Published online Jul 27, 2021. doi: 10.4240/wjgs.v13.i7.702
Peer-review started: February 8, 2021
First decision: March 30, 2021
Revised: April 12, 2021
Accepted: July 2, 2021
Article in press: July 2, 2021
Published online: July 27, 2021
Processing time: 164 Days and 14.7 Hours
Coronavirus disease 2019 (COVID-19), an infectious condition caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread worldwide since its first description in Wuhan in December 2019. Even though respiratory manifestations are the most prevalent and responsible for disease morbidity and mortality, extrapulmonary involvement has progressively gained relevance. In particular, gastrointestinal (GI) signs and symptoms, reported in up to two-thirds of patients with COVID-19, might represent the first and, in some cases, the only disease presentation. Their presence has been associated in some studies with an increased risk of a severe disease course. Proposed pathogenic mechanisms explaining GI tract involvement are either direct viral access to intestinal cells via angiotensin-converting enzyme 2 or indirect damage of the intestinal wall through mesenteric ischemia induced by the hypercoagulable state associated with COVID-19 infection. Although not typical of SARS-CoV-2 infection, several small bowel manifestations have been described in infected patients who underwent any form of abdominal imaging. The radiological findings were mainly reported in patients with abdominal symptoms, among which abdominal pain was the most common.
To discuss small bowel radiological manifestations of SARS-CoV-2 infection in abdominal imaging studies.
Bibliographical searches were performed in PubMed, using the following keywords: “COVID-19” AND “imaging” AND “gastrointestinal” OR “abdominal” OR “small bowel”.
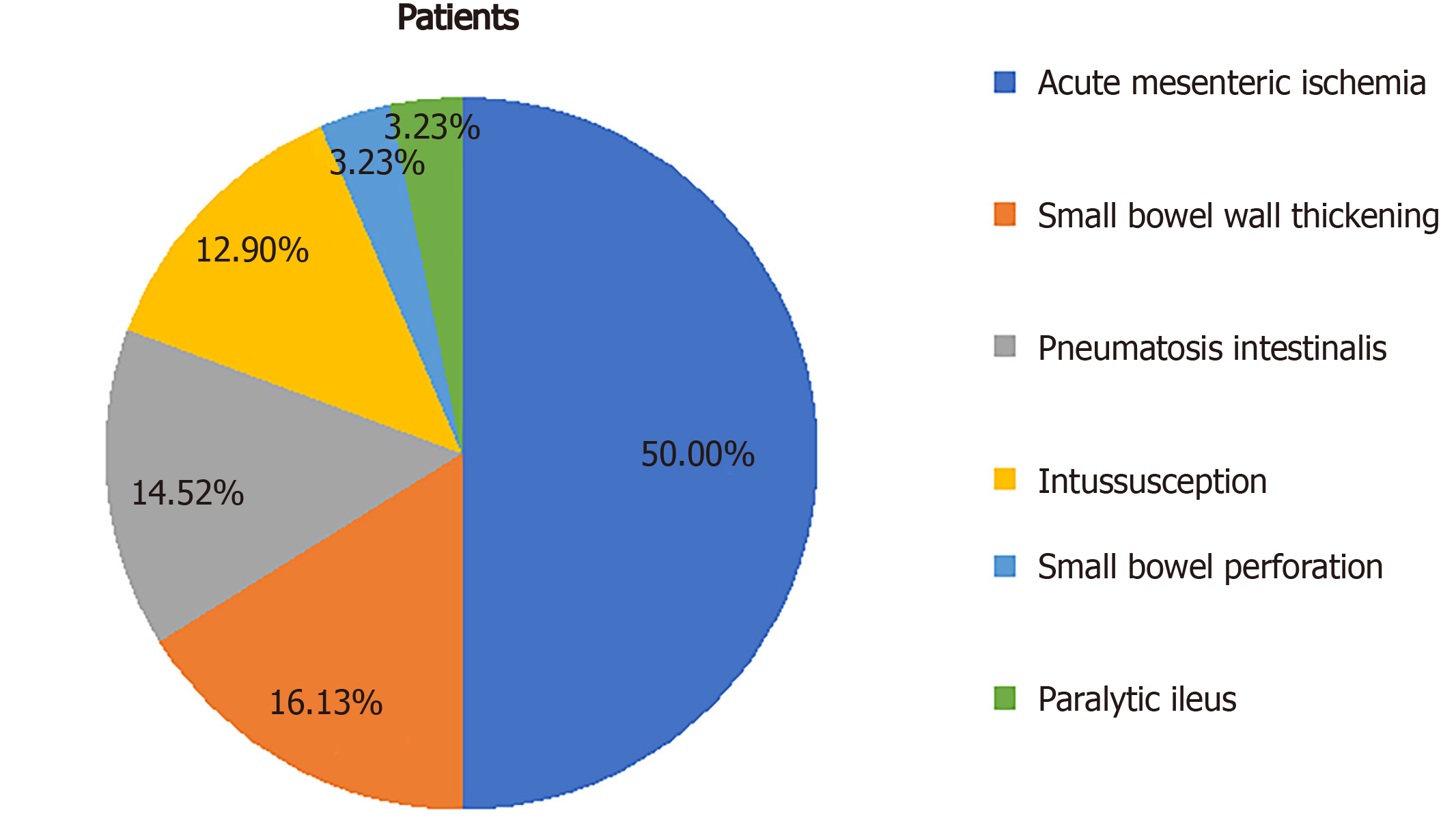
Of 62 patients with described radiologic small bowel alterations, mesenteric ischemia was diagnosed in 31 cases (50%), small bowel wall thickening in 10 cases (16%), pneumatosis in nine cases (15%), intussusception in eight cases (13%), pneumoperitoneum in two cases (3%) and paralytic ileus in two cases (3%). We also reported mesenteric adipose tissue hypertrophy and lymph nodes enlargement in a young woman.
So far it is difficult to establish whether these manifestations are the direct consequence of SARS-CoV-2 infection or collateral findings in infected patients, but their recognition would be pivotal to set a closer follow-up and to reduce missed diagnoses.
Core Tip: Gastrointestinal manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have been increasingly reported and corresponding abdominal imaging findings have been recognized. The present review includes case reports, case series, and retrospective studies discussing small bowel radiological manifestations of SARS-CoV-2 infection in abdominal imaging studies. Out of 62 patients, the most commonly reported finding was mesenteric ischemia, with a prevalence of 50%. Other less frequent features were small bowel wall thickening (16%), pneumatosis intestinalis (15%), intussusception (13%), pneumoperitoneum (3%), and paralytic ileus (3%). We also report a patient with mesenteric adipose tissue hypertrophy and lymph node enlargement.
- Citation: Pirola L, Palermo A, Mulinacci G, Ratti L, Fichera M, Invernizzi P, Viganò C, Massironi S. Acute mesenteric ischemia and small bowel imaging findings in COVID-19: A comprehensive review of the literature. World J Gastrointest Surg 2021; 13(7): 702-716
- URL: https://www.wjgnet.com/1948-9366/full/v13/i7/702.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i7.702
Coronavirus disease 2019 (COVID-19) is an infectious condition caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), firstly isolated in December 2019 in Wuhan, China[1]. As of February 8, 2021, there have been more than one hundred million confirmed cases of COVID-19, with more than two million deaths[2]. The most common COVID-19 symptoms include fever (85.6%), cough (65.7%), fatigue (42.4%) and dyspnea (21.4%)[3]. Although respiratory tract manifestations are the most prevalent and responsible for disease morbidity and mortality, extrapulmonary involvement has progressively gained relevance. In particular, gastrointestinal (GI) symptoms are described in a significant proportion of infected patients, being reported in up to two-thirds of patients with COVID-19[4]. Several studies suggest that GI symptoms might not only be the initial presentation of SARS-CoV-2 infection but are also the only manifestation reported during disease course in about 10% of cases[5]. Patients presenting with GI manifestations alone have a delayed diagnosis[6]. Furthermore, in some studies the presence of GI symptoms has been associated with an increased risk of severe clinical course, but the real impact of GI involvement on disease outcome is still a matter of debate[6,7]. The most commonly reported GI symptoms were loss of appetite (21%), diarrhea (9%), nausea or vomiting (6%), and abdominal pain (3%)[6]. It has been widely described and accepted in the literature that SARS-CoV-2 exploits angiotensin-converting enzyme 2 (ACE2) to gain access to single cells[8]. Hence, it is possible to assume that cells with higher expression of ACE2, such as enterocytes in the GI tract[9], are more susceptible to infection[10]. Several reports have evaluated the pulmonary radiological findings in COVID-19, which facilitate disease recognition and add to the understanding of its pathogenic mechanisms in the lung[11]. However, despite the broad spectrum and the high prevalence of GI symptoms, only a few studies have assessed the abdominal radiological findings in COVID-19 patients, and only a few of those have focused on small bowel alterations. Furthermore, the pathogenic mechanisms behind the described findings remain unclear, highlighting the importance of collecting further evidence on the topic. In this review, we analyzed the radiological small bowel findings in patients with COVID-19 and evaluated their impact on the whole clinical picture of the disease.
Bibliographical searches were performed in PubMed, using the keywords “COVID-19” AND “imaging” AND “gastrointestinal” OR “abdominal” OR “small bowel”. PubMed was used to search for all relevant articles published since the first description of SARS-CoV-2 infection, which occurred in December 2019, until the end of January 2021. Reference lists from studies selected from the electronic search were manually searched to identify further relevant reports. Reference lists from all available review articles, primary studies, and proceedings of major meetings were also considered. Articles published as abstracts were included. Only English-language papers were included.
Thirty-nine articles describing small bowel imaging findings in patients with COVID-19 were identified. The review included 28 case reports, six case series, and five retrospective studies discussing radiological manifestations of SARS-CoV-2 infection in abdominal imaging studies. Several small bowel radiological manifestations have been described in infected patients, with a prevalence ranging from 3% to 21%[12,13]. They were mainly reported in patients with abdominal symptoms, among which abdominal pain was the most common. Of the 62 patients with small bowel radiological findings, acute mesenteric ischemia (AMI) was the most prevalent condition, diagnosed in 31 cases (50%). The characteristics of COVID-19 patients with AMI are shown in Table 1. Other radiological findings (Figure 1) were small bowel wall thickening in 10 cases (16%), pneumatosis in nine (15%), intussusception in eight (13%), pneumoperitoneum in two (3%), and paralytic ileus in two (3%). A summary of those findings is shown in Table 2. In addition, we reported mesenteric adipose tissue hypertrophy and lymph node enlargement in a young woman.
| Ref. | Patients, n | Age in yr | Gender | GI symptoms | Respiratory symptoms | Relevant laboratory test | Imaging modality | Radiological findings | Treatment | Outcome |
| de Barry et al[17] | 1 | 79 | F | Abdominal pain, diarrhea | Yes | WBC 12.6 × 109/L; CRP 125 mg/L | CT | Mesenteric ischemia; upper mesenteric vein and portal thrombosis; upper mesenteric artery and jejunal artery thrombosis | Laparotomy with segmental resection; thrombolysis and thrombectomy | Dead 4 d after surgery |
| Beccara et al[18] | 1 | 52 | M | Abdominal pain, diarrhea, vomiting | Yes | WBC 30 × 109/L; CRP 222 mg/L | CT | Mesenteric ischemia; upper mesenteric artery thrombosis | Laparotomy with segmental resection; anticoagulant; antiaggregant | NR |
| Ofosu et al[19] | 1 | 55 | M | NR | Yes | WBC 9.5 × 109/L; CRP 3 mg/L; D-dimer 440 ng/mL | CT | Mesenteric ischemia; portal vein thrombosis | Anticoagulant | Discharged |
| La Mura et al[20] | 1 | 72 | M | NR | No | WBC 19.76 × 109/L (n 81%); CRP 172.3 mg/L | CT | Mesenteric ischemia; portal vein thrombosis | Anticoagulant | Discharged |
| Del Hoyo et al[21] | 1 | 61 | F | Abdominal pain, vomiting | No | CRP 9.43 mg/L; D-dimer 43.998 ng/mL | CT | Mesenteric ischemia; spleen-portal vein and hepatic vein thrombosis | Anticoagulant | Dead |
| Karna et al[22] | 1 | 61 | F | Abdominal pain, distension | Yes | NR | CT | Mesenteric ischemia; upper mesenteric artery thrombosis | Laparotomy with segmental resection; anticoagulant; antiaggregant | Dead 36 h after surgery |
| Cheung et al[23] | 1 | 55 | M | Abdominal pain, diarrhea, nausea | NR | NR | CT | Mesenteric ischemia; upper mesenteric artery thrombosis | NR | NR |
| de Roquetaillade et al[24] | 1 | NR | NR | NR | NR | NR | CT | Mesenteric ischemia | NR | NR |
| Vartanoglu et al[25] | 5 | NR | M | NR | Yes | WBC 8.67 × 109/L (mean); CRP 970 mg/L (mean); D-dimer 447 ng/mL (mean); Fib 6245 mg/dL (mean) | NR | Mesenteric ischemia | NR | 1 patient dead |
| Fraissé et al[26] | 3 | NR | NR | NR | NR | NR | NR | Mesenteric ischemia | NR | NR |
| Ignat et al[27] | 1 | 28 | F | Abdominal pain, vomiting | No | NR | CT | Mesenteric ischemia; upper mesenteric vein and portal vein thrombosis | Laparotomy with segmental resection | NR |
| Pang et al[28] | 1 | 30 | M | Abdominal pain, vomiting | No | D-dimer 20000 ng/mL; Fib 465 mg/dL | CT | Mesenteric ischemia; upper mesenteric vein thrombosis | Laparotomy with segmental resection; anticoagulant | Discharged |
| Bianco et al[29] | 1 | 59 | M | Abdominal pain, nausea | Yes | NR | CT | Mesenteric ischemia; peritoneal free fluid | Laparotomy with segmental resection | Dead 4 d after surgery |
| Norsa et al[30] | 3 | 79 | F | Abdominal pain | NR | D-dimer 8 × ULN | CT | Mesenteric ischemia | NR | NR |
| 62 | M | Abdominal pain, vomiting | NR | D-dimer 76 × ULN | CT | Mesenteric ischemia; upper mesenteric vein thrombosis | NR | Dead | ||
| 83 | F | Abdominal pain | NR | D-dimer 3 × ULN | CT | Mesenteric ischemia | NR | Dead | ||
| Collange et al[31] | 1 | 56 | M | NR | NR | D-dimer 2260 ng/mL; Fib 113 mg/dL | CT | Mesenteric ischemia; mesenteric intravenous air | Laparotomy with segmental resection; anticoagulant | NR |
| Vulliamy et al[32] | 1 | 75 | M | Abdominal pain, vomiting | Yes | WBC 18 × 109/L; D-dimer 32000 ng/mL | CT | Mesenteric ischemia; embolic occlusion of upper mesenteric artery | Laparotomy with segmental resection; thrombectomy | NR |
| Rodriguez-Nakamura et al[33] | 2 | 45 | M | Abdominal pain | Yes | WBC 16.6 × 109/L (n 86%); CRP 367 mg/L; D-dimer 1450 ng/mL; Fib 579 mg/dL | CT | Mesenteric ischemia; upper mesenteric thrombosis | Laparotomy with segmental resection; anticoagulant | Discharged |
| 42 | F | Abdominal pain | Yes | WBC 18.8 × 109/L (n 83.5%); CRP 239 mg/L; D-dimer 14.407 ng/mL; Fib 338 mg/dL | CT | Mesenteric ischemia; mesenteric veins and portal vein thrombosis | Laparotomy with segmental resection | Dead 48 h after surgery | ||
| E English et al[34] | 1 | 40 | M | Abdominal distension | Yes | Fib 548 mg/dL | CT | Mesenteric ischemia | Laparotomy and laparoscopy with segmental resection | Dead 48 h after surgery |
| Helms et al[35] | 1 | NR | NR | NR | NR | NR | CT | Mesenteric ischemia | NR | NR |
| Mitchell et al[36] | 1 | 69 | M | Abdominal pain, constipation, eructation | NR | NR | CT | Mesenteric ischemia; upper mesenteric artery thrombosis | Laparotomy with segmental resection; thrombolysis and thrombectomy | Discharged |
| Azouz et al[37] | 1 | 56 | M | Abdominal pain, vomiting | No | NR | CT | Mesenteric ischemia; upper mesenteric thrombosis | Laparotomy with segmental resection; anticoagulant | NR |
| Franco-Moreno et al[38] | 1 | 27 | M | Abdominal pain | Yes | WBC 18 × 109/L (n 85%); CRP 245 mg/L; D-dimer 9530 ng/mL; Fib > 500 mg/dL | CT | Mesenteric ischemia; portal vein thrombosis | Anticoagulant | Discharged |
| Ref. | Patients, n | Age in yr | Gender | GI symptoms | Imaging modality | Radiological findings | Other relevant information |
| Hellinger et al[40] | 1 | 64 | NR | Abdominal pain, nausea, vomiting, diarrhea, fever | CT | Small bowel thickening and hyperemia | - |
| Bhayana et al[12] | 5 | 18-99 | NR | Abdominal pain, nausea, vomiting, diarrhea, fever | CT | Small bowel thickening | 100% of patients needed ICU |
| Goldberg-Stein et al[13] | 2 | NR | NR | NR | CT | Small bowel thickening | - |
| Periyakaruppan et al[41] | 1 | NR | M | Abdominal pain, diarrhea | CT | Small bowel thickening | Complete recovery after 2 d from I.V. immunoglobulin infusion |
| Guo et al[42] | 1 | 29 | M | Diarrhea, fever | CT | Small bowel thickening | - |
| Bhayana et al[12] | 4 | 18-99 | NR | Abdominal pain, nausea, vomiting, diarrhea, fever | CT | Pneumatosis intestinalis | 20% of patients needed ICU.100% of patients underwent laparotomy |
| Tirumani et al[48] | 1 | NR | NR | NR | CT | Pneumatosis intestinalis; portal venous gas | - |
| Kielty et al[49] | 1 | 47 | M | Vomiting | CT | Pneumatosis intestinalis; peritoneal free fluid | The patient needed ICU. No need of surgery. Discharged |
| Di Grezia et al[50] | 3 | NR | NR | NR | CT | Pneumatosis intestinalis | Needed surgical intervention with open abdomen and negative pressure therapy (no need of intestinal resection) |
| Makrinioti et al[54] | 2 | 10-mo | F | Crying, vomiting, red currant jelly-like stool | US | Ileocolic intussusception | Dead |
| 10-mo | F | Bilious vomiting, red currant jelly-like stool | NR | Intussusception | Recovered after surgery | ||
| Rajalakshmi et al[55] | 1 | 8-mo | M | Vomiting, blood-stained stools | US | Intussusception | Recovered after surgery |
| Moazzam et al[56] | 1 | 4-mo | M | Abdominal pain | US | Intussusception with "doughnut sign" | Recovered after surgery |
| Bazuaye-Ekwuyasi et al[57] | 1 | 9-mo | M | Abdominal pain, vomiting, decreased oral intake, and blood-streaked stool | RX | Intussusception with "target" sign | Recovered after surgery |
| US | |||||||
| Cai et al[58] | 1 | 10-mo | F | Vomiting, currant jelly-like stool | US | Intussusception | Dead |
| Martínez-Castaño et al[59] | 1 | 6-mo | M | Vomiting, abdominal cramps, currant jelly stools | US | Ileocolic intussusception | Discharged |
| Lu et al[60] | 1 | 10-mo | NR | NR | NR | Intussusception | Dead |
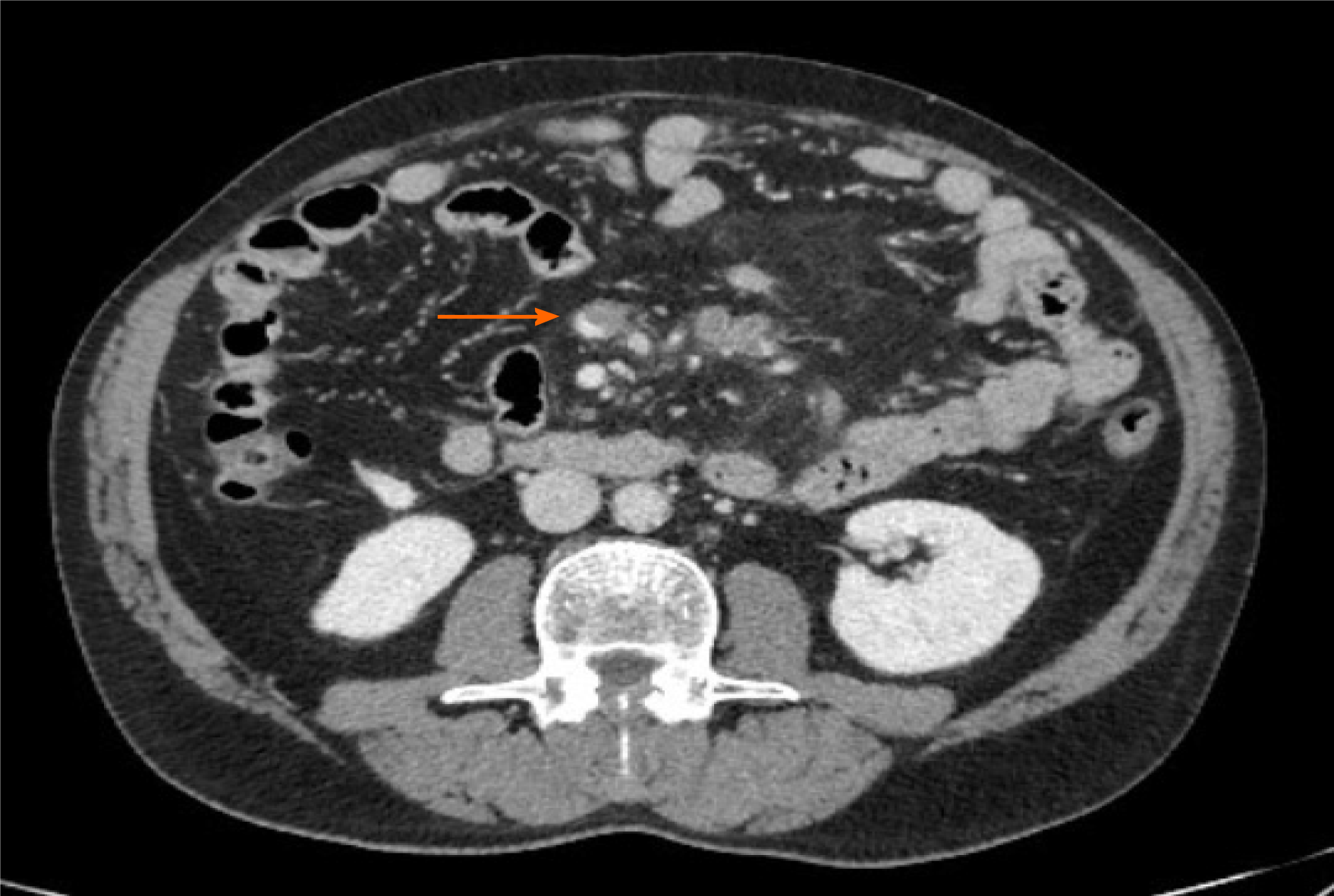
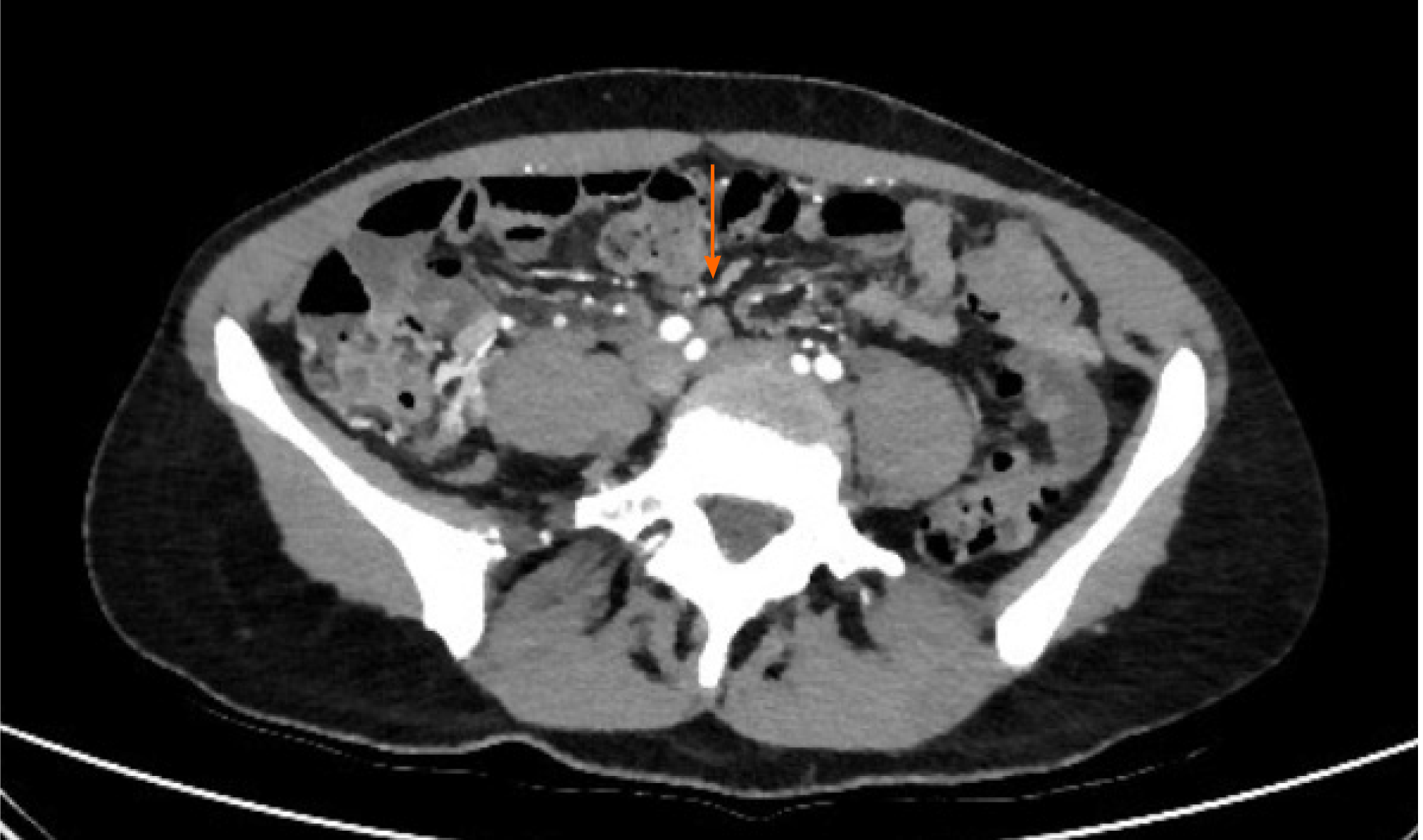
AMI is a pathological condition characterized by a sudden decline in blood flow through the mesenteric vessels, resulting in a discrepancy between the metabolic need of the visceral organs and actual oxygen delivery[14]. If untreated, it may lead to small bowel wall necrosis, with a mortality rate of up to 80%[15]. Among the causes of AMI, the most prevalent are acute mesenteric artery embolism, nonocclusive mesenteric ischemia[16], and acute mesenteric vein thrombosis. So far, 22 papers have been published describing mesenteric ischemia in 31 COVID-19 patients, including 17 case reports, two case series, and three retrospective studies[17-38]. The characteristics and radiological findings of COVID-19 patients with AMI are reported in Table 1. All but one patient underwent abdominal contrast-enhanced computed tomography (CT) scan after the onset of GI symptoms such as nausea, vomiting, and/or abdominal pain. Among the vascular findings, the most frequent were nine cases with thrombosis of the upper mesenteric artery or jejunal artery and nine cases of thrombosis of splanchnic veins, of which seven involved the portal vein, five involved the superior mesenteric vein (Figure 2), and one involved splenic and hepatic veins. Other findings described in association with AMI were the presence of liquid in the peritoneal cavity (two cases), bowel distension (one case), mesenteric intravenous air (one case), and splenic infarction (one case). From a pathological perspective, it is of interest that none of the patients had evidence of a systemic atherosclerotic disorder or other pathologic conditions that could possibly explain the findings. Treatment of AMI was reported in 17 cases. Thirteen (76%) were treated by segmental resection of small bowel, and anticoagulant therapy alone was administered in four cases. Of the nineteen patients with a reported outcome, nine (47%) died.
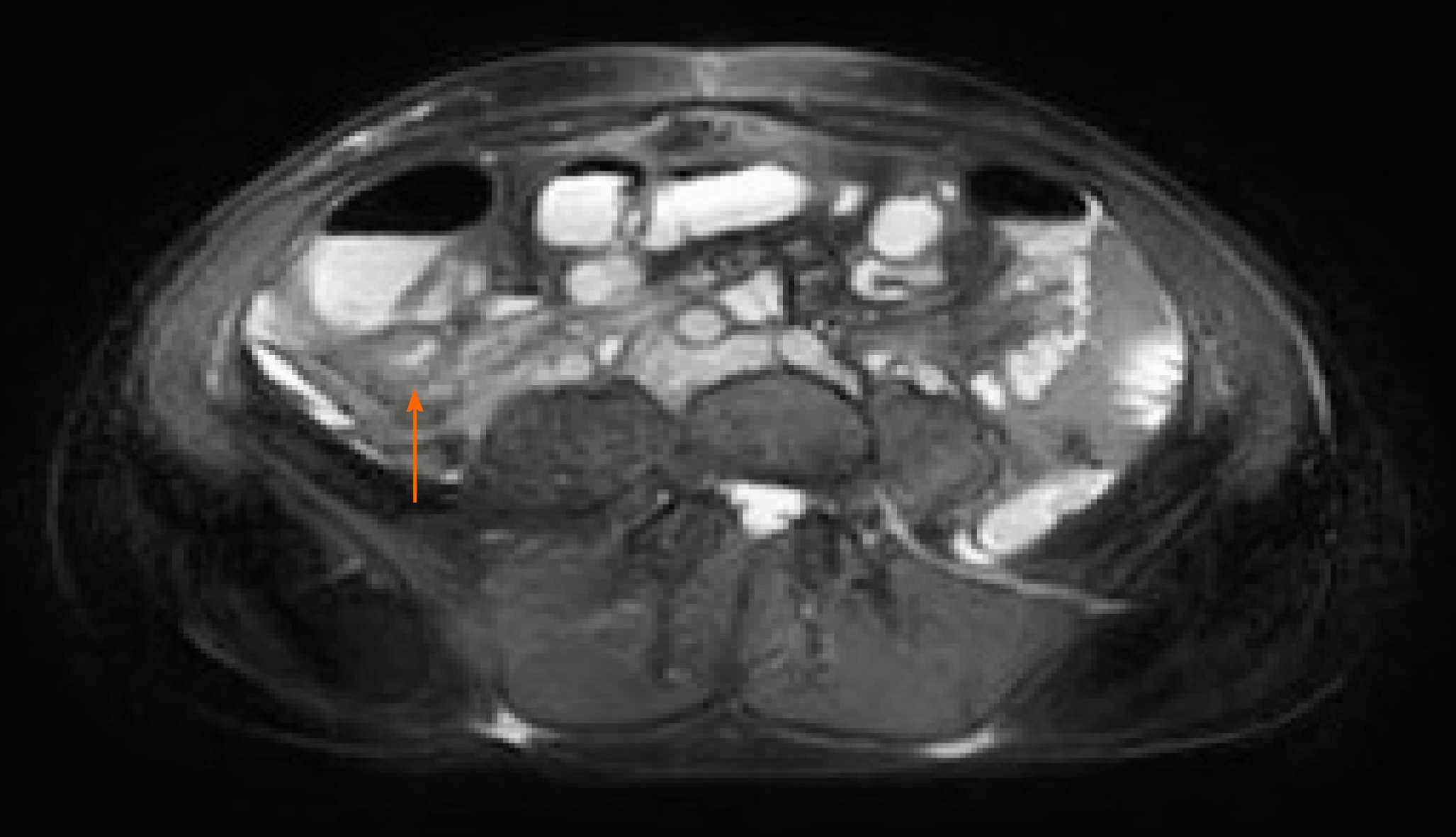
Thickening of the small bowel wall may occur secondary to neoplastic, inflammatory, infectious, or ischemic conditions[39]. It was first described by Hellinger et al[40] in a patient presenting at the emergency room with abdominal symptoms, and this condition has been subsequently reported in other COVID-19 patients (Figure 3). At the time of writing, we identified five papers, three case reports and two retrospective studies, describing ten patients with radiological evidence of small bowel wall thickening. In a study of 412 patients admitted to the hospital for SARS-CoV-2 infection, 42 underwent abdominal CT scans. In five of those patients (11.9%) small bowel thickening, reported as a single-wall thickness greater than 3 mm in distended loops or greater than 5 mm in collapsed loops[12], was described. In a report including 141 COVID-19 patients who underwent abdominopelvic CT within 14 d of diagnosis, GoldbergStein et al[13] reported a small bowel thickening prevalence of 2.5%. In addition, diffuse mural wall thickening of the ileum was reported in a single case study of a 11-year-old boy with SARS-CoV-2 infection, who presented to the emergency room with fever, diarrhea, and abdominal pain, without respiratory symptoms[41]. Lastly, Guo et al[42] described segmental wall thickening involving a segment of jejunum in a 29-year-old man presenting with diarrhea and fever. The cause of small bowel thickening in SARS-CoV-2 infected patients is still unclear. It may be a manifestation of local inflammation and edema secondary to direct or indirect viral damage of the bowel wall. Another hypothesis is that may be linked to hypercoagulability secondary to viral infection, which may promote formation of fibrin clots in the microcirculation, leading to ischemia and edema.
Pneumatosis intestinalis is a radiological sign reflecting the presence of gas within the intestinal wall, most commonly in the mucosa or submucosa. Pneumatosis can occur in any part of the digestive tract, and may even be accompanied by the presence of gas in the portal or mesenteric vein[43]. The clinical relevance of pneumatosis intestinalis varies widely and ranges from a benign to life-threatening condition depending on the underlying cause[44]. This pathologic finding has been previously described in association with viral infections, such as cytomegalovirus, adenovirus, rotavirus, and norovirus, and the proposed pathogenic mechanism was direct mucosal damage caused by viral activity[44-47].
So far, four papers, one case report, one case series, and two retrospective studies, have described pneumatosis intestinalis in nine patients with SARS-CoV-2 infection. Bhayana et al[12] described pneumatosis intestinalis of the small bowel in four of 42 COVID-19 patients (9.5%) with abdominal CT scans. The percentage reached 20% in patients admitted to the intensive care unit. All patients underwent laparotomic exploration. Two cases showed frank signs of bowel necrosis, with bowel resection performed in one. Fibrin clots were detected in arterioles adjacent to the necrotic mucosa in histological samples. In a retrospective study, Tirumani et al[48] described small bowel pneumatosis with portal venous gas in one of 73 SARS-CoV-2 infected patients with abdominal CT scans. Pneumatosis intestinalis of the small bowel was also reported in a small study of three cases and in a single-patient case report. Complete resolution was reported following conservative treatment in a patient with widespread pneumatosis affecting the jejunum, proximal ileum, and caecum, for which surgery was deemed to be associated with unacceptably high morbidity[49]. In the remaining three patients, an open abdomen with negative pressure therapy was successfully performed without the need of intestinal resection[50]. The rationale behind this approach lies in the capability of either increasing gastrointestinal arterial and venous blood flow through intra-abdominal pressure reduction and dampening intestinal cytokine release in the peritoneal cavity, which might also prevent the deterioration of lung function[12,51,52].
Intussusception is the invagination of a segment of the bowel within a more distal one[53]. It is the most common cause of bowel obstruction in infants, usually occurring between 4 and 10 mo of age[53]. On the contrary, intussusception in adults is a rare disease, accounting for less than 5% of bowel obstruction episodes[53]. In both children and adults, intussusception usually involves the ileum, with ileocecal valve invagination into the cecum being the most common localization. Furthermore, 90% of cases of intussusception in adults are secondary to a well-defined pathological condition, such as inflammatory bowel disease, postoperative tractions, Meckel’s diverticulum, benign and malignant lesions, metastatic neoplasms, or even an iatrogenic cause (e.g., intestinal tubes, jejunostomy feeding tubes, or gastric surgery)[53]. Conversely, in the pediatric age group, approximately 90% of cases are idiopathic. In the literature, viral infection was cited as a possible cause of intussusception in children[54]. Indeed, local immune activation and mesenteric adenitis may trigger enhanced peristaltic activity, consequently leading to the invagination of the proximal bowel segment into the distal one[54].
So far, eight cases of intussusception were reported in children between 4 and 10 mo of age with laboratory-confirmed SARS-CoV-2 infection in one case series, five case reports, and one retrospective study[54-60]. The diagnosis was made mainly by ultrasound (US) through the presence of typical findings of intussusception, such as the doughnut sign (i.e. concentric alternating hyperechoic and hypoechoic rings); as expected, in all cases except one in which the site of intussusception was not specified, the ileocolic tract was involved. Three patients died of complications. To date, it has not been possible to establish an association between SARS-CoV-2 infection and intussusception. Testing for viral pathogens, including SARS-CoV-2, may be considered in infants with symptoms consistent with intussusception or with typical radiological findings.
Pneumoperitoneum, an indirect sign of small bowel perforation, refers to the presence of free air in the abdominal cavity, often suspected through abdominal radiography and confirmed on CT scans[61]. It has been less frequently reported than the previously mentioned radiologic features. One case report and one retrospective study described small bowel perforations in two patients with SARS-CoV-2 infection without any other possible explanation other than viral infection. Both cases were then confirmed by laparoscopic exploration[12,62]. The clinical outcome was not specified in one study. In the other, despite prompt orotracheal-intubation and surgery, the patient developed refractory septic shock and died[12,62]. As for the previously described findings, the disease pathogenesis is unknown. However, it is likely that small bowel perforation occurred as a consequence of either viral action on the intestinal mucosa or as a consequence of ischemic small bowel necrosis.
Paralytic ileus is defined as a temporary functional cessation of propulsive contractions of the gastrointestinal tract, with subsequent upstream gut dilation and accumulation of secretions and gas within the lumen[63]. Diagnosis of paralytic ileus is established by the coexistence of a clinical suspect and the support of radiological imaging. Many conditions may cause paralytic ileus, the most common being abdominal or retroperitoneal surgery. Other causes include the use of opioids, intra-abdominal infections, bleeding, hypokalemia, and the absence of enteral nutrition[64]. In patients with SARS-CoV-2 infection, one case of paralytic ileus of the small bowel was reported in a retrospective study, and another, involving both the small and the large bowel was described in a case report[13,65].
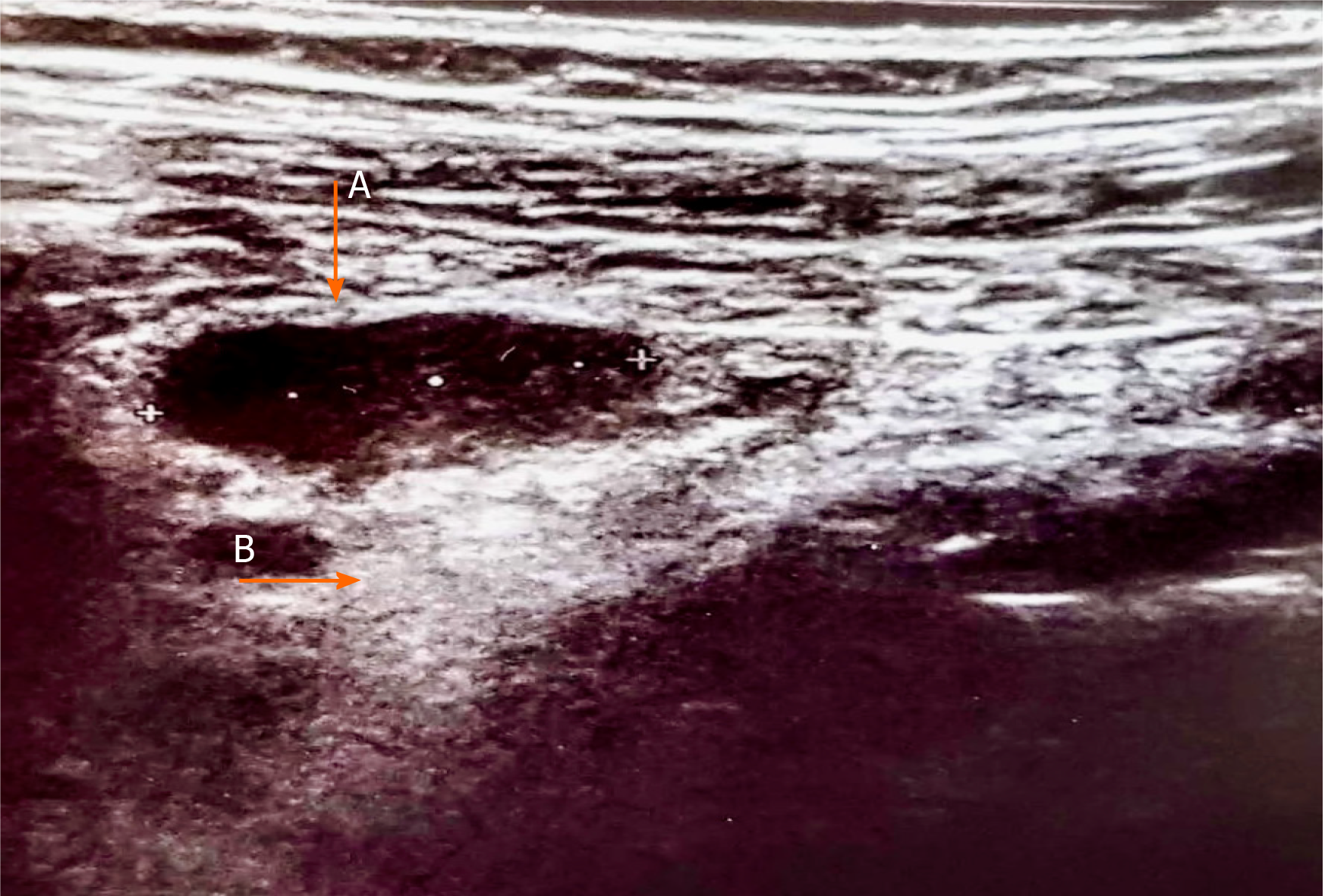
Even though there are very few data available in literature at the time of writing, small bowel involvement in SARS-CoV-2 infection may also lead to mesenteric activation and lymph nodes enlargement[66]. We report those alterations in a 34-year-old woman with SARS-CoV-2 infection presenting abdominal pain, vomiting, and diarrhea. Abdominal US showed enlarged abdominal lymph nodes with a maximum short-axis diameter of 17 mm, and mesenteric adipose tissue hypertrophy (Figure 4). Abdominal CT confirmed the presence of multiple mesenteric enlarged lymph nodes associated with adipose tissue hypertrophy (Figure 5).
In this review, we focused on the small bowel radiological findings in COVID-19 patients, as described so far mainly in patients presenting with GI symptoms. Although no specific aspect was identified, some radiological features have been reported in a significant proportion of patients with GI symptoms, reaching up to 21% of prevalence. Among them, AMI was the most frequent. AMI is an uncommon cause of abdominal pain, accounting for 0.09%-0.2% of all acute surgical admissions to emergency departments[15]. Acute mesenteric arterial embolism caused by atrial fibrillation, thrombosis of the superior mesenteric artery (SMA) in a background of pre-existing chronic atherosclerotic disease, and nonocclusive mesenteric ischemia secondary to SMA vasoconstriction, are the major causes of AMI. Hypercoagulability, including that induced by inherited disorders such as Factor V Leiden and prothrombin mutations, protein S and C deficiency, and others, promote mesenteric venous thrombosis and have been described as causative agents of AMI[15].
Interestingly, enteric involvement has been described in other beta coronavirus infections such as SARS and Middle East respiratory syndrome, but a strong relationship with AMI has never been reported. Of note, all cases of AMI reported during COVID-19 infection were subsequent to direct vascular obstruction, thus ruling out the possibility of nonocclusive mesenteric ischemia[67,68]. It has been widely reported that SARS-CoV-2 infection is associated with an increased risk of various thromboembolic complications[69]. Indeed, SARS-CoV-2 infection induces a hypercoagulable state through systemic inflammation, endothelial activation, and hypoxia. The tropism of SARS-CoV-2 for the vascular endothelium may be explained by the expression of the ACE2, the target receptor for viral entry into cells, and endothelial damage induces massive release of Von Willebrand factor, further increasing the risk of thrombosis[70]. Overall, both hemodynamic alterations consequent to vascular thrombosis and direct enterocyte damage might contribute to the development of small bowel ischemia and necrosis[70], although their exact contribution to the development of wall ischemia is still to be determined. Studies of histological samples are required to further explain the pathophysiology behind this manifestation. The mortality rate of AMI in COVID-19 is still unknown, yet it is likely to contribute significantly to an increased burden of disease. It is thus of the utmost importance to raise awareness among clinicians to recognize the typical signs of mesenteric ischemia, as early diagnosis and timely intervention are essential to decrease the mortality curve. Jung et al[71] recently described the role of contrast-enhanced ultrasonography (CEUS) to detect abdominal microcirculatory disorders in severe cases of COVID-19. CEUS has good sensibility in detecting areas of reduced micro-vascularization, even in the early stages[71]. Small bowel wall thickening was reported in 16% of cases. The cause is still unclear, but it may be a manifestation of local inflammation and edema secondary to direct viral damage of the bowel wall. Pneumatosis intestinalis is a radiological finding described in 15% of cases. The etiology in patients with COVID-19 remains unclear. Direct viral mucosal damage, intestinal ischemia, or atrophy of the lymphoid follicles with secondary increased mucosal permeability[72] were considered as possible pathogenic mechanisms.
Other reported imaging findings include intussusception (13%), pneumoperitoneum (3%), and paralytic ileus (3%). We also reported mesenteric enlarged lymph nodes and mesenteric adipose tissue hypertrophy in a young woman with COVID-19. It is possible that, in addition to direct viral damage of enterocytes, SARS-CoV-2 infection could promote a local immune activation with cytokines release, leading to the described alterations[66].
This review has some limitations. First, it is mainly based on case series and few retrospective studies, thus the real prevalence of the reported radiological findings may actually be significantly higher, as only a proportion of patients with GI symptoms undergo imaging studies. Further investigation of abdominal imaging abnormalities in COVID-19 patients is a topic for future research, and could help to decrease missed diagnosis, encourage closer follow-up, and decrease morbidity and mortality.
So far, the small number of reported cases does not allow to conclusively ascribe these manifestations to the direct action of SARS-CoV-2; nevertheless, it is important to exclude this infection in the current diagnostic workup of patients presenting with gastrointestinal symptoms.
Coronavirus disease 2019 (COVID-19) is an infectious disease with predominant respiratory symptoms. Yet extrapulmonary manifestations have been increasingly recognized in COVID-19 patients. In particular, gastrointestinal (GI) symptoms are reported in up to two-thirds of patients and might be the only manifestations in some cases.
Given the high prevalence of gastrointestinal involvement of COVID-19 and the unclear association with disease clinical outcome, we believe that it could be of interest to deeply investigate small bowel involvement in severe acute respiratory syndrome coronavirus 2 infection.
To analyze and to summarize small bowel radiological features described in COVID-19 patients, and possibly clarify their impact on the clinical management of COVID-19 patients presenting with GI symptoms.
A literature search of the PubMed electronic database was conducted using the MeSH terms “COVID-19”, “imaging” and “gastrointestinal” or “abdominal” or “small bowel”. The search was limited to English-language papers. All available case reports, case series and retrospective studies between December 2019 and January 2021 were included.
AMI is the major radiological finding in COVID-19 patients with small bowel involvement (50%). Less common findings are thickening of the small bowel wall, pneumatosis intestinalis, intussusception, and paralytic ileus. Furthermore, we described a case of mesenteric adipose tissue hypertrophy and enlarged lymph nodes associated to COVID-19.
Gastrointestinal involvement in COVID-19 patients is highly prevalent. The most frequent small bowel alteration is AMI, a condition associated with high mortality. Raised awareness and prompt identification of small bowel involvement in COVID 19-patients could be essential to improve clinical management and clinical outcome, mainly in case of AMI.
Further investigation of abdominal imaging abnormalities in COVID-19 patients may be a topic for future research and could help in reducing missed diagnoses and benefit overall morbidity and mortality.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: de Melo FF, Sabelnikova EA, Shivaji UN S-Editor: Zhang H L-Editor: Filipodia P-Editor: Li JH
| 1. | Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6893] [Cited by in RCA: 7497] [Article Influence: 1499.4] [Reference Citation Analysis (0)] |
| 2. | WHO. Coronavirus Disease (COVID-19) Dashboard. [cited 8 February 2021]. Available from: https://COVID19.who.int/. |
| 3. | Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, Wu Y, Sun L, Xu Y. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Virol. 2020;127:104371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 4. | Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, Shen L, Chan WW. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology. 2020;159:765-767.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 5. | COVIDSurg Collaborative. Global guidance for surgical care during the COVID-19 pandemic. Br J Surg. 2020;107:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 495] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 6. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 754] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 7. | Vespa E, Pugliese N, Colapietro F, Aghemo A. Stay (GI) Healthy: COVID-19 and Gastrointestinal Manifestations. Tech Innov Gastrointest Endosc. 2021;23:179-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14268] [Article Influence: 2853.6] [Reference Citation Analysis (0)] |
| 9. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1994] [Article Influence: 398.8] [Reference Citation Analysis (1)] |
| 10. | Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1286] [Cited by in RCA: 1530] [Article Influence: 306.0] [Reference Citation Analysis (0)] |
| 11. | Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S, Shan H, Jacobi A, Chung M. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295:200463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1728] [Cited by in RCA: 1597] [Article Influence: 319.4] [Reference Citation Analysis (1)] |
| 12. | Bhayana R, Som A, Li MD, Carey DE, Anderson MA, Blake MA, Catalano O, Gee MS, Hahn PF, Harisinghani M, Kilcoyne A, Lee SI, Mojtahed A, Pandharipande PV, Pierce TT, Rosman DA, Saini S, Samir AE, Simeone JF, Gervais DA, Velmahos G, Misdraji J, Kambadakone A. Abdominal Imaging Findings in COVID-19: Preliminary Observations. Radiology. 2020;297:E207-E215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 227] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 13. | Goldberg-Stein S, Fink A, Paroder V, Kobi M, Yee J, Chernyak V. Abdominopelvic CT findings in patients with novel coronavirus disease 2019 (COVID-19). Abdom Radiol (NY). 2020;45:2613-2623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Lawson RM. Mesenteric Ischemia. Crit Care Nurs Clin North Am. 2018;30:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, Ben-Ishay O, Rubinstein C, Balogh ZJ, Civil I, Coccolini F, Leppaniemi A, Peitzman A, Ansaloni L, Sugrue M, Sartelli M, Di Saverio S, Fraga GP, Catena F. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2017;12:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 16. | Fan BE. COVID-19-Associated Thromboembolic Events Causing Acute Mesenteric Ischaemia. Acad Radiol. 2020;27:1788-1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | de Barry O, Mekki A, Diffre C, Seror M, El Hajjam M, Carlier RY. Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. Radiol Case Rep. 2020;15:1054-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | A Beccara L, Pacioni C, Ponton S, Francavilla S, Cuzzoli A. Arterial Mesenteric Thrombosis as a Complication of SARS-CoV-2 Infection. Eur J Case Rep Intern Med. 2020;7:001690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 19. | Ofosu A, Ramai D, Novikov A, Sushma V. Portal Vein Thrombosis in a Patient With COVID-19. Am J Gastroenterol. 2020;115:1545-1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | La Mura V, Artoni A, Martinelli I, Rossio R, Gualtierotti R, Ghigliazza G, Fusco S, Ierardi AM, Andrisani MC, Carrafiello G, Peyvandi F. Acute Portal Vein Thrombosis in SARS-CoV-2 Infection: A Case Report. Am J Gastroenterol. 2020;115:1140-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Del Hoyo J, López-Muñoz P, Fernández-de la Varga M, Garrido-Marín A, Valero-Pérez E, Prieto M, Aguilera V. Hepatobiliary and Pancreatic: A fatal case of extensive splanchnic vein thrombosis in a patient with Covid-19. J Gastroenterol Hepatol. 2020;35:1853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Karna ST, Panda R, Maurya AP, Kumari S. Superior Mesenteric Artery Thrombosis in COVID-19 Pneumonia: an Underestimated Diagnosis-First Case Report in Asia. Indian J Surg. 2020;1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Cheung S, Quiwa JC, Pillai A, Onwu C, Tharayil ZJ, Gupta R. Superior Mesenteric Artery Thrombosis and Acute Intestinal Ischemia as a Consequence of COVID-19 Infection. Am J Case Rep. 2020;21:e925753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | de Roquetaillade C, Chousterman BG, Tomasoni D, Zeitouni M, Houdart E, Guedon A, Reiner P, Bordier R, Gayat E, Montalescot G, Metra M, Mebazaa A. Unusual arterial thrombotic events in Covid-19 patients. Int J Cardiol. 2021;323:281-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 25. | Vartanoglu Aktokmakyan T, Tokocin M, Meric S, Celebi F. Is Mesenteric Ischemia In COVID-19 Patients A Surprise? Surg Innov. 2021;28:236-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Fraissé M, Logre E, Pajot O, Mentec H, Plantefève G, Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020;24:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 27. | Ignat M, Philouze G, Aussenac-Belle L, Faucher V, Collange O, Mutter D, Pessaux P. Small bowel ischemia and SARS-CoV-2 infection: an underdiagnosed distinct clinical entity. Surgery. 2020;168:14-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 28. | Pang JHQ, Tang JH, Eugene-Fan B, Lee CL, Low JK. A Peculiar Case of Small Bowel Stricture in a Coronavirus Disease 2019 Patient with Congenital Adhesion Band and Superior Mesenteric Vein Thrombosis. Ann Vasc Surg. 2021;70:286-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Bianco F, Ranieri AJ, Paterniti G, Pata F, Gallo G. Acute intestinal ischemia in a patient with COVID-19. Tech Coloproctol. 2020;24:1217-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Norsa L, Bonaffini PA, Indriolo A, Valle C, Sonzogni A, Sironi S. Poor Outcome of Intestinal Ischemic Manifestations of COVID-19. Gastroenterology. 2020;159:1595-1597.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Collange O, Tacquard C, Delabranche X, Leonard-Lorant I, Ohana M, Onea M, Anheim M, Solis M, Sauer A, Baloglu S, Pessaux P, Ohlmann P, Kaeuffer C, Oulehri W, Kremer S, Mertes PM. Coronavirus Disease 2019: Associated Multiple Organ Damage. Open Forum Infect Dis. 2020;7:ofaa249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Vulliamy P, Jacob S, Davenport RA. Acute aorto-iliac and mesenteric arterial thromboses as presenting features of COVID-19. Br J Haematol. 2020;189:1053-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 33. | Rodriguez-Nakamura RM, Gonzalez-Calatayud M, Martinez Martinez AR. Acute mesenteric thrombosis in two patients with COVID-19. Two cases report and literature review. Int J Surg Case Rep. 2020;76:409-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | E English W, Banerjee S. Coagulopathy and mesenteric ischaemia in severe SARS-CoV-2 infection. ANZ J Surg. 2020;90:1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1669] [Cited by in RCA: 2050] [Article Influence: 410.0] [Reference Citation Analysis (0)] |
| 36. | Mitchell JM, Rakheja D, Gopal P. SARS-CoV-2-related Hypercoagulable State Leading to Ischemic Enteritis Secondary to Superior Mesenteric Artery Thrombosis. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Azouz E, Yang S, Monnier-Cholley L, Arrivé L. Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID-19. Intensive Care Med. 2020;46:1464-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 38. | Franco-Moreno A, Piniella-Ruiz E, Montoya-Adarraga J, Ballano-Franco C, Alvarez-Miguel F, Peinado-Martinez C, Landete-Hernandez E, Saez-Vaquero T, Ulla-Anes M, Torres-Macho J. Portal vein thrombosis in a patient with COVID-19. Thromb Res. 2020;194:150-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Macari M, Balthazar EJ. CT of bowel wall thickening: significance and pitfalls of interpretation. AJR Am J Roentgenol. 2001;176:1105-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 40. | Hellinger JC, Sirous R, Hellinger RL, Krauthamer A. Abdominal presentation of COVID-19. Appl Radiol. 2020;49:24-26. |
| 41. | Periyakaruppan M, Kumar S, Kandasamy S, Sangaralingam T, Srinivasan S, Thiagarajan A, Ganapathy N. COVID Abdomen: SARS-CoV-2 Infection Presenting as 'Acute Abdomen' in a Child. Indian J Pediatr. 2021;88:299-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Guo Y, Hu X, Yu F, Chen J, Zheng W, Liu J, Zeng P. Abdomen CT findings in a COVID-19 patient with intestinal symptoms and possibly false negative RT-PCR before initial discharge. Quant Imaging Med Surg. 2020;10:1158-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Heye T, Bernhard M, Mehrabi A, Kauczor HU, Hosch W. Portomesenteric venous gas: is gas distribution linked to etiology and outcome? Eur J Radiol. 2012;81:3862-3869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Khalil PN, Huber-Wagner S, Ladurner R, Kleespies A, Siebeck M, Mutschler W, Hallfeldt K, Kanz KG. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res. 2009;14:231-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 261] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 46. | Kim MJ, Kim YJ, Lee JH, Lee JS, Kim JH, Cheon DS, Jeong HS, Koo HH, Sung KW, Yoo KH, Choe YH. Norovirus: a possible cause of pneumatosis intestinalis. J Pediatr Gastroenterol Nutr. 2011;52:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Balasuriya HD, Abeysinghe J, Cocco N. Portal venous gas and pneumatosis coli in severe cytomegalovirus colitis. ANZ J Surg. 2018;88:113-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Tirumani SH, Rahnemai-Azar AA, Pierce JD, Parikh KD, Martin SS, Gilkeson R, Ramaiya NH. Are asymptomatic gastrointestinal findings on imaging more common in COVID-19 infection? Abdom Radiol (NY). 2021;46:2407-2414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Kielty J, Duggan WP, O'Dwyer M. Extensive pneumatosis intestinalis and portal venous gas mimicking mesenteric ischaemia in a patient with SARS-CoV-2. Ann R Coll Surg Engl. 2020;102:e145-e147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Di Grezia M, Fransvea P, Santullo F, Tirelli F, Fico V, Mirco P, Cozza V, La Greca A, Sganga G. Intra-abdominal hypertension as a trigger of "gut failure" in SARS-CoV-2 infection: Effect of open abdomen (OA) and negative pressure therapy (NPT) on respiratory and gastrointestinal (GI) function. Med Hypotheses. 2020;144:109954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Kubiak BD, Albert SP, Gatto LA, Snyder KP, Maier KG, Vieau CJ, Roy S, Nieman GF. Peritoneal negative pressure therapy prevents multiple organ injury in a chronic porcine sepsis and ischemia/reperfusion model. Shock. 2010;34:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 52. | Rossi M, Sganga G, Mazzone M, Valenza V, Guarneri S, Portale G, Carbone L, Gatta L, Pioli C, Sanguinetti M, Montalto M, Glieca F, Fadda G, Schiavello R, Silveri NG. Cardiopulmonary bypass in man: role of the intestine in a self-limiting inflammatory response with demonstrable bacterial translocation. Ann Thorac Surg. 2004;77:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Marinis A, Yiallourou A, Samanides L, Dafnios N, Anastasopoulos G, Vassiliou I, Theodosopoulos T. Intussusception of the bowel in adults: a review. World J Gastroenterol. 2009;15:407-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 428] [Cited by in RCA: 507] [Article Influence: 31.7] [Reference Citation Analysis (2)] |
| 54. | Makrinioti H, MacDonald A, Lu X, Wallace S, Jobson M, Zhang F, Shao J, Bretherton J, Mehmood T, Eyre E, Wong A, Pakkiri L, Saxena A, Wong G. Intussusception in 2 Children With Severe Acute Respiratory Syndrome Coronavirus-2 Infection. J Pediatric Infect Dis Soc. 2020;9:504-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Rajalakshmi L, Satish S. Unusual presentation of COVID-19 as intussusception. Indian J Pract Pediatr. 2020;22:236. |
| 56. | Moazzam Z, Salim A, Ashraf A, Jehan F, Arshad M. Intussusception in an infant as a manifestation of COVID-19. J Pediatr Surg Case Rep. 2020;59:101533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | Bazuaye-Ekwuyasi EA, Camacho AC, Saenz Rios F, Torck A, Choi WJ, Aigbivbalu EE, Mehdi MQ, Shelton KJ, Radhakrishnan GL, Radhakrishnan RS, Swischuk LE. Intussusception in a child with COVID-19 in the USA. Emerg Radiol. 2020;27:761-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Cai X, Ma Y, Li S, Chen Y, Rong Z, Li W. Clinical Characteristics of 5 COVID-19 Cases With Non-respiratory Symptoms as the First Manifestation in Children. Front Pediatr. 2020;8:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 59. | Martínez-Castaño I, Calabuig-Barbero E, Gonzálvez-Piñera J, López-Ayala JM. COVID-19 Infection Is a Diagnostic Challenge in Infants With Ileocecal Intussusception. Pediatr Emerg Care. 2020;36:e368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y, Wu C, Liu H, Liu D, Shao J, Peng X, Yang Y, Liu Z, Xiang Y, Zhang F, Silva RM, Pinkerton KE, Shen K, Xiao H, Xu S, Wong GWK; Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382:1663-1665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1629] [Cited by in RCA: 1703] [Article Influence: 340.6] [Reference Citation Analysis (0)] |
| 61. | Lee CH. Images in clinical medicine. Radiologic signs of pneumoperitoneum. N Engl J Med. 2010;362:2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Corrêa Neto IJF, Viana KF, Silva MBS da, da Silva LM, de Oliveira G, da Silva Cecchini AR, Sá Rolim A, Robles L. Perforated acute abdomen in a patient with COVID-19: an atypical manifestation of the disease. J Coloproctology. 2020;40:269-272. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Chapuis PH, Bokey L, Keshava A, Rickard MJ, Stewart P, Young CJ, Dent OF. Risk factors for prolonged ileus after resection of colorectal cancer: an observational study of 2400 consecutive patients. Ann Surg. 2013;257:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 64. | Beach EC, De Jesus O. Ileus. 2021 Feb 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] |
| 65. | Ibrahim YS, Karuppasamy G, Parambil JV, Alsoub H, Al-Shokri SD. Case Report: Paralytic Ileus: A Potential Extrapulmonary Manifestation of Severe COVID-19. Am J Trop Med Hyg. 2020;103:1600-1603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 66. | Calinescu AM, Vidal I, Grazioli S, Lacroix L, Wildhaber BE. Beware of Too Aggressive Approach in Children With Acute Abdomen During COVID-19 Outbreak! Ann Surg. 2020;272:e244-e245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS, Cescon DW, Poutanen SM, Detsky AS. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 964] [Cited by in RCA: 994] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 68. | Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, Lam WK, Seto WH, Yam LY, Cheung TM, Wong PC, Lam B, Ip MS, Chan J, Yuen KY, Lai KN. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 727] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 69. | Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1535] [Article Influence: 307.0] [Reference Citation Analysis (0)] |
| 70. | Parry AH, Wani AH, Yaseen M. Acute Mesenteric Ischemia in Severe Coronavirus-19 (COVID-19): Possible Mechanisms and Diagnostic Pathway. Acad Radiol. 2020;27:1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 71. | Jung EM, Stroszczynski C, Jung F. Contrast enhanced ultrasonography (CEUS) to detect abdominal microcirculatory disorders in severe cases of COVID-19 infection: First experience. Clin Hemorheol Microcirc. 2020;74:353-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 72. | Devgun P, Hassan H. Pneumatosis cystoides intestinalis: a rare benign cause of pneumoperitoneum. Case Rep Radiol. 2013;2013:353245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (1)] |