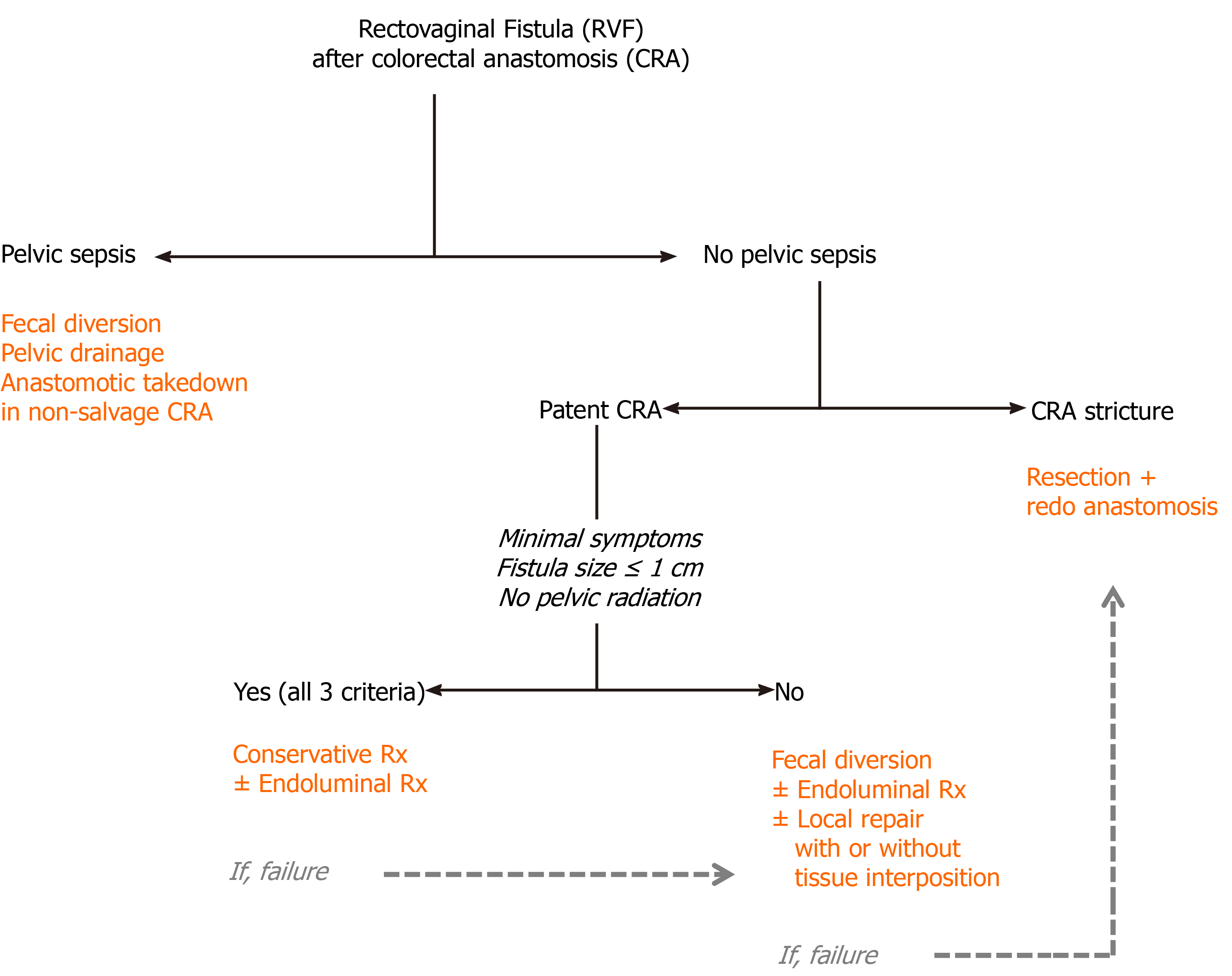
The decision to manage RVF after low anterior resection for rectal malignancy could primarily be made according to the presence of pelvic sepsis and diverting stoma, quality of colorectal anastomosis and surrounding tissue, size and location of the fistula, time to occurrence and symptoms of the patients. However, other host factors (e.g., age, physical condition, comorbidity, and continence status), tumor factors (e.g., tumor clearance, cancer staging, and requirement of adjuvant therapy), and previous surgical corrections of the fistula could significantly impact the decision-making process. Based on the available literature, we have proposed a decision-making algorithm for the management of RVF after low anterior resection for rectal malignancy in Figure 2.
RVF is a challenging but potentially preventable complication after rectal cancer surgery. With the limited availability of clinical data and management strategies in the literature, we comprehensively summarize its incidence, risk factors, clinical evaluation, and treatment options in this review. We also propose a decision-making algorithm for RVF after low anterior resection for rectal malignancy, which could be rational and applicable to most medically-fit patients with such a condition.
The authors would like to thank Dr. Hokierti C for providing medical illustrations to this article.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chakrabarti S, Yang TY S-Editor: Liu M L-Editor: A P-Editor: Xing YX
| 1. | Lohsiriwat V, Arsapanom D, Prapasrivorakul S, Iramaneerat C, Riansuwan W, Boonnuch W, Lohsiriwat D. A 20-year experience of rectovaginal fistula management in a tertiary university hospital in Thailand. J Med Assoc Thai. 2017;100 Suppl:S19-S25. |
| 2. | Corte H, Maggiori L, Treton X, Lefevre JH, Ferron M, Panis Y. Rectovaginal Fistula: What Is the Optimal Strategy? Ann Surg. 2015;262:855-60; discussion 860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Antonsen HK, Kronborg O. Early complications after low anterior resection for rectal cancer using the EEA stapling device. A prospective trial. Dis Colon Rectum. 1987;30:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Rex JC Jr, Khubchandani IT. Rectovaginal fistula: complication of low anterior resection. Dis Colon Rectum. 1992;35:354-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Matthiessen P, Hansson L, Sjödahl R, Rutegård J. Anastomotic-vaginal fistula (AVF) after anterior resection of the rectum for cancer--occurrence and risk factors. Colorectal Dis. 2010;12:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 6. | Watanabe J, Ota M, Kawaguchi D, Shima H, Kaida S, Osada S, Kamimukai N, Kamiya N, Ishibe A, Watanabe K, Matsuyama R, Akiyama H, Ichikawa Y, Oba M, Endo I. Incidence and risk factors for rectovaginal fistula after low anterior resection for rectal cancer. Int J Colorectal Dis. 2015;30:1659-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Zheng H, Guo T, Wu Y, Li C, Cai S, Liu F, Xu Y. Rectovaginal fistula after low anterior resection in Chinese patients with colorectal cancer. Oncotarget. 2017;8:73123-73132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Huang MJ, Ye DX, Lin Y, Lu XR, Lin HM, Chi P, Huang Y. A nomogram for predicting rectovaginal fistula after low anterior resection for rectal cancer. Surg Today. 2020;50:1206-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Woo IT, Park JS, Choi GS, Park SY, Kim HJ, Lee HJ. Optimal strategies of rectovaginal fistula after rectal cancer surgery. Ann Surg Treat Res. 2019;97:142-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Barugola G, Bertocchi E, Leonardi A, Almoudaris AM, Ruffo G. Post surgical rectovaginal fistula: who really benefits from stoma diversion? Updates Surg. 2021;73:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Kim CW, Kim JH, Yu CS, Shin US, Park JS, Jung KY, Kim TW, Yoon SN, Lim SB, Kim JC. Complications after sphincter-saving resection in rectal cancer patients according to whether chemoradiotherapy is performed before or after surgery. Int J Radiat Oncol Biol Phys. 2010;78:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Rivadeneira DE, Ruffo B, Amrani S, Salinas C. Rectovaginal fistulas: current surgical management. Clin Colon Rectal Surg. 2007;20:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Komori K, Kinoshita T, Oshiro T, Ouchi A, Ito S, Abe T, Senda Y, Misawa K, Ito Y, Natsume S, Higaki E, Okuno M, Hosoi T, Nagao T, Kunitomo A, Oki S, Takano J, Suenaga Y, Maeda S, Dei H, Numata Y, Shimizu Y. Surgical Strategy for Rectovaginal Fistula After Colorectal Anastomosis at a High-volume Cancer Center According to Image Type and Colonoscopy Findings. Anticancer Res. 2019;39:5097-5103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Emoto S, Nozawa H, Yoneyama S, Murono K, Kaneko M, Sasaki K, Otani K, Nishikawa T, Tanaka T, Hata K, Kiyomatsu T, Kawai K, Omata K, Noguchi T, Masuda K, Sakata H, Tajima Y, Hidemura A, Suzuki H, Ishimaru M, Watanabe T. Rectovaginal fistula after low anterior resection for rectal cancer healed by nonoperative treatment. Int J Surg Case Rep. 2017;41:121-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Tsutsumi N, Yoshida Y, Maehara Y, Kohnoe S. Rectovaginal fistula following double-stapling anastomosis in low anterior resection for rectal cancer. Hepatogastroenterology. 2007;54:1682-1683. [PubMed] |
| 16. | Kondo W, Ribeiro R, Trippia CH, Zomer MT. Spontaneous healing of a rectovaginal fistula developing after laparoscopic segmental bowel resection for intestinal deep infiltrating endometriosis. Case Rep Obstet Gynecol. 2013;2013:837903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Hiraki M, Tanaka T, Kanai T, Shimamura T, Ikeda O, Yasunaga M, Ogata S, Kitahara K. The treatment for refractory rectovaginal fistula after low anterior resection with estriol, polyglycolic acid sheets and primary closure: A case report. Int J Surg Case Rep. 2020;75:483-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Dohgomori H, Arikawa K, Nobori M, Tonari M. Hyperbaric oxygenation for rectovaginal fistula: a report of two cases. J Obstet Gynaecol Res. 1999;25:343-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | García-Arranz M, Herreros MD, González-Gómez C, de la Quintana P, Guadalajara H, Georgiev-Hristov T, Trébol J, Garcia-Olmo D. Treatment of Crohn's-Related Rectovaginal Fistula With Allogeneic Expanded-Adipose Derived Stem Cells: A Phase I-IIa Clinical Trial. Stem Cells Transl Med. 2016;5:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Lamazza A, Fiori E, Sterpetti AV, Schillaci A, De Cesare A, Lezoche E. Endoscopic placement of self-expandable metallic stents for rectovaginal fistula after colorectal resection: a comparison with proximal diverting ileostomy alone. Surg Endosc. 2016;30:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Lamazza A, Fiori E, Schillaci A, Sterpetti AV, Lezoche E. Treatment of rectovaginal fistula after colorectal resection with endoscopic stenting: long-term results. Colorectal Dis. 2015;17:356-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Shibata, Mizuguchi, Takeda, Miyashita. Successful closure of a rectovaginal fistula following low anterior resection by endoscopic fibrin glue application. Colorectal Dis. 1999;1:42-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Artifon EL, Silva GL, Furuya CK, Buch M, Bonini L. Endoscopic stent combined with endovaginal clipping for resolution of rectovaginal fistula after colorectal anastomotic dehiscence. Gastrointest Endosc. 2014;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Tong Y, Trilling B, Sage PY, Girard E, Faucheron JL. Short-term outcomes of the over-the-scope clip proctology system for rectovaginal fistula repair: a prospective study. Tech Coloproctol. 2019;23:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | van Vledder MG, Doornebosch PG, de Graaf EJ. Transanal endoscopic surgery for complications of prior rectal surgery. Surg Endosc. 2016;30:5356-5363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Lowry AC, Thorson AG, Rothenberger DA, Goldberg SM. Repair of simple rectovaginal fistulas. Influence of previous repairs. Dis Colon Rectum. 1988;31:676-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 114] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Maggiori L, Blanche J, Harnoy Y, Ferron M, Panis Y. Redo-surgery by transanal colonic pull-through for failed anastomosis associated with chronic pelvic sepsis or rectovaginal fistula. Int J Colorectal Dis. 2015;30:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Cutait DE, Cutait R, Ioshimoto M, Hyppólito da Silva J, Manzione A. Abdominoperineal endoanal pull-through resection. A comparative study between immediate and delayed colorectal anastomosis. Dis Colon Rectum. 1985;28:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |