Published online Jul 27, 2021. doi: 10.4240/wjgs.v13.i7.633
Peer-review started: February 8, 2021
First decision: May 4, 2021
Revised: May 16, 2021
Accepted: June 15, 2021
Article in press: June 15, 2021
Published online: July 27, 2021
Processing time: 164 Days and 14.1 Hours
Pancreatic and peripancreatic collections are the main local complications of acute pancreatitis with a high incidence. In the early phase, most acute pancreatic and peripancreatic collections can resolve spontaneously with supportive treatment. However, in some cases, they will develop into pancreatic pseudocyst (PPC) or walled-off necrosis (WON). When causing symptoms or coinfection, both PPC and WON may require invasive intervention. Compared to PPC, which can be effectively treated by endoscopic ultrasound-guided transmural drainage with plastic stents, the treatment of WON is more complicated and challenging, particularly in the presence of infected necrosis. In the past few decades, with the development of minimally invasive interventional technology especially the progression of endoscopic techniques, the standard treatments of those severe complications have undergone tremendous changes. Currently, based on the robust evidence from randomized controlled trials, the step-up minimally invasive approaches have become the standard treatments for WON. However, the pancreatic fistulae during the surgical step-up treatment and the stent-related complications during the endoscopic step-up treatment should not be neglected. In this review article, we will mainly discuss the indications of PPC and WON, the timing for intervention, and minimally invasive treatment, especially endoscopic treatment. We also introduced our preliminary experience in endoscopic gastric fenestration, which may be a promising innovative method for the treatment of WON.
Core Tip: Conservative treatment is suitable for most pancreatic pseudocysts. The endoscopic ultrasound-guided transmural drainage with plastic stents may be the preferred therapy for pancreatic pseudocysts if they are symptomatic. Combined transpapillary drainage is not routinely suggested. Walled-off necrosis requires invasive intervention except for sterile asymptomatic walled-off necrosis. The timing of intervention is debatable in the era of minimal invasion. The endoscopic step-up approach is the preferred treatment for walled-off necrosis, whereas percutaneous drainage followed by minimally invasive surgery is an important alternative therapy. The innovative approach of endoscopic gastric fenestration without any stent may be a promising procedure in selected patients.
- Citation: Xiao NJ, Cui TT, Liu F, Li W. Current status of treatments of pancreatic and peripancreatic collections of acute pancreatitis. World J Gastrointest Surg 2021; 13(7): 633-644
- URL: https://www.wjgnet.com/1948-9366/full/v13/i7/633.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i7.633
Acute pancreatitis, one of the most common gastrointestinal emergencies, is a potentially fatal disease. Treatment of acute pancreatitis has improved considerably in the past few decades, moving toward multidisciplinary and minimally invasive approaches. In the early phase of acute pancreatitis, the current international consensus is that supportive care should be adopted, including goal-directed intravenous hydration, nutrition support, and etiological treatment[1-3]. Notwithstanding the improvements in initial management and critical care, local complications of acute pancreatitis are still common, especially in severe acute pancreatitis[4,5].
According to the 2012 revised Atlanta classification[6], the main local complications of acute pancreatitis are defined as pancreatic and peripancreatic collections, which are divided into four subtypes: acute peripancreatic fluid collections, acute necrotic collections, pancreatic pseudocyst (PPC), and walled-off necrosis (WON). Acute peripancreatic fluid collections and acute necrotic collections usually develop in the early phase of interstitial edematous pancreatitis or necrotic pancreatitis. They may remain sterile and then resolve spontaneously over time. Therefore, unless the infection is suspected, most of them do not require invasive intervention. PPC and WON ordinarily mature 4 wk after the onset of acute pancreatitis, and they are usually encapsulated by a well-defined inflammatory wall. The distinctions between PPC and WON are that PPC occurs in interstitial edematous pancreatitis, which contains homogenous liquid without solid components, while WON arises from necrotic pancreatic and/or peripancreatic tissues and contains varying amounts of fluid with solid necrotic debris. The majority of those matured collections, especially the PPC, will subside spontaneously with supportive care. But some of them can be symptomatic and troublesome, particularly in the presence of infections, leading to significant morbidity and mortality and requiring advanced invasive intervention.
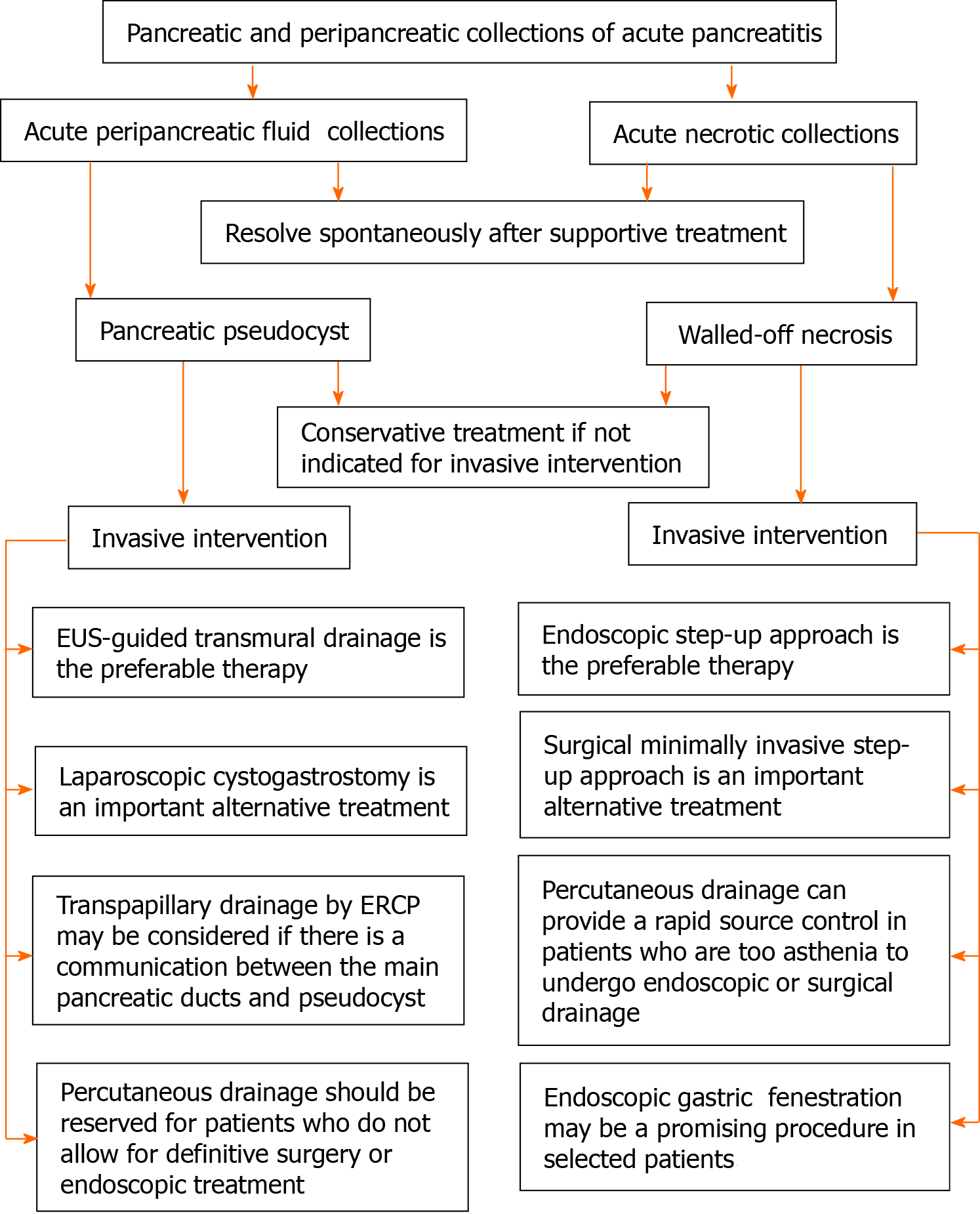
Currently, the most commonly used invasive interventions for the treatment of PPC or WON have shifted from open surgery to minimally invasive interventions, which mainly include percutaneous drainage, laparoscopic surgery, and endoscopic intervention. However, issues such as who should undergo the invasive intervention, when to perform the procedure, and which approach should be chosen remains open for discussion. In this review article, we will mainly discuss the indications of PPC and WON, the timing of intervention, and minimally invasive treatments respectively, especially focusing on the endoscopic approach that has made considerable progress in recent years. Furthermore, we also introduced the preliminary experience of endoscopic gastric fenestration (EGF) in the treatment of infected WON. The marked difference between PPC and WON has potential clinical implications and determines the differential treatment algorithms. Therefore, we separately demonstrated the current status of treatment of PPC and WON and concluded an algorithm of treatment of pancreatic and peripancreatic collections (Figure 1).
The reported incidence of PPC in acute pancreatitis ranged from 6.3% to 16.8%[7-9], and more than half of all pseudocysts can resolve spontaneously[10]. In a prospective multicenter study, about 84.2% of PPC disappeared (5/19) or decreased in size (11/19) without drainage[9], and even a larger pseudocyst with a diameter of 160mm could regress spontaneously[10]. In addition, the complications during prolonged observation of PPC in the expectation of spontaneous resolution were infrequent and treatable. Even though some centers once held the position statement that pseudocysts with a size above 60 mm and do not resolve within 6 wk should be treated[8,11,12], the indication of treating pseudocyst based on size and duration should be abandoned due to the knowledge of the natural history of PPC. According to the American College of Gastroenterology guidelines[13], asymptomatic PPC do not warrant intervention regardless of size, location, and/or extension. The strategy of ‘wait and see’ for them seems suitable. General indications for pseudocyst interventions should be those that caused symptoms (abdominal pain, early satiety), presented concomitant complications (infection, bleeding, rupture), led to an obstruction of surrounding organs, or progressively increased in size on serial imaging. In addition, when it is difficult to distinguish PPC from pancreatic cystic tumors through noninvasive examinations, those cystic lesions should be intervened by a surgical or endoscopic approach without delay to make a clear diagnosis and then determine the appropriate management.
Nowadays, many approaches are available to treat pseudocysts, mainly including percutaneous, endoscopic, and surgical drainage. Percutaneous drainage was once one of the commonly used initial minimally invasive management methods of symptomatic PPC. Compared with endoscopic drainage, percutaneous drainage is associated with a lower treatment success rate and a higher residual collection rate, which means that patients undergoing percutaneous drainage may need additional interventions (such as endoscopic drainage or surgery) to assist in achieving clinical success[11]. Additionally, percutaneous drainage is associated with high recurrence and reintervention[8,14] and may lead to external pancreatic fistulae[11]. Therefore, it is not a preferred approach in the management of PPC now and should be reserved for patients with comorbidities who do not allow for definitive surgery or endoscopic treatment.
Decades ago, open pancreatic cystogastrostomy was used as a standard treatment for PPC. However, with the emergence of minimally invasive interventions including laparoscopic surgery or endoscopic drainage, it is no longer the first-line therapy. Laparoscopic cystogastrostomy is associated with a smoother and quicker recovery with lower postoperative morbidity (10% vs 60%) and shorter hospital stay (6.2 d vs 11.0 d) compared with open surgery in a case-matched comparative study[15]. Similarly, there is no evidence that open pancreatic cystogastrostomy for pseudocyst is of superior efficacy or safety comparing to endoscopic drainage in a randomized controlled trial (RCT)[16]. Furthermore, patients in the endoscopic treatment group were associated with a shorter hospital stay, lower cost, and better physical and mental status[16-18]. A systematic review contained six comparative studies of 342 patients, which confirmed that there were no significant differences in the treatment success rates, adverse events, and recurrence rates between surgery and endoscopic treatments, but the endoscopic group had shorter hospital stays and lower treatment costs[19]. When it comes to the comparison between laparoscopic surgery and endoscopic drainage, both techniques were similar in terms of mortality, overall success rate, recurrence rate, and postoperative complications[20]. The relative contraindications of laparoscopic cystogastrostomy include peritoneal adhesions and the adverse surgical risks of general anesthesia, in which case endoscopic drainage will be unrestricted. A recent meta-analysis[21] and systematic review[22] separately compared percutaneous, endoscopic, and surgical pseudocyst drainage, both results support that endoscopic drainage is the preferred approach due to the comparable clinical success, adverse events rates, and reduced hospital stay.
The majority of endoscopic drainage procedures were transmural using two double-pigtailed plastic stents (7F or 10F). The stent size and number were not significantly associated with treatment success in a retrospective study[23]. Clinically, endoscopists also attempt to drain the PPC with metal stents due to their relatively larger diameter. Despite the theoretical advantage, the practical efficacy of metal stents is debatable for endoscopic drainage of PPC. In some studies, no significant difference was reported between plastic stents and metal stents in terms of technical success rate, treatment success, reintervention, and adverse events. Moreover, plastic stents are less expensive[23-25]. But the other studies had shown that drained with metal stents may improve clinical outcomes in PPC[26,27]. Those conflicting results may be continuously present, and large-sized RCTs are needed for a better conclusion. Currently, in the context of the high success rate (more than 90%) of diverse stents[27], we recommend the double-pigtail plastic stent as the preferred choice in the endoscopic transmural drainage for PPC, given their excellent safety profile and relatively lower cost.
Transpapillary drainage by endoscopic retrograde cholangiopancreatography might be added if there is a communicating tract between the pseudocyst and the main pancreatic duct. However, the incremental benefit is very limited in the case of pancreatic duct disruption. In a retrospective multicenter study[28] comparing transmural drainage alone to a combination of transmural and transpapillary drainage, the technical success rate was 97% for transmural drainage and 44% for combination (mainly caused by failed transpapillary drainage). Adverse events were not significantly different between transmural drainage and the combination group. Much more than this, the short- and long-term follow-up outcomes of those two groups showed no significant difference in neither symptomatic resolution nor radiologic resolution. Based on those results and a meta-analysis[29] that showed that the combination method had no superior advantage to transmural drainage alone in terms of clinical success, recurrence, or complications, the authors argued that transpapillary drainage had no added benefit on treatment outcomes in patients already undergoing transmural drainage of PPC. Given the relatively low technical success rate of combination as well as no additional benefit, it is reasonable to avoid executing transpapillary drainage routinely in the treatment of PPC. This approach may be considered when the initial simple transmural drainage failed, and communication between the main pancreatic ducts and pseudocyst existed.
With the development of therapeutic endoscopic ultrasound, transmural drainage under the real-time guidance of endoscopic ultrasound (EUS) has been popular and becoming the mainstay for the treatment of PPC. Compared with traditional blinding transmural drainage, EUS-guided transmural drainage has a higher technical success, especially in lesions without luminal compression[30]. Additionally, puncture under the real-time guidance of EUS can effectively decrease iatrogenic bleeding, making the complications less severe. Even so, the overall complication rate of endoscopic transmural drainage is still about 15%[21,31], and adverse events mainly consist of bleeding, infection, stent migration, and perforation. Bleeding is a potential deadly adverse condition that may need additional interventional radiology-guided embolization or surgery to stanch. When using plastic stents, about 1% of patients undergoing transmural drainage may experience bleeding, and it is more common when using the lumen-apposing metal stents[32].
In short, invasive intervention is rarely needed in the treatment of PPC of acute pancreatitis. Only persistent symptomatic, continuously increasing in size, or complicated PPC should be considered as indications. The timing of intervention can be delayed as long as possible if the lesion is stable. Both endoscopic drainage and laparoscopic cystogastrostomy are suitable approaches when intervention is required. As the minimum invasive internal drainage, the EUS-guided transmural drainage with plastic stents may be the preferred therapy when it can be reached endoscopically, considering its excellent efficacy, acceptable complication rate, and less expensive cost. Meanwhile, combined transpapillary drainage is not suggested routinely. Nonetheless, many factors (such as anatomical location, available technology, and the individual patient’s circumstances) limit the use of endoscopic transmural drainage. In those cases, percutaneous or laparoscopic intervention may be suitable alternatives.
Comparing with pseudocysts, WON associated with necrosis of pancreatic and/or peripancreatic tissue is more threatening and more susceptible to infection. Patients with pancreatic necrosis or secondary WON may face a complicated, prolonged intensive care unit stay and invasive interventions. This condition is associated with a high risk of multiple organ dysfunction syndromes and a high mortality rate of up to 20%–30%[4,33,34]. Therefore, the guidelines[33] recommended a multidisciplinary team, including gastroenterologists, surgeons, interventional radiologists, and other specialists to provide optimal management.
Although the acute necrotizing pancreatitis is quite serious and difficult to manage, the sterile pancreatic and/or peripancreatic necrosis can be initially treated with supportive care because more than 50% of pancreatic or peripancreatic collections will resolve spontaneously over time[4,35]. Hence, sterile asymptomatic lesions do not require invasive intervention, regardless of their size, location, or extension[13]. Moreover, early drainage of aseptic lesions may increase the risk of iatrogenic infection, which is an intractable issue in the background of pancreatitis-related systematic inflammatory response syndrome. Therefore, the invasive interventions for sterile lesions should be reserved for patients with related symptoms in the late phase (more than 4 wk) when the collections were finely encapsulated.
Patients with infected necrotic tissue are virtually always indicated for immediate broad-spectrum or targeted antibiotic therapy[35]. To completely control the infection, invasive interventions are usually unavoidable to empty the abscess and/or remove the infected necrotic tissue. In the era of surgical necrosectomy as the standard treatment for necrosis, postponing intervention until the WON was matured facilitated safe interventions and lower complication rates or mortality[36]. Guidelines[6,13,33,37,38] also recommended that surgical timing of debridement should be delayed at least 4 wk, except if there is a strong indication. For instance, abdominal compartment syndrome, which is a lethal complication of acute necrotizing pancreatitis with a mortality of about 50% and prolonged exposure to high intra-abdominal pressure may result in irreversible damage to organ function. With the development of minimally invasive interventions, the step-up approach mainly consists of percutaneous catheter drainage or endoscopic transluminal drainage, followed by video-assisted retroperitoneal debridement (VARD) or endoscopic necrosectomy is the current standard treatment if there are indications[1,33,38].
The timing for minimally invasive intervention in necrotizing pancreatitis is controversial in the era of the step-up approach. Although guidelines used to recommend that invasive intervention should be postponed, if possible, at least 4 wk to allow the collections to become walled-off[38]. This recommendation stemmed from the relationship between early open necrosectomy and high mortality, aimed to prevent these already critically ill patients from the “extra hit” of open surgery in the early phase. As the standard treatment shifted, postponing minimally invasive intervention until the walled-off formation may not be a prerequisite for safe and successful management in patients without persistent organ failure[39]. In contrast, it has been hypothesized that early drainage of infected necrosis might lessen systematic inflammatory response syndrome[40]. Additionally, in an international survey about the timing of intervention of necrotizing pancreatitis, 55% of expert pancreatologists stated that typically postponed drainage was used after treatment of antibiotics and support care, whereas the other 45% performed immediate catheter drainage after infected necrosis was diagnosed[41].
A retrospective study[42] of 193 patients showed that there was no difference in procedure-related complications between early and standard intervention time. However, the mortality of the early intervention group is higher (13% vs 4%). This result may reflect the severity of illness rather than the consequence of intervention timing because infections (91% vs 39%) and other complications were more common in the early intervention group. Most of the initial interventions involved in this study are endoscopic transmural drainage, which may contribute to the relatively low all-cause mortality rate (7.8%). Based on these results, the authors believe that endoscopy may be performed early in the setting of clinical deterioration and suspected infections.
Even so, based on our limited experience, it is suggested that patients with infected necrotizing pancreatitis should be treated with a primarily antibiotic administration and support care. If the symptoms are ameliorated, then the invasive interventions may be postponed until the necrosis becomes the walled-off form. Furthermore, invasive interventions may be obviated in some cases. Early debridement should be avoided except for strong indications like abdominal compartment syndrome. Currently, further advanced evidence about the timing of minimally invasive intervention is needed. The forthcoming results of an RCT (POINTER trial)[43] comparing immediate with postponed primary percutaneous drainage until necrosis encapsulation are expected to shed further insight into the ideal timing of primary percutaneous drainage.
Since an RCT (PANTER trial)[44,45] showed that in the treatment of necrotizing pancreatitis, the minimally invasive step-up approach was superior to the open necrosectomy in both short- and long-term outcomes, the standard treatment has shifted from open surgical treatment to the step-up and minimally invasive approaches[1,33,46]. Afterward, many minimally invasive techniques are available to drain and/or debride the infected pancreatic or peripancreatic necrotic tissue, which can be generally classified based on the method of visualization (radiologic, endoscopic, hybrid, or other) and route (peroral, transpapillary or transmural, percutaneous retroperitoneal, and percutaneous transperitoneal, with or without transmural puncture)[47]. Currently, the most frequently used approaches are: (1) percutaneous drainage; (2) minimally invasive surgery; and (3) endoscopic drainage and necrosectomy.
The procedure of percutaneous drainage is comparatively handy. Moreover, it can provide a rapid and effective source control in patients who are too asthenic to undergo endoscopic or surgical drainage. In fact, prospective studies and systematic review have shown that primary percutaneous drainage alone can help 35.0%-55.7% of patients with WON to obviate further surgical intervention[35,45,48]. Percutaneous drainage is of definite advantage when the lesions are located at the paracolic gutters or pelvis where it is beyond the reach of endoscopic drainage. Percutaneous drainage also facilitates bedside lavation, and the catheter tract can act as a potential passageway for subsequent VARD. One major drawback of percutaneous drainage is the high incidence of pancreatic fistulae. It can be as high as 32% compared to 2% in the endoscopic approach[49]. Although most of the pancreatic fistulae can be handled by endoscopic retrograde cholangiopancreatography with pancreatic sphincterotomy or stent placement, it may decrease the life quality, which should not be neglected. The combined percutaneous and endoscopic approach may affect avoidance of pancreatic fistulae[50]. Additionally, to improve drainage efficiency in our center, an innovative method of three-dimensional visualization technology was used to assist percutaneous drainage, which can provide an optimal puncture path based on the precise anatomical evaluation[51].
Minimally invasive surgery mainly included VARD and laparoscopic transgastric debridement. Since the surgical minimally invasive step-up approach was introduced in the PANTER trial[45], percutaneous drainage or endoscopic drainage, followed by VARD had been the mainstream for treating infected necrotizing pancreatitis. In addition, it was proved in an RCT (TENSION trial)[49] that there was no difference in major complication rates or mortality between endoscopic and surgical minimally invasive step-up approaches. However, those results may be because pancreatic fistulae were not defined as a major complication in this trial. Moreover, compared with minimally invasive surgery of VARD or, if not feasible, laparoscopic transgastric debridement, the endoscopic step-up approach reduced the proinflammatory response as well as the composite major complications in another small sample RCT (PENGUIN trial)[52]. Similar results were verified by a later RCT (MISER trial)[53] that enrolled 66 patients with infected necrotizing pancreatitis, in which the rate of systematic inflammatory response syndrome decreased after endoscopic intervention but increased after the surgical minimally invasive intervention. Furthermore, the MISER trial also showed that the endoscopic approach, as compared with the surgical minimally invasive approach, reduced the rate of major complications (11.8% vs 40.6%), particularly less likelihood of the enteral or pancreatic-cutaneous fistulae (0% vs 28.1%). Again, the endoscopic approach shortened the procedural duration, lowered costs, and increased the quality of life in this trial[53]. With those results of RCTs and other cohort studies, the endoscopic step-up approach is now recommended as the first-line therapy in the majority of patients with infected necrotizing pancreatitis.
Endoscopic drainage and/or necrosectomy emerged two decades ago and have now evolved into the preferred approach with the development of relative technique and equipment (particularly the novel stents)[33,54,55]. Transgastric and transduodenal procedures are two main passages to achieve drainage. Although the transgastric passage is more often used, the safety and success rate of these two procedures are not significantly different. Decision-making usually depends on the anatomical relationship between the location or extension of WON and the stomach or duode
Numerous studies of plastic stents, lumen-apposing metal stents (LAMS), and other different subtypes of metal stents have been mushrooming in recent years, which is one of the most conspicuous characteristics in the endoscopic intervention. Traditionally, plastic stents with a double pigtail shape were indwelled to drain the collections and minimize migration risk, but the main limitation associated with plastic stents is the frequent occlusions due to the small-caliber diameter. Thus, endoscopists usually place two or more stents, enduring this technically difficult and time-consuming procedure, to ensure appropriate drainage. Even so, inefficient drainage and the need for subsequent reintervention is not uncommon in plastic stent drainage[27,57].
Then, the fully covered self-expandable metal stents (FCSEMS) were considered owing to a larger luminal diameter. Though the clinical success rate may be superior to plastic stents, the straight-shape stent, which was originally designed for bile duct drainage, was associated with higher rates of stent migration. Thus, many modified FCSEMSs with antimigratory design were introduced. In a prospective randomized study, a newly designed FCSEMS (BONA-Soo stent) with a flare at the proximal end and a 90-degree angulation flap at the distal end is comparable to plastic stents in terms of technical feasibility, efficacy, and safety in EUS-guided pancreatic and peripancreatic collection drainage[58].
A LAMS with a bilateral flanged, wide-lumen, short length design was utilized specifically for pancreatic and peripancreatic collection drainage, providing a double-head buckle to fasten the connection and allowing repeated access to the WON for endoscopic necrosectomy. Additionally, the matched cautery-enhanced delivery systems decrease technical difficulty and shorten the procedural time. Even though some studies show LAMS might have a slight advantage over plastic stents in terms of clinical success[27,59,60], more studies have shown that there were no differences between LAMS and plastic stents regarding the technical success rate, clinical success, recurrence in drainage of pancreatic, and peripancreatic collections[24,25,60-62]. In contrast, the bleeding risk (including pseudoaneurysm bleeding, which is life-threatening and may require immediate interventional radiology-guided coil embolization or open surgery) is higher in the LAMS group[32,61,62]. Moreover, in an RCT, non-superiority of LAMS over plastic stents was observed, and stent-related adverse events and procedure costs of LAMS were higher[63]. A systematic review including 17 studies (881 patients) also proved that no difference in the overall treatment success, adverse events, and recurrence between metal stents (including FCSEMS and LAMS) and plastic stents[64].
Therefore, the debate on the choice of stents for WON in EUS-guided drainage is still intense. This situation may reflect the fact that no stent is perfectly suitable for endoscopic drainage. Thus, we explored EGF as an innovative alternative therapy without any stents. This procedure is as follows. We first selected appropriate sites for gastric fenestration with the guidance of EUS. Then the gastric wall was incised layer by layer as in endoscopic submucosal dissection until gastric muscularis propria and adherent WON wall were both penetrated. The advantage of EGF is that the diameter of the fenestration site can be safely expanded to 2-3 cm according to the spatial orientations of WON and with the guidance of EUS, which greatly exceeds the caliber of LAMS, allowing more effective drainage or subsequent necrosectomy. In addition, it evades stent-related adverse complications and may be less expensive. Recently, 5 patients attempted to undergo this procedure in our center, and 4 of them were successful. The preliminary experiences of this procedure suggest that EGF is a promising intervention in patients with WON, perhaps outperforming endoscopic LAMS placement if the WON is adherent to the gastric wall[65].
In summary, WON is more troublesome than PPC, especially when infection develops. The sterile asymptomatic lesions can be treated by conservative support, but the symptomatic lesions may require invasive intervention, particularly when the coexisting infection is suspected. The timing of intervention is debatable. Although the postponed intervention was pragmatic experience in the era of open surgery, it remains reasonable to hold the statement in the era of minimally invasive intervention before strong evidence to support early intervention. The endoscopic step-up approach is the preferred approach to treat WON, whereas percutaneous drainage followed by VARD is an important alternative therapy. Given the considerable stent-related problems like bleeding and stent clogging, the innovative approach of EGF without any stents may be a promising procedure in selected patients.
Pancreatic and peripancreatic collections are the most frequent local complications associated with acute pancreatitis. The acute peripancreatic fluid collections and acute necrotic collections in the early phase usually can be treated with supportive care. In contrast, PPC and WON may require invasive intervention when they are symptomatic or suspected of infection. The treatment of PPC is relatively simple. Currently, the EUS-guided transmural drainage with plastic stents is the preferred approach, which has high efficacy and safety. The treatment of WON is more complex and challenging. With the development of the minimally invasive intervention, both morbidity and mortality of WON have been dramatically reduced. Currently, the step-up approaches are the standard treatment for symptomatic WON. Given the high risk of pancreatic fistulae in percutaneous drainage or surgical minimally invasive step-up approach, the endoscopic step-up approach is now the most preferred intervention if the lesions can be reached endoscopically. However, this procedure is still full of challenges due to the stent-related problems. Therefore, innovative approaches to improve the prognosis of WON are still thirstily needed.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aseni P, Hayashi K S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LL
| 1. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 582] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 2. | Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial Medical Treatment of Acute Pancreatitis: American Gastroenterological Association Institute Technical Review. Gastroenterology. 2018;154:1103-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 3. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 561] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 4. | Manrai M, Kochhar R, Gupta V, Yadav TD, Dhaka N, Kalra N, Sinha SK, Khandelwal N. Outcome of Acute Pancreatic and Peripancreatic Collections Occurring in Patients With Acute Pancreatitis. Ann Surg. 2018;267:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Bai X, Jin M, Zhang H, Lu B, Yang H, Qian J. Evaluation of Chinese updated guideline for acute pancreatitis on management of moderately severe and severe acute pancreatitis. Pancreatology. 2020;20:1582-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4330] [Article Influence: 360.8] [Reference Citation Analysis (45)] |
| 7. | Kim KO, Kim TN. Acute pancreatic pseudocyst: incidence, risk factors, and clinical outcomes. Pancreas. 2012;41:577-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Tan JH, Zhou L, Cao RC, Zhang GW. Identification of risk factors for pancreatic pseudocysts formation, intervention and recurrence: a 15-year retrospective analysis in a tertiary hospital in China. BMC Gastroenterol. 2018;18:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Cui ML, Kim KH, Kim HG, Han J, Kim H, Cho KB, Jung MK, Cho CM, Kim TN. Incidence, risk factors and clinical course of pancreatic fluid collections in acute pancreatitis. Dig Dis Sci. 2014;59:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Rasch S, Nötzel B, Phillip V, Lahmer T, Schmid RM, Algül H. Management of pancreatic pseudocysts-A retrospective analysis. PLoS One. 2017;12:e0184374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Keane MG, Sze SF, Cieplik N, Murray S, Johnson GJ, Webster GJ, Thorburn D, Pereira SP. Endoscopic versus percutaneous drainage of symptomatic pancreatic fluid collections: a 14-year experience from a tertiary hepatobiliary centre. Surg Endosc. 2016;30:3730-3740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 12. | Tan JH, Chin W, Shaikh AL, Zheng S. Pancreatic pseudocyst: Dilemma of its recent management (Review). Exp Ther Med. 2021;21:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1382] [Article Influence: 115.2] [Reference Citation Analysis (3)] |
| 14. | Akshintala VS, Saxena P, Zaheer A, Rana U, Hutfless SM, Lennon AM, Canto MI, Kalloo AN, Khashab MA, Singh VK. A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc. 2014;79:921-928; quiz 983.e2, 983.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Khaled YS, Malde DJ, Packer J, Fox T, Laftsidis P, Ajala-Agbo T, De Liguori Carino N, Deshpande R, O'Reilly DA, Sherlock DJ, Ammori BJ. Laparoscopic versus open cystgastrostomy for pancreatic pseudocysts: a case-matched comparative study. J Hepatobiliary Pancreat Sci. 2014;21:818-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 331] [Article Influence: 27.6] [Reference Citation Analysis (1)] |
| 17. | Zhao X, Feng T, Ji W. Endoscopic versus surgical treatment for pancreatic pseudocyst. Dig Endosc. 2016;28:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Saul A, Ramirez Luna MA, Chan C, Uscanga L, Valdovinos Andraca F, Hernandez Calleros J, Elizondo J, Tellez Avila F. EUS-guided drainage of pancreatic pseudocysts offers similar success and complications compared to surgical treatment but with a lower cost. Surg Endosc. 2016;30:1459-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Farias GFA, Bernardo WM, De Moura DTH, Guedes HG, Brunaldi VO, Visconti TAC, Gonçalves CVT, Sakai CM, Matuguma SE, Santos MELD, Sakai P, De Moura EGH. Endoscopic versus surgical treatment for pancreatic pseudocysts: Systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e14255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 20. | Garg PK, Meena D, Babu D, Padhan RK, Dhingra R, Krishna A, Kumar S, Misra MC, Bansal VK. Endoscopic versus laparoscopic drainage of pseudocyst and walled-off necrosis following acute pancreatitis: a randomized trial. Surg Endosc. 2020;34:1157-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Szakó L, Mátrai P, Hegyi P, Pécsi D, Gyöngyi Z, Csupor D, Bajor J, Erőss B, Mikó A, Szakács Z, Dobszai D, Meczker Á, Márta K, Rostás I, Vincze Á. Endoscopic and surgical drainage for pancreatic fluid collections are better than percutaneous drainage: Meta-analysis. Pancreatology. 2020;20:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Teoh AY, Dhir V, Jin ZD, Kida M, Seo DW, Ho KY. Systematic review comparing endoscopic, percutaneous and surgical pancreatic pseudocyst drainage. World J Gastrointest Endosc. 2016;8:310-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Bang JY, Wilcox CM, Trevino JM, Ramesh J, Hasan M, Hawes RH, Varadarajulu S. Relationship between stent characteristics and treatment outcomes in endoscopic transmural drainage of uncomplicated pancreatic pseudocysts. Surg Endosc. 2014;28:2877-2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Shin HC, Cho CM, Jung MK, Yeo SJ. Comparison of Clinical Outcomes between Plastic Stent and Novel Lumen-apposing Metal Stent for Endoscopic Ultrasound-Guided Drainage of Peripancreatic Fluid Collections. Clin Endosc. 2019;52:353-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Bang JY, Hasan MK, Navaneethan U, Sutton B, Frandah W, Siddique S, Hawes RH, Varadarajulu S. Lumen-apposing metal stents for drainage of pancreatic fluid collections: When and for whom? Dig Endosc. 2017;29:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 26. | Sharaiha RZ, DeFilippis EM, Kedia P, Gaidhane M, Boumitri C, Lim HW, Han E, Singh H, Ghumman SS, Kowalski T, Loren D, Kahaleh M, Siddiqui A. Metal versus plastic for pancreatic pseudocyst drainage: clinical outcomes and success. Gastrointest Endosc. 2015;82:822-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Yoon SB, Lee IS, Choi MG. Metal versus plastic stents for drainage of pancreatic fluid collection: A meta-analysis. United European Gastroenterol J. 2018;6:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Yang D, Amin S, Gonzalez S, Mullady D, Hasak S, Gaddam S, Edmundowicz SA, Gromski MA, DeWitt JM, El Zein M, Khashab MA, Wang AY, Gaspar JP, Uppal DS, Nagula S, Kapadia S, Buscaglia JM, Bucobo JC, Schlachterman A, Wagh MS, Draganov PV, Jung MK, Stevens T, Vargo JJ, Khara HS, Huseini M, Diehl DL, Keswani RN, Law R, Komanduri S, Yachimski PS, DaVee T, Prabhu A, Lapp RT, Kwon RS, Watson RR, Goodman AJ, Chhabra N, Wang WJ, Benias P, Carr-Locke DL, DiMaio CJ. Transpapillary drainage has no added benefit on treatment outcomes in patients undergoing EUS-guided transmural drainage of pancreatic pseudocysts: a large multicenter study. Gastrointest Endosc. 2016;83:720-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Amin S, Yang DJ, Lucas AL, Gonzalez S, DiMaio CJ. There Is No Advantage to Transpapillary Pancreatic Duct Stenting for the Transmural Endoscopic Drainage of Pancreatic Fluid Collections: A Meta-Analysis. Clin Endosc. 2017;50:388-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 31. | Panamonta N, Ngamruengphong S, Kijsirichareanchai K, Nugent K, Rakvit A. Endoscopic ultrasound-guided versus conventional transmural techniques have comparable treatment outcomes in draining pancreatic pseudocysts. Eur J Gastroenterol Hepatol. 2012;24:1355-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Lang GD, Fritz C, Bhat T, Das KK, Murad FM, Early DS, Edmundowicz SA, Kushnir VM, Mullady DK. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: comparison of efficacy and adverse event rates. Gastrointest Endosc. 2018;87:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 33. | Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology. 2020;158:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 417] [Article Influence: 83.4] [Reference Citation Analysis (2)] |
| 34. | Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current Concepts in Severe Acute and Necrotizing Pancreatitis: An Evidence-Based Approach. Gastroenterology 2019; 156: 1994-2007. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 35. | van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Dejong CH, van Eijck CH, van Ramshorst B, Schaapherder AF, van der Harst E, Hofker S, Nieuwenhuijs VB, Brink MA, Kruyt PM, Manusama ER, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, Cuesta MA, Wahab PJ, Gooszen HG; Dutch Pancreatitis Study Group. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 466] [Article Influence: 33.3] [Reference Citation Analysis (2)] |
| 36. | Besselink MG, Verwer TJ, Schoenmaeckers EJ, Buskens E, Ridwan BU, Visser MR, Nieuwenhuijs VB, Gooszen HG. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg. 2007;142:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 37. | Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N, Sartelli M, Wolbrink D, van Goor H, Baiocchi G, Ansaloni L, Biffl W, Coccolini F, Di Saverio S, Kluger Y, Moore E, Catena F. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 426] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 38. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1039] [Article Influence: 86.6] [Reference Citation Analysis (6)] |
| 39. | Guo Q, Li A, Xia Q, Lu H, Ke N, Du X, Zhang Z, Hu W. Timing of intervention in necrotizing pancreatitis. J Gastrointest Surg. 2014;18:1770-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | van Grinsven J, van Santvoort HC, Boermeester MA, Dejong CH, van Eijck CH, Fockens P, Besselink MG; Dutch Pancreatitis Study Group. Timing of catheter drainage in infected necrotizing pancreatitis. Nat Rev Gastroenterol Hepatol. 2016;13:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | van Grinsven J, van Brunschot S, Bakker OJ, Bollen TL, Boermeester MA, Bruno MJ, Dejong CH, Dijkgraaf MG, van Eijck CH, Fockens P, van Goor H, Gooszen HG, Horvath KD, van Lienden KP, van Santvoort HC, Besselink MG; Dutch Pancreatitis Study Group. Diagnostic strategy and timing of intervention in infected necrotizing pancreatitis: an international expert survey and case vignette study. HPB (Oxford). 2016;18:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Trikudanathan G, Tawfik P, Amateau SK, Munigala S, Arain M, Attam R, Beilman G, Flanagan S, Freeman ML, Mallery S. Early (<4 Weeks) Versus Standard (≥ 4 Weeks) Endoscopically Centered Step-Up Interventions for Necrotizing Pancreatitis. Am J Gastroenterol. 2018;113:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 43. | van Grinsven J, van Dijk SM, Dijkgraaf MG, Boermeester MA, Bollen TL, Bruno MJ, van Brunschot S, Dejong CH, van Eijck CH, van Lienden KP, Boerma D, van Duijvendijk P, Hadithi M, Haveman JW, van der Hulst RW, Jansen JM, Lips DJ, Manusama ER, Molenaar IQ, van der Peet DL, Poen AC, Quispel R, Schaapherder AF, Schoon EJ, Schwartz MP, Seerden TC, Spanier BWM, Straathof JW, Venneman NG, van de Vrie W, Witteman BJ, van Goor H, Fockens P, van Santvoort HC, Besselink MG; Dutch Pancreatitis Study Group. Postponed or immediate drainage of infected necrotizing pancreatitis (POINTER trial): study protocol for a randomized controlled trial. Trials. 2019;20:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Hollemans RA, Bakker OJ, Boermeester MA, Bollen TL, Bosscha K, Bruno MJ, Buskens E, Dejong CH, van Duijvendijk P, van Eijck CH, Fockens P, van Goor H, van Grevenstein WM, van der Harst E, Heisterkamp J, Hesselink EJ, Hofker S, Houdijk AP, Karsten T, Kruyt PM, van Laarhoven CJ, Laméris JS, van Leeuwen MS, Manusama ER, Molenaar IQ, Nieuwenhuijs VB, van Ramshorst B, Roos D, Rosman C, Schaapherder AF, van der Schelling GP, Timmer R, Verdonk RC, de Wit RJ, Gooszen HG, Besselink MG, van Santvoort HC; Dutch Pancreatitis Study Group. Superiority of Step-up Approach vs Open Necrosectomy in Long-term Follow-up of Patients With Necrotizing Pancreatitis. Gastroenterology. 2019;156:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 45. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1036] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 46. | Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016;375:1972-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 556] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 47. | Loveday BP, Petrov MS, Connor S, Rossaak JI, Mittal A, Phillips AR, Windsor JA; Pancreas Network of New Zealand. A comprehensive classification of invasive procedures for treating the local complications of acute pancreatitis based on visualization, route, and purpose. Pancreatology. 2011;11:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG; Dutch Pancreatitis Study Group. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg. 2011;98:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 49. | van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, van Eijck CH, Erkelens WG, van Goor H, van Grevenstein WMU, Haveman JW, Hofker SH, Jansen JM, Laméris JS, van Lienden KP, Meijssen MA, Mulder CJ, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TE, Scheepers JJ, Schepers NJ, Schwartz MP, Seerden T, Spanier BWM, Straathof JWA, Strijker M, Timmer R, Venneman NG, Vleggaar FP, Voermans RP, Witteman BJ, Gooszen HG, Dijkgraaf MG, Fockens P; Dutch Pancreatitis Study Group. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018;391:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 474] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 50. | Ross AS, Irani S, Gan SI, Rocha F, Siegal J, Fotoohi M, Hauptmann E, Robinson D, Crane R, Kozarek R, Gluck M. Dual-modality drainage of infected and symptomatic walled-off pancreatic necrosis: long-term clinical outcomes. Gastrointest Endosc. 2014;79:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 51. | Wang PF, Liu ZW, Cai SW, Su JJ, He L, Feng J, Xin XL, Lu SC. Usefulness of three-dimensional visualization technology in minimally invasive treatment for infected necrotizing pancreatitis. World J Gastroenterol. 2018;24:1911-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM, Poley JW, van Ramshorst B, Vleggaar FP, Boermeester MA, Gooszen HG, Weusten BL, Timmer R; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 498] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 53. | Bang JY, Arnoletti JP, Holt BA, Sutton B, Hasan MK, Navaneethan U, Feranec N, Wilcox CM, Tharian B, Hawes RH, Varadarajulu S. An Endoscopic Transluminal Approach, Compared With Minimally Invasive Surgery, Reduces Complications and Costs for Patients With Necrotizing Pancreatitis. Gastroenterology. 2019;156:1027-1040.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 54. | Arvanitakis M, Dumonceau JM, Albert J, Badaoui A, Bali MA, Barthet M, Besselink M, Deviere J, Oliveira Ferreira A, Gyökeres T, Hritz I, Hucl T, Milashka M, Papanikolaou IS, Poley JW, Seewald S, Vanbiervliet G, van Lienden K, van Santvoort H, Voermans R, Delhaye M, van Hooft J. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. 2018;50:524-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 290] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 55. | ASGE Standards of Practice Committee; Muthusamy VR, Chandrasekhara V, Acosta RD, Bruining DH, Chathadi KV, Eloubeidi MA, Faulx AL, Fonkalsrud L, Gurudu SR, Khashab MA, Kothari S, Lightdale JR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Yang J, Cash BD, DeWitt JM. The role of endoscopy in the diagnosis and treatment of inflammatory pancreatic fluid collections. Gastrointest Endosc. 2016;83:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 56. | Chacaltana Mendoza A, Li Salvatierra B, Llatas Perez J, Diaz Rios R, Vera Calderon A. [Efficacy and safety of echoendoscopy drainage of liquid peripancreatic collections in a reference hospital]. Rev Gastroenterol Peru. 2020;40:46-51. [PubMed] |
| 57. | Ang TL, Kongkam P, Kwek AB, Orkoonsawat P, Rerknimitr R, Fock KM. A two-center comparative study of plastic and lumen-apposing large diameter self-expandable metallic stents in endoscopic ultrasound-guided drainage of pancreatic fluid collections. Endosc Ultrasound. 2016;5:320-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Lee BU, Song TJ, Lee SS, Park DH, Seo DW, Lee SK, Kim MH. Newly designed, fully covered metal stents for endoscopic ultrasound (EUS)-guided transmural drainage of peripancreatic fluid collections: a prospective randomized study. Endoscopy. 2014;46:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 59. | Hammad T, Khan MA, Alastal Y, Lee W, Nawras A, Ismail MK, Kahaleh M. Efficacy and Safety of Lumen-Apposing Metal Stents in Management of Pancreatic Fluid Collections: Are They Better Than Plastic Stents? Dig Dis Sci. 2018;63:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 60. | Al Lehibi A, Al Jabri A, Abbarh S, Al Balkhi A, Al Otaibi N, Almasoudi T, Al Mtawa A, AlGhamdi A, Al Eid A, Al Ghamdi A, Al Khathlan A, Qutub A, Al Sayari K, Ahmad S. Peripancreatic fluid collections, plastic stents, and different sub-types of metal stents: Where does the evidence land? Saudi J Gastroenterol. 2021;27:85-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Park CH, Park SW, Nam E, Jung JH, Jo JH. Comparative efficacy of stents in endoscopic ultrasonography-guided peripancreatic fluid collection drainage: A systematic review and network meta-analysis. J Gastroenterol Hepatol. 2020;35:941-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Brimhall B, Han S, Tatman PD, Clark TJ, Wani S, Brauer B, Edmundowicz S, Wagh MS, Attwell A, Hammad H, Shah RJ. Increased Incidence of Pseudoaneurysm Bleeding With Lumen-Apposing Metal Stents Compared to Double-Pigtail Plastic Stents in Patients With Peripancreatic Fluid Collections. Clin Gastroenterol Hepatol. 2018;16:1521-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 63. | Bang JY, Navaneethan U, Hasan MK, Sutton B, Hawes R, Varadarajulu S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut. 2019;68:1200-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 64. | Bang JY, Hawes R, Bartolucci A, Varadarajulu S. Efficacy of metal and plastic stents for transmural drainage of pancreatic fluid collections: a systematic review. Dig Endosc. 2015;27:486-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 65. | Liu F, Wu L, Wang XD, Xiao JG, Li W. Endoscopic gastric fenestration of debriding pancreatic walled-off necrosis: A pilot study. World J Gastroenterol. 2020;26:6431-6441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |