Published online May 27, 2021. doi: 10.4240/wjgs.v13.i5.476
Peer-review started: January 29, 2021
First decision: March 6, 2021
Revised: March 13, 2021
Accepted: April 28, 2021
Article in press: April 28, 2021
Published online: May 27, 2021
Processing time: 111 Days and 22.6 Hours
The treatment of hepatocellular carcinoma (HCC) ≥ 10 cm remains a challenge.
To consolidate the role of surgical resection for HCC larger than 10 cm.
Eligible HCC patients were identified from the Chang Gung Research Database, the largest multi-institution database, which collected medical records of all patients from Chang Gung Memorial Foundation. The surgical outcome of HCC ≥ 10 cm (L-HCC) was compared to that of HCC < 10 cm (S-HCC) (model 1). The survival of L-HCC after either liver resection or transarterial chemoembolization (TACE) was also analyzed (model 2). The long-term risks of all-cause mortality and recurrence were assessed to consolidate the role of surgery for L-HCC.
From January 2004 to July 2015, a total of 32403 HCC patients were identified from the Chang Gung Research Database. Among 3985 patients who received liver resection, 3559 (89.3%) had S-HCC, and 426 had L-HCC. The L-HCC patients had a worse disease-free survival (0.27 for L-HCC vs 0.40 for S-HCC) and overall survival (0.18 for L-HCC vs 0.45 for S-HCC) than the S-HCC after liver resection (both P < 0.001). However, the surgical and long-term outcome of resected L-HCC had improved dramatically in the recent decades. After adjusting for covariates, surgery could provide a better outcome for L-HCC than TACE (adjusted hazard ratio of all-cause mortality: 0.46, 95% confidence interval: 0.38-0.56 for surgery). Subgroup analysis stratified by different stages showed similar trend of survival benefit among L-HCC patients receiving surgery.
Our study demonstrated an improving surgical outcome for HCC larger than 10 cm. Under selected conditions, surgery is better than TACE in terms of disease control and survival and should be performed. Due to inferior survival, a subclassification within T1 stage should be considered. Future studies are mandatory to confirm our findings.
Core Tip: By analyzing the data from one of the largest clinical databases worldwide, the current study demonstrated an improving surgical outcome for hepatocellular carcinoma ≥ 10 cm. Under selected conditions, surgery is better than transarterial chemoembolization in terms of disease control and survival. Due to inferior survival for HCC ≥ 10 cm, a subclassification within T1 stage should be considered.
- Citation: Lee CW, Yu MC, Wang CC, Lee WC, Tsai HI, Kuan FC, Chen CW, Hsieh YC, Chen HY. Liver resection for hepatocellular carcinoma larger than 10 cm: A multi-institution long-term observational study. World J Gastrointest Surg 2021; 13(5): 476-492
- URL: https://www.wjgnet.com/1948-9366/full/v13/i5/476.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i5.476
Hepatocellular carcinoma (HCC) is a lethal malignancy of liver and ranks as one of the most common causes of cancer-related death globally[1-3]. Liver resection remains as an effective treatment for HCC, but the underlying chronic liver injury has hampered the feasibility of surgery[4-11]. Fortunately, with improvements in preoperative preparation, operative techniques, surgical instruments, and perioperative care, the mortality rate of this challenging operation has been greatly reduced[4,12-14]. According to a recent study, the 30-d and in-hospital mortality rates after hepatectomy for HCC were both below 3%[15,16]. As a result, liver resection is recommended by most guidelines as one of the standard curative treatments for HCC.
Among published guidelines, the Barcelona Clinic Liver Cancer (BCLC) staging system is acknowledged by major academic societies worldwide. Both the Taiwan Liver Cancer Association, the American Association for the Study of Liver Diseases, and the European Association for the Study of Liver have recognized BCLC system to guide the treatment for patients with HCC[17-19]. According to BCLC, liver resection is the preferred treatment for patients with BCLC stage 0 and A diseases. Asymptomatic large (> 5 cm) HCC without major vascular invasion or extrahepatic spread, on the other hand, should receive transarterial chemoembolization (TACE) since it is categorized as intermediate stage disease (BCLC B)[20,21]. However, major guidelines have not excluded liver resection for HCC greater than 5 cm[17,18], and there were also studies addressing the surgical outcome of large HCC[22,23]. The best management for large HCC, particularly those greater than 10 cm, remains undetermined as a result. Studies comprising larger sample size and longer follow-up are therefore required to recommend treatment guidelines for these large HCC.
The Chang Gung Research Database contains all medical records of the Chang Gung Memorial Foundation since year 2000 and has become one of the largest clinical databases worldwide[24-26]. The current study, by utilizing the data from the Chang Gung Research Database (CGRD) and comparing them with our previous results, aimed to consolidate the role of liver resection for HCC ≥ 10 cm.
The CGRD, which collected the clinical information from seven Chang Gung memorial hospitals in Taiwan, was the primary data source of the current study. With more than 280000 admissions by 10070 beds, 8500000 outpatient visits, and 500000 emergency visits each year, the CGRD has become an excellent database for various kinds of clinical studies[24-26]. For cancer patients, it contains comprehensive cancer registry maintained in a prospective manner. The information obtained from the cancer registry is manually validated with a high completeness rate[27,28]. Both the International Classification of Diseases, 9th and 10th revision, Clinical Modification codes and the International Classification of Diseases, 3rd edition are used in the CGRD. For personal privacy, the individual identity is protected by encryption. The medical information is prospectively digitalized and stored in the CGRD and is amenable for researchers to perform large-scale retrospective analysis.
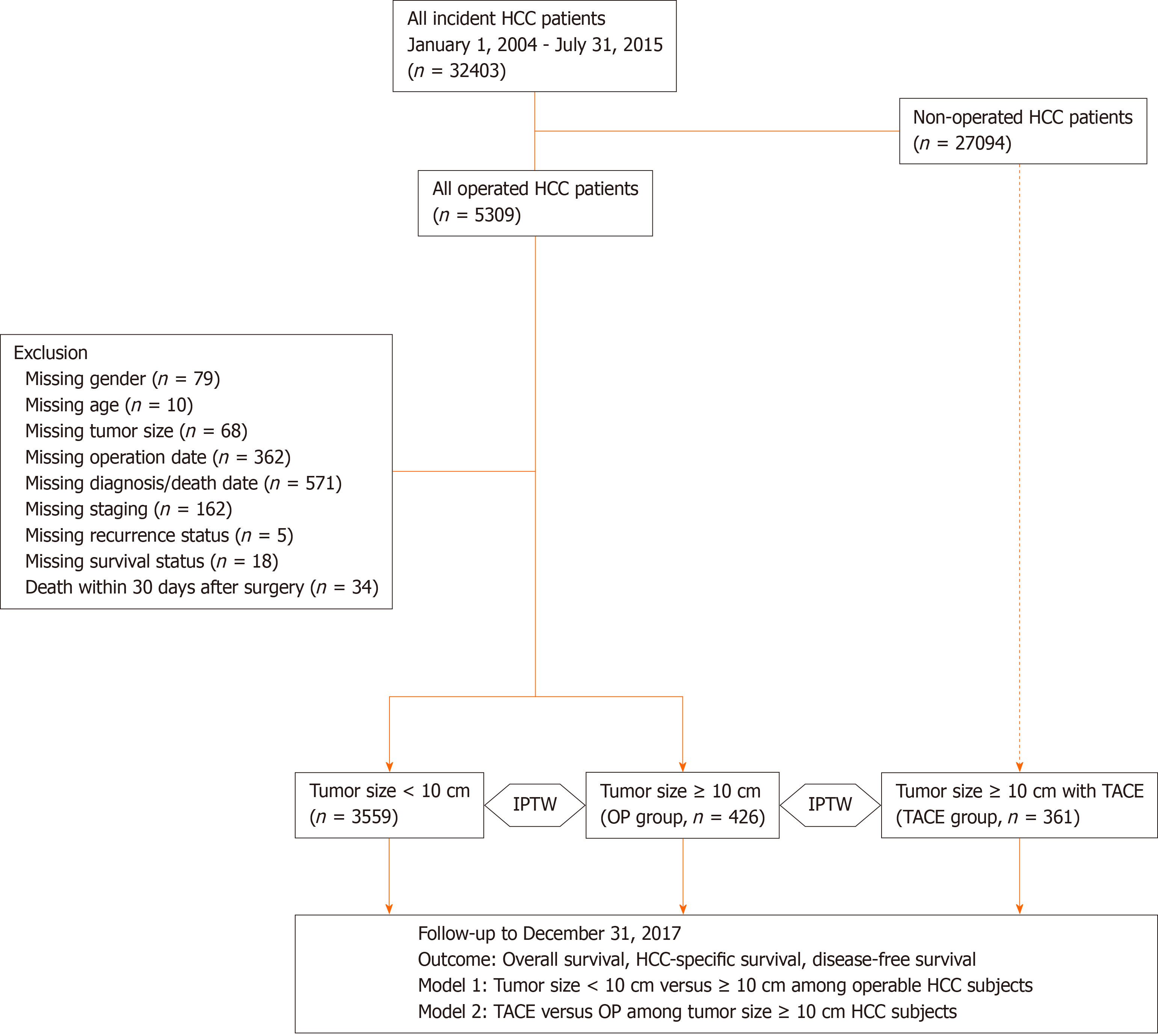
Figure 1 is the flow diagram of the current study. The diagnosis of HCC in Taiwan is made when two of the following criteria are met: α-fetoprotein (AFP) ≥ 400 ng/mL, positive findings on multi-phasic magnetic resonance imaging or computed tomography liver scan, and pathological confirmation, according to the recommendations from the American Association for the Study of Liver Diseases and the Gastroenterological Society in Taiwan[17,19]. HCC patients diagnosed from January 2004 to July 2015 were retrieved from the CGRD database (n = 32403). The first date of definite diagnosis for HCC was set as the index date. These eligible subjects were followed until December 2017. Two models were designed for outcome analysis: Model 1, patients with HCC ≥ 10 cm (L-HCC) or HCC < 10 cm (S-HCC) receiving curative-intent liver resection; model 2, patients with HCC ≥ 10 cm receiving either curative-intent liver resection or TACE as the primary treatment. The surgical indications and techniques were based on our previous publications[15,16,29]. Patients who underwent liver transplantation for HCC were excluded from the current study. The primary outcome was overall survival (OS), while HCC-related survival and disease-free survival (DFS) were the secondary outcomes. OS was calculated from the index date to the date of death or the end of year 2017. HCC-related survival spanned the period between the index date and the date of liver-cause mortality. The liver-causes included tumor recurrence, metastasis, and complications of decompensated liver cirrhosis. DFS defined the period between the index date and the date of the first documented clinical recurrence or the end of year 2017. In addition, the evolution of surgical outcome for HCC ≥ 10 cm from separate cohorts of different era was also examined. Tumor was staged by the American Joint Committee on Cancer (AJCC) Tumor Node Metastasis staging system for HCC, 6th and 7th edition[30-32]. The study was approved by the Institutional Review Board of the Chang Gung Memorial Hospital, No. 202000608B0.
The demographic features were presented as either continuous numbers with mean ± SD or counts with proportion (in percentage), and all covariates were compared by either t-test or chi-square statistics according to the nature of the covariates. To eliminate the potential confounding bias originating from heterogeneous baseline features and disproportionate case numbers, inverse-probability of treatment weighting between different groups was adopted[33,34]. The following covariates were considered: Gender, age, lifestyle, co-morbidities, viral hepatitis, tumor size, tumor stage, BCLC stage, and biochemical profiles including AFP, albumin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, direct bilirubin, indocyanine green retention test at 15 min, and prothrombin time with international normalize ratio. These covariates were selected to generate the propensity score. Then 1/ps was assigned as the weight of L-HCC subjects receiving liver resection (model 1 and 2), while 1/1-ps was assigned as the weight of S-HCC subjects receiving surgery (model 1) or L-HCC subjects receiving TACE (model 2). To estimate the survival, Kaplan-Meier curves with log-rank tests were used, and, after applying inverse-probability of treatment weighting (IPTW) to each subject, cox-regression analysis was employed to assess the hazard ratio (HR). Both the unadjusted and adjusted HR were acquired. Subgroup analysis was performed to investigate further the outcome in different stages of HCC. All statistics were analyzed by STATA software version 16.0 (StataCorp. 2019. Stata Statistical Software: Release 16, College Station, TX, USA: StataCorp LLC.), and the statistical significance was defined as a P value of less than 0.05.
We first identified 32403 patients diagnosed to have HCC from the CGRD. Among them, 5309 patients received liver resection for their HCC. After excluding patients with missing data and 30-d surgical mortality, a total of 3985 patients were enrolled into our final analysis. Among them, 3559 (89.3%) patients had smaller HCC (S-HCC), and 426 (10.7%) had HCC larger than 10 cm (L-HCC). As for the remaining 27069 non-operated HCC patients, 361 (13.3%) patients underwent TACE-based treatment for their large HCC (Figure 1). Tables 1 and 2 summarized the demographic data of HCC patients receiving liver resection. The mean age of HCC diagnosis was 58.7-years-old, and 77% were male. Hepatitis B virus (HBV) infection was the most common cause (52%), followed by chronic hepatitis C virus (HCV) infection (28%). Earlier stage HCC (AJCC stage I and II) accounted for around 80% of all operated patients.
| Variables | Total | S-HCC, < 10 cm | L-HCC, ≥ 10 cm | P value | |
| Number1 | 3985 (100) | 3559 (89) | 426 (11) | ||
| Gender | Male | 3061 (77) | 2716 (76) | 345 (81) | 0.031 |
| Age | ≤ 20 | 9 (< 1) | 4 (< 1) | 5 (1) | < 0.001 |
| 21-40 | 292 (7) | 240 (7) | 52 (12) | ||
| 41-60 | 1668 (42) | 1478 (42) | 190 (45) | ||
| ≥ 61 | 2016 (51) | 1837 (52) | 179 (42) | ||
| Diabetes | Yes | 831 (21) | 764 (21) | 67 (16) | 0.006 |
| Hypertension | Yes | 1172 (29) | 1053 (30) | 119 (28) | 0.480 |
| HBV surface antigen | Positive | 2074 (52) | 1849 (52) | 225 (53) | 0.740 |
| Anti-HCV antibody | Positive | 1135 (28) | 1091 (31) | 44 (10) | < 0.001 |
| Non-HBV non-HCV | Yes | 986 (25) | 815 (23) | 171 (40) | < 0.001 |
| Cigarette smoking | Yes | 380 (10) | 330 (9) | 50 (12) | 0.100 |
| Alcohol consumption | Yes | 322 (8) | 277 (8) | 45 (11) | 0.047 |
| Betel nut | Yes | 109 (3) | 93 (3) | 16 (4) | 0.170 |
| AJCC 6th (2002-2009) | 1771 | 1577 | 194 | ||
| Stage I | 1035 (58) | 973 (62) | 62 (32) | < 0.001 | |
| Stage II | 347 (20) | 307 (19) | 40 (21) | ||
| Stage III | 364 (21) | 279 (18) | 85 (44) | ||
| Stage IV | 25 (1) | 18 (1) | 7 (4) | ||
| AJCC 7th (2010-2015) | 2214 | 1982 | 232 | ||
| Stage I | 1064 (48) | 1031(52) | 33 (14) | < 0.001 | |
| Stage II | 783 (35) | 717 (36) | 66 (28) | ||
| Stage III | 341 (15) | 220 (11) | 121 (52) | ||
| Stage IV | 26 (1) | 14 (1) | 12 (5) |
| Variables | Total | S-HCC, < 10 cm | L-HCC, > 10 cm | P value | |
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| Tumor size | cm | 4.41 ± 3.98 | 3.36 ± 2.14 | 13.14 ± 4.95 | < 0.001 |
| Age | yr | 58.7 ± 12.4 | 59.1 ± 12.1 | 55.7 ± 14.3 | < 0.001 |
| Alpha-fetoprotein | ng/mL | 6156.3 ± 60012 | 2400.7 ± 19087 | 40531.8 ± 178925 | < 0.001 |
| Albumin | g/dL | 3.8 ± 0.6 | 3.8 ± 0.6 | 3.5 ± 0.7 | < 0.001 |
| Alkaline phosphatase | U/L | 97.9 ± 104.4 | 90.1 ± 75.6 | 161.1 ± 221.4 | < 0.001 |
| AST | U/L | 81.3 ± 158.1 | 76.4 ± 140.0 | 121.8 ± 261.2 | < 0.001 |
| ALT | U/L | 85.1 ± 144.8 | 84.2 ± 133.5 | 92.2 ± 217.0 | 0.290 |
| Bilirubin-direct | mg/dL | 0.4 ± 0.8 | 0.4 ± 0.8 | 0.4 ± 0.6 | 0.400 |
| Bilirubin-total | mg/dL | 1.1 ± 1.3 | 1.1 ± 1.4 | 1.1 ± 1.0 | 0.680 |
| ICG-15 | % | 9.7 ± 7.9 | 9.9 ± 8.1 | 8.0 ± 5.8 | < 0.001 |
| PT | seconds | 11.8 ± 2.1 | 11.7 ± 2.0 | 12.0 ± 2.6 | 0.019 |
When comparing HCC of different sizes, L-HCC patients were significantly younger than their S-HCC counterparts (55.7 vs 59.1 years, P < 0.001). There were considerably more male and less diabetes mellitus in the L-HCC group (P = 0.031 and 0.006, respectively). While the prevalence of HBV infection was comparable between the two groups, there was significantly less HCV infection in the L-HCC group (L-HCC vs S-HCC, 10% vs 31%, P < 0.001). The non-viral cause, on the other hand, accounted for a remarkably greater proportion of L-HCC patients (40% vs 23%, P < 0.001). As for the underlying liver function, the serum levels of aspartate aminotransferase and alkaline phosphatase and prothrombin time were higher in the L-HCC group (All P < 0.05), while that of albumin was lower in the L-HCC group (P < 0.001). The serum level of AFP, on the other hand, was significantly higher in the L-HCC group P < 0.001). The levels of alanine aminotransferase and bilirubin were similar between the two groups. As for the disease severity, L-HCC was far more advanced in terms of AJCC stage in both eras (Table 1).
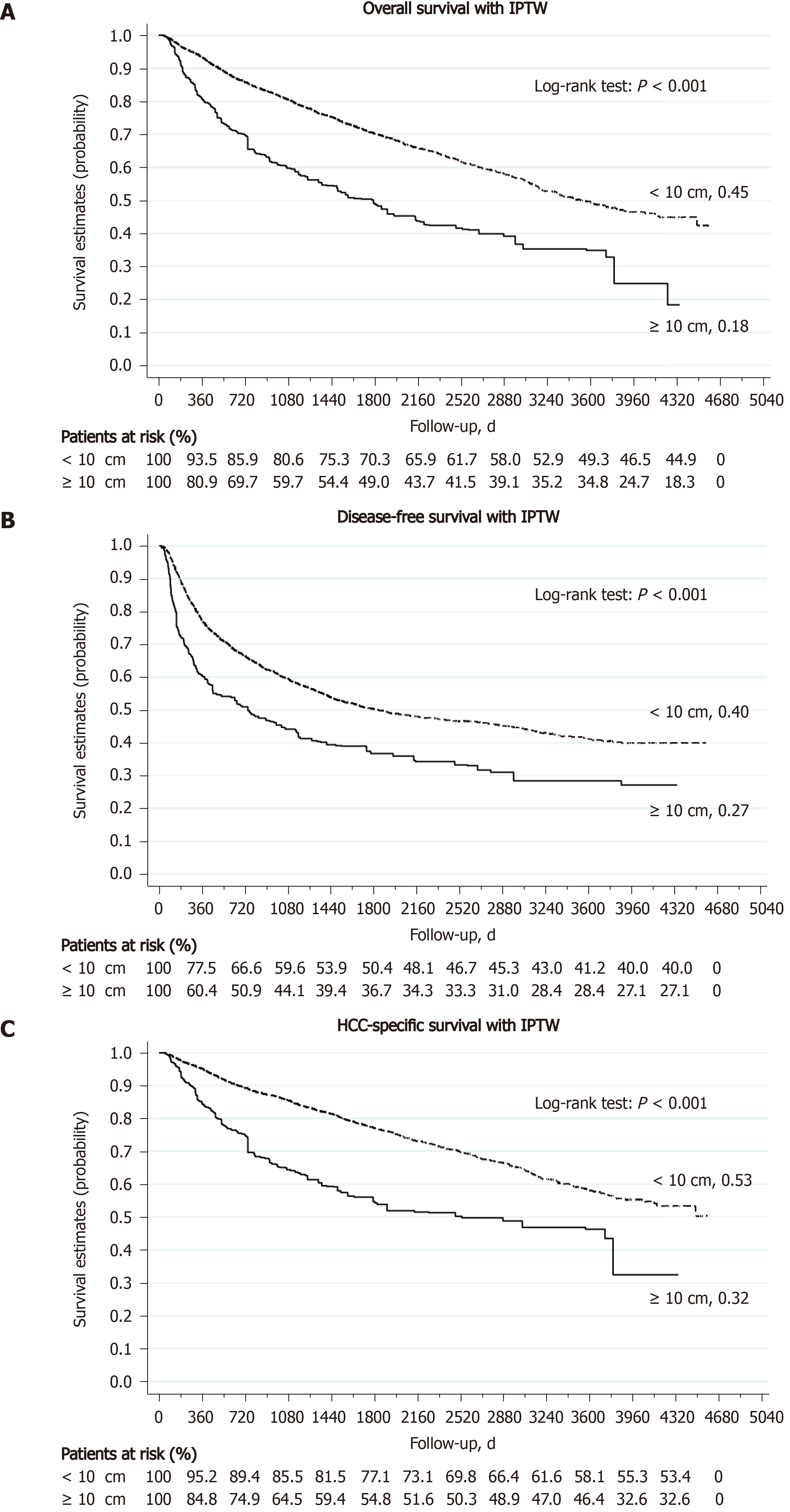
The 30-d surgical mortality rate was 2.1% in the L-HCC group and 0.7% in the S-HCC group (P = 0.003). After excluding these patients with surgical mortality, the L-HCC (426 patients) and S-HCC (3559 patients) groups were followed for a mean of 1175 d and 1733 d, respectively. Around 60% of L-HCC patients developed tumor recurrence during postoperative follow-up, compared to 44% in the S-HCC group (P < 0.001). Local recurrence was the most common pattern of HCC recurrence in both groups (L-HCC, 51.2%; S-HCC, 70.7%); however, distant metastasis occurred more frequently in the L-HCC group (L-HCC, 18.4%; S-HCC, 5.5%, P < 0.001). The 1-, 3-, and 5-year DFS rate was 48.5%, 36.1%, and 31.1% in the L-HCC group and 78.3%, 61.7%, and 55.3% in the S-HCC group (all P < 0.001) (Table 3). Kaplan-Meier analysis found that the L-HCC group had a significantly worse DFS than the S-HCC group (median DFS, 329 d in the L-HCC and 1024 d in the S-HCC, log-rank P < 0.001) (Figure 2). At the end of this study, 41.1% of L-HCC patients were still alive, compared to 68.1% in the S-HCC group. Liver-related causes remained the most common reason of death in both groups (45.8% and 22.3% of total, respectively). The 1-, 3-, and 5-year OS rate was 75.8%, 53.3%, and 46.2% in the L-HCC group and 94.2%, 83.0%, and 75.6% in the S-HCC group (all P < 0.001) (Table 3). Kaplan-Meier analysis found that the L-HCC group had a significantly worse OS than the S-HCC group (median OS, 801.5 d in the L-HCC and 1579 d in the S-HCC, log-rank P < 0.001) (Figure 2, with IPTW; Supplementary Figure 1, without IPTW).
| S-HCC, < 10 cm | L-HCC, ≥ 10 cm | P-value | |||
| Surgical mortality at 30-d | 25 (0.7) | 9 (2.1) | 0.003 | ||
| Patient number1 | 3559 (100) | 426 (100) | |||
| Follow-up times in d, mean ± SD | 1733.1 ± 1071.5 | 1175.3 ± 1034.2 | < 0.001 | ||
| Recurrence status | < 0.001 | ||||
| Yes | 1568 (44.1) | 256 (60.1) | |||
| No | 1735 (48.7) | 101 (23.7) | |||
| Never disease free | 256 (7.2) | 69 (16.2) | |||
| Recurrence pattern | < 0.001 | ||||
| Local2 | 1109 (70.7) | 131 (51.2) | |||
| Regional3 | 88 (5.6) | 10 (3.9) | |||
| Combined4 | 63 (4.0) | 36 (14.1) | |||
| Distant | 87 (5.5) | 47 (18.4) | |||
| Death without recurrence | 221 (14.1) | 32 (12.5) | |||
| Disease free survival in d, median (IQR) | 1024 (413-1907) | 329 (121-1244) | < 0.001 | ||
| 1-yr DFS rate | 2585 (78.3) | 173 (48.5) | < 0.001 | ||
| 3-yr DFS rate | 2037 (61.7) | 129 (36.1) | < 0.001 | ||
| 5-yr DFS rate | 1828 (55.3) | 111 (31.1) | < 0.001 | ||
| Final status | < 0.001 | ||||
| Alive | 2424 (68.1) | 175 (41.1) | |||
| Death - liver cause | 795 (22.3) | 195 (45.8) | |||
| Death - other cause | 340 (9.6) | 56 (13.1) | |||
| Overall survival (d) [median (IQR)] | 1579 (871-2455) | 801.5 (362-1818) | < 0.001 | ||
| 1-yr OS rate | 3354 (94.2) | 323 (75.8) | < 0.001 | ||
| 3-yr OS rate | 2954 (83.0) | 227 (53.3) | < 0.001 | ||
| 5-yr OS rate | 2689 (75.6) | 197 (46.2) | < 0.001 | ||
Before applying IPTW, the L-HCC group had a significantly higher risk of tumor recurrence than the S-HCC group [adjusted HR (aHR), 1.85, 95% confidence interval (CI), 1.60-2.13, P < 0.001] (Table 4). The risk of tumor recurrence was still significantly higher in the L-HCC group after matched analysis by applying IPTW (aHR, 1.73, 95%CI, 1.40-2.15, P < 0.001). Subgroup analysis revealed that, for patients in the same stage, the L-HCC group was associated with a significantly higher risk of tumor recurrence than the S-HCC group (aHR, 1.62, 95%CI, 1.02-2.56, P = 0.042 for stage I; aHR, 1.70, 95%CI, 1.28-2.27, P < 0.001 for stage II; aHR, 2.14, 95%CI, 1.74-2.63, P < 0.001 for stage III). As for the risk of death, the L-HCC group had a significantly higher risk of all-cause mortality than the S-HCC group both without and with IPTW analysis (aHR, 1.95, 95%CI, 1.68-2.26, P < 0.001, and aHR, 2.07 95%CI, 1.70-2.51, P < 0.001, respectively). In subgroup analysis, the risk of all-cause mortality was still significantly higher in the L-HCC group (stage I to stage III, all P < 0.05). Similarly, the risk of liver-cause mortality was significantly higher in the L-HCC group both without and with IPTW analysis (aHR, 2.16, 95%CI, 1.82-2.56, P < 0.001, and aHR, 2.15 95%CI, 1.73-2.67, P < 0.001, respectively). With IPTW analysis, all stage I to stage III L-HCC patients were found to have a significantly higher risk of liver-cause mortality than the S-HCC patients in the same stage (all P < 0.05).
| Without IPTW | With IPTW | |||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Recurrence | ||||||||
| Entire cohort | 2.24 (1.96-2.56) | < 0.001 | 1.85 (1.60-2.13) | < 0.001 | 1.60 (1.28-2.01) | < 0.001 | 1.73 (1.40-2.15) | < 0.001 |
| Within stage I | 1.43 (0.87-2.37) | < 0.001 | 1.62 (1.02-2.56) | 0.042 | ||||
| Within stage II | 1.64 (1.23-2.20) | 0.160 | 1.70 (1.28-2.27) | < 0.001 | ||||
| Within stage III | 1.98 (1.61-2.43) | 0.001 | 2.14 (1.74-2.63) | < 0.001 | ||||
| Within stage IV | 1.46 (0.56-3.83) | < 0.001 | 1.93 (0.63-5.91) | 0.251 | ||||
| All-cause mortality | ||||||||
| Entire cohort | 2.70 (2.36-3.10) | < 0.001 | 1.95 (1.68-2.26) | < 0.001 | 1.99 (1.59-2.48) | < 0.001 | 2.07 (1.70-2.51) | < 0.001 |
| Within stage I | 2.34 (1.49-3.67) | < 0.001 | 2.47 (1.64-3.74) | < 0.001 | ||||
| Within stage II | 1.81 (1.22-2.69) | 0.003 | 1.80 (1.31-2.49) | < 0.001 | ||||
| Within stage III | 1.78 (1.44-2.20) | < 0.001 | 1.89 (1.52-2.35) | < 0.001 | ||||
| Within stage IV | 0.71 (0.27-1.86) | 0.490 | 1.31 (0.58-2.96) | 0.510 | ||||
| Liver-cause mortality | ||||||||
| Entire cohort | 3.16 (2.70-3.70) | < 0.001 | 2.16 (1.82-2.56) | < 0.001 | 2.09 (1.61-2.72) | < 0.001 | 2.15 (1.73-2.67) | < 0.001 |
| Within stage I | 2.36 (1.31-4.26) | 0.004 | 2.15 (1.43-3.24) | < 0.001 | ||||
| Within stage II | 1.95 (1.21-3.15) | 0.006 | 1.88 (1.29-2.72) | 0.001 | ||||
| Within stage III | 2.00 (1.58-2.55) | < 0.001 | 2.12 (1.67-2.69) | < 0.001 | ||||
| Within stage IV | 0.67 (0.24-1.92) | 0.460 | 0.96 (0.37-2.48) | 0.932 | ||||
Table 5 compared the treatment results between the current study and those of the same institute conducted more than 20 years ago[22]. Between 1982 and 2001, which was the first era, there were 211 patients operated for their L-HCC. The mean age of diagnosis was 47.8 years in the first era, which was a lot younger than that of the current study (55.7 years, the second era). Male patients constituted the majority of these L-HCC in both eras (around 80%). Interestingly, while the proportion of chronic HCV infection remained similar (11.6% and 10.3% in the first and second eras, respectively), HBV infection declined dramatically from 81.9% in the first era to 52.8% in the second era. The 30-d, or surgical mortality rate, improved from 4.3% in the first era to 2.1% in the second era. As for the oncological outcome, the 1-, 3-, and 5-year DFS rates improved from 32.9%, 18.8%, and 12.7%, respectively, in the first era to 48.5%, 36.1%, and 31.1%, respectively, in the second era. The 1-, 3-, and 5-year OS rates were also much prolonged in the second era (48.1%, 24.0%, and 16.7%, respectively, in the first era, and 75.8%, 53.3%, and 46.2%, respectively, in the second era).
| Yeh etal[27]: 1st era | Current study: 2nd era | |
| Number of HCC ≥ 10 cm | 211 | 426 |
| Study period | 1982-2001 | 2002-2015 |
| Study design | Retrospective | Institutional-based cohort with IPTW |
| Age in yr | 47.8 ± 14.3 | 55.7 ± 14.3 |
| Gender, male % | 78% | 81% |
| HBV infection, positive | 81.9% | 52.8% |
| HCV infection, positive | 11.6% | 10.3% |
| Size in cm | 13.9 ± 3.4 | 13.14 ± 4.95 |
| Surgical mortality at 30-d | 4.3% | 2.1% |
| 1-yr DFS rate | 32.9% | 48.5% |
| 3-yr DFS rate | 18.8% | 36.1% |
| 5-yr DFS rate | 12.7% | 31.1% |
| 1-yr OS rate | 48.1% | 75.8% |
| 3-yr OS rate | 24.0% | 53.3% |
| 5-yr OS rate | 16.7% | 46.2% |
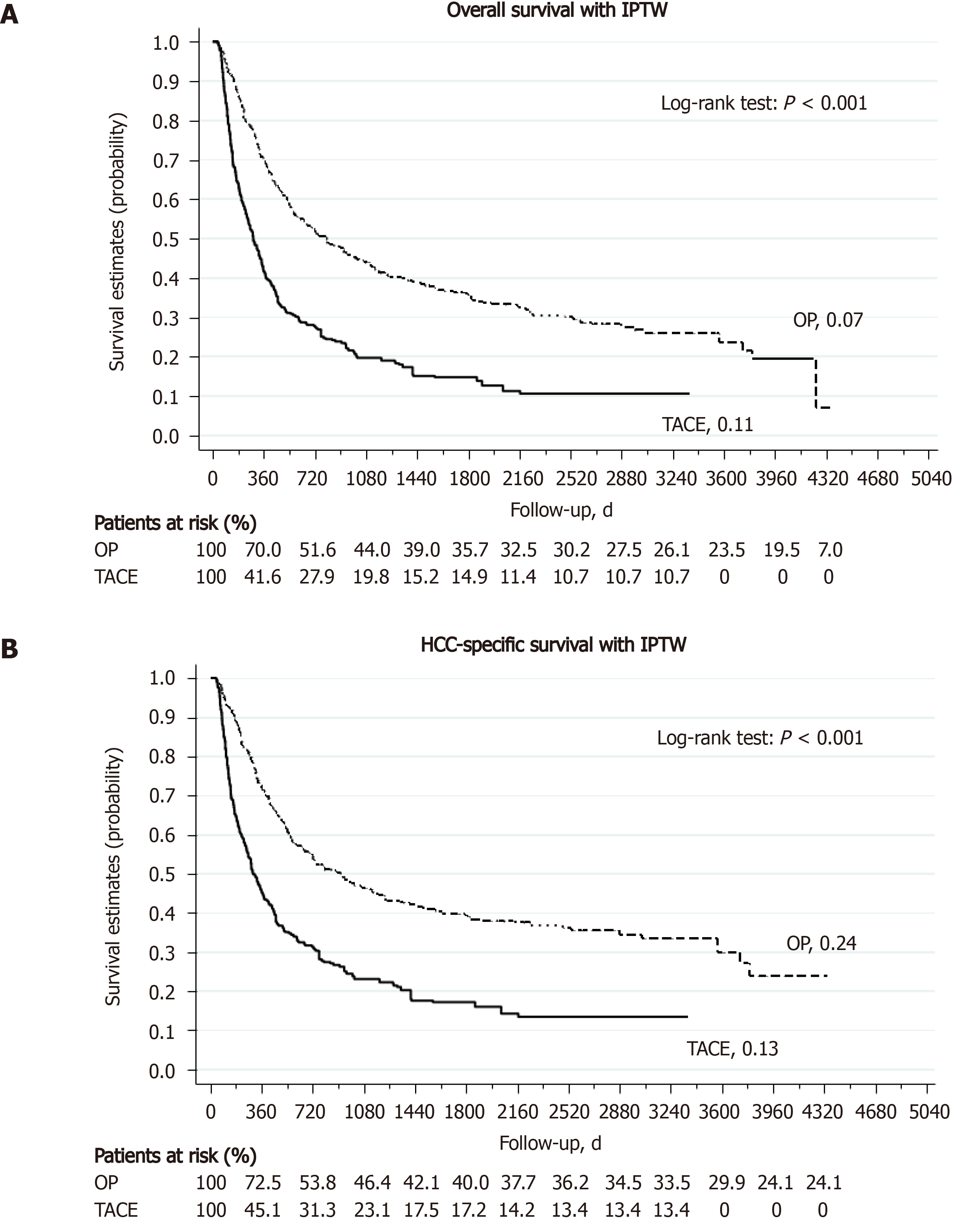
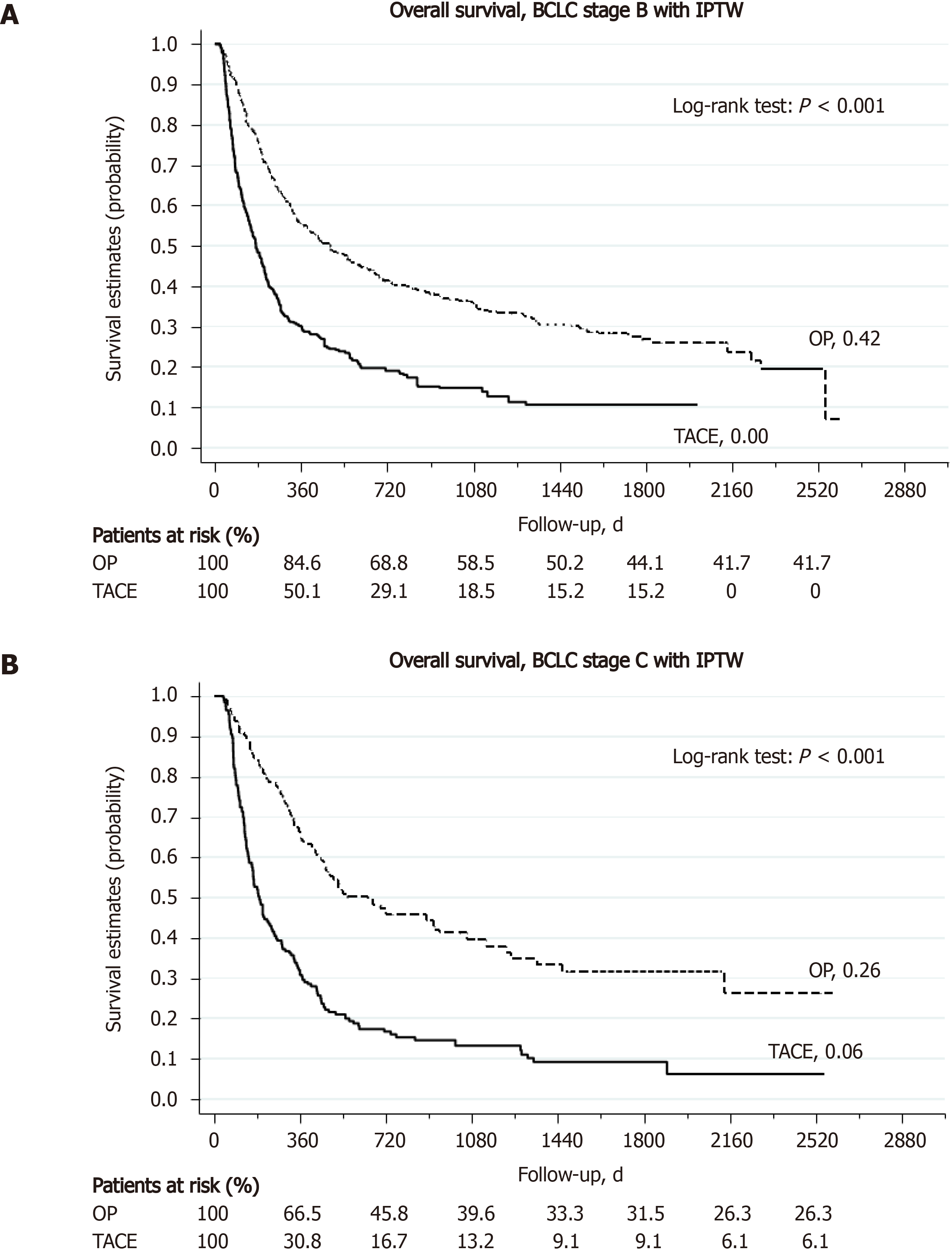
A total of 361 patients received TACE as the primary treatment for their large HCC (TACE group). As shown in Supplementary Table 2, the TACE group was significantly older with more HCV infection than the hepatectomy group (all P < 0.05). While the gender distribution (male, 81%) and tumor size (13.1 cm) were comparable between the two groups, the TACE group was far more advanced than the hepatectomy group in terms of AJCC staging and BCLC staging (P < 0.001). Furthermore, the degree of liver impairment in terms of liver biochemistry was also more severe in the TACE group. Supplementary Table 4 summarizes the treatment outcome of L-HCC after either hepatectomy or TACE. The 30-d and 90-d mortality rates were significantly higher in the TACE group than in the hepatectomy group (30-d, 6.5% vs 2.1%, P = 0.002; 90-d, 27.5% vs 7.8%, P < 0.001). More than 90% of patients in the TACE group could not achieve disease-free during their treatments, as compared to only 16.2% in the hepatectomy group (P < 0.001). At the end of this study, 41.1% of hepatectomy group were still alive, compared to 13.9% in the TACE group. Liver-related causes remained the most common cause of death in both groups (45.8% and 72.3% of total, respectively). The 1-, 3-, and 5-year OS rate was 75.8%, 53.3%, and 46.2% in the hepatectomy group and 36.0%, 17.5%, and 15.0% in the TACE group (all P < 0.001) (Supplementary Table 4). Kaplan-Meier analysis found that the TACE group had a significantly worse OS than the hepatectomy group (median OS, 801.5 d in the hepatectomy group and 243 d in the TACE group, P < 0.001) (Figure 3, with IPTW; Supplementary Figure 2, without IPTW). Subgroup analysis showed that the hepatectomy group still enjoyed a significantly better OS than the TACE group in either BCLC stage B/C patients or AJCC stage I to stage IV patients (Figure 4 and Supplementary Figure 3, respectively). Regarding the risk of death, the hepatectomy group had a significantly reduced risk of all-cause mortality than the TACE group both without and with IPTW matched analysis (aHR, 0.37, 95%CI, 0.30-0.47, P < 0.001, and aHR, 0.38 95%CI, 0.30-0.48, P < 0.001, respectively). In subgroup analysis, the risk of all-cause mortality was still significantly lower for the hepatectomy group in both BCLC stage B/C patients and AJCC stage I to IV patients (all P < 0.05). Similarly, the risk of liver-cause mortality was significantly reduced in the hepatectomy group both without and with IPTW analysis (aHR, 0.35, 95%CI, 0.27-0.44, P < 0.001, and aHR, 0.35 95%CI, 0.28-0.45, P < 0.001, respectively). With IPTW matched analysis, the hepatectomy group could achieve a significantly lower risk of liver-cause mortality in both BCLC stage B and C patients (all P < 0.001) (Table 6).
| Without IPTW | With IPTW | |||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| All-cause mortality | ||||||||
| Entire cohort | 0.33 (0.28-0.40) | < 0.001 | 0.46 (0.38-0.55) | < 0.001 | 0.49 (0.39-0.61) | < 0.001 | 0.46 (0.38-0.56) | < 0.001 |
| Within stage I | 0.39 (0.20-0.80) | 0.009 | 0.37 (0.19-0.73) | 0.004 | ||||
| Within stage II | 0.30 (0.16-0.54) | < 0.001 | 0.24 (0.13-0.45) | < 0.001 | ||||
| Within stage III | 0.50 (0.41-0.62) | < 0.001 | 0.50 (0.40-0.62) | < 0.001 | ||||
| Within stage IV | 0.31 (0.14-0.67) | 0.003 | 0.30 (0.09-0.93) | 0.037 | ||||
| Liver-cause mortality | ||||||||
| Entire cohort | 0.32 (0.26-0.39) | < 0.001 | 0.45 (0.37-0.56) | < 0.001 | 0.48 (0.37-0.61) | < 0.001 | 0.46 (0.37-0.56) | < 0.001 |
| Within stage I | 0.33 (0.16-0.71) | 0.004 | 0.27 (0.12-0.60) | 0.001 | ||||
| Within stage II | 0.30 (0.16-0.56) | < 0.001 | 0.22 (0.10-0.47) | < 0.001 | ||||
| Within stage III | 0.50 (0.39-0.63) | < 0.001 | 0.50 (0.40-0.63) | < 0.001 | ||||
| Within stage IV | 0.30 (0.13-0.70) | 0.005 | 0.29 (0.09-1.00) | 0.050 | ||||
| All-cause mortality | ||||||||
| Entire cohort | 0.33 (0.28-0.40) | < 0.001 | 0.37 (0.30-0.47) | < 0.001 | 0.38 (0.30-0.48) | < 0.001 | 0.38 (0.30-0.48) | < 0.001 |
| BCLC stage A | NA | NA | NA | NA | ||||
| BCLC stage B | 0.33 (0.23-0.48) | < 0.001 | 0.30 (0.20-0.44) | < 0.001 | ||||
| BCLC stage C | 0.40 (0.30-0.53) | < 0.001 | 0.41 (0.31-0.54) | < 0.001 | ||||
| BCLC stage D | NA | NA | NA | NA | ||||
| Liver-cause mortality | ||||||||
| Entire cohort | 0.32 (0.26-0.39) | < 0.001 | 0.35 (0.27-0.44) | < 0.001 | 0.36 (0.28-0.45) | <0 .001 | 0.35 (0.28-0.45) | < 0.001 |
| BCLC stage A | NA | NA | NA | NA | ||||
| BCLC stage B | 0.32 (0.21-0.47) | < 0.001 | 0.29 (0.19-0.43) | < 0.001 | ||||
| BCLC stage C | 0.37 (0.27-0.49) | < 0.001 | 0.38 (0.28-0.51) | < 0.001 | ||||
| BCLC stage D | NA | NA | NA | NA | ||||
The best treatment strategy for HCC larger than 10 cm has not been clearly defined. Due to its large size, both liver transplantation and radiofrequency ablation are not recommended. TACE has become the suggested primary treatment for these unfavorable diseases as a result[17-20]. Nevertheless, the response of these large HCC to TACE is generally poor[35-37]. Liver resection for HCC larger than 10 cm is thus still preserved by surgeons worldwide. However, most articles regarding surgical treatment for HCC larger than 10 cm had limited case numbers[22,38-49]. To our knowledge, the current study is one of the largest series in the literature to address the efficacy of surgery for HCC larger than 10 cm.
According to the current study, more than 30% of L-HCC patients still remained disease-free and more than 45% of them were still alive at 5 years after the operation. This group of patients, although shorter than the S-HCC group, can survive for a median of more than 2.2 years after the curative operation. Even though the surgical mortality rate for L-HCC was higher than that of the S-HCC group, it was still in the acceptable range of around 2%. Furthermore, when compared to the patients receiving TACE, patients with large HCC undergoing surgery were much more likely to achieve disease-free and enjoyed a significantly longer OS. To eliminate further the influence imposed by various confounding factors, we have applied the IPTW method in subgroup analysis and found that, for patients with comparable liver functional reserve, liver resection led to a significantly lower risk of death than TACE for either solitary HCC larger than 10 cm (AJCC stage I and II), HCC larger than 10 cm with daughter nodules or major vascular invasion, or ruptured HCC larger than 10 cm (AJCC stage III). Since the BCLC staging system is also prognostic for HCC and is adopted by various treatment guidelines[17-19], we compared surgery with TACE in subgroup analysis and discovered that liver resection is still remarkably better than TACE in terms of death in BCLC B patients. This finding is similar to previous articles showing that surgery would be a better treatment modality for large HCC; furthermore, instead of dealing with HCC larger than 5 cm[50,51], we compared and confirmed that surgery is better for HCC larger than 10 cm.
Moreover, the current study has demonstrated the evolutional surgical outcome of HCC larger than 10 cm between two different eras. Since the surgeries were operated by the same group of surgeons, the results should be rather representative. We have shown that the risk of death from surgery had improved dramatically from 4.3% in late 1990s to only 2% in year 2000s. The long-term oncological outcome is also improving. This may be attributed to the advancement of preoperative preparation, surgical technique, perioperative care, and postoperative surveillance. In addition, we discovered that there was less HBV infection in the second era. It may be due to our nationwide HBV vaccination, effective antiviral therapy, and increasing incidence of non-alcoholic fatty liver disease. As a result, with acceptable performance status and liver functional reserve (i.e. European Cooperative Oncology Group 0-1, Child-Pugh A, indocyanine green retention test at 15 min < 10%, absence of Vp4 invasion, and future liver remnant > 30%), we suggest liver resection should be performed for single huge HCC larger than 10 cm or huge HCC with limited daughter nodules confined in the same lobe.
The current study discovered that patients with L-HCC were generally younger with less HCV infection and diabetes mellitus. Since younger HCC patients have been demonstrated to have lower rates of HCV infection and cirrhosis, it may explain the demographic differences observed[22]. However, it is also likely that the carcinogenesis of L-HCC is different from that of the smaller ones. The non-viral cause, such as non-alcoholic fatty liver disease, might have significant roles in the pathogenesis of L-HCC. This assumption can be supported by our finding that non-viral cause accounted for 40% of L-HCC in the present study, as compared to only 23% of S-HCC (P < 0.001). Further studies are warranted to unravel the causal relationships between these associations.
Despite acceptable outcome, the current study still identified inferior surgical results of L-HCC than that of S-HCC. The L-HCC patients had significantly higher risks of tumor recurrence and death than the S-HCC patients in all stage I to III patients. There was also more distant recurrence in the L-HCC group. We believe this inferior outcome may be due to either capsular invasion, absent or incomplete capsule, or occult metastasis when the L-HCC was going to be resected. Frequent postoperative follow-up is thus mandatory and routine adjuvant TACE or even systemic therapy should be considered for L-HCC after surgery. Furthermore, the findings discovered in the current study also prompt the necessity to stratify further the Tumor Node Metastasis staging system, since there was an apparent survival difference among stage I HCC patients with different tumor sizes. As suggested by a recent study, patients with a single HCC between 5 cm and 8 cm can be allocated into a new BCLC stage between early and intermediate stage, while patients with a single HCC larger than 8 cm can be ascribed to intermediate stage[44], we recommend there should be a subcategory within T1 stage to precisely predict patient outcome. The exact cutoff value warrants further investigations.
Despite remarkable findings, the current study still has several limitations. As mentioned above, since the most appropriate treatment modality for huge HCC has not been established, the management disposition (i.e. surgery or TACE for L-HCC) was based on the discretion of individual physician in the current study. As a result, the background demographics and biochemical profiles were heterogeneous between the surgical and TACE group. This heterogeneity was a significant weakness and rendered the statistics biased. Secondly, since the current study was generated from the hospital-based database and cancer registry, more descriptive variables such as performance status, ascites, encephalopathy, bilobar involvement, major vessel involvement, the volume of future liver remnant, and pathologic details were inaccessible. It may thus interfere with the final analysis. Our interpretation should therefore be rather cautious and not be extrapolated. Thirdly, since the current study was conducted in the largest tertiary care center in Taiwan, referral bias could be encountered. Further larger scale nationwide cohort studies are thus warranted to validate our findings.
In conclusion, our institutional-based observational study based on the CGRD had demonstrated an improving surgical outcome for HCC larger than 10 cm. With acceptable performance status and liver functional reserve, we suggest liver resection should be conducted for single huge HCC larger than 10 cm or huge HCC with limited daughter nodules confined in the same lobe. Due to its inferior survival, we suggest a subclassification within T1 stage to predict precisely patient outcome. Future studies are mandatory to confirm our findings.
The treatment of hepatocellular carcinoma (HCC) larger than 10 cm remains challenging. The Chang Gung Research Database (CGRD) contains all medical records of the Chang Gung Memorial Foundation and has become one of the largest clinical databases worldwide. By utilizing the data from CGRD, we attempted to analyze the outcome of HCC larger than 10 cm.
Owing to advancement in surgical technique and perioperative care, the surgical risks associated with liver resection are decreasing in the recent decades. However, the surgical outcome regarding HCC larger than 10 cm has not been updated.
We aimed to consolidate the role of surgical resection for HCC larger than 10 cm. The survival outcomes between surgery and transarterial chemoembolization (TACE) were also compared.
Eligible HCC patients were identified from the CGRD, and two models were adopted: The surgical outcome between HCC ≥ 10 cm (L-HCC) and HCC < 10 cm (S-HCC) (model 1); the survival of L-HCC after either liver resection or TACE (model 2). To eliminate the potential confounding bias originating from heterogeneous baseline features and disproportionate case numbers, inverse-probability of treatment weighting between different groups was adopted.
Although worse than the S-HCC, the surgical and long-term oncological outcome of L-HCC had improved in the recent decades. Moreover, surgery could provide a better survival outcome for L-HCC than TACE.
With acceptable performance status and liver functional reserve, we suggest liver resection should be conducted for HCC larger than 10 cm. Due to its inferior survival, T1 stage should be further sub-divided to predict precisely patient outcome.
The current study demonstrated the inferior survival of L-HCC. The necessity of adjuvant therapy following liver resection for L-HCC should thus be determined by further randomized controlled trials.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kneteman NM S-Editor: Liu M L-Editor: Filipodia P-Editor: Li JH
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21363] [Article Influence: 2136.3] [Reference Citation Analysis (3)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20504] [Article Influence: 2050.4] [Reference Citation Analysis (20)] |
| 3. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 939] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 4. | Wei AC, Tung-Ping Poon R, Fan ST, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Gozzetti G, Mazziotti A, Cavallari A, Bellusci R, Bolondi L, Grigioni W, Bragaglia R, Grazi GL, De Raffele E. Clinical experience with hepatic resections for hepatocellular carcinoma in patients with cirrhosis. Surg Gynecol Obstet. 1988;166:503-510. [PubMed] |
| 6. | Bismuth H, Houssin D, Ornowski J, Meriggi F. Liver resections in cirrhotic patients: a Western experience. World J Surg. 1986;10:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 153] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Thompson HH, Tompkins RK, Longmire WP Jr. Major hepatic resection. A 25-year experience. Ann Surg. 1983;197:375-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 212] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Fortner JG, Kim DK, Maclean BJ, Barrett MK, Iwatsuki S, Turnbull AD, Howland WS, Beattie EJ Jr. Major hepatic resection for neoplasia: personal experience in 108 patients. Ann Surg. 1978;188:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 132] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Howat JM. Major hepatic resections in infancy and childhood. Gut. 1971;12:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Lai EC, Fan ST, Lo CM, Chu KM, Liu CL, Wong J. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg. 1995;221:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 319] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 11. | Matsumata T, Kanematsu T, Shirabe K, Sonoda T, Furuta T, Sugimachi K. Decreased morbidity and mortality rates in surgical patients with hepatocellular carcinoma. Br J Surg. 1990;77:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Lin HM, Lei LM, Zhu J, Li GL, Min J. Risk factor analysis of perioperative mortality after ruptured bleeding in hepatocellular carcinoma. World J Gastroenterol. 2014;20:14921-14926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg. 2011;35:2073-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 559] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 15. | Hsu HY, Yu MC, Lee CW, Tsai HI, Sung CM, Chen CW, Huang SW, Lin CY, Jeng WJ, Lee WC, Chen MF. RAM score is an effective predictor for early mortality and recurrence after hepatectomy for hepatocellular carcinoma. BMC Cancer. 2017;17:742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Lee CW, Tsai HI, Sung CM, Chen CW, Huang SW, Jeng WJ, Wu TH, Chan KM, Yu MC, Lee WC, Chen MF. Risk factors for early mortality after hepatectomy for hepatocellular carcinoma. Medicine (Baltimore). 2016;95:e5028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3233] [Article Influence: 461.9] [Reference Citation Analysis (1)] |
| 18. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6038] [Article Influence: 862.6] [Reference Citation Analysis (3)] |
| 19. | Surveillance group; Diagnosis group; Staging group; Surgery group; Local ablation group; TACE/TARE/HAI group; Target therapy/systemic therapy group; Radiotherapy group; Prevention group; Drafting group. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2018;117:381-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4092] [Article Influence: 584.6] [Reference Citation Analysis (6)] |
| 21. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 865] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 22. | Yeh CN, Lee WC, Chen MF. Hepatic resection and prognosis for patients with hepatocellular carcinoma larger than 10 cm: two decades of experience at Chang Gung memorial hospital. Ann Surg Oncol. 2003;10:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Cho YB, Lee KU, Lee HW, Cho EH, Yang SH, Cho JY, Yi NJ, Suh KS. Outcomes of hepatic resection for a single large hepatocellular carcinoma. World J Surg. 2007;31:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Shao SC, Chan YY, Kao Yang YH, Lin SJ, Hung MJ, Chien RN, Lai CC, Lai EC. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 2019;28:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 25. | Tsai MS, Lin MH, Lee CP, Yang YH, Chen WC, Chang GH, Tsai YT, Chen PC, Tsai YH. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed J. 2017;40:263-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 26. | Liu JM, Lin CC, Liu KL, Lin CF, Chen BY, Chen TH, Sun CC, Wu CT. Second-line Hormonal Therapy for the Management of Metastatic Castration-resistant Prostate Cancer: a Real-World Data Study Using a Claims Database. Sci Rep. 2020;10:4240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Chiang CJ, You SL, Chen CJ, Yang YW, Lo WC, Lai MS. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol. 2015;45:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 28. | Chiang CJ, Wang YW, Lee WC. Taiwan's Nationwide Cancer Registry System of 40 years: Past, present, and future. J Formos Med Assoc. 2019;118:856-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 29. | Lee CW, Chan KM, Tsai HI, Hsieh YC, Lin CY, Kuo YC, Hsu HY, Yu MC. Hepatic resection for hepatocellular carcinoma in the octogenarian: is it justified? Aging (Albany NY). 2019;11:1537-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Greene FL, Sobin LH. A worldwide approach to the TNM staging system: collaborative efforts of the AJCC and UICC. J Surg Oncol. 2009;99:269-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Lei HJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Wu CW. Prognostic value and clinical relevance of the 6th Edition 2002 American Joint Committee on Cancer staging system in patients with resectable hepatocellular carcinoma. J Am Coll Surg. 2006;203:426-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Chun YH, Kim SU, Park JY, Kim DY, Han KH, Chon CY, Kim BK, Choi GH, Kim KS, Choi JS, Ahn SH. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur J Cancer. 2011;47:2568-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661-3679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1759] [Cited by in RCA: 2860] [Article Influence: 286.0] [Reference Citation Analysis (0)] |
| 34. | Mansournia MA, Altman DG. Inverse probability weighting. BMJ. 2016;352:i189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 295] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 35. | Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P'eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 175] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Harada T, Matsuo K, Inoue T, Tamesue S, Nakamura H. Is preoperative hepatic arterial chemoembolization safe and effective for hepatocellular carcinoma? Ann Surg. 1996;224:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Yoon HM, Kim JH, Kim EJ, Gwon DI, Ko GY, Ko HK. Modified cisplatin-based transcatheter arterial chemoembolization for large hepatocellular carcinoma: multivariate analysis of predictive factors for tumor response and survival in a 163-patient cohort. J Vasc Interv Radiol. 2013;24:1639-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Liau KH, Ruo L, Shia J, Padela A, Gonen M, Jarnagin WR, Fong Y, D'Angelica MI, Blumgart LH, DeMatteo RP. Outcome of partial hepatectomy for large (> 10 cm) hepatocellular carcinoma. Cancer. 2005;104:1948-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Shrager B, Jibara GA, Tabrizian P, Schwartz ME, Labow DM, Hiotis S. Resection of large hepatocellular carcinoma (≥10 cm): a unique western perspective. J Surg Oncol. 2013;107:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Hanazaki K, Kajikawa S, Shimozawa N, Shimada K, Hiraguri M, Koide N, Adachi W, Amano J. Hepatic resection for hepatocellular carcinoma in diameter of > or = 10 cm. Hepatogastroenterology. 2002;49:518-523. [PubMed] |
| 41. | Young AL, Malik HZ, Abu-Hilal M, Guthrie JA, Wyatt J, Prasad KR, Toogood GJ, Lodge JP. Large hepatocellular carcinoma: time to stop preoperative biopsy. J Am Coll Surg. 2007;205:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Yang L, Xu J, Ou D, Wu W, Zeng Z. Hepatectomy for huge hepatocellular carcinoma: single institute's experience. World J Surg. 2013;37:2189-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Chen JH, Wei CK, Lee CH, Chang CM, Hsu TW, Yin WY. The safety and adequacy of resection on hepatocellular carcinoma larger than 10 cm: A retrospective study over 10 years. Ann Med Surg (Lond). 2015;4:193-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Zhong JH, Pan LH, Wang YY, Cucchetti A, Yang T, You XM, Ma L, Gong WF, Xiang BD, Peng NF, Wu FX, Li LQ. Optimizing stage of single large hepatocellular carcinoma: A study with subgroup analysis by tumor diameter. Medicine (Baltimore). 2017;96:e6608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Wang L, Liu Z, Liu X, Zeng Y, Liu J. The hepatectomy efficacy of huge hepatocellular carcinoma and its risk factors: A meta analysis. Medicine (Baltimore). 2017;96:e9226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Yang J, Li C, Wen TF, Yan LN, Li B, Wang WT, Yang JY, Xu MQ. Is hepatectomy for huge hepatocellular carcinoma (≥ 10 cm in diameter) safe and effective? Asian Pac J Cancer Prev. 2014;15:7069-7077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Fang Q, Xie QS, Chen JM, Shan SL, Xie K, Geng XP, Liu FB. Long-term outcomes after hepatectomy of huge hepatocellular carcinoma: A single-center experience in China. Hepatobiliary Pancreat Dis Int. 2019;18:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Li C, Wang MD, Lu L, Wu H, Yu JJ, Zhang WG, Pawlik TM, Zhang YM, Zhou YH, Gu WM, Wang H, Chen TH, Han J, Xing H, Li ZL, Lau WY, Wu MC, Shen F, Yang T. Preoperative transcatheter arterial chemoembolization for surgical resection of huge hepatocellular carcinoma (≥ 10 cm): a multicenter propensity matching analysis. Hepatol Int. 2019;13:736-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002;194:592-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Stevens CL, Awad A, Abbas SM, Watters DAK. Systematic review and meta-analysis of hepatic resection vs transarterial chemoembolization for solitary large hepatocellular carcinoma. HPB (Oxford). 2017;19:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Liu PH, Su CW, Hsu CY, Hsia CY, Lee YH, Huang YH, Lee RC, Lin HC, Huo TI. Solitary Large Hepatocellular Carcinoma: Staging and Treatment Strategy. PLoS One. 2016;11:e0155588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |