Published online Jun 27, 2021. doi: 10.4240/wjgs.v13.i6.597
Peer-review started: March 15, 2021
First decision: May 4, 2021
Revised: May 4, 2021
Accepted: May 19, 2021
Article in press: May 19, 2021
Published online: June 27, 2021
Processing time: 95 Days and 3.4 Hours
The benefits of laparoscopic approach for right colectomy have been well established. However, the technical difficulty to construct the intra-corporeal anastomosis is still cumbersome.
To analyze the results of 3D and 2D laparoscopic right colectomy and to compare it to the published series through a systematic review and meta-analysis.
A retrospective study with propensity score matching analysis of patients undergoing laparoscopic right colectomy at Umbria2 Hospitals from January 2014 to March 2020 was performed. A systematic review was accomplished comparing 2D and 3D right colectomy.
In the personal series 47 patients of the 2D group were matched to 47 patients of the 3D group. The 3D group showed a favorable trend in terms of mean operative time (170.7 ± 32.9 min vs 183.8 ± 35.4 min; P = 0.053) and a significant lower anastomotic time (16.9 ± 2.3 min vs 19.6 ± 2.9 min, P < 0.001). The complete mesocolic excision (CME) subgroups analysis showed a shorter anastomotic time (16.5 ± 1.8 min vs 19.9 ± 3.0 min; P < 0.001) and operative time (175.0 ± 38.5 min vs 193.7 ± 37.1 min; P = 0.063) in the 3D group. Six studies and our series were included in the meta-analysis with 551 patients (2D group: 291; 3D group: 260).The pooled analysis demonstrated a significant difference in favour of the 3D group regarding the operative time (P < 0.001) and the anastomotic time (P < 0.001) while no differences were identified between groups in terms of blood loss (P = 0.827), LNH yield (P = 0.243), time to first flatus (P = 0.333), postoperative complications (P = 0.718) and length of stay (P = 0.835).
The meta-analysis results showed that 3D laparoscopic right colectomy shortens operative and anastomotic time without affecting the standard lymphadenectomy. In our series, the advantage of the 3D system becomes evident when CME and/or more complex associated procedure are requested significantly reducing both the total operative and the anastomotic time.
Core Tip: The technological improvements introducing the three-dimensional vision in laparoscopic systems provided some of the advantages of robotic platform; thus, 3D laparoscopic surgery has emerged as a competitive alternative to the robotic one. Recently, we compared robotic surgery and 3D laparoscopy for right colectomy with complete mesocolic excision and intra-corporeal anastomosis. Now, we undertook the present study with the aim to appraise our whole experience in the use of 3D laparoscopic system in right colectomy. In addition, we performed a meta-analysis in order to compare our results to the literature ones in the attempt to increase the statistical power and level of evidence.
- Citation: Costa G, Fransvea P, Lepre L, Rondelli F, Costa A, Campanelli M, Lisi G, Mastrangeli MR, Laracca GG, Garbarino GM, Ceccarelli G. 2D vs 3D laparoscopic right colectomy: A propensity score-matching comparison of personal experience with systematic review and meta-analysis. World J Gastrointest Surg 2021; 13(6): 597-619
- URL: https://www.wjgnet.com/1948-9366/full/v13/i6/597.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i6.597
According to estimates from World Health Organization and from other national cancer institutes and registers, colorectal cancer (CRC) is the third most common cancer and represents the second most common cause of cancer related death[1,2]. It has been reported that right-sided tumor exhibits peculiar features such as the fact that it is more frequently observed in older patients and detected at an advanced stage[3].
The benefits of laparoscopic approach for patients with CRC have been well established even in the elderly and in advanced stage[4-7]. The minimally invasive surgery is associated with less intra-operative blood loss, better pulmonary function, reduced postoperative pain, quicker return of bowel function, a shorter hospital stay, and a lower incisional hernia incidence with similar oncologic results when compared with standard open surgery[8-10]. However, with specific regard to some technical aspects of right colectomy, the difficulty to construct the intra-corporeal anastomosis questioned the adoption of laparoscopic approach to perform such colonic resection as well as it occurred in other surgical fields[11-15]. Although nowadays a side-to-side stapled intra-corporeal anastomosis gained popularity[14,16,17], it still remains technically demanding requiring advanced skills. Indeed, the anastomotic leak rate seems to be higher even when performed by experienced surgeons[18].
The robotic platform with his advantages in 3D vision and with the Endowrist© technology overcame the drawbacks of standard laparoscopy offering the same beneficial effects of minimally invasive approach[19-24]. Despite these undoubted advantages, the use of robotic approach might be restricted because limited resources and high costs[24,25].
The technological improvements introducing the three-dimensional vision in laparoscopic systems provided some of the advantages of robotic platform; thus, 3D laparoscopic surgery has emerged as a competitive alternative to the robotic one. In a recent paper, we compare robotic surgery and 3D laparoscopy for right colectomy with complete mesocolic excision (CME) and intra-corporeal anastomosis[26]. Given our experience in minimally invasive colorectal surgery and driven to such previous effort, we wanted to undertake the present study with the aim to critically appraise our whole experience in the use of 3D laparoscopic system in right colectomy making a comparison with the 2D one. In addition, we performed a systematic review of published series on this issue and carried out a meta-analysis of available data in order to compare our results to the literature ones in the attempt to increase the statistical power and level of evidence.
We performed a retrospective observational clinical study. Medical charts of patients who underwent right colectomy by mean of minimally invasive approach at Umbria2 Local Health Service Hospitals in Spoleto and Foligno from January 2014 to March 2020 were reviewed. Patients were retrieved from the theatre electronic databanks using the International Classification of Diseases versions 9 (ICD-9™) [codes: 45.72 to 45.74 and 54.21 and/or 0.39]. As reported by others, only procedures performed by qualified colorectal surgeons with adequate laparoscopic experience were considered [27]. Procedure performed by novice surgeons or residents were excluded. We further selected adenocarcinoma or neuroendocrine tumour (NET) confirmed by pathological examination limited to the following tumor locations: cecum, ascending colon and hepatic flexure. Exclusion criteria included malignant lymphoma or other non-cancer cases, and emergency procedures. Locally advanced tumor as well as hepatic metastases or concomitant conditions requiring surgical treatment were not considered exclusion criteria. With regard to the surgical approach only 2D or 3D laparoscopic procedures were finally considered for analysis. The STROBE (Streng
All clinical records were reviewed in terms of demographics and clinical variables, procedure details, peri-operative outcomes and oncologic data. Demographics variables included: age, gender distribution, body mass index (BMI), ASA classification, comorbidity, tumor size (considered as the greatest dimension reported in any pre-operative work-up study), and staging. Comorbidity was recorded if the condition was being medically treated at the time of admission, or if previous treatment for the condition was described in the admission report and were consi
Procedure details included operative time, anastomotic time, whether or not the CME was carried out, time to first flatus, length of hospital stay (LOS), 30-d post-operative complication arranged by Clavien-Dindo (C-D)[30]. The occurrence of intraoperative complications and the conversion rate were also recorded. A senior staff surgeon (G. Costa) blinded to operative surgeon and patient’s postoperative course graded the complexity level of any additional procedure as previously described[26]. No specific enhanced recovery after surgery (ERAS) protocol was adopted. Oncologic data included TNM classification, number of retrieved lymph nodes, number of positive lymph nodes and lymph node ratio. The TNM 8th edition of UICC classification system was adopted for staging the tumors.
A formal institutional review board approval was not required because of the un-interventional retrospective design; however, a signed consent for the data treatment and storage for scientific purpose was obtained from all patients at hospital admission.
All the right colectomies were performed with the same surgical technique both with 2D or 3D placing 4 ports: in left hypochondrium, in umbilicus, in right iliac fossa, and in left flank respectively. The 2D laparoscopic procedures were performed using the IMAGE1 Camera-System (Storz, Tuttlingen, Germany) or the Olympus CH-S200-XZ-EB (Olympus Surgical Technologies Europe, Hamburg, Germany). The three-dimensional (3D) procedures were performed using the 3D-HD Viking Camera System (Conmed, Utica, New York, United States).
No formal protocol for the allocation of patients to either group was established. The key steps of the surgical procedure were elsewhere described[26]. Briefly, a bottom-up approach with transection of the ileocolic vessels as close as possible to the root and a medial-to-lateral mobilization of the colon were performed. Intra-corporeal ileocolic side-to-side anastomosis was constructed in isoperistaltic manner with a stapler and the entero-colotomy was sutured in continuous double layer with two separate knotless barbed suture. The mesenteric window was left opened and the specimen was extracted with an endobag through the enlarged periumbilical port incision[31].
Propensity scores were calculated by bivariate logistic regression, including the following variables that might be considered as potential baseline confounders between the groups: sex, age, BMI, size of tumor, CME yes or not, complexity grade of concomitant procedure. We matched propensity scores 1:1 with the use of the nearest neighbor methods without replacement by using the closest calipers width to achieve the maximum number of cases without statistical differences in confounders variables. In this instance the caliper width was set at 0.2.
Statistical analysis was performed with SPSS software version 21 (IBM Analytics Italy, Segrate, MI) integrated with SPSS R Essentials for R Statistical Software version 2.14.2 (Foundation for Statistical Computing, Vienna, Austria). The distribution of continuous variables is reported as mean and standard deviation and/or as median with range and/or 25%-75% Interquartile Range (IQR 25%-75%) when of clinical relevance. Categorical variables are presented as numbers and percentages. Pre-matching and post-matching data were compared between the two groups. Differences were analysed using the Mann–Whitney U test for continuous variables. Qualitative data were compared using the Chi-square test with or without Yates’ correction or the Fisher’s exact test when necessary. All statistical analysis was performed with the two-sided method. Statistical significance was considered with P values of less than 0.05. G-Power for MacOSX version 3.1 was used to carry out a post hoc analysis for the χ2 test and t- test in order to evaluate the power estimation aimed at assessing the adequacy of the CME subgroups sample sizes.
Literature search strategy: A systematic review was accomplished according to the PRISMA statement[32] in order to identify articles comparing 2D and 3D system vision in performing right colectomy. In this paper a literature search was carried out through MEDLINE (PubMed), Embase, WebOfScience, Scopus, and The Cochrane Library from January 1980 to 31 October 2020. The following keywords and/or medical subject heading (MeSH) terms were used in combination: “2D”, “two-dimensional”, “3D”, “three-dimensional”, “laparoscopy”, “colon”, “colorectal surgery”, and “right colectomy”. A manually search has also been performed in Google Scholar and in the reference lists of relevant articles to find potential additional studies. The search was carried out by using English language terms but no restriction was adopted to exclude any paper neither by language nor by study type. Records retrieved have been managed by Mendeley Desktop version 1.19.4.
Inclusion and exclusion criteria: Studies were selected according to the PICOS criteria[33] (population, intervention, comparison, outcomes, and study design). More specifically, only studies reporting a comparison of the use of 2D and 3D systems on adult patients undergoing laparoscopic right colectomy were considered. At least one peri-operative outcome of interest should be reported. Studies comparing 3D robotic vision to 2D laparoscopic vision were excluded. Any paper was excluded from the quantitative study whenever it was not possible to quantify the number of patients or the outcomes of interest in each group as well as case series without control group, case report, technical note or paper related to video. Whenever the same group of authors presented multiple papers through years, all the papers were considered, but only the most informative or highest quality study was included. Paper in Chinese language[34] has been purchased from the journal web site and translated by an on-line translator. The translation has been further checked by a Chinese language teacher.
Data extraction and quality assessment: According to the eligibility criteria in order to minimize selection bias, two pairs of reviewers (P.F.–L.L. and G.M.G–F.R.) independently reviewed each paper and assessed the quality of the studies by using the Newcastle-Ottawa Scale. In addition, two reviewers (P.F and G.M.G.) indepen
The following general demographic informations were identified and collected when available: age, gender distribution, body mass index (BMI), ASA classification, and tumour size and/or staging. The following surgical outcomes were considered: operating time, anastomotic time, blood loss, lymph nodes harvested, intraoperative complications, conversion to open approach, time to first flatus, LOS, 30-d post-operative morbidity, and mortality. Intraoperative and/or postoperative complications were reported both as quantitative and qualitative whenever possible.
Continuous variables were analysed by the weighted mean difference (WMD) and 95% confidence interval (CI). Categorical variables were evaluated using the odds ratio (OR) and 95%CI. When variables were reported in the papers as median and range or interquartile range, they have been converted to mean and standard deviation (SD) according to well established method.
The degrees of heterogeneity between the studies were assessed by the I2 value. We considered an I2 value of 40% or lower as trivial or not important heterogeneity and an I2 value of 75% or higher as considerable heterogeneity. When I2 value was higher than 50%, pooled estimates were obtained using a random effects model with the generic inverse variance method. As regard to p value of Q index (chi-square test of heterogeneity) a P < 0.10 was considered significant otherwise a conventional level of P < 0.05 was accepted as statistically significant. All statistical analysis and forest and funnel plot regarding meta-analysis was carried out and generated using the Jamovi Software (Version 1.2.22) integrated with the plug-in module for the R Statistical software. (The Jamovi project (2019) retrieved from https://www.jamovi.org and R Core Team (2018). R: A Language and enviroment for statistical computing retrieved from https://cran.r-project.org/).
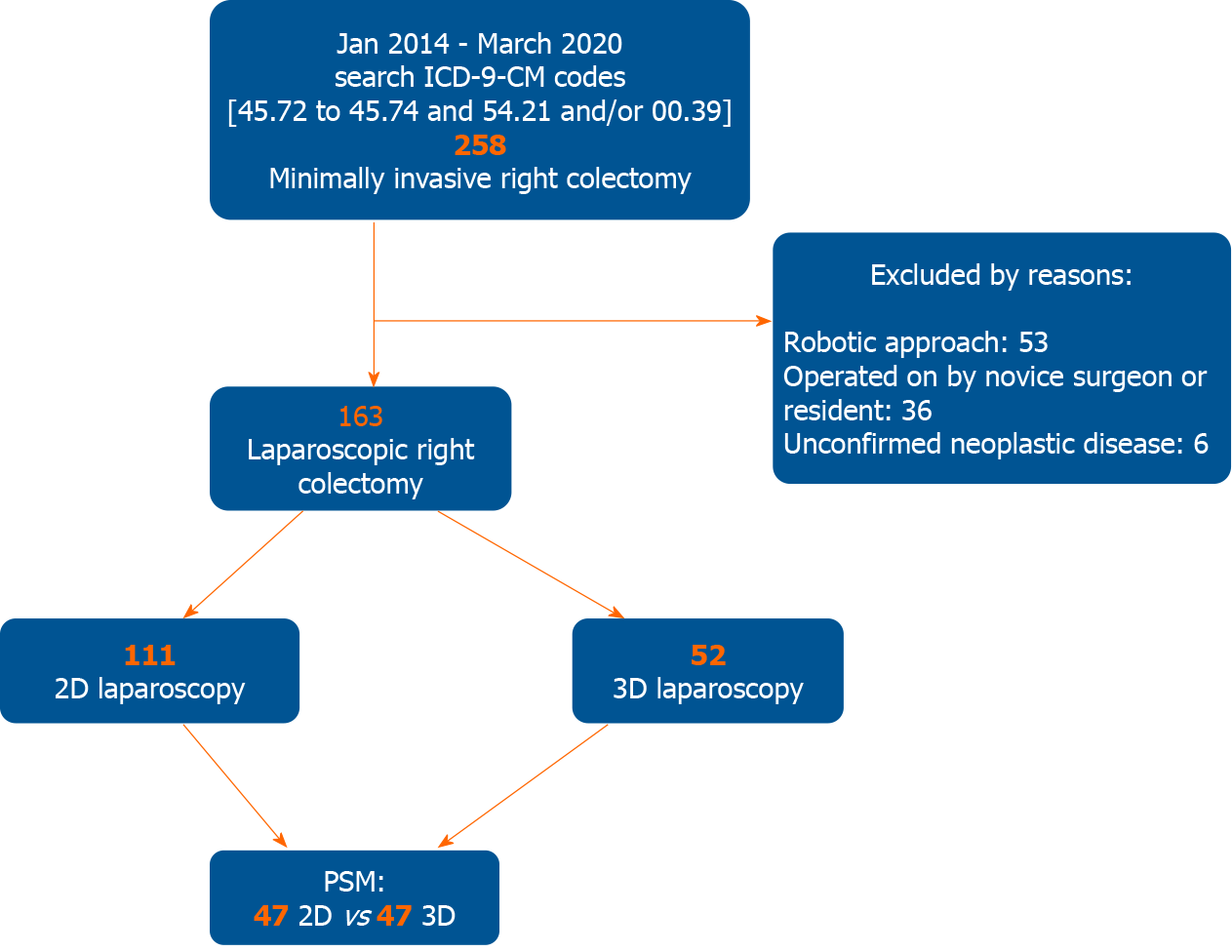
A total of 258 patients undergoing minimally invasive right colectomy for cancer in the study period were selected for this study. Of these, 163 patients fulfilled the inclusion criteria. One hundred-eleven were operated with a 2D system and 52 by mean of a 3D system. (Figure 1) The mean age was 68.6 ± 12.9 years for the 2D group and 73.2 ± 12.5 years for the 3D laparoscopic group (P = 0.006).
The mean operative time was 185.3 ± 48.6 min in the 2D group and 169.8 ± 32.4 in the 3D laparoscopic group. The difference was almost statistically significant (P = 0.087). The mean anastomotic time was 19.3 ± 2.9 min in the 2D group while was 16.9 ± 2.3 min in the 3D one (P < 0.001). About the operative time, it must be considered that CME was accomplished in 41 cases (36.9%) in the 2D group and in 30 cases (57.7%) in the 3D group. The difference was statistically significant (P = 0.013). Moreover, 40 patients (36.0%) in the 2D group and 25 (48.1%) in the 3D one had an associate procedure. The difference did not reach statistical significance. In the 2D cohort 14 cholecystectomies, 3 cholecystectomies with adhesiolysis, 9 adhesiolysis, 1 adhesiolysis plus ileal resection, 7 hepatic wedge resections, 1 hepatic wedge resection plus ileal resection, 1 ileal resection, 1 Liver cyst deroofing, 1 annessectomy, 1 uterine fibroid removal, and 1 abdominal wall repair for incisional hernia were performed; in the 3D group 8 cholecystectomies, 2 cholecystectomies with adhesiolysis, 10 adhesiolysis, 1 adhesiolysis plus ileal resection, 3 hepatic wedge resections, and 1 Limited resection of the pancreatic tail with splenectomy were performed. The latter was performed because of an incidental pancreatic mass. Definitive pathological examination revealed a papillary cystic neoplasm.
Mean level of complexity of associated procedures resulted comparable between the two groups (4.06 ± 0.89 in 2D group and 3.92 ± 0.79 in 3D group). No intraoperative complications occurred either in the 2D group or in the 3D one. Conversion was required in 10 patients (9.0%) in the 2D group and in 1 patient (1.9%) in the 3D group [P = 0.176; odds ratio: 5.050, 95%CI: 0.629-40.500). In the two-dimensional group the causes of conversion were: dense matted adhesions in 5 cases, gross locally advanced tumour in 3 cases, oncological safety because of metachronous pancreatic NET in 1 case, and failure of intraoperative localization of endoscopic tattooing in 1 case. The cause of conversion in the three-dimensional group was a gross locally advanced tumour requiring ureteral resection with end-to-end anastomosis over a double-J stent.
The mean time to first flatus was 3.21 ± 1.26 d in the 2D group and 3.25 ± 1.08 d in the 3D group (P = 0.606). The length of post-operative stay in 2D and 3D group was 8.36 ± 5.89 d and 7.69 ± 2.17 d (P = 0.858), respectively.
Post-operative complications occurred in 25 patients (22.5%) in 2D group: 13 patients had C-D grade I complication (canalization delay with vomiting, anemia, pneumonia, urinary retention, and wound infection); 7 patients had C-D grade II complication (mild respiratory insufficiency, atrial fibrillation, transitory ischaemic attack, prolonged postoperative ileus, intra-abdominal bleeding which required blood transfusion); 3 patients had C-D grade III complication (upper GI bleeding treated by endoscopic haemostasis, anastomotic leakage, small bowel obstruction); and 2 patients had C-D grade IV complication (acute renal failure requiring dialysis, myocardial infarction).
Post-operative complications occurred in 12 patients (23.1%) in 3D laparoscopic group: 6 patients had C-D grade I complication (canalization delay with vomiting, anemia, transient lymphorrhea, anastomotic bleeding not requiring blood transfusion, pneumonia, and wound infection); 3 patients had C-D grade II complication (intra-abdominal bleeding which required transfusion, atrial fibrillation); 2 patients had C-D grade III complication (one of these as a consequence of accidental removal of drain on postoperative day three, developed a fluid collection due to ‘biochemical leak’ [former defined as grade A pancreatic fistula] requested a percutaneous drainage; the other complication was arrhythmia requiring pacemaker implantation); and 1 patient had C-D grade IV complication (ischaemic stroke). The difference in overall morbidity was not statistically significant (P = 0.937).
The overall mortality rates in 2D and 3D groups were 1.8% (2 patients) and 1.9% (1 patient) respectively. In all cases, mortality was due to non-surgical complications.
The total number of retrieved lymph nodes was slightly greater in the 2D group (19.6 ± 6.6 vs 18.8 ± 7.4), however the difference did not turn out to be statistically significant (P = 0.400).
After the propensity score-matching (PSM) procedure, 47 patients of the 2D group and 47 patients of the 3D group were selected for the analysis. Conversion was required in 1 patient (2.1%) in the 2D group and in 1 patient (2.1%) in the 3D group (P = 1.000). In the two-dimensional group the cause of conversion was the search for oncological adequacy because of metachronous pancreatic NET; the cause of conversion in the three-dimensional group has been previously described. There were no differences in any of the analyzed variables between the two groups, except for the anastomotic time (19.6 ± 2.9 min in the 2D group and 16.9 ± 2.3 min in the 3D one) (P < 0.001). Again, the difference in mean operative time was almost statistically significant (183.8 ± 35.4 min in the 2D group and 170.7 ± 32.9 min in the 3D one; P = 0.053). The total number of retrieved lymph nodes was slightly greater in the 3D group (18.9 ± 7.3 vs 17.8 ± 5.2). The difference was not statistically significant (P = 0.654). Although slightly higher after 3D surgery, the overall morbidity rates were comparable between the two groups without statistical significance (25.5% vs 21.3%; P = 0.626; OR = 0.788, 95%CI: 0.302-2.050). Demographic characteristics, procedure details, post-operative course, and oncologic data of patients pre and post propensity matching study are shown in Tables 1-3.
| Before propensity score matching | After propensity score matching | |||||
| 2D, n = 111 | 3D, n = 52 | P value | 2D, n = 47 | 3D, n = 47 | P value | |
| Age (yr), mean ± SD | 68.6 ± 12.9 | 73.2 ± 12.5 | 0.006 | 71.8 ± 12.3 | 72.8 ± 12.4 | 0.538 |
| Sex, n (%) | 0.252 | 1.000 | ||||
| M | 64 (57.7) | 25 (48.1) | 25 (53.2) | 25 (53.2) | ||
| F | 47 (42.3) | 27 (51.9) | 22 (46.8) | 22 (46.8) | ||
| BMI, mean ± SD | 25.0 ± 3.1 | 24.7 ± 2.9 | 0.498 | 24.4 ± 2.2 | 24.9 ± 3.0 | 0.661 |
| ASA, n (%) | 0.645 | 0.997 | ||||
| 1 | 21 (18.9) | 9 (17.3) | 7 (14.9) | 7 (14.9) | ||
| 2 | 58 (52.3) | 23 (44.2) | 23 (48.9) | 22 (46.8) | ||
| 3 | 31 (27.9) | 19 (36.5) | 16 (34.0) | 17 (36.2) | ||
| 4 | 1 (0.9) | 1 (1.9) | 1 (2.1) | 1 (2.1) | ||
| CACI (median, range) | 4 (0-16) | 5 (0-12) | - | 5 (0-9) | 5 (0-12) | - |
| CACI, mean ± SD | 4.00 ± 3.22 | 4.78 ± 3.17 | 0.165 | 4.27 ± 2.78 | 4.66 ± 2.92 | 0.635 |
| Tumor size (cm), mean ± SD | 4.31 ± 1.44 | 4.21 ± 1.90 | 0.426 | 4.42 ± 1.43 | 4.13 ± 1.87 | 0.223 |
| Before propensity score matching | After propensity score matching | |||||
| 2D, n = 111 | 3D, n = 52 | P value | 2D, n = 47 | 3D, n = 47 | P value | |
| Type of right colectomy, n (%) | 0.013 | 0.836 | ||||
| CME | 41 (36.9) | 30 (57.7) | 24 (51.1) | 25 (53.2) | ||
| No CME | 70 (63.1) | 22 (42.3) | 23 (48.9) | 22 (46.8) | ||
| Operative time (min), mean ± SD | 185.3 ± 48.6 | 169.8 ± 32.4 | 0.087 | 183.8 ± 35.4 | 170.7 ± 32.9 | 0.053 |
| Anastomotic time (min), mean ± SD | 19.3 ± 2.9 | 16.9 ± 2.3 | < 0.001 | 19.6 ± 2.9 | 16.9 ± 2.3 | < 0.001 |
| Associated procedures, n (%) | 40 (36.0) | 25 (48.1) | 0.143 | 20 (42.6) | 22 (46.8) | 0.678 |
| Complexity of associated procedures, mean ± SD | 4.06 ± 0.89 | 3.92 ± 0.79 | 0.615 | 3.82 ± 0.82 | 3.91 ± 0.82 | 0.682 |
| Conversion, n (%) | 10 (9.0) | 1 (1.9 ) | 0.178 | 1 (2.1) | 1 (2.1) | 1.000 |
| Time to first flatus (d), mean ± SD | 3.21 ± 1.26 | 3.25 ± 1.08 | 0.606 | 3.46 ± 0.99 | 3.31 ± 1.10 | 0.375 |
| Hospital Stay (d, median) | 7 (3-60) | 7 (4-15) | - | 7 (3-24) | 7 (4-15) | - |
| Hospital Stay (d), mean ± SD | 8.36 ± 5.89 | 7.69 ± 2.17 | 0.858 | 8.08 ± 3.43 | 7.80 ± 2.24 | 0.877 |
| Postoperative complications, n (%) | 0.937 | 0.626 | ||||
| Yes | 25 (22.5) | 12 (23.1) | 10 (21.3) | 12 (25.5) | ||
| No | 86 (77.5) | 40 (76.9) | 37 (78.7) | 35 (74.5) | ||
| Clavien-Dindo Classification, n (%) | 0.729 | 0.781 | ||||
| I-II | 20 (80.0) | 9 (75.0) | 8 (80.0) | 9 (75.0) | ||
| III-IV | 5 (20.0) | 3 (25.0) | 2 (20.0) | 3 (25.0) | ||
| Type of complication, n (%) | ||||||
| Anastomotic bleeding | 0 (0.0) | 1 (1.9) | 0 (0.0) | 0 (0.0) | ||
| Anastomotic leak | 1 (0.9) | 0 (0.0) | 1 (2.1) | 0 (0.0) | ||
| Intra-abdominal bleeding | 0 (0.0) | 1 (1.9) | 0 (0.0) | 0 (0.0) | ||
| Canalization delay | 12 (10.8) | 4 (7.7) | 5 (10.6) | 4 (8.4) | ||
| Neurological | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (2.1) | ||
| Cardiac | 2 (1.8) | 2 (3.8) | 1 (2.1) | 1 (2.1) | ||
| Pulmonary | 4 (3.6) | 2 (3.8) | 2 (4.2) | 2 (4.2) | ||
| Urinary | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Wound infection | 1 (0.9) | 1 (1.9) | 1 (2.1) | 1 (2.1) | ||
| Other | 3 (2.7) | 5 (9.6) | 1 (2.1) | 5 (10.6) | ||
| 30 d mortality, n (%) | 2 (1.8) | 1 (1.9) | 1.000 | 1 (2.1) | 1 (2.1) | 1.000 |
| Before propensity score matching | After propensity score matching | |||||
| 2D, n = 111 | 3D, n = 52 | P value | 2D, n = 47 | 3D, n = 47 | P value | |
| T-stage, n (%) | 0.340 | 0.921 | ||||
| Adenoma | 7 (6.3) | 8 (15.4) | 6 (12.8) | 8 (17.0) | ||
| pTis | 5 (4.5) | 1 (1.9) | 3 (6.4) | 1 (2.1) | ||
| pT1 | 14 (12.6) | 8 (15.4) | 8 (17.0) | 7 (14.9) | ||
| pT2 | 24 (21.6) | 7 (13.5) | 8 (17.0) | 7 (14.9) | ||
| pT3 | 49 (44.1) | 25 (48.1) | 18 (38.3) | 21 (44.7) | ||
| pT4a | 7 (6.3) | 1 (1.9) | 2 (4.3) | 1 (2.1) | ||
| pT4b | 5 (4.5) | 2 (3.8) | 2 (4.3) | 2 (4.3) | ||
| N-stage, n (%) | 0.518 | 0.899 | ||||
| pN0 | 81 (73.0) | 42 (80.8) | 36 (76.6) | 37 (78.7) | ||
| pN1 | 22 (19.8) | 8 (15.4) | 8 (17.0) | 8 (17.0) | ||
| pN2 | 8 (7.2) | 2 (3.8) | 3 (6.4) | 2 (4.3) | ||
| Retrieved nodes, mean ± SD | 19.6 ± 6.6 | 18.8 ± 7.4 | 0.400 | 17.8 ± 5.2 | 18.9 ± 7.3 | 0.654 |
| Positive nodes, mean ± SD | 2.80 ± 1.91 | 2.30 ± 2.00 | 0.232 | 2.63 ± 1.80 | 2.30 ± 2.00 | 0.478 |
| Node ratio, mean ± SD | 0.15 ± 0.13 | 0.14 ± 0.16 | 0.346 | 0.15 ± 0.15 | 0.14 ± 0.17 | 0.574 |
A total of 71 patients underwent CME. Of these, 41 (36.9%) were operated with a 2D system and 30 (57.7%) by mean of a 3D system. The difference was statistically significant (P=0.013 [effect size w 0.430; power 0.995]). Before propensity matching, the mean age was 70.1 ± 12.6 years for the 2D group and 74.7 ± 11.4 years for the 3D laparoscopic group [P = 0.037 (effect size w 0.383; power 0.336)]. The mean operative time was 209.0 ± 40.0 min in the 2D group and 172.6 ± 37.1 in the 3D laparoscopic group [P < 0.001 (effect size w 1.066; power 0.989)]. The mean anastomotic time was 20.2 ± 2.6 min in the 2D group while was 16.6 ± 1.8 min in the 3D one [P < 0.001 (effect size w 1.488; power 0.999)]. There were no differences between the two groups in any of the other analyzed variables.
After the PSM procedure, 24 patients of the 2D group and 25 patients of the 3D group were available for the comparative analysis. The mean operative time was 193.7 ± 37.1 min in the 2D group and 175.0 ± 38.5 in the 3D laparoscopic group. The difference was almost statistically significant [P = 0.063 (effect size w 1.211; power 0.981)]. The mean anastomotic time was 19.9 ± 3.0 min in the 2D group while was 16.5 ± 1.8 min in the 3D one [P < 0.001 (effect size w 1.437; power 0.997)]. No differences between the two groups were found in any of the other analyzed variables. Features of CME patients are shown in Table 4.
| Before propensity score matching | After propensity score matching | |||||
| 2D, n = 41 | 3D, n = 30 | P value | 2D, n = 24 | 3D, n = 5 | P value | |
| Age (yr), mean ± SD | 70.1 ± 12.6 | 74.7 ± 11.4 | 0.037 | 70.8 ± 13.1 | 74.2 ± 11.2 | 0.352 |
| Sex, n (%) | 0.258 | 0.199 | ||||
| M | 26 (63.4) | 15 (50.0) | 10 (41.7) | 15 (60.0) | ||
| F | 15 (36.6) | 15 (50.0) | 14 (58.3) | 10 (40.0) | ||
| BMI, mean ± SD | 24.6 ± 3.0 | 24.4 ± 2.8 | 0.620 | 23.9 ± 2.0 | 24.7 ± 2.9 | 0.610 |
| CACI, median (range) | 4 (0-16) | 5 (0-12) | - | 4.5 (0-9) | 4 (0-12) | - |
| CACI, mean ± SD | 3.95 ± 2.78 | 5.23 ± 3.34 | 0.250 | 4.04 ± 2.89 | 5.08 ± 2.94 | 0.420 |
| Tumor size (cm), mean ± SD | 4.26 ± 1.71 | 4.52 ± 1.88 | 0.478 | 4.33 ± 1.52 | 4.42 ± 1.77 | 0.840 |
| Operative time (min), mean ± SD | 208.0 ± 40.0 | 172.6 ± 37.1 | < 0.001 | 193.7 ± 37.1 | 175.0 ± 38.5 | 0.063 |
| Anastomotic time (min), mean ± SD | 20.2 ± 2.6 | 16.6 ± 1.8 | < 0.001 | 19.9 ± 3.0 | 16.5 ± 1.8 | < 0.001 |
| Associated procedures, n (%) | 17 (41.5) | 17 (56.7) | 0.205 | 10 (41.7) | 14 (56.0) | 0.316 |
| Complexity of associated procedures, mean ± SD | 3.80 ± 0.69 | 4.08 ± 0.85 | 0.420 | 3.75 ± 0.84 | 4.10 ± 0.92 | 0.355 |
| Time to first flatus (d), mean ± SD | 3.53 ± 1.58 | 3.23 ± 1.04 | 0.492 | 3.45 ± 1.06 | 3.36 ± 1.07 | 0.681 |
| Hospital stay (d, median) | 7 (3-60) | 7 (6-15) | - | 7 (3-24) | 7 (6-15) | - |
| Hospital Stay (d), mean ± SD | 8.90 ± 8.89 | 8.13 ± 2.44 | 0.231 | 8.16 ± 4.29 | 8.44 ± 2.55 | 0.333 |
| Postoperative complications, n (%) | 0.646 | 0.376 | ||||
| Yes | 9 (26.7) | 8 (22.0) | 5 (20.8) | 8 (32.0) | ||
| No | 32 (77.5) | 22 (76.9) | 19 (79.2) | 17 (68.0) | ||
| Clavien-Dindo Classification, n (%) | 0.893 | 0.835 | ||||
| I-II | 7 (80.0) | 6 (75.0) | 4 (80.0) | 6 (75.0) | ||
| III-IV | 2 (25.0) | 2 (22.2) | 1 (20.0) | 2 (25.0) | ||
| 30-d mortality, n (%) | 0 | 0 | - | 0 | 0 | - |
| Retrieved nodes, mean ± SD | 23.7 ± 6.3 | 22.7 ± 7.1 | 0.571 | 22.0 ± 3.4 | 23.9 ± 6.4 | 0.415 |
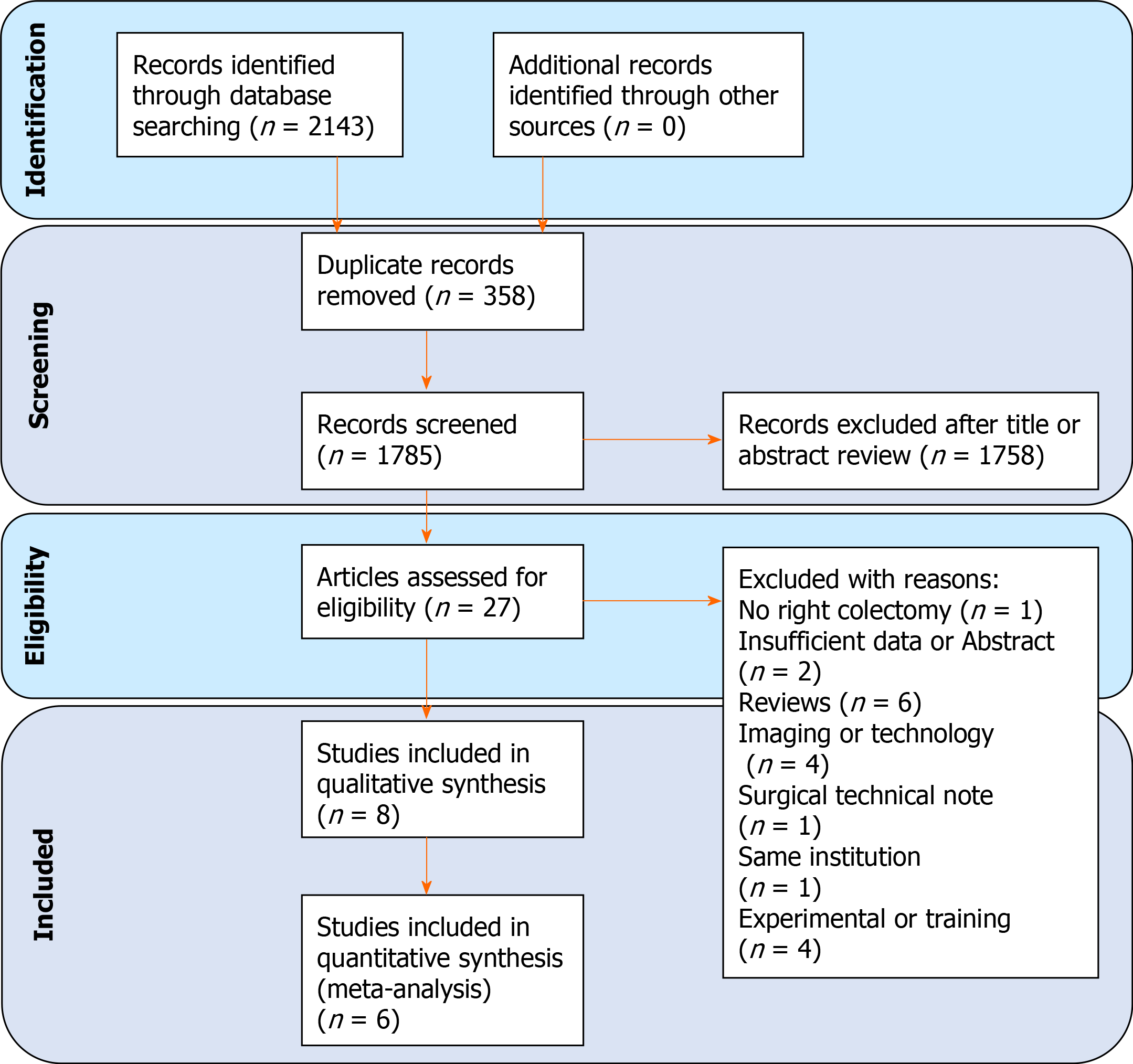
Using the described search strategy, 2143 items were identified. After removing duplicates and screening titles and abstract, 27 full text papers were evaluated. Nine-teen papers were further eliminated with reasons thus 7 studies were considered eligible (Figure 2). One retrospective study conducted in Korea and one prospective randomized study conducted in China have been included only in the qualitative analysis because the outcomes of interest were not reported separately for right and left-sided colorectal cancer[35,36]. Finally, our PSM series and six relevant studies were selected which enrolled 551 patients (2D group = 291; 3D group = 260)[34,37-41]. With regard to the retrieved studies, three of these were conducted in China, and three in Italy. Three studies were prospective non randomized, two studies were retrospective with control group, and one was retrospective case-matched. Five studies were conducted in a single centre while one had a double-centre design. All studies recruited patients between 2013 and 2018, five of these were conducted in a short period of time, almost all in a two-year period and only one in the entire long period. Papers were published between 2016 and 2020. The overall quality of studies was deemed as acceptable [Newcastle-Ottawa Scale mean 6.5 (range 4-8)]. The age was reported in all the studies and the mean age in the 2D and 3D group was 64.8 ± 6.4 years and 65.3 ± 6.2 years, respectively. When reported, gender ratio, BMI, tumour location and size, and tumour staging were comparable without statistically significant differences between the two groups. The baseline characteristics of included studies are summarized in Tables 5 and 6.
| Ref. | Year | Country | Type of study | Recruitment period | Level of evidence | 2D (%) | 3D (%) | Male gender (%) | AGE (yr) (Mean or median) | BMI (Mean or median) | NOS | |||
| 2D | 3D | 2D | 3D | 2D | 3D | |||||||||
| Currò et al[37] | 2016 | Italy | Prospective non randomized Single center | 2014-2015 | 2b | 25 | 25 | 14 (53.8) | 12 (48.0) | 68 (43-75) | 69 (40-78) | 30 (24-35) | 31 (23-34) | 7 |
| Tao et al[38] | 2016 | China | Retrospective Single center | 2014-2015 | 2b | 31 | 27 | 20 (64.5) | 16 (59.2) | 55 (37-71) | 57 (35-74) | 23.9 ± 3.6 | 22.7 ± 4.2 | 7 |
| Ji et al[34] | 2017 | China | Retrospective Single center | 2015-2017 | 2b | 39 | 37 | 27 (69.2) | 33 (89.2) | 68 ± 4 | 62 ± 6 | NA | NA | 6 |
| Su et al[39] | 2019 | China | Prospective non randomized Single center | 2016-2018 | 2b | 54 | 43 | 30 (55.6) | 29 (67.4) | 56.0 ± 10.9 | 58.3 ± 10.6 | 23.8 ± 2.9 | 24.4 ± 3.0 | 8 |
| Zuccaro et al[40] | 2019 | Italy | Prospective observational Single center | 2015-2018 | 2b | 42 | 28 | NA | NA | 69.5 (49-89) | 69.25 (48-90) | NA | NA | 4 |
| Bracale et al[41] | 2020 | Italy | Retrospective case matched Two centers | 2013-2018 | 2b | 53 | 53 | 28 (52.8) | 28 (52.8) | 68.6 ± 9.5 | 68.3 ± 11.7 | 26.7 ± 4.8 | 26.8 ± 4.7 | 7 |
| Ref. | Currò et al[37] | Tao et al[38] | Ji et al[34] | Su et al[39] | Zuccaro et al[40] | Bracale et al[41] | |||||||
| n | 25 | 25 | 31 | 27 | 39 | 37 | 54 | 43 | 42 | 28 | 53 | 53 | |
| 2D | 3D | 2D | 3D | 2D | 3D | 2D | 3D | 2D | 3D | 2D | 3D | ||
| Cancer site | Caecum | NA | NA | 7 (22.6) | 6 (22.2) | NS | NA | NA | NA | NA | 21 | 18 | |
| Ascendingcolon | NA | NA | 16 (51.6) | 14 (51.9) | 33 | 28 | NA | NA | 22 | 17 | |||
| Transverse colon | NA | NA | 8 (25.8) | 7 (25.9) | 6 | 5 | NA | NA | 10 | 18 | |||
| Descending colon | NA | NA | NA | NA | 10 | 15 | NA | NA | NA | NA | |||
| Tumour size, cm (mean or median) | NA | NA | 5.7 ± 2.4 | 5.2 ± 2.7 | > 3 cm 34 (91.9) | > 3 cm 32 (82.1) | 3.5 ± 1.1 | 3.9 ± 1.9 | NA | NA | NA | NA | |
| TNM or T stage, n (%) | 0-I | T1-212 | T1-210 | 5 (16.1) | 4 (14.8) | NA | NA | 5 (9.3) | 5 (11.6) | NA | NA | 10 (18.8) | 13 (24.5) |
| II | 15 (48.4) | 14 (51.9) | NA | NA | 20 (37.0) | 18 (41.9) | NA | NA | 29 (54.8) | 27 (51.0) | |||
| III | T3-4 13 | T3-4 15 | 11 (35.5) | 9 (33.3) | NA | NA | 29 (53.7) | 20 (46.5) | NA | NA | 14 (26.4) | 13 (24.5) | |
| Previous abdominal surgery [Yes, n (%)] | NA | NA | NA | NA | NA | NA | 15 (27.8) | 11 (25.6) | NA | NA | 12 (22.6) | 12 (22.6) | |
| Operative time (min) (mean or median) | 110 (100–130) | 105 (95–120) | 152.2 ± 28.9 | 130.5 ± 27.6 | 185 ± 22 | 177 ± 19 | 127.1 ± 36.6 | 131.9 ± 42.3 | 208 (100-350) | 167 (95-370) | 153.2 ± 52.4 | 131.0 ± 51.0 | |
| Anastomotic time (min) | 30 (24-36) | 25 (20-28) | ECA | ECA | 44 ± 5 | 38 ± 7 | 15.0 ± 3.0 | 13.2 ± 3.0 | NS | NS | 21.7 ± 6.2 | 19.2 ± 5.9 | |
| Blood loss, mL (mean or median) | NA | NA | 84.7 ± 22.3 | 80.8 ± 29.0 | 95 ± 35 | 105 ± 25 | 48.0 ± 45.5 | 54.7 ± 48.4 | 102 (50-500) | 53.2 (20-250) | NA | NA | |
| Harvested nodes (mean or median) | NA | NA | 19.3 ± 5.6 | 20.4 ± 5.7 | 21.5 ± 3.5 | 19.5 ± 2.5 | 22.3 ± 9.4 | 21.1 ± 7.7 | 23 (11-36) | 25 (10-48) | 21 ± 7 | 23 ± 11 | |
| Morbidity, n (%) | 0 | 1 (4.0) | 3 (9.6) | 4 (14.8) | NA | NA | 6 (14.0) | 6 (11.1) | NA | NA | 15 (28.3) | 12 (22.6) | |
| Mortality, n (%) | 0 | 0 | 0 | 0 | NA | NA | 0 | 0 | NA | NA | 0 | 0 | |
| Time to first flatus (d) (mean or median) | NA | NA | 3 (1-4) | 3 (1-4) | NA | NA | 3.3 ± 0.9 | 3.1 ± 0.7 | NA | NA | 2 ± 1 | 2 ± 1 | |
| LOS (d) (mean or median) | NA | NA | 9 (6-21) | 8 (6-14) | NA | NA | 6.6 ± 0.9 | 6.9 ± 1.1 | 10.9 (6-51) | 9 (6-14) | 6 ± 1 | 6 ± 2 | |
Neither intraoperative complications nor conversions occurred in the six included studies as well as in the study of Yoon et al[35] and in the study of Wang et al[36] In our PSM series conversion was required in 1 patient in the 2D group (2.1%) and in 1 patient in the 3D one (2.1%) (P = 1.000). The causes of conversion have been extensively described above in the personal series results section.
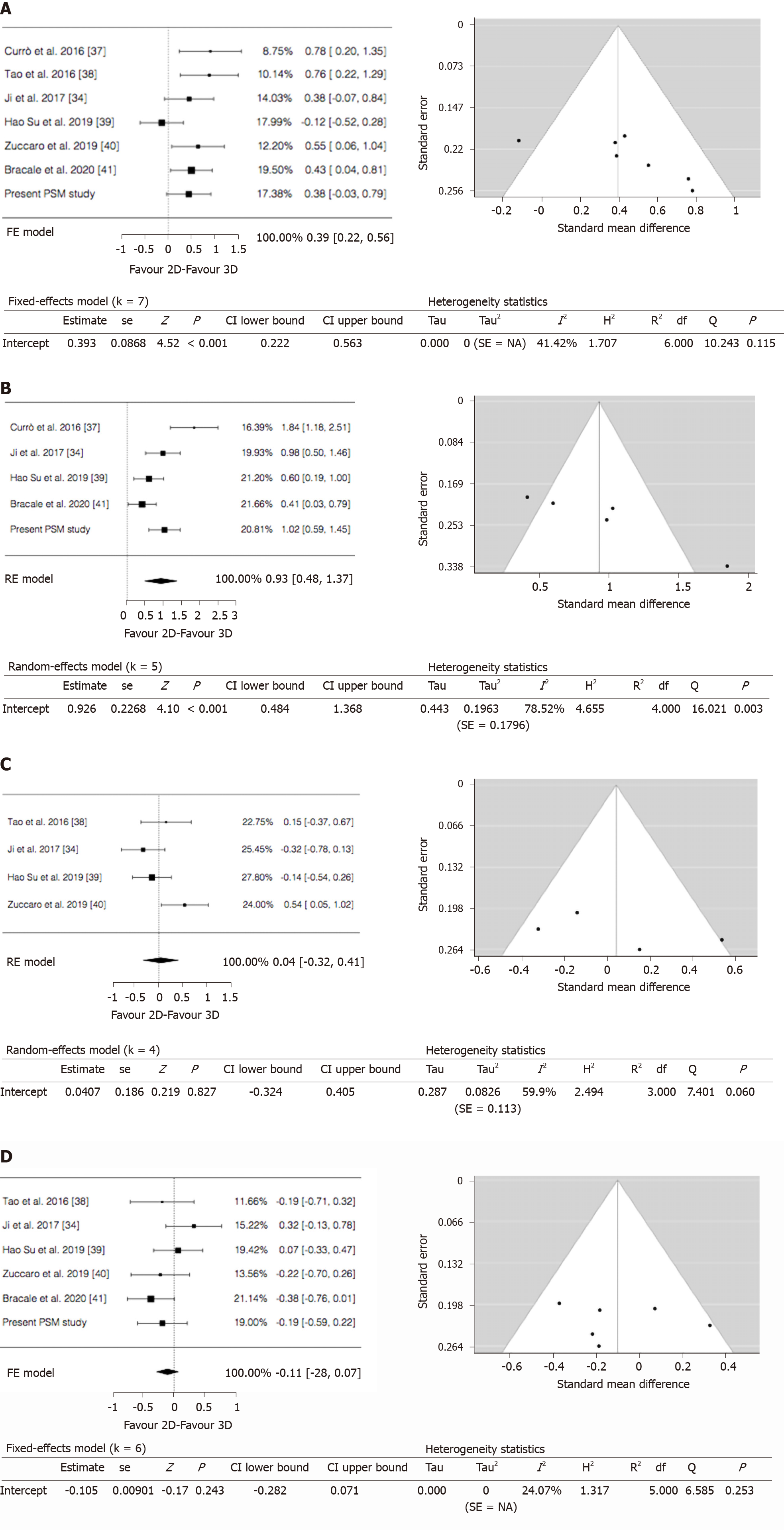
Operative time: All the included studies reported the operative time. The pooled analysis demonstrated a difference in favour of the 3D group (WMD = 0.393; 95%CI: 0.222-0.563; P < 0.001). Heterogeneity among the studies was moderate (I2 = 41.42%; P = 0.115). Publication bias assessment was performed by analyzing funnel plot asymmetry with Egger’s linear regression test (Y Intercept 2.286; P = 0.022) and with Begg and Mazumdar’s rank correlation test (Kendall’s Tau 0.619; P = 0.069 (Figure 3A).
Anastomotic time: Intracorporeal anastomosis has been performed by 5 authors and by us. However, data regarding anastomotic time were provided only in 4 of the included studies and in our series. The pooled analysis of 423 patients showed a significantly shorter anastomotic time in the 3D group (WMD = 0.926; 95%CI: 0.484-1.368; P < 0.001). Heterogeneity among the studies was considerable (I2 = 78.52%; P = 0.003) thus a random-effect model was used. Publication bias assessment was performed by analyzing funnel plot asymmetry with Egger’s linear regression test (Y Intercept 3.748; P < 0.001) and with Begg and Mazumdar’s rank correlation test (Kendall’s Tau 0.800; P = 0.083) (Figure 3B).
Blood loss: Four studies with 301 patients compared the blood loss. The results showed that the blood loss amount between the 2 groups was superimposable (WMD = 0.040; 95%CI: -0.324 to 0.405; P = 0.827). Heterogeneity among the studies was substantial (I2 = 59.90%; P = 0.060); a random-effect model was used. Publication bias assessment was performed by analyzing funnel plot asymmetry with Egger’s linear regression test (Y Intercept 1.024; P = 0.306) and with Begg and Mazumdar’s rank correlation test (Kendall’s Tau 0.333; P = 0.750) (Figure 3C).
LN harvested: Five studies and our series reported the number of harvested nodes allowing a pooled analysis of 501 patients. The results showed that the total LNH between the 2 groups was similar (WMD = -0.105; 95%CI: -0.282 to 0.071; P = 0.243). Heterogeneity among the studies was trivial (I2 =24.07%; P = 0.253). Publication bias assessment was performed by analyzing funnel plot asymmetry with Egger’s linear regression test (Y Intercept 0.455; P = 0.649) and with Begg and Mazumdar’s rank correlation test (Kendall’s Tau 0.200; P = 0.719) (Figure 3D).
Qualitative descriptive analysis of postoperative complications showed in the included study is summarized in Table 7. All but our series reported no mortality. In our PSM series mortality occurred in 1 patient (2.1%) in the 3D group and in 1 (2.1%) patient in the 2D one. In both cases mortality was not surgical related.
| Patient included | 2D, n = 163 | 3D, n = 148 |
| Postoperative complications | ||
| Yes | 37 (22.7) | 32 (21.6) |
| No | 126 (77.3) | 116 (78.4) |
| Type of complication | ||
| Anastomotic bleeding | 2 (1.2) | 5 (3.3) |
| Anastomotic leak | 4 (2.4) | 2 (1.3) |
| Postoperative anemia | 3 (1.8) | 3 (2.0) |
| Canalization delay | 12 (7.3) | 4 (2.7) |
| Bowel obstruction | 0 (0.0) | 1 (0.6) |
| Wound infection | 8 (4.9) | 6 (4.0) |
| Abdominal infection | 1 (0.6) | 1 (0.6) |
| Pulmonary | 4 (2.4) | 4 (2.7) |
| Other and/or NS | 14 (8.5) | 14 (9.4) |
| 30-d mortality | 2 (1.2) | 1 (0.6) |
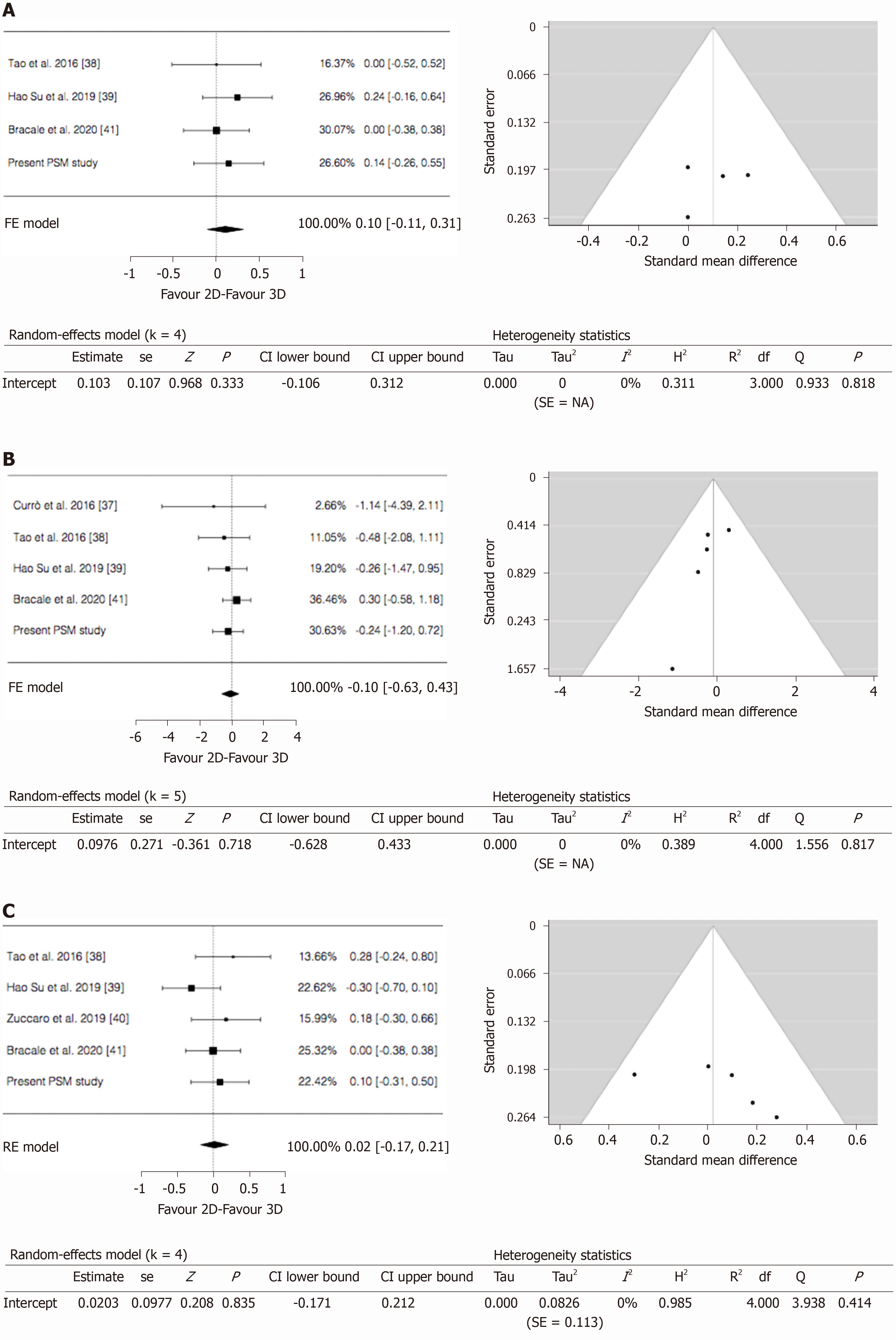
Time to first flatus: Our series along with 3 studies with 355 patients focused on this item. The results showed similar mean time to first flatus between the 2 groups (WMD = 0.103; 95%CI: -0.106 to 0.312; P = 0.333). No heterogeneity among the studies was found (I2 = 0%; P = 0.818). Publication bias assessment was performed by analyzing funnel plot asymmetry with Egger’s linear regression test (Y Intercept -0.253; P = 0.800) and with Begg and Mazumdar’s rank correlation test (Kendall’s Tau 0.000; P = 1.000) (Figure 4A).
Morbidity: From 4 studies and our series, 405 participants were enrolled to assess postoperative complications between the 2 groups. The results showed there was no statistically significant difference in postoperative complications between the 2 groups (OR = -0.097; 95%CI: -0.628 to 0.433; P = 0.718). No heterogeneity existed among the studies (I2 = 0%; P = 0.817). Publication bias assessment was performed by analyzing funnel plot asymmetry with Egger’s linear regression test (Y Intercept -0.970; P = 0.332) and with Begg and Mazumdar’s rank correlation test (Kendall’s Tau -0.800; P = 0.083) (Figure 4B).
Length of stay: Four studies and our series with 501 patients were analyzed for postoperative hospital stay between 2D and 3D groups. The results showed there was no statistically significant difference in length of stay between the 2 groups (WMD = 0.020; 95%CI: -0.171 to 0.212; P = 0.835). No heterogeneity among the studies was detected (I2 = 0%; P = 0.414). Publication bias assessment was performed by analyzing funnel plot asymmetry with Egger’s linear regression test (Y Intercept 1.295; P = 0.195) and with Begg and Mazumdar’s rank correlation test (Kendall’s Tau 0.800; P = 0.083) (Figure 4C).
Papers concerning systematic literature review with meta-analysis are considered among the best scientific contributions having high level of evidence even for non RCTs. For this reason, nowadays many authors publish meta-analysis carrying out very powerful statistical work managing data elsewhere retrieved. As a consequence, there is an evident plethora of redundant meta-analysis especially as regard to general surgery. Given our convincement of this abused methodology, we tried to innovate the very concept of writing such type of paper by adding our series to the literature data according to what described by other authors within various medical fields [42-46].
In the last decades, technological advances like high-definition (HD) cameras, dedicated instruments and articulating staplers, improved the safety and feasibility of laparoscopic procedures. As a consequence, a large diffusion of minimally invasive approach providing for the execution of more complex and demanding operations has been seen. Nevertheless, laparoscopic surgery is more difficult to learn and requires different psychomotor skills than open surgery: the surgeons work in a three-dimensional space, but are guided by two-dimensional images. This limitation can be challenging, especially with regard to manoeuvres requiring precision and dexterity such as suture and knotting. The 3D laparoscopy attempts to resolve this perceptual problem bringing the stereoscopic vision back to the surgeon.
In an evaluation regarding stereopsis in surgeons, Biddle revealed that 74%-83% of surgeons possessed high-grade stereopsis while 2%-14% had reduced stereopsis[47]. Another study found that 10% of the evaluated surgeons did not have measurable stereopsis[48]. The implication of these two researches was that at least approximately 10% of surgeons would not be able to appreciate the depth perception despite the 3D vision. On the other hand, Honeck[49] showed that visual misinterpretation in two-dimensional laparoscopy was responsible for 97% of errors during laparoscopic surgery, while Sun et al[50] found that the improved depth perception provided by 3D laparoscopy improves the quality of laparoscopic surgery, and may also improve patient safety.
However, the oncological and technical advantage of 3D laparoscopy over 2D is a matter of debate[51,52]. The majority of studies comparing 2D and 3D were conducted in experimental and teaching setting while studies regarding clinical trial are sparse, heterogeneous, and deemed qualitative inadequate[53].
With regard to the use of 3D laparoscopy in gastrointestinal surgery, a meta-analysis of Zhao et al[54] showed that of 3D imaging in gastric cancer surgery could shorten operative time and reduce blood loss while it had no clear advantages in colorectal cancer patients. Same results were reported by Pantalos[55].
In this article, we reported our experience and performed a systematic review and meta-analysis comparing the surgical outcome and postoperative recovery between 2D and 3D laparoscopic right colectomy. The results showed that in terms of conversion rate and of postoperative recovery such as return to bowel function and length of hospital stay as well as with regard to the overall morbidity rate no differences were found between the two groups of patients. Mortality rate was not considerable because only one patient in the pooled analysis died for unrelated surgical causes.
With regard to operative time, the prospective randomized trial regarding all colorectal procedure included in qualitative evaluation[36] and the pooled analysis revealed a reduction in 3D laparoscopy while our series showed that such reduction was really clinically almost statistically detectable only when CME was carried out. Bracale et al[41] suggested that a shorter operative time is likely to translate into benefits for patients due to a lower rate of pulmonary complications. Moreover, this reduction might be associated with cost savings which might compensate for the higher costs of purchase of the 3D systems. The shorter operative time in 3D laparoscopy is probably related to better depth perception and better depiction of anatomical structures and this is consistent with the reports of the authors who have shown that the use of stereoscopic cameras improves manual actions[56].
However, in order to evaluate at its best the 2D and 3D laparoscopy in right colectomy two items, among others, are of paramount relevance: what concerns the anastomosis and the number of lymph nodes harvested.
Our series did not show any statistically significant difference between the 3D approach compared to the 2D one in terms of anastomotic leakage and/or bleeding and such findings have been confirmed by the pooled results of meta-analysis and by Yoon et al[35] and Wang et al[36].
Deepening into more technical aspects, in our personal series we found that 3D laparoscopy improves surgical performance in terms of reducing the time to perform the intra-corporeal ileo-colic anastomosis. Although statistically significant, it is important to note that such difference accounted for about four minutes. The same occurrence has been seen in the majority of the studies included in the meta-analysis where the anastomotic time difference was very small. Moreover, the publication bias exists along with a substantial heterogeneity of the studies. As stated by other authors, our experience has led us to observe how the advantage of 3D vision is perceived especially by novice surgeons compared to senior experienced surgeons. A comparative trial is ongoing, but preliminary results showed that the anastomotic time was really significantly lower when the anastomosis has been performed by novice surgeon. As 3D imaging can ameliorate depth perception, spatial location, hand-eye coordination, and precision during surgery, we agree with the hypothesis that these improvements can reflect on the learning curve allowing young surgeon to quickly achieve good result while it does not give the same advantages for experienced surgeons[36]. These findings are corroborated by the literature data demonstrating how both difficult and easy tasks were completed with greater precision and shorter performance time when medical students were working under 3D vision rather than 2D vision. Conversely, in the same setting, advanced laparoscopic surgeons, although faster and more accurate than medical students, did not show any significant difference in performance time and precision for easy task under both 3D and 2D vision, but were faster during difficult tasks as suturing and stitching[56]. Spille et al[57] evaluated preferences among different levels of experience. A total of 277 subjects from three subgroups (students, residents and specialists) were required to perform four laparoscopic tasks with both 3D and 2D laparoscopies and they were asked to fill in a questionnaire afterwards. Overall, 68.8% of the participants preferred 3D to 2D laparoscopy and this was consistent within all three subgroups.
As cited above, the lymphadenectomy is other pivotal concern in oncological surgery because it is reported that the number of harvested LNs affects either disease free or overall survival regardless of nodal status[58-62]. Therefore, complete mesocolic excision and central vessel ligation in Western countries, similar to D3 Lymphadenectomy in Asia, have been suggested for right colon cancer. However, to date, the improving in oncological outcome of CME compared to standard surgery has not been definitively stated yet[63-66]. Although no international surgical society has ever recommended right colectomy with CME as “gold standard”, we decided to perform such procedure in as many cases as possible thinking that the conventional right colectomy could not always correctly stage the tumour as it has also been demonstrated recently by Nesgaar et al[67]. Despite an extensive policy, even if in the recent years the rate of CME is increasing, only about 40% of our patients underwent such complex procedure. Several authors reported that lymphadenectomy performed with a 3D vision could have some advantages because it minimize lack of depth perception and spatial orientation especially when lymphadenectomy is performed around major vessels[68,69]. In this regard, we have included in the qualitative analysis the report of Yoon et al[35] who investigated the role of 3D laparoscopic in extended lymphadenectomy both for right- and left-sided colorectal cancer. The paper has the limitation that the results were cumulatively reported; however, the study showed that the 3D system did not reduce the operative time and it appeared to be beneficial only in reducing blood loss and in increasing the number of harvested nodes. On the contrary, Su et al[39], also performing D3 dissection in all patients, reported that there were no differences between the 2D and 3D groups in terms of the operation time, of the blood loss, and of the number of lymph nodes retrieved. In our subgroup of CME patients, we have found that the 3D laparoscopy significantly reduces both operative and anastomotic time and we were able to confirm that it slightly increases the number of LNs harvested. However, within this context, our cumulative data along with pooled meta-analysis results showed that the same LN yield was seen in the 2D and 3D laparoscopy group. The same results are shown in the randomized trial of Wang et al[36] which, however, has the same limitations of the study of Yoon et al[35] about reporting cumulative results for right- and left-sided cancer. As a consequence, although the long-term oncological outcomes were not reported in our series and was not an outcome of interest and therefore not a focus of the present meta-analysis, it might be argued that the 3D vision itself does not impact on survival.
Finally, as for the whole intra-operative aspect concerning the surgeon's perception, we can state that during the 3D laparoscopy, the surgeons may experience initial visual fatigue and headache due to the use of the glasses for 3D vision. Such discomfort improves over time as the operative experience progresses, but we recognize that it might be a limitation in the adoption of 3D laparoscopy even due to the recent advent of 4K technology.
As it clearly descends from the above, there are several limitations in the present paper concerning both our series and meta-analysis. With regard to our experience, the study has some limitations like the retrospective fashion and that it encompasses a relatively small number of patients. However, it is important to consider that the propensity score model allowed us to compare two similar groups and the post-hoc analysis of CME groups demonstrated an adequate power. Being only qualified surgeons’ experience, on the one hand, there may be a criticism about the lack of reproducibility due to different skills and learning curve in laparoscopic surgery among different surgeons; on the other hand, the bias due to the different ability between operators could be limited.
Moreover, there are evident limitations even in the meta-analysis. Firstly, all the studies included in the quantitative analysis were conducted in only two countries such as Italy and China and the majority were retrospective enrolling a small sample size of patients. It is well known that such researches have been considered underpowered and may limit the conclusions on the efficacy of one technique over another. Secondly, publication bias is present and a considerable degree of heterogeneity was observed precisely in the two unique outcomes of interest found statistically different i.e. the operative time and the anastomotic time. Although a random effect model was used, the results must be considered prudently.
The studies that showed superiority of a 3D over a 2D imaging system were conducted primarily using experimental models and, albeit their results are superior with 3D laparoscopy, do not necessarily reflect the complexity of surgery in real life. Present systematic review with meta-analysis would show that surgery for right colon cancer may benefit from the use of the three-dimensional laparoscopy by reducing operative and anastomotic time while it does not affect the lymphadenectomy. Although the 3D system seems to offer better depth perception and subjectively determines less physical strain compared to 2D vision, on the basis of our series, 3D imaging seems to limit its impact on the technical performance and outcomes of standard laparoscopic right colectomy when the surgeon is experienced in open and/or 2D laparoscopy. For this reason, the 3D system seems to allow to shorten the learning curve and to make easier some technical gestures in surgeons with less experience e.g., by reducing the numbers of repetitions and errors. In addition, it could be stated that the value of the 3D laparoscopy becomes better evident when CME has been carried out and/or when more complex associated procedures are requested. In such instances the 3D vision is really more effective in reducing both the total operative time and the anastomotic time facilitating some movements such as dissection around major vessels and suturing and also giving greater safety even to experienced surgeons by achieving an easier identification of anatomic landmarks.
The benefits of laparoscopic approach for right colectomy have been well reported. However, there are some critical surgical steps that are still debated such as intracorporeal anastomosis, central vein ligation (CVL) and complete mesocolic excision (CME). The introduction of the three-dimensional (3D) vision in laparoscopic systems provided some of the advantages of robotic platform; thus, 3D laparoscopic surgery has emerged as a competitive alternative to the robotic one in order to overcome the technical issues of the two-dimensional laparoscopic right colectomy.
In a recent paper, we compare robotic surgery and 3D laparoscopy for right colectomy with CME and intra-corporeal anastomosis. Given our experience in minimally invasive colorectal surgery and driven to such previous effort, we wanted to undertake the present study with the aim to critically appraise our whole experience in the use of 3D laparoscopic system in right colectomy making a comparison with the 2D one. Moreover, we decided to carry out a meta-analysis of available data in order to compare our results to the literature ones in the attempt to increase the statistical power and level of evidence.
The aim of this study is to analyze the results of 3D and 2D laparoscopic right colectomy and to compare it to the published series through a systematic review and meta-analysis.
Personal series: A retrospective study with propensity score matching analysis of patients undergoing laparoscopic right colectomy at Umbria2 Hospitals from January 2014 to March 2020 was performed. Inclusion criteria were adenocarcinoma or neuroendocrine tumour (NET) confirmed by pathological examination limited to the following tumour locations: cecum, ascending colon and hepatic flexure. Exclusion criteria were: malignant lymphoma or other non-cancer cases, and emergency procedures. Locally advanced tumor as well as hepatic metastases or concomitant conditions requiring surgical treatment were not considered exclusion criteria. Propensity scores were calculated by bivariate logistic regression, including the following variables: sex, age, BMI, size of tumor, CME yes or not, complexity grade of concomitant procedure. We matched propensity scores 1:1 with the use of the nearest neighbor methods without replacement. The caliper width was set at 0.2. A CME subgroups analysis was also performed. Meta-analysis: A systematic review was carried out through MEDLINE (PubMed), Embase, Web of Science, Scopus, and The Cochrane Library from January 1980 to 31 October 2020. The following keywords and/or medical subject heading (MeSH) terms were used in combination: “2D”, “two-dimensional”, “3D”, “three-dimensional”, “laparoscopy”, “colon”, “colorectal surgery”, and “right colectomy”. At least one peri-operative outcome of interest should be reported. Studies comparing 3D robotic vision to 2D laparoscopic vision were excluded.
Forty-seven patients of the 2D group were matched to 47 patients of the 3D group. The 3D group showed a favorable trend in terms of mean operative time (170.7 ± 32.9 min vs 183.8 ± 35.4 min; P = 0.053) and a significant lower anastomotic time (16.9 ± 2.3 min vs 19.6 ± 2.9 min, P < 0.001). The CME subgroups analysis showed a shorter anastomotic time (16.5 ± 1.8 min vs 19.9 ± 3.0 min; P < 0.001) and operative time (175.0 ± 38.5 vs 193.7 ± 37.1 min; P = 0.063) in the 3D group. Six studies and our series were included in the meta-analysis with 551 patients (2D group: 291; 3D group: 260).The pooled analysis demonstrated a significant difference in favour of the 3D group regarding the operative time (P < 0.001) and the anastomotic time (P < 0.001) while no differences were identified between groups in terms of blood loss (P = 0.827), LNH yield (P = 0.243), time to first flatus (P = 0.333), postoperative complications (P = 0.718) and length of stay (P = 0.835).
The advantage of the 3D system becomes evident when CME and/or more complex associated procedure are requested significantly reducing both the total operative and the anastomotic time. 3D laparoscopic right colectomy has shorter operative and anastomotic time without affecting the standard lymphadenectomy.
The 3D system seems to allow to shorten the learning curve and to overcome some technical issues of the classic 2D laparosocpy. The value of the 3D laparoscopy becomes better evident when CME has been carried out and/or when more complex associated procedures are requested. Further researches are needed to validate those results.
The authors are grateful to Professor Alessandro Listuzzi (Professor at Foreign Languages Teaching Center of Carabinieri Army, Via Carlo Alberto Dalla Chiesa, Rome 00192, Italy) for his kind assistance in checking the translation of the article in Chinese of Ji and colleagues[34].
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kawakami M S-Editor: Gong ZM L-Editor: A P-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55806] [Article Influence: 7972.3] [Reference Citation Analysis (132)] |
| 2. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3268] [Article Influence: 653.6] [Reference Citation Analysis (2)] |
| 3. | Feng Z, Shi X, Zhang Q, Zhang X, Li X, Chen Z, Liu D, Sun B, Zuo Y, Ren S. Analysis of clinicopathological features and prognosis of 1315 cases in colorectal cancer located at different anatomical subsites. Pathol Res Pract. 2019;215:152560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Antoniou SA, Antoniou GA, Koch OO, Pointner R, Granderath FA. Laparoscopic colorectal surgery confers lower mortality in the elderly: a systematic review and meta-analysis of 66,483 patients. Surg Endosc. 2015;29:322-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Ngu JC, Kuo LJ, Teo NZ. Minimally invasive surgery in the geriatric patient with colon cancer. J Gastrointest Oncol. 2020;11:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Liu Q, Luo D, Lian P, Yu W, Zhu J, Cai S, Li Q, Li X. Reevaluation of laparoscopic surgery's value in pathological T4 colon cancer with comparison to open surgery: A retrospective and propensity score-matched study. Int J Surg. 2018;53:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Blackmore AE, Wong MT, Tang CL. Evolution of laparoscopy in colorectal surgery: an evidence-based review. World J Gastroenterol. 2014;20:4926-4933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Parker JM, Feldmann TF, Cologne KG. Advances in Laparoscopic Colorectal Surgery. Surg Clin North Am. 2017;97:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Pascual M, Salvans S, Pera M. Laparoscopic colorectal surgery: Current status and implementation of the latest technological innovations. World J Gastroenterol. 2016;22:704-717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Fujii S, Ishibe A, Ota M, Yamagishi S, Watanabe J, Suwa Y, Kunisaki C, Endo I. Long-term results of a randomized study comparing open surgery and laparoscopic surgery in elderly colorectal cancer patients (Eld Lap study). Surg Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Lim S, Ghosh S, Niklewski P, Roy S. Laparoscopic Suturing as a Barrier to Broader Adoption of Laparoscopic Surgery. JSLS. 2017;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Li YS, Meng FC, Lin JK. Procedural and post-operative complications associated with laparoscopic versus open abdominal surgery for right-sided colonic cancer resection: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e22431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Fabozzi M, Cirillo P, Corcione F. Surgical approach to right colon cancer: From open technique to robot. State of art. World J Gastrointest Surg. 2016;8:564-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (2)] |
| 14. | Anania G, Agresta F, Artioli E, Rubino S, Resta G, Vettoretto N, Petz WL, Bergamini C, Arezzo A, Valpiani G, Morotti C, Silecchia G; SICE CoDIG (Colon Dx Italian Group). Laparoscopic right hemicolectomy: the SICE (Società Italiana di Chirurgia Endoscopica e Nuove Tecnologie) network prospective trial on 1225 cases comparing intra corporeal versus extra corporeal ileo-colic side-to-side anastomosis. Surg Endosc. 2020;34:4788-4800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Arezzo A, Passera R, Ferri V, Gonella F, Cirocchi R, Morino M. Laparoscopic right colectomy reduces short-term mortality and morbidity. Results of a systematic review and meta-analysis. Int J Colorectal Dis. 2015;30:1457-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Cleary RK, Kassir A, Johnson CS, Bastawrous AL, Soliman MK, Marx DS, Giordano L, Reidy TJ, Parra-Davila E, Obias VJ, Carmichael JC, Pollock D, Pigazzi A. Intracorporeal versus extracorporeal anastomosis for minimally invasive right colectomy: A multi-center propensity score-matched comparison of outcomes. PLoS One. 2018;13:e0206277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Emile SH, Elfeki H, Shalaby M, Sakr A, Bassuni M, Christensen P, Wexner SD. Intracorporeal versus extracorporeal anastomosis in minimally invasive right colectomy: an updated systematic review and meta-analysis. Tech Coloproctol. 2019;23:1023-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Allaix ME, Degiuli M, Bonino MA, Arezzo A, Mistrangelo M, Passera R, Morino M. Intracorporeal or Extracorporeal Ileocolic Anastomosis After Laparoscopic Right Colectomy: A Double-blinded Randomized Controlled Trial. Ann Surg. 2019;270:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 19. | Andolfi C, Umanskiy K. Appraisal and Current Considerations of Robotics in Colon and Rectal Surgery. J Laparoendosc Adv Surg Tech A. 2019;29:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Rausa E, Kelly ME, Asti E, Aiolfi A, Bonitta G, Bonavina L. Right hemicolectomy: a network meta-analysis comparing open, laparoscopic-assisted, total laparoscopic, and robotic approach. Surg Endosc. 2019;33:1020-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Solaini L, Cavaliere D, Pecchini F, Perna F, Bazzocchi F, Avanzolini A, Marchi D, Checcacci P, Cucchetti A, Coratti A, Piccoli M, Ercolani G. Robotic versus laparoscopic right colectomy with intracorporeal anastomosis: a multicenter comparative analysis on short-term outcomes. Surg Endosc. 2019;33:1898-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Genova P, Pantuso G, Cipolla C, Latteri MA, Abdalla S, Paquet JC, Brunetti F, de'Angelis N, Di Saverio S. Laparoscopic versus robotic right colectomy with extra-corporeal or intra-corporeal anastomosis: a systematic review and meta-analysis. Langenbecks Arch Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Mushtaq HH, Shah SK, Agarwal AK. The Current Role of Robotics in Colorectal Surgery. Curr Gastroenterol Rep. 2019;21:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Addison P, Agnew JL, Martz J. Robotic Colorectal Surgery. Surg Clin North Am. 2020;100:337-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Felder SI, Ramanathan R, Russo AE, Jimenez-Rodriguez RM, Hogg ME, Zureikat AH, Strong VE, Zeh HJ, Weiser MR. Robotic gastrointestinal surgery. Curr Probl Surg. 2018;55:198-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Ceccarelli G, Costa G, Ferraro V, De Rosa M, Rondelli F, Bugiantella W. Robotic or three-dimensional (3D) laparoscopy for right colectomy with complete mesocolic excision (CME) and intracorporeal anastomosis? A propensity score-matching study comparison. Surg Endosc. 2021;35:2039-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Park Y, Yong YG, Yun SH, Jung KU, Huh JW, Cho YB, Kim HC, Lee WY, Chun HK. Learning curves for single incision and conventional laparoscopic right hemicolectomy: a multidimensional analysis. Ann Surg Treat Res. 2015;88:269-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2924] [Cited by in RCA: 3457] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 29. | Brusselaers N, Lagergren J. The Charlson Comorbidity Index in Registry-based Research. Methods Inf Med. 2017;56:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 243] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 30. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24805] [Article Influence: 1181.2] [Reference Citation Analysis (0)] |
| 31. | Casciola L, Codacci-Pisanelli M, Ceccarelli G, Bartoli A, Di Zitti L, Patriti A. A modified umbilical incision for specimen extraction after laparoscopic abdominal surgery. Surg Endosc. 2008;22:784-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47144] [Article Influence: 2946.5] [Reference Citation Analysis (0)] |
| 33. | McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston R V, Thomas J. Defining the criteria for including studies and how they will be grouped for the synthesis. In: Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd 2019; 33-65. [DOI] [Full Text] |
| 34. | Ji F, Fang X, Fei B. [Comparative study of 3D and 2D laparoscopic surgery for gastrointestinal tumors]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:509-513. [PubMed] |
| 35. | Yoon J, Kang SI, Kim MH, Kim MJ, Oh HK, Kim DW, Kang SB. Comparison of Short-Term Outcomes Between 3D and 2D Imaging Laparoscopic Colectomy with D3 Lymphadenectomy for Colon Cancer. J Laparoendosc Adv Surg Tech A. 2019;29:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Wang Z, Liang J, Chen J, Mei S, Liu Q. Three-Dimensional (3D) Laparoscopy Versus Two-Dimensional (2D) Laparoscopy: A Single-Surgeon Prospective Randomized Comparative Study. Asian Pac J Cancer Prev. 2020;21:2883-2887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Currò G, Lazzara S, La Malfa G, Giovanni P, De Leo E, Fortugno A, Navarra G, Curro G, Lazzara S, La Malfa G, Giovanni P, De Leo E, Fortugno A, Navarra G. Three-dimensional (3D) versus two-dimensional (2D) laparoscopic oncological colorectal surgery: A single-surgeon prospective randomized comparative study. Eur J Surg Oncol. 2016;42:S206. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Tao K, Liu X, Deng M, Shi W, Gao J. Three-Dimensional Against 2-Dimensional Laparoscopic Colectomy for Right-sided Colon Cancer. Surg Laparosc Endosc Percutan Tech. 2016;26:324-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Su H, Jin W, Wang P, Bao M, Wang X, Zhao C, Wang X, Zhou Z, Zhou H. Comparing short-time outcomes of three-dimensional and two-dimensional totally laparoscopic surgery for colon cancer using overlapped delta-shaped anastomosis. Onco Targets Ther. 2019;12:669-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Zuccaro M, Fiscon V. Impact of three dimensional laparoscopic surgery on right colon resection for cancer. Eur J Surg Oncol. 2019;45:e75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Bracale U, Merola G, Rizzuto A, Pontecorvi E, Silvestri V, Pignata G, Pirozzi F, Cuccurullo D, Sciuto A, Corcione F. Does a 3D laparoscopic approach improve surgical outcome of mininvasive right colectomy? A retrospective case-control study. Updates Surg. 2020;72:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Neumann PA, Reischl S, Berg F, Jäger C, Friess H, Reim D, Ceyhan GO. Meta-analysis and single-center experience on the protective effect of negative suction drains on wound healing after stoma reversal. Int J Colorectal Dis. 2020;35:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Gruber LM, Strajina V, Bancos I, Murad MH, Dy BM, Young WF, Farley DR, Lyden ML, Thompson GB, McKenzie TJ. Not all adrenal incidentalomas require biochemical testing to exclude pheochromocytoma: Mayo clinic experience and a meta-analysis. Gland Surg. 2020;9:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Trimboli P, Fulciniti F, Paone G, Barizzi J, Piccardo A, Merlo E, Mazzucchelli L, Giovanella L. Risk of Malignancy (ROM) of Thyroid FNA Diagnosed as Suspicious for Malignancy or Malignant: an Institutional Experience with Systematic Review and Meta-Analysis of Literature. Endocr Pathol. 2020;31:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Borgognoni L, Sestini S, Gerlini G, Brandani P, Chiarugi C, Gelli R, Giannotti V, Crocetti E. Sentinel Lymph Node Status is a Main Prognostic Parameter Needful for the Correct Staging of Patients with Melanoma Thicker than 4 mm: Single-Institution Experience and Literature Meta-Analysis. J Invest Surg. 2019;32:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Chahoud J, Msaouel P, Campbell MT, Bathala T, Xiao L, Gao J, Zurita AJ, Shah AY, Jonasch E, Sharma P, Tannir NM. Nivolumab for the Treatment of Patients with Metastatic Non-Clear Cell Renal Cell Carcinoma (nccRCC): A Single-Institutional Experience and Literature Meta-Analysis. Oncologist. 2020;25:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 47. | Biddle M, Hamid S, Ali N. An evaluation of stereoacuity (3D vision) in practising surgeons across a range of surgical specialities. Surgeon. 2014;12:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Fergo C, Burcharth J, Pommergaard HC, Rosenberg J. Age is highly associated with stereo blindness among surgeons: a cross-sectional study. Surg Endosc. 2016;30:4889-4894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Honeck P, Wendt-Nordahl G, Rassweiler J, Knoll T. Three-dimensional laparoscopic imaging improves surgical performance on standardized ex-vivo laparoscopic tasks. J Endourol. 2012;26:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Sun CC, Chiu AW, Chen KK, Chang LS. Assessment of a three-dimensional operating system with skill tests in a pelvic trainer. Urol Int. 2000;64:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Yim C, Lo CH, Lau MH, Fan R, Lai HM, Foo DCC. Three-dimensional laparoscopy: is it as good as it looks?–a review of the literature. Ann Laparosc Endosc Surg. 2017;2:131-131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Curtis NJ, Conti JA, Dalton R, Rockall TA, Allison AS, Ockrim JB, Jourdan IC, Torkington J, Phillips S, Allison J, Hanna GB, Francis NK. 2D versus 3D laparoscopic total mesorectal excision: a developmental multicentre randomised controlled trial. Surg Endosc. 2019;33:3370-3383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Sørensen SM, Savran MM, Konge L, Bjerrum F. Three-dimensional versus two-dimensional vision in laparoscopy: a systematic review. Surg Endosc. 2016;30:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 54. | Zhao B, Lv W, Mei D, Luo R, Bao S, Huang B, Lin J. Comparison of short-term surgical outcome between 3D and 2D laparoscopy surgery for gastrointestinal cancer: a systematic review and meta-analysis. Langenbecks Arch Surg. 2020;405:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Pantalos G, Patsouras D, Spartalis E, Dimitroulis D, Tsourouflis G, Nikiteas N. Three-dimensional Versus Two-dimensional Laparoscopic Surgery for Colorectal Cancer: Systematic Review and Meta-analysis. In Vivo. 2020;34:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Storz P, Buess GF, Kunert W, Kirschniak A. 3D HD versus 2D HD: surgical task efficiency in standardised phantom tasks. Surg Endosc. 2012;26:1454-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 57. | Spille J, Wenners A, von Hehn U, Maass N, Pecks U, Mettler L, Alkatout I. 2D Versus 3D in Laparoscopic Surgery by Beginners and Experts: A Randomized Controlled Trial on a Pelvitrainer in Objectively Graded Surgical Steps. J Surg Educ. 2017;74:867-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Backes Y, Elias SG, Bhoelan BS, Groen JN, van Bergeijk J, Seerden TCJ, Pullens HJM, Spanier BWM, Geesing JMJ, Kessels K, Kerkhof M, Siersema PD, de Vos Tot Nederveen Cappel WH, van Lelyveld N, Wolfhagen FHJ, Ter Borg F, Offerhaus GJA, Lacle MM, Moons LMG; Dutch T1 CRC Working Group. The prognostic value of lymph node yield in the earliest stage of colorectal cancer: a multicenter cohort study. BMC Med. 2017;15:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Kobayashi H, Enomoto M, Higuchi T, Uetake H, Iida S, Ishikawa T, Ishiguro M, Kato S, Sugihara K. Clinical significance of lymph node ratio and location of nodal involvement in patients with right colon cancer. Dig Surg. 2011;28:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Raoof M, Nelson RA, Nfonsam VN, Warneke J, Krouse RS. Prognostic significance of lymph node yield in ypN0 rectal cancer. Br J Surg. 2016;103:1731-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Choi HK, Law WL, Poon JT. The optimal number of lymph nodes examined in stage II colorectal cancer and its impact of on outcomes. BMC Cancer. 2010;10:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Li Destri G, Barchitta M, Pesce A, Latteri S, Bosco D, Di Cataldo A, Agodi A, Puleo S. Predictive Value of the Number of Harvested Lymph Nodes and Cut-Off for Lymph Node Ratio in the Prognosis of Stage II and III Colorectal Cancer Patients. J Invest Surg. 2019;32:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 63. | Dai Q, Tu S, Dong Q, Chen B. Laparoscopic Complete Mesocolic Excision Versus Noncomplete Mesocolic Excision: A Systematic Review and Meta-analysis. Surg Laparosc Endosc Percutan Tech. 2020;31:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Gao Z, Wang C, Cui Y, Shen Z, Jiang K, Shen D, Wang Y, Zhan S, Guo P, Yang X, Liu F, Shen K, Liang B, Yin M, Xie Q, Wang Y, Wang S, Ye Y. Efficacy and Safety of Complete Mesocolic Excision in Patients With Colon Cancer: Three-year Results From a Prospective, Nonrandomized, Double-blind, Controlled Trial. Ann Surg. 2020;271:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 65. | Wang C, Gao Z, Shen K, Shen Z, Jiang K, Liang B, Yin M, Yang X, Wang S, Ye Y. Safety, quality and effect of complete mesocolic excision vs non-complete mesocolic excision in patients with colon cancer: a systemic review and meta-analysis. Colorectal Dis. 2017;19:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 66. | Alhassan N, Yang M, Wong-Chong N, Liberman AS, Charlebois P, Stein B, Fried GM, Lee L. Comparison between conventional colectomy and complete mesocolic excision for colon cancer: a systematic review and pooled analysis : A review of CME versus conventional colectomies. Surg Endosc. 2019;33:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 67. | Nesgaar JM, Stimec BV, Bakka AO, Edwin B, Bergamaschi R, Ignjatovic D. Right Colectomy with Extended D3 Mesenterectomy: Anterior and Posterior to the Mesenteric Vessels. Surg Technol Int. 2019;35:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Kanemitsu Y, Komori K, Kimura K, Kato T. D3 Lymph Node Dissection in Right Hemicolectomy with a No-touch Isolation Technique in Patients With Colon Cancer. Dis Colon Rectum. 2013;56:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 69. | Kim NK, Kim YW, Han YD, Cho MS, Hur H, Min BS, Lee KY. Complete mesocolic excision and central vascular ligation for colon cancer: Principle, anatomy, surgical technique, and outcomes. Surg Oncol. 2016;25:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |