Published online May 27, 2021. doi: 10.4240/wjgs.v13.i5.493
Peer-review started: January 24, 2021
First decision: March 29, 2021
Revised: March 30, 2021
Accepted: April 23, 2021
Article in press: April 23, 2021
Published online: May 27, 2021
Processing time: 116 Days and 10 Hours
Endoscopic drainage remains the treatment of choice for unresectable or inoperable malignant distal biliary obstruction (MDBO).
To compare the safety and efficacy of plastic stent (PS) vs self-expanding metal stent (SEMS) placement for treatment of MDBO.
This meta-analysis was developed according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. A comprehensive search was performed in MEDLINE, Cochrane, Embase, Latin American and Caribbean Health Sciences Literature, and grey literature to identify randomized clinical trials (RCTs) comparing clinical success, adverse events, stent dysfunction rate, reintervention rate, duration of stent patency, and mean survival. Risk difference (RD) and mean difference (MD) were calculated and heterogeneity was assessed with I2 statistic. Subgroup analyses were performed by SEMS type.
Twelve RCTs were included in this study, totaling 1005 patients. There was no difference in clinical success (RD = -0.03, 95% confidence interval [CI]: -0.01, 0.07; I2 = 0%), rate of adverse events (RD = -0.03, 95%CI: -0.10, 0.03; I2 = 57%), and mean patient survival (MD = -0.63, 95%CI: -18.07, 19.33; I2 = 54%) between SEMS vs PS placement. However, SEMS placement was associated with a lower rate of reintervention (RD = -0.34, 95%CI: -0.46, -0.22; I2 = 57%) and longer duration of stent patency (MD = 125.77 d, 95%CI: 77.5, 174.01). Subgroup analyses revealed both covered and uncovered SEMS improved stent patency compared to PS (RD = 152.25, 95%CI: 37.42, 267.07; I2 = 98% and RD = 101.5, 95%CI: 38.91, 164.09; I2 = 98%; respectively). Stent dysfunction was higher in the covered SEMS group (RD = -0.21, 95%CI: -0.32, -0.1; I² = 205%), with no difference in the uncovered SEMS group (RD = -0.08, 95%CI: -0.56, 0.39; I² = 87%).
While both stent types possessed a similar clinical success rate, complication rate, and patient-associated mean survival for treatment of MDBO, SEMS were associated with a longer duration of stent patency compared to PS.
Core Tip: Bile duct or pancreatic malignancies that result in malignant distal biliary obstruction (MDBO) are often associated with a poor prognosis. Currently, palliative treatment via endoscopic biliary drainage is considered the treatment of choice for unresectable or inoperable MDBO. In this systematic review and meta-analysis of randomized controlled trials, we compared the efficacy and safety of plastic stent (PS) vs self-expanding metal stent (SEMS) placement. We concluded that SEMS placement had a longer duration of patency, lower rate of reintervention, and lower rate of stent dysfunction compared to PS in patients with MDBO. There was no difference in outcomes of clinical success, mean patient survival, and overall adverse events.
- Citation: Scatimburgo MVCV, Ribeiro IB, de Moura DTH, Sagae VMT, Hirsch BS, Boghossian MB, McCarty TR, dos Santos MEL, Franzini TAP, Bernardo WM, de Moura EGH. Biliary drainage in inoperable malignant biliary distal obstruction: A systematic review and meta-analysis. World J Gastrointest Surg 2021; 13(5): 493-506
- URL: https://www.wjgnet.com/1948-9366/full/v13/i5/493.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i5.493
While malignant bile duct tumors are uncommon, estimated to have an incidence of 8000 new intra- and extrahepatic cases per year according to the American Cancer Society[1], these neoplasms are associated with a very poor overall prognosis. Similarly, malignant pancreatic lesions, which may also result in malignant distal biliary obstruction (MDBO), portend a poor prognosis. In many cases, these lesions have no curative perspective by the time of diagnosis. Thus, palliative treatment methods to achieve bile duct clearance play a major role, providing a longer life expectancy and improved quality of life[2].
Endoscopic stenting, percutaneous transhepatic bile duct drainage (PTBD), and surgical bile derivation (i.e. surgical bypass) are established methods to achieve bile duct drainage. The emergence of metallic stents was a turning point in endoscopic treatments, especially in chronic and palliative patients, being diffusely used in various situations such as emergencies of the colon[3], stomach/duodenum[4,5] and drainage of the bile ducts[6]. Endoscopic biliary stenting was first described by Soehendra[7] in 1979, and today is considered the treatment of choice in the palliative care of unresectable or inoperable MDBO. Additionally, endoscopic biliary drainage may be considered an alternative or as a combined approach method to PTBD[8]. Endoscopic drainage is associated with a decreased mortality and lower complication rate, as well as a higher clinical success rate, compared to a traditional surgical approach; however, there does appear to be a higher rate of recurrent biliary obstruction[8,9].
Two types of stents may be utilized to achieve successful endoscopic biliary drainage: Plastic stent (PS) and self-expanding metal stent (SEMS) placement. Each of these stent types possess different characteristics regarding stent patency, need for reintervention, potential for stent dysfunction, and other adverse events. There is great deal of discussion in the literature, in randomized clinical trials (RCTs)[10-21] and previous meta-analyses[22-28], on which type of stent may be a preferred treatment for malignant biliary obstruction; however, these studies often include multiple locations for bile duct obstruction – making the conclusions challenging to interpret or implement in routine clinical practice. As such, this systematic review and meta-analysis compared the safety and efficacy of PS vs SEMS placement for patients with unresectable MDBO.
This study was performed in conformity with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and was registered in the International Prospective Register of Systematic Reviews under file number CRD42020191234. The study was approved by the Ethics Committee of Hospital das Clínicas, Faculty of Medicine, University of São Paulo.
We screened all studies comparing PS vs SEMS placement among patients with inoperable MDBO, due to unrespectability or poor patient status (after evaluation by the surgeon or anesthesiologist). No restrictions were set for publication date or language.
A comprehensive search was performed in MEDLINE, Cochrane, Embase, Latin American and Caribbean Health Sciences Literature (LILACS), and grey literature, from their inception to December 2020. Search strategies are available in Supplementary material, Appendix 1.
Studies meeting the eligibility criteria were selected by two reviewers, initially by title and abstract and later the relevant articles were selected for full-text review. Any disagreements were resolved by consultation with a third reviewer. For the evaluation of adverse events, data related to any disorder attributed to deployment of the stent were excluded. Complications related to the procedure were considered: Cholecystitis, bleeding, pancreatitis, perforation, and hepatic abscess. To evaluate stent dysfunction, data related to obstruction, kinking, and/or migration of the stent were extracted. This outcome was identified when there was a direct report of these data, or through the interpretation of data related to cholestasis and cholangitis described in the RCTs. Thus, only the dysfunction of the first stent placed was considered.
In this systematic review and meta-analysis, clinical success was defined as successful stent placement with a documented decrease in bilirubin post-procedure of ≥ 20% in relation to the value before the procedure and clinical improvement. Reintervention was evaluated by defining the replacement of a dysfunctional stent through any procedure necessary to drain the bile duct, whether endoscopic or percutaneous. For the evaluation of duration of stent patency, the time between deployment of the stent and the first clinical or laboratory manifestation of the dysfunction of the stent was evaluated, with the need for a new reintervention, calculated in days. In this outcome, the average duration of patency of the first stent deployed in the patient was evaluated, being disregarded the other reinterventions. For the survival evaluation, the global average survival of the patients was evaluated, calculated in days.
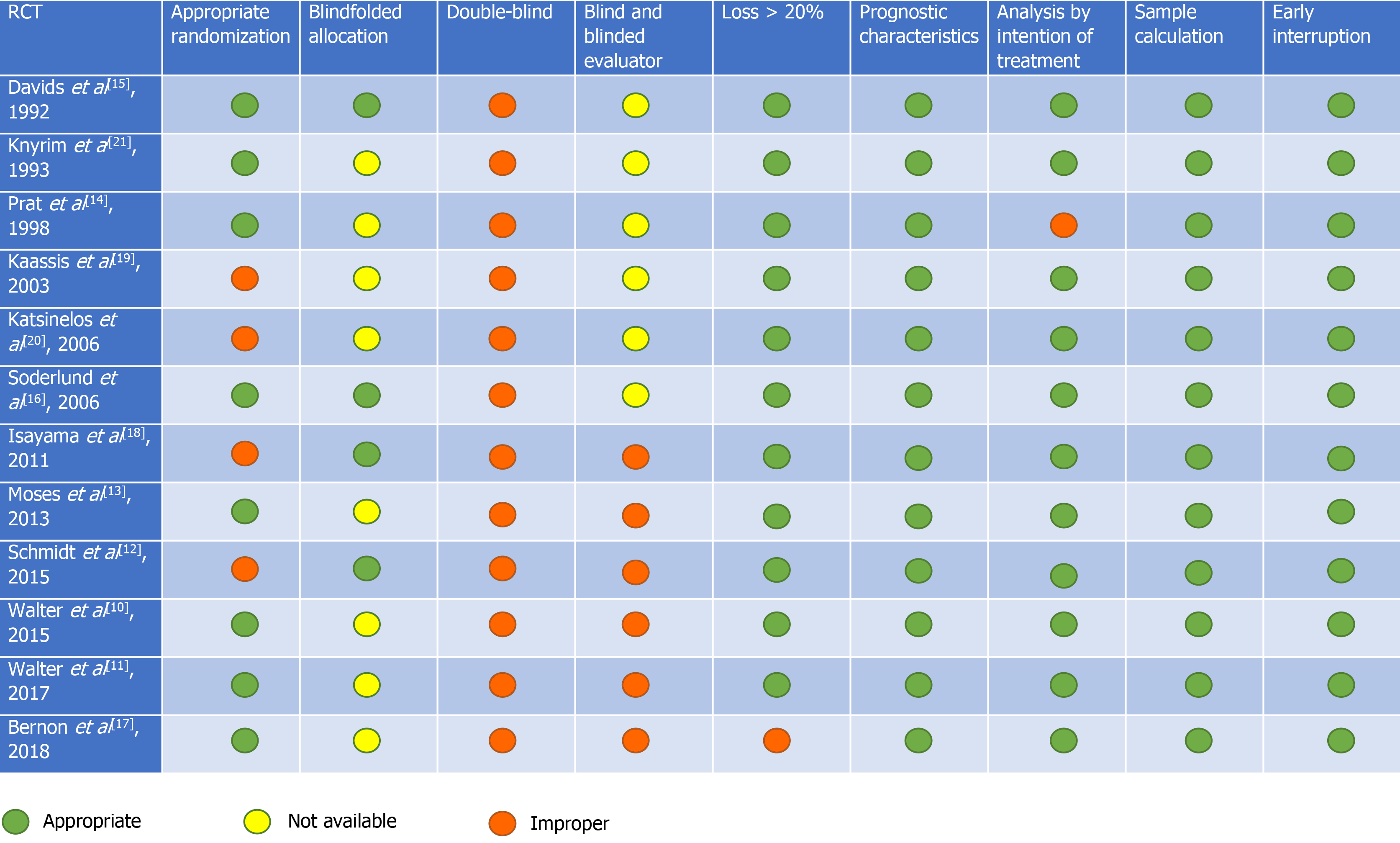
Risk of bias was evaluated through individual RCTs by Cochrane's risk assessment tool for randomized trials, available as ROB-II[29]. The items evaluated were as follows: Randomization, blindfolded allocation, double-blind and blinded evaluator, loss > 20%, analysis by intention of treatment, prognostic characteristics, sample calculation, analyzed outcomes, and early interruption. Each item evaluated was classified as low, moderate, or high bias risk. The quality of the evidence was analyzed using the Recommendation Classification, Development, and Evaluation (GRADE) working group[30].
The data from the selected works were analyzed through the software Review Manager version 5.4 (RevMan 5.4). For dichotomous endpoints, the difference was calculated by the risk difference (RD), using the Cochrane Mantel-Haenszel test, with 95% confidence interval (CI). A fixed effects model was used in cases where the I2 was less than 50% and a random effects model was used when the I2 was greater than 50%. P < 0.05 was considered statistically significant. For continuous variables, the inverse variance test was applied. To calculate the difference between the measures, the difference of the mean was used through calculations among the mean, standard deviation, and sample size of each group. In RCTs where the standard deviation was not reported, it was calculated using the mean, the interval reported in the outcome, and the sample size.
The inconsistency index was evaluated through I2, in which it is possible to observe the presence of heterogeneity. The I2 varies from 0% to 100%, and when it presents as heterogeneity > 50%, we consider it high and > 75% is considered very high. The sensitivity test (Egger) was performed whenever the heterogeneity was high in the search for publication bias (outlier)[31].
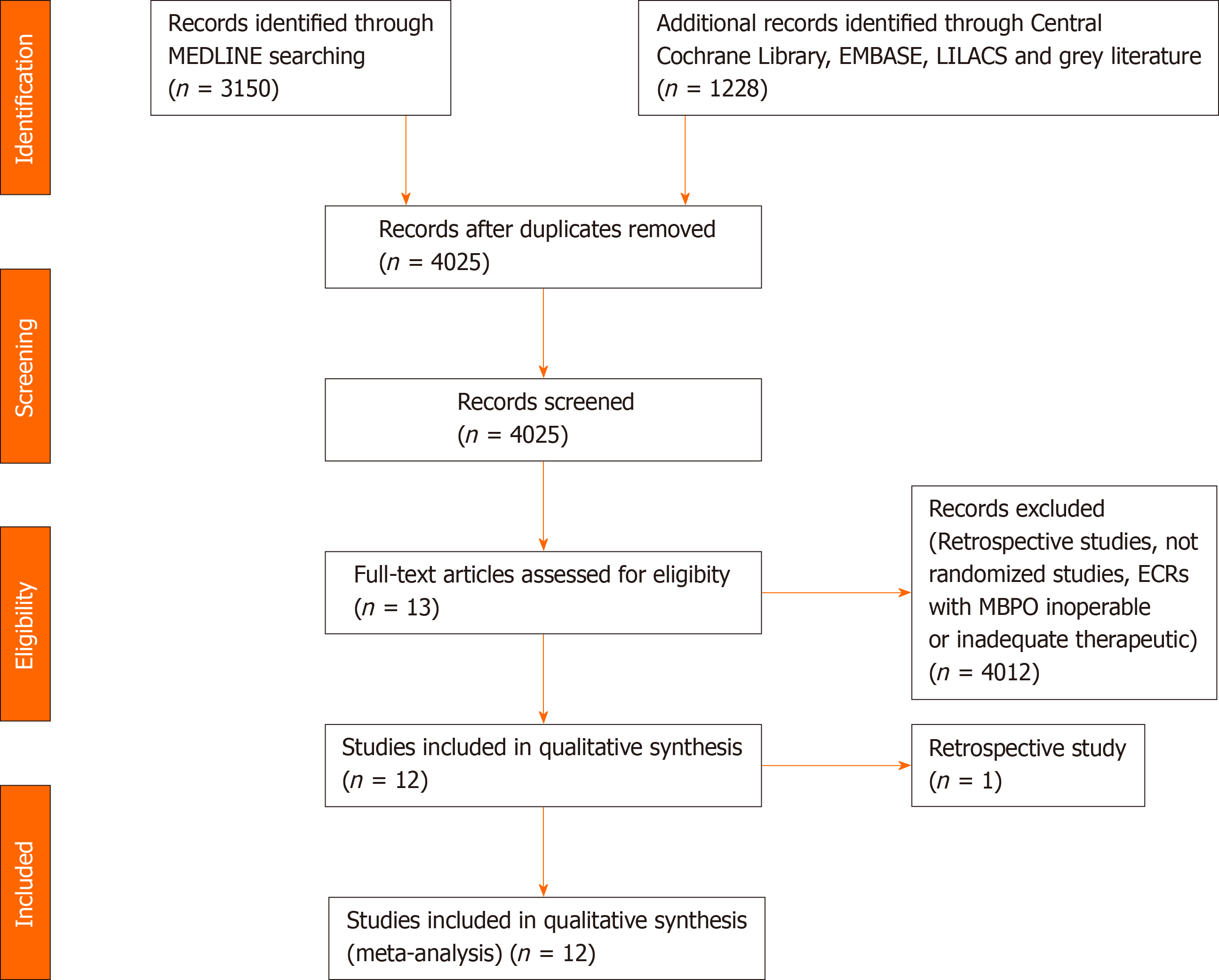
The search strategy identified 4378 articles. After excluding the duplicates, retrospective studies, and applying the eligibility criteria, 12 RCTs were selected[10-21] (Figure 1).
Twelve randomized clinical trials were selected[10-21], totaling 1005 patients. This population provides several etiological profiles for the cause of biliary obstruction (Supplementary Table 1). In two studies[10,11] there was no division of data between uncovered SEMS and partially or fully covered SEMS, but only one[10] result was discriminated between the two types of SEMS.
Moreover, Prat et al[14] described three groups, and in group 2 (G2) the PS was changed every 3 mo. Taking into account that certain outcomes would not be reliably evaluated, we chose to exclude this group from our review, considering only the population of group G1 (in which PS was changed only if dysfunctional) and G3 (in which SEMS was changed only if dysfunctional). Regarding the PS, only one study[20] specified the type of stent between straight and pigtail, having used straight PS. Regarding the diameter of the stent, the most cited diameter was 10 mm for SEMS and 10 Fr for PS. Table 1 shows the type of specific stent used in each group and the outcomes evaluated in each study.
| Author | Patients | P - palliative disease | I - intervention | C - control | O - outcomes |
| Davids et al[15], 1992 | 105 | MBDO | 49 | 56 | Stent dysfunction, mean survival, duration of patency, complications, reinterventions, clinical success |
| 8-10 mm wallstent | 10 Fr PS straight | ||||
| Knyrim et al[21], 1993 | 62 | MBDO | 31 | 31 | Complications, stent dysfunctions, reinterventions, clinical success |
| 8 mm wallstent ou strecket stent (1 or 2) | 11,5 Fr PS If it fails à 14 Fr percutaneous technique | ||||
| Prat et al[14], 1998 | 1051 | MBDO | 34 | 2 groups: 33/34 | Stent dysfunction, reinterventions |
| 10 mm wallstent | 11,5 Fr PS | ||||
| Kaassis et al[19], 2003 | 118 | MBDO | 59 | 59 | Stent dysfunction, reinterventions, complications |
| 10 mm wallstent | 10 Fr Tannenbaum PS | ||||
| Katsinelos et al[20], 2006 | 47 | MBDO | 23 | 24 | Stent dysfunction, Mean survival, complications, reinterventions, duration of patency, clinical success |
| 10 mm uSEMS | 10 Fr Tannenbaum PS straight | ||||
| Soderlund et al[16], 2006 | 100 | MBDO | 49 | 51 | Mean survival, reinterventions, duration of patency, complications, mean survival, clinical success |
| 10 mm cSEMS | 10 Fr PS | ||||
| Isayama et al[18], 2011 | 120 | MBDO | 60 | 60 | Mean survival, complication, stent dysfunction, reinterventions, duration of patency |
| cSEMS | 10 Fr Stent Double Layer | ||||
| Moses et al[13], 2013 | 85 | MBDO | 42 | 43 | Mean survival, duration of patency, complications, stent dysfunction, clinical success |
| 10 mm pcSEMS | 10 Fr Amsterdam PS | ||||
| Schmidt et al[12], 2015 | 37 | MBDO | 19 | 18 | Stent dysfunction, complications. |
| 10 mm uSEMS, cSEMS or pcSEMS | 10 Fr ViaDuct Stent (PS wing) | ||||
| Walter et al[10], 2015 | 2401 | MBDO | uSEMS: 75 / pcSEMS: 73 | 73 | Stent dysfunction, mean survival, complications, duration of patency, clinical success |
| 10 mm uSEMS or pcSEMS Wallstent RX | 10 Fr PS Polyethylene or polyurethan | ||||
| Walter et al[11], 2017 | 2401 | MBDO | uSEMS: 75 / pcSEMS: 73 | 73 | Stent dysfunction, duration of patency, mean survival |
| 10 mm uSEMS or pcSEMS Wallstent RX | 10 Fr PS Polyethylene or polyurethan | ||||
| Bernon et al[17], 2018 | 40 | MBDO | 19 | 21 | Stent dysfunction, duration of patency, complications, clinical success |
| 10 mm uSEMS | 10 Fr PS polyethylene |
Two studies[10,11] were carried out by the same author and had the same population number. Thus, in the analysis of the oldest article, we used only the outcomes that were not cited in Walter et al[11]. In the other endpoints, the most updated data were used, so that there was no data overlapping.
In the risk of bias analysis, it was not possible to evaluate the presence of double-blinding due to the study characteristics. The risk of bias analysis for each individualized study is shown in Figure 2. In two studies[10,11], the randomization of 240 patients was performed before endoscopic retrograde cholangiopancreatography (ERCP); however, after examination, only 219 were included in the studies. Patients presenting with intrahepatic obstruction, absence of stenosis, duodenal involvement, benign stenosis, candidates for surgery, prior bile duct surgery, and those who did not have an informed consent form were excluded. Four studies[10,11,15,16] associated percutaneous drainage with endoscopic drainage, when there was failure to place the stent only through the endoscopic route.
The quality of evidence was evaluated by GRADE criteria, and very low levels of evidence were found for complications, stent dysfunction, reinterventions (dichotomous variables), and duration of stent patency; low for mean survival and reinterventions (in the analysis of continuous variables); moderate for drainage success. The Supplementary material, Appendix 2 demonstrates the table GRADE.
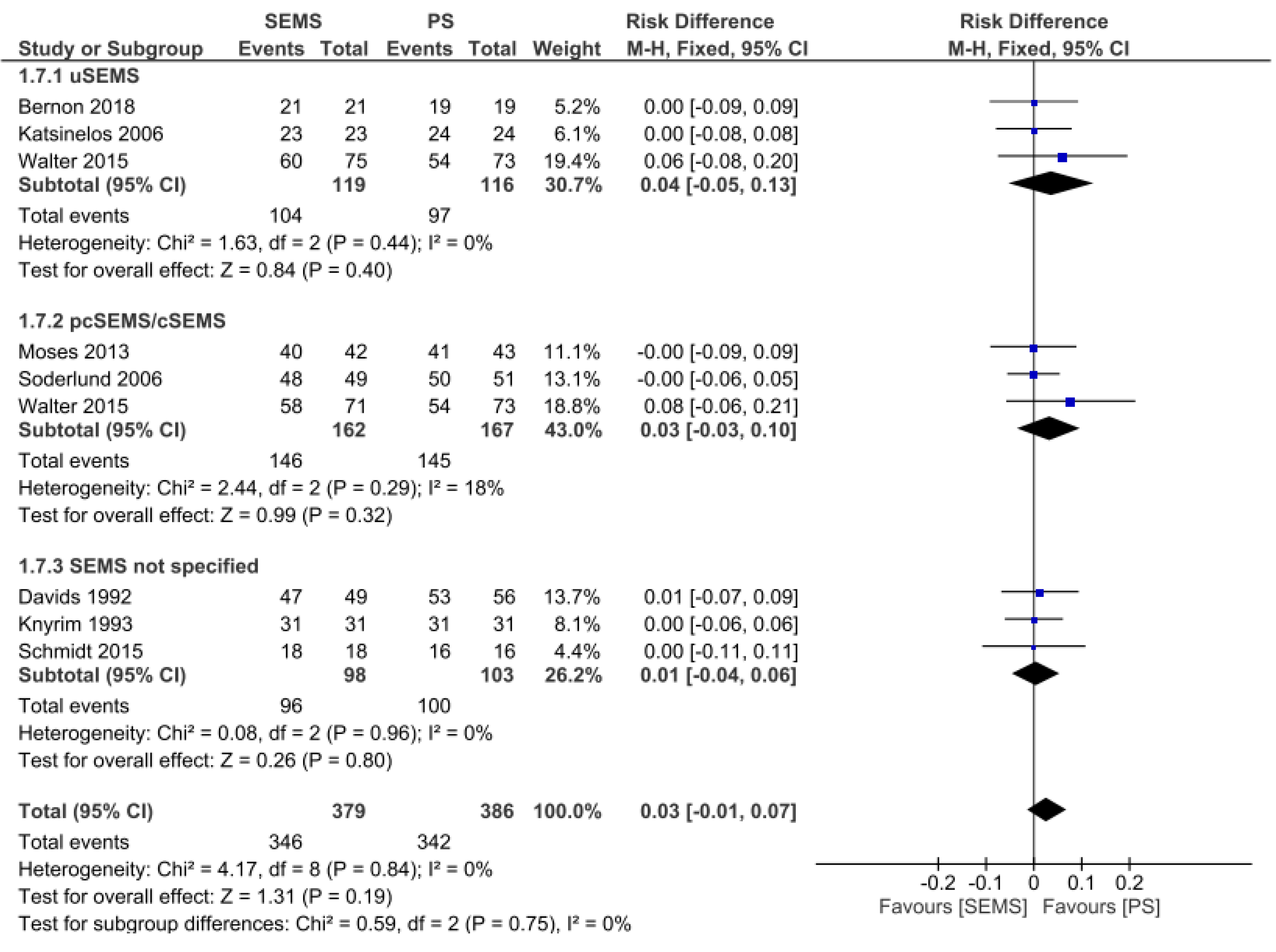
Clinical Success: Clinical success was evaluated in eight studies[10,12,13,15-17,20,21], totaling 765 patients (379 in the SEMS group and 386 in the PS group). There was no difference between the two groups (RD = 0.03, 95%CI = -0.01, 0.07; I2 = 0%; P = 0.19). There was also no difference in the uncovered SEMS (RD = 0.04, 95%CI: -0.05,0.13; I2 = 0%; P = 0.40), partially/fully covered SEMS (RD = 0.03, 95%CI: -0.03, 0.10; I2 = 18%; P = 0.32), and SEMS not specified subgroups (RD = 0.01, 95%CI: -0.04, 0.06; I2 = 0%; P = 0.80) (Figure 3).
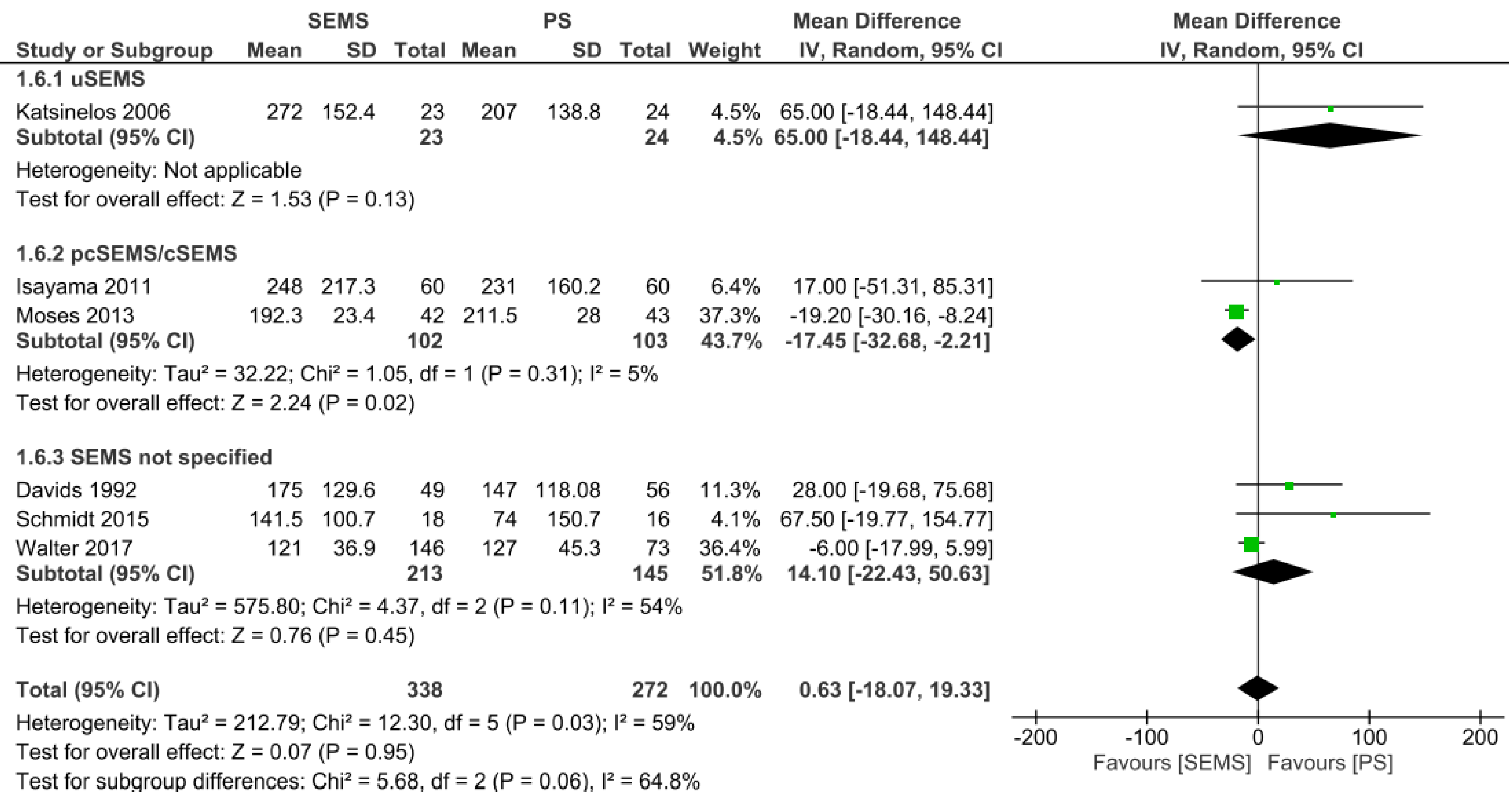
Mean survival: The mean survival analysis was performed in days, and documented in six studies[11-13,15,18,20], evaluating a total of 610 patients: 338 patients in the SEMS group and 272 patients in the PS group. There was no difference between the two groups (mean difference [MD] = 0.63, 95%CI: -18.07, 19.33; I² = 59%; P = 0.95). Regarding the subgroups, uncovered SEMS (MD = 65 d, 95%CI: -18.44, 148.44; I2 = not applicable; P = 0.13) and SEMS not otherwise specified (MD = 14.10 d, 95%CI: -22.43, 50.63; I² = 54%; P = 0.45) were not different from PS placement. However, partially or fully covered SEMS revealed an increase in mean survival (MD = -17.45 d, 95%CI: -32.68, -2.21; I² = 5%; P = 0.02) (Figure 4).
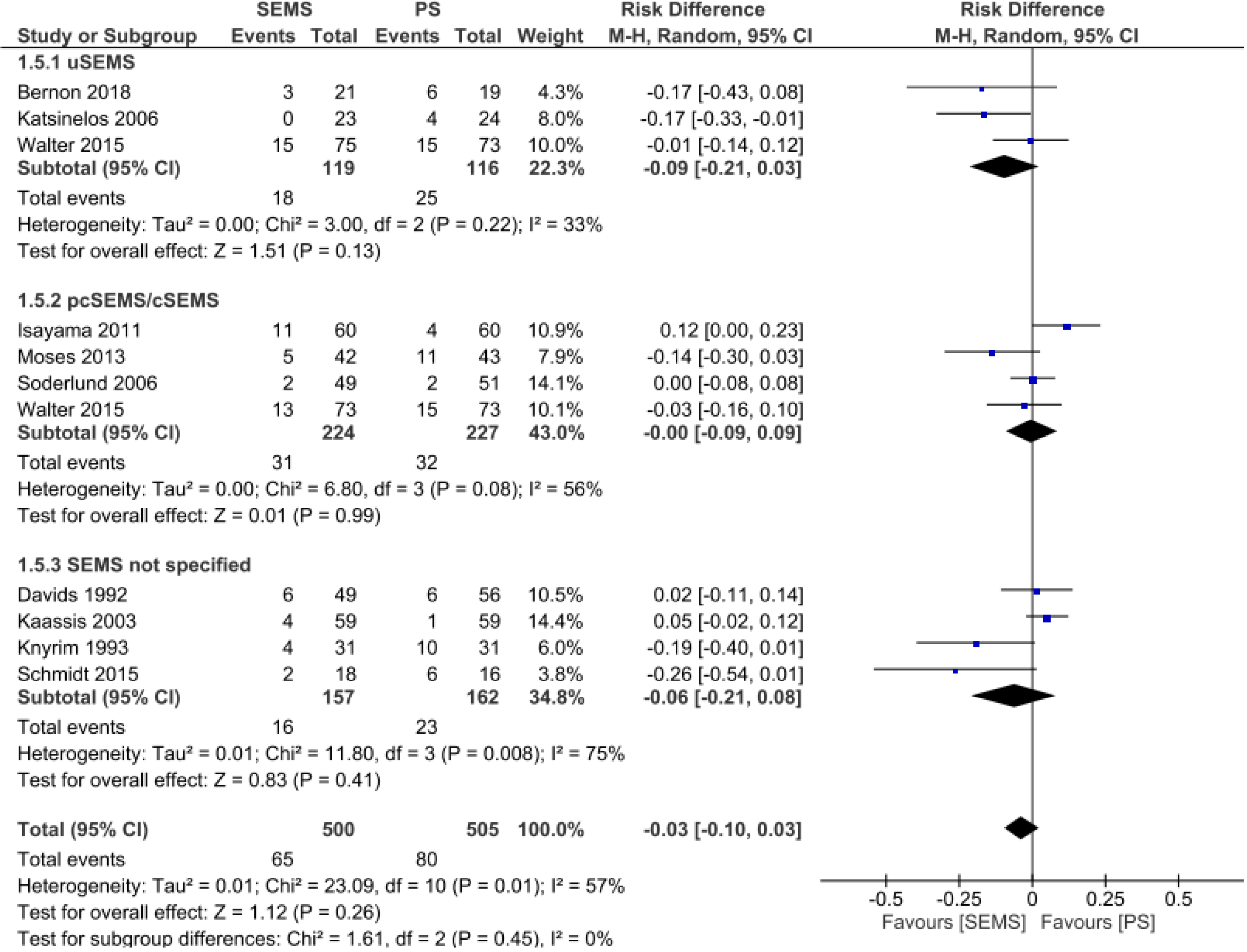
Adverse events: Analyses of 10 studies[10,12,13,15-21] totaling 505 patients in the PS group and 500 patients in the SEMS group. There was no difference between the two groups (RD = -0.03, 95%CI: -0.10, 0.03; P = 0.26). Subgroup analyses revealed no differences by specific SEMS type (uncovered SEMS: RD = -0.09, 95%CI: -0.21, 0.03, I² = 33; P = 0.13; partially/fully covered SEMS: RD = -0.00, 95%CI: -0.09, 0.09, I² = 56%; P = 0.99; and SEMS not otherwise specified RD = -0.06, 95%CI: -0.21, 0.08, I² = 75%; P = 0.41) (Figure 5).
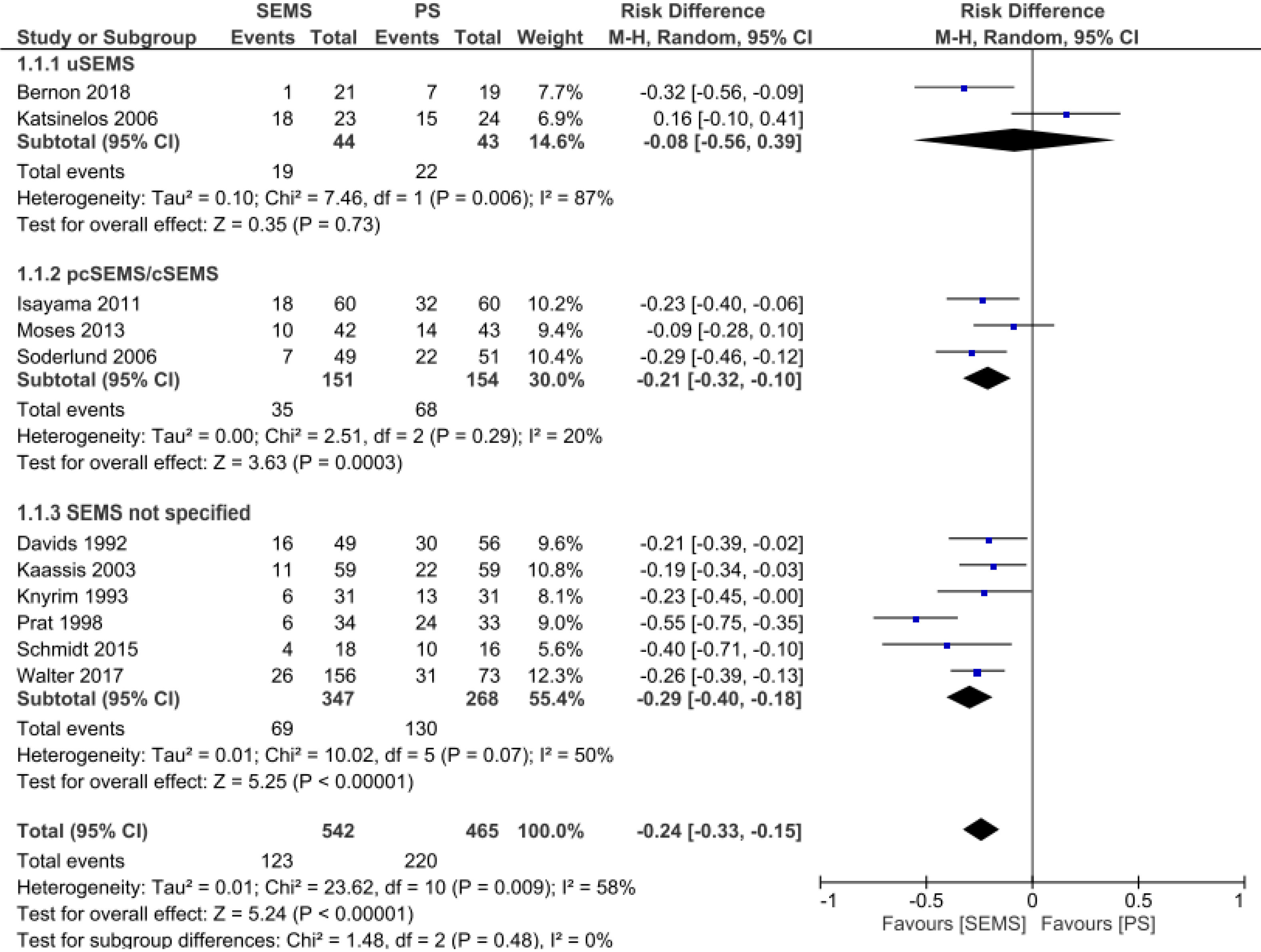
Stent dysfunction: In the stent dysfunction analysis, it was possible to extract data from 11 studies[11-21] totaling 465 patients in the PS group and 542 patients in the SEMS group. The rate of stent dysfunction was 24% lower in the SEMS group (RD = -0.24, 95%CI: -0.33, -0.15; I² = 58%; P < 0.00001). Performing a subgroup analysis by type of SEMS revealed no difference in stent dysfunction rate between uncovered SEMS and PS placement (RD = -0.08, 95%CI: -0.56, 0.39; I² = 87%; P = 0.73). In the other two subgroups there was a statistically significant difference. In the partial/fully covered SEMS subgroup (n = 151 patients), the stent dysfunction rate was 21% lower than in the PS group (n = 154 patients) (RD = -0.21, 95%CI: -0.32, -0.1; I² = 20%; P = 0.0003). In the SEMS not specified subgroup (n = 347 patients), there was 29% less dysfunction than in the PS group (n = 268 patients) (Figure 6).
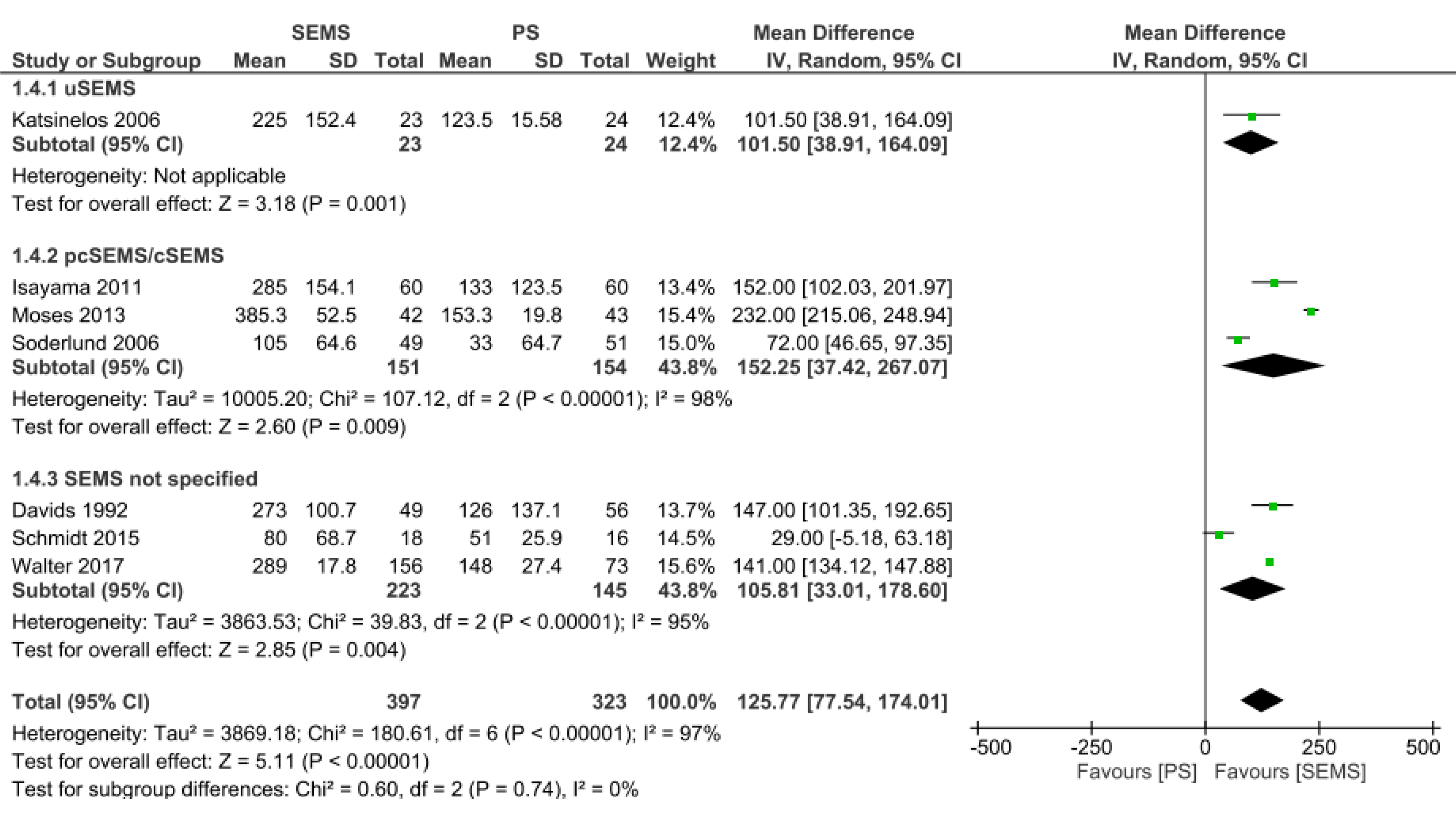
Stent patency: Data from seven studies[11-13,15,16,18,20] were evaluated in a total of 720 patients: 397 in the SEMS group and 323 in the PS group. The duration of patency was longer in the SEMS group (MD = 125.77, 95%CI: 77.5, 174.01; I² = 97%; P < 0.00001). In all subgroups, there was a longer time for stent dysfunction compared to PS. Twenty-three patients were evaluated in the uncovered SEMS subgroup (MD = 101.5, 95%CI: 38.91, 164.09; P = 0.001), 151 patients in the partially or fully covered SEMS subgroup (MD = 152.25, 95%CI: 37.42, 267.07; I2 = 98%; P = 0.009), and 223 patients in the SEMS subgroup not specified (MD: 105.81, 95%CI: 33.01, 178.60; I² = 95%; P = 0.004) (Figure 7).
Reintervention: The reintervention analyses were divided into two analyses: one evaluating studies in which the result was expressed in dichotomous variables and the other in continuous variables.
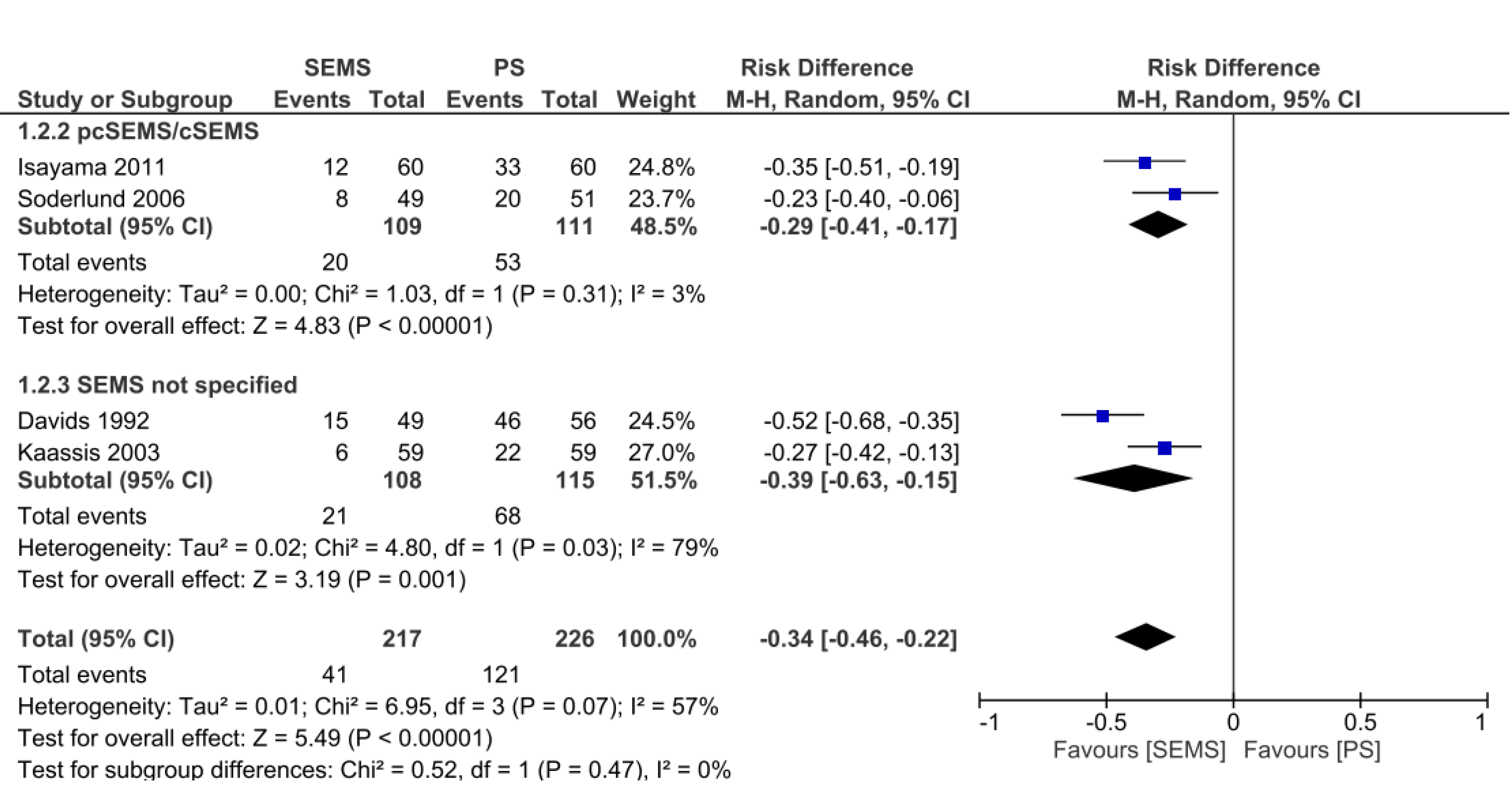
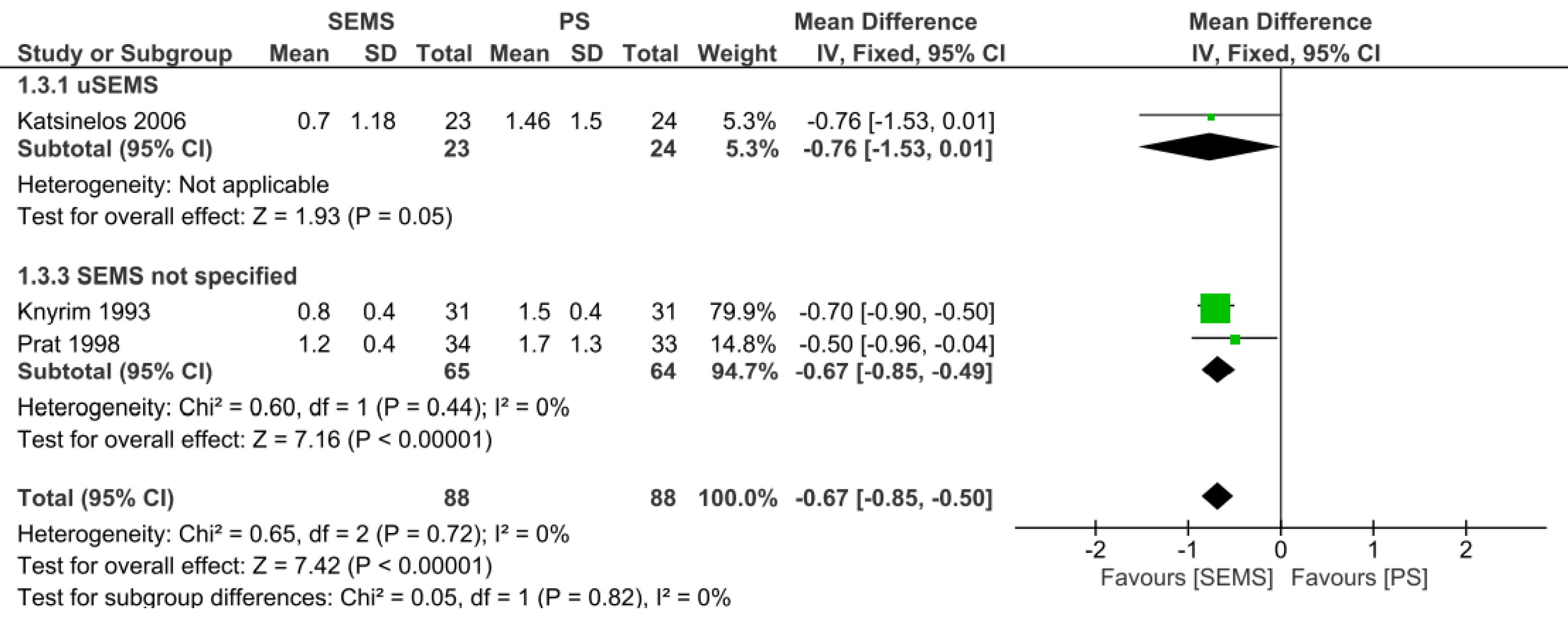
In the analysis of dichotomous variables, it was possible to evaluate four studies[15,16,18,19], presenting 226 patients in the PS group and 217 patients in the SEMS group, totaling 443 patients. The reintervention rate was 34% lower in the SEMS group, with statistical difference (RD = -0.34, 95%CI: -0.46, -0.22; I² = 57%; P < 0.00001). In both the covered SEMS subgroup and the SEMS not specified subgroup, there was a lower reintervention rate than in the PS group (RD = -0.29, 95%CI: -0.41, -0.17; I² = 3%; P < 0.00001 and RD = -0.39, 95%CI: -0.63, -0.15; I² = 79%; P = 0.001, respectively) (Figure 8). In the analyses of continuous variables, three studies[14,20,21] were evaluated, with 88 patients in each group. The reintervention rate was 67% lower in the SEMS group (MD = -0.67, 95%CI: -0.85, -0.50; I² = 0%; P < 0.00001). The uncovered SEMS subgroup revealed no difference vs the PS group (RD = -0.76, 95% C, -1.53, 0.01; I² = not applicable; P = 0.05); however, the SEMS not specified subgroup had a reintervention rate 67% lower than in the PS group (RD = -0.67, 95%CI: -0.85, -0.49; I2 = 0%; P < 0.00001) (Figure 9).
Based on the results of this systematic review and meta-analysis, both PS and SEMS types had a similar clinical success rate, complication rate, and patient-associated mean survival for treatment of MDBO. However, our study also demonstrated that SEMS was associated with a longer duration of stent patency compared to PS as well as a lower rate of reintervention. On subgroup analyses, both covered and uncovered SEMS improved stent patency compared to PS, although stent dysfunction was higher among patients who received treatment with a partially or fully covered SEMS.
In this study, we found that SEMS was associated with a longer duration of patency, lower rate of stent dysfunction, and decreased need for reintervention. This may be explained by two factors. First, SEMS is self-expanding and reaches a larger diameter when compared to PS placement, allowing for a greater flow and consequently better drainage of the bile duct. Furthermore, SEMS has less surface for bacterial multiplication and fixation, which may lead to the formation of biofilm and deposition of bile sludge, responsible for earlier obstruction of the PS[14,20,21].
In the subgroup of uncovered metal stents, the main cause of obstruction was internal tumor growth ("ingrowth"), making replacement extremely challenging in cases of obstruction. In the subgroups of partially covered or covered metal stents, due to their covering, the main complication is migration. This is due to the fact that this type of stent applies a greater expandable force that, associated with tumor growth, leads to its migration. However, partially or fully covered SEMS allow for a greater possibility of stent removal or replacement in case of failure/clogging compared to uncovered SEMS[14,20].
Regarding survival, there was no difference between SEMS and PS; however, when analyzing the subgroups, the covered SEMS placement outperformed PS. MDBO leads to late symptoms and diagnosis, consequently reducing survival, regardless of the form of palliative treatment performed. However, some factors favor the choice between the two types of stent. It should be noted that in those patients who have a life expectancy longer than 120 to 180 days, the use of SEMS is indicated, due to the longer duration of patency and lower reintervention rate, since it would reduce the need for numerous procedures, resulting in a better quality of life[2,8,9]. In five included RCTs[10,12,14,19] the main factors for choosing between PS and SEMS were tumors larger than 30 mm and the presence of hepatic metastasis[32]. According to these studies, these factors may significantly reduce the patient’s survival, favoring the deployment of PS, because of its lower initial cost.
Regarding complications, there was no statistical difference between SEMS and PS. This may be explained by the fact that ERCP is a therapeutic procedure that can present complications regardless of the type of stent used. The main complications reported in the studies were pancreatitis, infections (cholangitis, cholecystitis, or liver abscess), perforation, and bleeding. In most cases, the complications were diagnosed and treated early; however, sometimes they may appear later (7 to 10 days after the procedure), as in the case of infections or bleeding. In our study, it was not possible to carry out an analysis by type of complication, since the RCT did not make this division[33].
It is important to acknowledge that there have been several previous meta-analyses[22-28] on this subject in the literature. However, in all of them, location was not isolated to the site of distal biliary obstruction (i.e. inclusion of both malignant proximal and distal biliary obstructions), thereby leading to greater bias, as different locations result in different tumor behaviors and associated morbidity/mortality. In addition, in five of these meta-analyses[23,24,26-28], comparison of percutaneous and endoscopic placed stents were performed, generating another bias, since these treatments produce different rates of complications, recurrence and associated mortality. Furthermore, two previous meta-analysis included retrospective studies[24,26], thereby allowing for the introduction of patient selection bias and significantly reducing the quality of evidence.
Therefore, we included and analyzed only RCTs among patients with confirmed non-operative MDBO. As such, this systematic review and meta-analysis is the first to analyze endoscopic biliary drainage as a specific treatment for MDBO. In additional to comparing plastic vs metal stent placement, this study also performed subgroup analysis by type of SEMS.
Yet despite this study’s strengths, this meta-analysis also had limitations. Despite inclusion of high-quality RCT data, significant heterogeneity existed for many outcomes included in this study. However, this is not surprising given the aggressive nature of these malignancies and palliative endoscopic treatment involved.
The use of SEMS provides for a longer duration of stent patency, lower reintervention rate, and lower rate of stent dysfunction when compared to the use of PS in patients with MDBO. With regard to mean survival analysis, there was no difference between SEMS vs PS placement; however, on subgroup analyses, partial or fully covered SEMS demonstrated an increased mean survival compared to PS. There was no difference between the methods in concern of clinical success and rate of complications.
The drainage endoscopic in patients with inoperable and/or irresectable malignant distal biliary obstruction (MDBO) has several advantages such as patient comfort, increased survival and fewer complications due to biliary obstruction. However, there are two types of stents, self-expanding metal stent (SEMS) and plastic stent (PS), leading to much discussion about which one to use, due to their different characteristics.
In many cases, MDBO have no curative perspective by the time of diagnosis. Therefore, palliative treatment for bile duct clearance plays a major role, since they provide longer life expectancy and better quality of life. Therefore, we wanted to compare the two types of stents commonly used in an attempt to understand which approach is best.
To perform a systematic review and meta-analysis of Randomized Controlled Trials comparing plastic vs metal stents in palliative treatment of MDBO.
We performed a systematic review and meta-analysis using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. Electronic searches were performer using MEDLINE, EMBASE, Central Cochrane, Latin American and Caribbean Health Sciences Literature databases. Only randomized control trials were included. The outcomes studied were stent dysfunction rate, reintervention rate, duration of patency, mean survival, complications, and clinical success.
Twelve randomized clinical trials were included in the final analysis with a total of 1005 patients, of whom 681 belonged to the SEMS group and 542 to the PS group. The SEMS group was divided into three subgroups, uncovered metal stent (uSEMS), partially/fully covered (pcSEMS/cSEMS) and the group in which the SEMS was not specified (SEMS not specified). SEMS had a lower dysfunction rate than the PS and in the analysis of the subgroups, uSEMS had no difference comparing to PS and pcSEMS/cSEMS was higher. Regarding reintervention, SEMS had a lower reintervention rate compared to PS. Concerning duration of patency, SEMS also showed advantage than PS. In the three subgroups of the SEMS, there was longer duration of patency. In the mean survival analysis, there was no difference between SEMS and PS, however, in the analysis of the subgroups, pcSEMS/cSEMS favored over the PS. Regarding complications rate and clinical success were similar in both groups, and have no significant difference.
Our study showed that SEMS presents a higher duration of patency, lower reintervention rate, and lower dysfunction rate when compared to the use of PS. There was no difference between the methods in concern of survival analysis, clinical success and rate of complications.
The use of SEMS was a great advent in palliative therapy for MDBO. In our study, the use of SEMS revealed even more benefits in most cases. We hope that this study can clarify its benefit and that more patients can be benefited in deciding the type of stent used from here.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Krishnan A, Taira K S-Editor: Liu M L-Editor: Filipodia P-Editor: Li JH
| 1. |
American Cancer Society.
Cancer Facts & Figures |
| 2. | Pu LZ, Singh R, Loong CK, de Moura EG. Malignant Biliary Obstruction: Evidence for Best Practice. Gastroenterol Res Pract. 2016;2016:3296801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Ribeiro IB, de Moura DTH, Thompson CC, de Moura EGH. Acute abdominal obstruction: Colon stent or emergency surgery? World J Gastrointest Endosc. 2019;11:193-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 4. | Minata MK, Bernardo WM, Rocha RS, Morita FH, Aquino JC, Cheng S, Zilberstein B, Sakai P, de Moura EG. Stents and surgical interventions in the palliation of gastric outlet obstruction: a systematic review. Endosc Int Open. 2016;4:E1158-E1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Upchurch E, Ragusa M, Cirocchi R. Stent placement versus surgical palliation for adults with malignant gastric outlet obstruction. Cochrane Database Syst Rev. 2018;5:CD012506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Visconti TAC, Bernardo WM, Moura DTH, Moura ETH, Gonçalves CVT, Farias GF, Guedes HG, Ribeiro IB, Franzini TP, Luz GO, Dos Santos MEDL, de Moura EGH. Metallic vs plastic stents to treat biliary stricture after liver transplantation: a systematic review and meta-analysis based on randomized trials. Endosc Int Open. 2018;6:E914-E923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Soehendra N, Reynders-Frederix V. [Palliative biliary duct drainage. A new method for endoscopic introduction of a new drain]. Dtsch Med Wochenschr. 1979;104:206-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 55] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 479] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 9. | Wang CC, Yang TW, Sung WW, Tsai MC. Current Endoscopic Management of Malignant Biliary Stricture. Medicina (Kaunas). 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Walter D, van Boeckel PG, Groenen MJ, Weusten BL, Witteman BJ, Tan G, Brink MA, Nicolai J, Tan AC, Alderliesten J, Venneman NG, Laleman W, Jansen JM, Bodelier A, Wolters FL, van der Waaij LA, Breumelhof R, Peters FT, Scheffer RC, Leenders M, Hirdes MM, Steyerberg EW, Vleggaar FP, Siersema PD. Cost Efficacy of Metal Stents for Palliation of Extrahepatic Bile Duct Obstruction in a Randomized Controlled Trial. Gastroenterology. 2015;149:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Walter D, van Boeckel PG, Groenen MJ, Weusten BL, Witteman BJ, Tan G, Brink MA, Nicolai J, Tan AC, Alderliesten J, Venneman NG, Laleman W, Jansen JM, Bodelier A, Wolters FL, van der Waaij LA, Breumelhof R, Peters FT, Scheffer RC, Steyerberg EW, May AM, Leenders M, Hirdes MM, Vleggaar FP, Siersema PD. Higher quality of life after metal stent placement compared with plastic stent placement for malignant extrahepatic bile duct obstruction: a randomized controlled trial. Eur J Gastroenterol Hepatol. 2017;29:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Schmidt A, Riecken B, Rische S, Klinger C, Jakobs R, Bechtler M, Kähler G, Dormann A, Caca K. Wing-shaped plastic stents vs. self-expandable metal stents for palliative drainage of malignant distal biliary obstruction: a randomized multicenter study. Endoscopy. 2015;47:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Moses PL, Alnaamani KM, Barkun AN, Gordon SR, Mitty RD, Branch MS, Kowalski TE, Martel M, Adam V. Randomized trial in malignant biliary obstruction: plastic vs partially covered metal stents. World J Gastroenterol. 2013;19:8638-8646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Prat F, Chapat O, Ducot B, Ponchon T, Pelletier G, Fritsch J, Choury AD, Buffet C. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 305] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 697] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 16. | Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Bernon MM, Shaw J, Burmeister S, Chinnery G, Hofmeyr S, Kloppers JC, Jonas E, Krige JE. Distal malignant biliary obstruction: a prospective randomised trial comparing plastic and uncovered self-expanding metal stents in the palliation of symptomatic jaundice. S Afr J Surg. 2018;56:30-34. [PubMed] |
| 18. | Isayama H, Yasuda I, Ryozawa S, Maguchi H, Igarashi Y, Matsuyama Y, Katanuma A, Hasebe O, Irisawa A, Itoi T, Mukai H, Arisaka Y, Okushima K, Uno K, Kida M, Tamada K. Results of a Japanese multicenter, randomized trial of endoscopic stenting for non-resectable pancreatic head cancer (JM-test): Covered Wallstent versus DoubleLayer stent. Dig Endosc. 2011;23:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Kaassis M, Boyer J, Dumas R, Ponchon T, Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF, Burtin P. Plastic or metal stents for malignant stricture of the common bile duct? Gastrointest Endosc. 2003;57:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 317] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 20. | Katsinelos P, Paikos D, Kountouras J, Chatzimavroudis G, Paroutoglou G, Moschos I, Gatopoulou A, Beltsis A, Zavos C, Papaziogas B. Tannenbaum and metal stents in the palliative treatment of malignant distal bile duct obstruction: a comparative study of patency and cost effectiveness. Surg Endosc. 2006;20:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Knyrim K, Wagner HJ, Pausch J, Vakil N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 358] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 22. | Moss AC, Morris E, Leyden J, MacMathuna P. Do the benefits of metal stents justify the costs? Eur J Gastroenterol Hepatol. 2007;19:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Hong WD, Chen XW, Wu WZ, Zhu QH, Chen XR. Metal versus plastic stents for malignant biliary obstruction: an update meta-analysis. Clin Res Hepatol Gastroenterol. 2013;37:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Sawas T, Al Halabi S, Parsi MA, Vargo JJ. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc 2015; 82: 256-267. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 25. | Zorrón Pu L, de Moura EG, Bernardo WM, Baracat FI, Mendonça EQ, Kondo A, Luz GO, Furuya Júnior CK, Artifon EL. Endoscopic stenting for inoperable malignant biliary obstruction: A systematic review and meta-analysis. World J Gastroenterol. 2015;21:13374-13385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 26. | Moole H, Jaeger A, Cashman M, Volmar FH, Dhillon S, Bechtold ML, Puli SR. Are self-expandable metal stents superior to plastic stents in palliating malignant distal biliary strictures? Med J Armed Forces India. 2017;73:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Almadi MA, Barkun A, Martel M. Plastic vs. Self-Expandable Metal Stents for Palliation in Malignant Biliary Obstruction: A Series of Meta-Analyses. Am J Gastroenterol. 2017;112:260-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 28. | Yuan TW, Liu HQ, Wang SB, Cao J. Comparison of plastic stents with self-expandable metal stents in palliative treatment of malignant biliary obstruction: a meta-analysis. Eur Rev Med Pharmacol Sci. 2017;21:2847-2857. [PubMed] |
| 29. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15131] [Article Influence: 2521.8] [Reference Citation Analysis (0)] |
| 30. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 14870] [Article Influence: 874.7] [Reference Citation Analysis (0)] |
| 31. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46435] [Article Influence: 2110.7] [Reference Citation Analysis (3)] |
| 32. | Matsumoto K, Takeda Y, Onoyama T, Kawata S, Kurumi H, Koda H, Yamashita T, Isomoto H. Endoscopic treatment for distal malignant biliary obstruction. Ann Transl Med. 2017;5:190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, Beyna T, Dinis-Ribeiro M, Hritz I, Mariani A, Paspatis G, Radaelli F, Lakhtakia S, Veitch AM, van Hooft JE. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 495] [Article Influence: 99.0] [Reference Citation Analysis (1)] |