Published online Apr 27, 2021. doi: 10.4240/wjgs.v13.i4.355
Peer-review started: January 12, 2021
First decision: February 11, 2021
Revised: February 11, 2021
Accepted: April 14, 2021
Article in press: April 14, 2021
Published online: April 27, 2021
Processing time: 97 Days and 23.9 Hours
The association of tuberculosis (TB) with anal fistulas can make its treatment quite difficult. The main challenge is timely detection of TB in anal fistulas and its proper management. There is little data available on diagnosis and management of TB in anal fistulas.
To detect TB in fistula-in-ano patients were analyzed in different methods utilized.
A retrospective analysis of different methods, polymerase chain-reaction (PCR), GeneXpert and histopathology (HPE), utilized to detect tuberculosis in fistula-in-ano patients, treated between 2014-2020, was performed. The sampling was done for tissue (fistula tract lining) and pus (when available). The detection rate of various tests to detect TB and prevalence rate of TB in simple vs complex fistulae were studied.
In 1336 samples (776 patients) tested, TB was detected in 133 samples (122 patients). TB was detected in 52/703 (7.4%) samples tested by PCR-tissue, in 77/331 (23.2%) samples tested by PCR-pus, 3/197 (1.5%) samples tested with HPE-tissue and 1/105 (0.9%) samples tested by GeneXpert. To detect TB, PCR-tissue was significantly better than HPE-tissue (52/703 vs 3/197 respectively) (P = 0.0012, significant, Fisher’s exact test) and PCR-pus was significantly better than PCR-tissue (77/331 vs 52/703 respectively) (P < 0.00001, significant, Fisher’s exact test). TB fistulas were more complex than non-tuberculous fistulas [78/113 (69%) vs 278/727 (44.3%) respectively] (P < 0.00001, significant, Fisher’s exact test) but the overall healing rate was similar in tuberculous and non-tuberculous fistula groups [90/102 (88.2%) vs 518/556 (93.2%) respectively] (P = 0.10, not significant, Fisher’s exact test).
This is the largest study of anorectal TB to be published. The detection of TB by polymerase chain-reaction was significantly higher than by histopathology and GeneXpert. Amongst polymerase chain-reaction, pus had a higher detection rate than tissue. TB fistulas were more complex than non-tuberculous fistulas but aggressive diagnosis and meticulous treatment led to comparable overall success rates in both groups.
Core Tip: This is the largest study of anorectal tuberculosis (TB) to be reported. A total of 1336 tissue and pus samples were tested in 776 patients over 6 years. Polymerase chain-reaction, GeneXpert and histopathology were utilized to detect TB in these samples. Polymerase chain-reaction was more sensitive than histopathology and GeneXpert to detect TB in anal fistulas. This is the first study in which these three methods have been compared in the same cohort. Pus was more sensitive than tissue samples to detect TB. TB fistula had a significantly higher proportion of complex fistulas but timely diagnosis and treatment led to a high success rate in these fistulas.
- Citation: Garg P, Goyal A, Yagnik VD, Dawka S, Menon GR. Diagnosis of anorectal tuberculosis by polymerase chain reaction, GeneXpert and histopathology in 1336 samples in 776 anal fistula patients. World J Gastrointest Surg 2021; 13(4): 355-365
- URL: https://www.wjgnet.com/1948-9366/full/v13/i4/355.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i4.355
Tuberculosis (TB) is quite common in several regions of the world. Though pulmonary TB is the most common and accounts for 80% cases of all TB cases, extrapulmonary TB is also not rare and occurs in 20% of patients[1,2]. Amongst extrapulmonary TB, perianal TB occurs in 0.7% of them[2-4].
Anal fistulas, especially the complex fistulas, are notorious for high recurrence rate and refractoriness to treatment[5]. There are various causes responsible for non-healing and recurrences of fistulas, and inability to detect TB is one of them[5]. If a coexisting TB infection in the fistula goes undetected, then the chances of fistula healing after surgery are quite low. Either the fistula will not heal or there would a recurrence after few weeks to months of surgery[5].
There are several diagnostic tests in vogue to detect TB in anal fistulas. The most common and one of the oldest methods is histopathology (HPE)[6-8]. Several new tests have also been developed in last three decades but most of them have fallen out of favor. The only tests which demonstrate better detection rate than HPE are polymerase chain-reaction (PCR) and GeneXpert[5,9,10].
However, there are only a few studies which have tested the efficacy of PCR or GeneXpert in detecting TB in anal fistulas[11]. Even fewer studies have compared HPE with PCR in detecting anorectal TB[9]. However, no study till date has compared all three tests, HPE, PCR and GeneXpert, in detecting TB in anal fistulas; the present study is the first to do this. Also, as per our literature search, this would be the largest study of anorectal TB to be published till date.
A retrospective analysis of all patients of anorectal sepsis (anal fistulas and perianal abscesses) whose sample (tissue or pus) was tested for TB between September 2014 to December 2020 were included in the study (Tables 1 and 2). The approval was obtained by the Indus International Hospital-Institute Ethics Committee and written consent was taken from every patient. The patients were informed about the purpose of the study, and the study was conducted in accordance with the Declaration of Helsinki. The patients were operated at Indus International hospital (affiliation no 1) while the preoperative work-up, postoperative follow-up and the data analysis was done at Garg Fistula Research Institute (affiliation no 2).
| Samples | PCR-Pus (Total No./positive for TB) | PCR-Tissue (Total No. /positive for TB) | HPE (Total No./positive for TB) | GeneXpert (Total No./positive for TB) |
| First surgery | 206/49 | 673/47 | 184/3 | 102/1 |
| Repeat samples in patients with high level of suspicion1 | 55/17 | 9/1 | 0 | 0 |
| Samples in patients with recurrence of fistula | 34/2 | 21/4 | 13/0 | 3/0 |
| Out-patient department (Patients not operated) | 36/9 | 0 | 0 | 0 |
| TOTAL/ Positive for TB 1336/133 (9.95%) | 331/77 (23.2%) | 703/52 (7.4%) | 197/3 (1.5%) | 105/1 (0.9%) |
| Test of significance | PCR-Pus (23.2%) vs PCR-Tissue (7.4%) P < 0.00001 (Fisher’s exact test) | |||
| PCR-Tissue (7.4%) vs HPE (1.5%) P = 0.0012 (Fisher’s exact test) | ||||
| PCR-Pus (23.2%) vs PCR-Tissue (7.4%) vs HPE (1.5%) P < 0.00001 (Chi square test) | ||||
| Detection of TB | Total patients operated for anal fistula (n = 113) |
| After first surgery | 89/113 (78.8%) |
| Repeat samples in patients with high level of suspicion1 | 18/113 (15.9%) |
| Samples in patients with Recurrence of fistula | 6/113 (5.3%) |
The tissue sample collected was the fistula tract epithelium and tract wall (wherever possible). The tissue was only collected intraoperatively. The pus sample was collected whenever it was feasible to collect at least 1 mL of pus, either preoperatively in the surgeon’s office or intraoperatively. In cases where the initial intraoperative test report was negative for TB but there was a high suspicion of TB based on clinical findings, then a postoperative sample of pus was also sent for testing during the follow-up period.
The tissue sample was tested for PCR, GeneXpert and/or HPE. The pus sample was tested for PCR and/or GeneXpert. In the initial phase, PCR and HPE tests were carried out for two years (2015-2017). However, after two years, HPE testing was stopped as the yield was quite poor. Then, for one year (in 2019), GeneXpert was done along with PCR in all the samples. Due to extremely low yield, GeneXpert test was also discontinued. Since the beginning of 2020, only PCR testing was done.
PCR: The paraffin-embedded specimen of pus or the tissue was used for real-time PCR. The spin column method was used for deoxyribonucleic acid extraction (QIAGEN, Dusseldorf, Germany). PCR targeting for both “genus-specific target for Mycobacteria” and “species-specific gene target for Mycobacterium TB” were used for diagnostic purposes.
The samples in which the PCR result was positive for both genus Mycobacteria and species Mycobacterium TB were labeled as “PCR-Mycobacterium TB” positive. Samples in which the PCR result was positive for the genus Mycobacteria but negative for species Mycobacterium TB were labeled as “PCR-non tubercular Mycobacterium or PCR-Mycobacteria other than TB” positive[9,12].
Histopathology: For HPE, two stains were utilized, Ziehl-Neelsen carbol-fuchsin and hematoxylin and eosin. The features which suggested mycobacterial disease on HPE were caseating necrosis, granuloma formation, epithelioid cells and Langerhans giant cells[12].
GeneXpert (cartridge-based nucleic acid amplification test): The sample was analyzed by Xpert-Mycobacterium tuberculosis/rifampicin (RIF) (Cepheid, Sunnyvale, CA, United States). This was an automated molecular diagnostic test-that identified Mycobacterium TB deoxyribonucleic acid and resistance to RIF simultaneously. The target was a Mycobacterium tuberculosis-specific sequence of the rpoB gene, which was labelled with molecular beacons for mutations within the rifampicin resistance determining region. The sample was diluted with the Xpert sample reagent (containing NaOH and isopropanol) in the ratio of 2:1 by taking 1 mL of resuspended sample and 2 mL Xpert sample reagent. The closed specimen container was shaken manually several times and incubated at room temperature for 15 min. The suspension was transferred to the test cartridge which was then inserted into the GeneXpert instrument. The cycle threshold (CTs) of 5 rpoB gene probes reported the presence or absence of Mycobacterium TB automatically. RIF resistance was reported automatically by calculation of a change in CT (∆CT) between the highest and the lowest signal of the five probes. RIF drug resistance was indicated by ∆CT greater or equal to 3.5. The results were reported in less than two hours.
A presumptive diagnosis of TB was made if any of the tests were positive for TB and standard anti-TB treatment was initiated. However, unlike TB culture which detects live mycobacteria, PCR detects live as well as dead mycobacteria. Therefore, false-positives were a risk with these tests. Hence, a positive TB report was correlated with the clinical picture before starting anti-TB treatment. The patients with complex fistula and a positive report were started on anti-TB treatment. Patients with a positive report in a simple fistula and no other symptoms were given the option of not taking anti-TB treatment. On the other hand, repeated samples were sent for testing in the patients with a negative report but with a high level of suspicion [non-healing of fistula, occurrence of new abscesses during treatment or delayed recurrences (fistula recurring 3-6 mo after the complete healing). This was done as none of the tests conducted during the study had 100% sensitivity. Therefore, the diagnosis missed in the first testing was detected on repeated tests in many cases.
The fistulas were also classified as per “St James University Hospital” (SJUH) classification[13] (Table 3). The fistulas were termed “simple” when they were amenable to fistulotomy safely without any risk to continence (Table 4)[14]. These were usually fistulas which involved less than one-thirds of the external sphincter. Fistulas were categorized as “complex” when they were not amenable to fistulotomy (Table 4). Performing fistulotomy would increase the risk of incontinence in these patients.
| Total patients operated = 740 | Non-TB fistula (n = 627) | TB fistula (n = 113) | P value |
| Sex (M/F) | 545/82 | 99/14 | 0.68 |
| Age ± 2SD (yr) | 38.6 ± 11.3 | 39.4 ± 11.2 | 0.17 |
| Follow-up: Median (Range) (mo) | 27 (2-75) | 34 (2-74) | 0.23 |
| Multiple tracts, n (%) | 408 (65.1) | 86 (76.1) | 0.022a |
| Associated abscess, n (%) | 164 (26.2) | 55 (48.7) | < 0.00001a |
| Horseshoe tract, n (%) | 133 (21.2) | 38 (33.6) | 0.0052a |
| Supralevator tract, n (%)) | 94 (15.0) | 21 (18.6) | 0.32b |
| Recurrent fistula, n (%) | 328 (52.3) | 74 (65.5) | 0.0103a |
| Fistula grading (SJUH Classification) Grade, n (%) | I- 85 (13.6) | I-7 (6.2) | 0.0038a |
| II-96 (15.3) | II-11 (9.7) | ||
| III-71 (11.3) | III- 4 (3.5) | ||
| IV-281 (44.8) | IV-70 (61.9) | ||
| V-94 (15.0) | V-21 (18.6) | ||
| Fistula healing-after first surgery | 499/5561 (89.7) | 78/1022 (76.5) | 0.0005a |
| Reoperations (healed) | 25 (19) | 16 (12) | 0.0001a |
| Fistula healing-overall | 518/5561 (93.2) | 90/1022 (88.2) | 0.101b |
| Total patients operated = 740 | Simple fistulas (fistulotomy possible) | Complex fistulas (fistulotomy not possible) | Significance (Fisher’s exact test) |
| Non-TB Fistula (n = 627) | 349 (55.7) | 278 (44.3) | P < 0.00001 (Significant) |
| TB Fistula (n = 113) | 35 (31) | 78 (69) |
The patients scheduled to get anti-TB treatment were initiated on standard four-drug anti-TB regimen (Isoniazid-5 mg/kg, Rifampicin-10 mg/kg, Pyrazinamide-15 mg/kg and Ethambutol-15 mg/kg body weight) for first two months (intensive phase). This was followed by the two-drug regimen (Isoniazid-5 mg/kg, Rifampicin-10 mg/kg) for next four months (maintenance phase). In patients who tested positive for TB and were suffering from complex anal fistulas, additional injection Streptomycin 15 mg/kg (maximum of 750 mg /d) intramuscular was administered during the first two months of the intensive four-drug regimen. Liver function tests were assessed before initiating anti-TB therapy and then on a monthly basis in every patient. The liver enzymes were monitored for any toxicity from isoniazid, rifampicin or pyrazinamide. In cases where the liver enzymes were raised more than twice normal, the three potentially hepatotoxic drugs (isoniazid, rifampicin or pyrazinamide) were withheld and replaced by second-line drugs [ofloxacin, streptomycin and ethambutol (if the patient was in maintenance phase of anti-TB treatment)]. The liver enzymes were regularly monitored and, once they returned to normal, the three withheld drugs were re-introduced stepwise (isoniazid followed by rifampicin followed by pyrazinamide) and the second line drugs were stopped.
The patients were meticulously followed-up regularly at the institute till the fistula healed completely. After that, they were meticulously followed-up telephonically or through personal messaging apps. Any recurrence of symptoms like pain, swelling or pus discharge, was promptly assessed by clinical examination and magnetic resonance imaging scan.
The categorical variables were compared by performing chi-squared or Fisher’s exact test. In the data, which was normally distributed, the continuous variables were tested by Student’s t-test when there were two samples and analysis of variance test was performed when there were more than two samples. In the data which was not distributed normally, Wilcoxon signed rank test was performed for paired samples and Mann-Whitney U test was applied for unpaired samples. The significant cut off point was set at P < 0.05.
A total of 1336 samples (tissue and pus) were tested in 776 anal fistula patients over a period of six years (Table 1). TB was detected in 133 samples in 122 patients (Table 2). Thus, 9.95% (133/1336) samples tested positive for TB in 15.7% (122/776) patients (Table 1). 36 out of 776 patients were not operated and only pus samples were collected preoperatively in these patients (Table 1).
In the remaining 740 patients who underwent surgery for fistula, the tissue sample was collected intraoperatively while the pus samples were collected preoperatively, intraoperatively and/or postoperatively (in patients with high levels of suspicion of TB). In these operated patients, 113/740 (15.3%) patients had TB while 627/740 (84.7%) did not have TB (Table 2).
TB was detected in 52/703 (7.4%) samples tested by PCR-tissue, in 77/331 (23.2%) samples tested by PCR-pus, 3/197 (1.5%) samples tested with HPE-tissue and 1/105 (0.9%) samples tested by GeneXpert (Table 1). To detect TB, PCR-tissue was significantly better than HPE-tissue (52/703 vs 3/197 respectively) (P = 0.0012, significant, Fisher’s exact test) and PCR-pus was significantly better than PCR-tissue (77/331 vs 52/703 respectively) (P < 0.00001, significant, Fisher’s exact test) (Table 1).
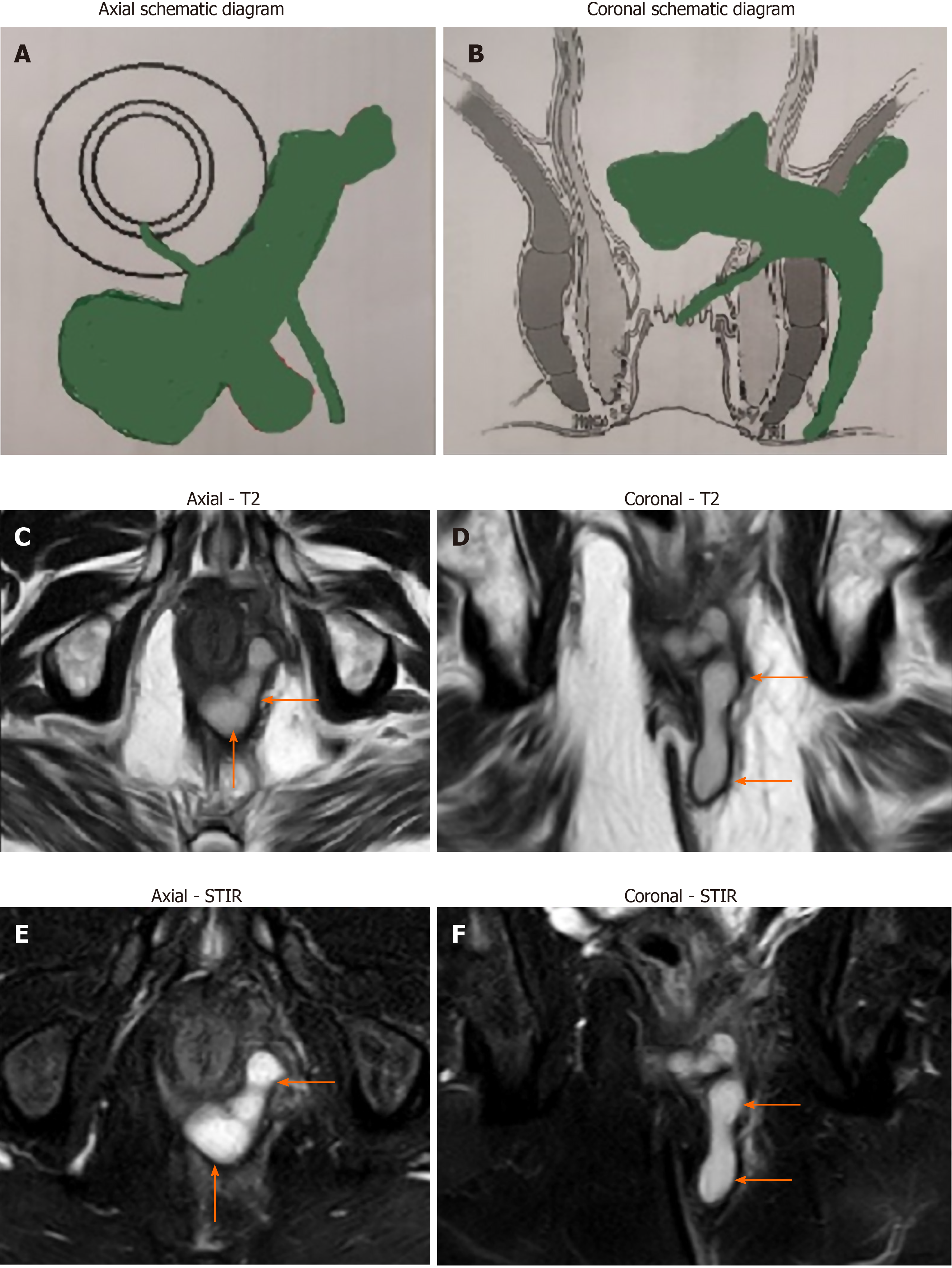
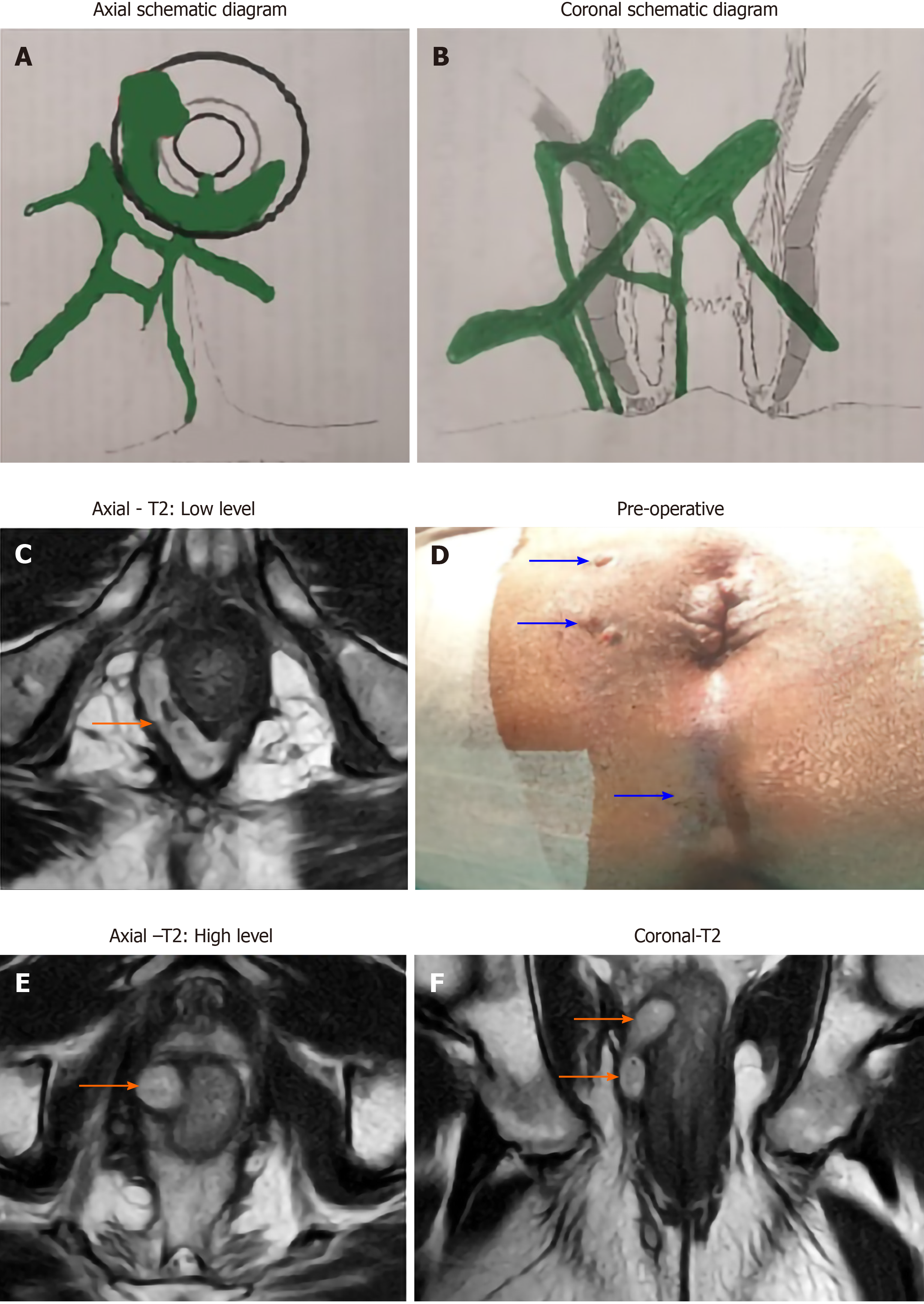
Amongst the patients operated for anal fistula (n = 740), both the groups (TB and non-TB fistula groups) were similar in age, sex and follow-up (Table 3). However, the various parameters of complex fistulas like multiple tracts (TB-76.1% vs non-TB-65.1%, P = 0.022, significant), associated abscess (TB-48.7% vs non-TB-26.2%, P < 0.00001, significant), horseshoe tracts (TB-33.6% vs non-TB-21.2%, P = 0.0052, significant) and recurrent fistulas (TB-65.5% vs non-TB-52.3%, P = 0.01, significant) were all significantly higher in TB group than non-TB group (Table 3). The only exception was presence of supralevator fistulas which were comparable in both the groups (TB-18.6% vs non-TB-15%, P = 0.32, not significant) (Table 3). Thus, overall, TB fistulas were significantly far more complex than non-TB fistulas [78/113 (69%) vs 278/727 (44.3%) respectively] (P < 0.00001, significant, Fisher’s exact test) (Table 4) (Figures 1 and 2). As per SJUH classification, the presence of fistulas of higher grade (III, IV and V) were significantly higher in the TB fistula group as compared to the non-TB fistula groups (TB-84.1% vs non-TB-71.1%) (P = 0.0038, significant, Fisher’s exact test) (Table 3) (Figures 1 and 2).
The fistula healing rate after the first surgery was significantly higher in the non-TB group than in the TB group (non-TB-89.7% vs TB-76.5%, P = 0.0005, significant) (Table 3). However, the overall fistula healing rate was comparable in both the groups (non-TB-93.2% vs TB-88.2%, P = 0.10, not-significant) (Table 3).
Out of 122 patients in whom TB was detected, 9 patients did not undergo surgery. In the remaining 113 patients who underwent operation for fistula, TB was detected in the first examined sample in 89/113 (78.8%) whereas in 18/113 (15.9%) patients with high levels of suspicion on clinical picture, TB was diagnosed by the repeat samples (tissue and/or pus) sent in the postoperative period (Table 2). In another 6/113 (5.3%) patients, TB was detected when the patient was operated again due to fistula recurrence (Table 2). Thus, in 24/113 (21.2%) patients, the diagnosis of TB was missed initially and multiple samples were needed to be tested to detect TB infection (Table 2). The cure rate was very good when TB could be detected and anti-tubercular therapy (ATT) was started before surgery or within six weeks (preferably three weeks) of surgery. However, if ATT was started more than six weeks after surgery, then recurrence was highly likely.
This study analyzed the efficacy of three modalities to detect TB in anal fistula patients in 1336 pus and tissue samples. This is not only the largest study conducted till date but is also the first study in which all three modalities, HPE, PCR and GeneXpert, have been compared in anorectal TB. This is also the first study in which GeneXpert has been utilized to detect TB in anal fistulas. The present study highlights few important points in diagnosis and management of anorectal TB. In this study, TB was detected in 13.7% (122/776) of fistula/abscess patients. This rate of prevalence of TB infection is within the range (0.3%-16%) of reported rates in endemic areas as published in previous studies[5,11,15-19].
The importance of accurate and timely diagnosis of TB in fistula patients cannot be overstated. A missed diagnosis of TB can lead to recurrence of fistula leading to substantial increase in physical morbidity, mental agony and financial burden. Not infrequently, patients have to undergo several unsuccessful operations when TB in a fistula goes undetected[5]. Therefore, timely identification of associated TB in anal fistulas holds immense importance. Unfortunately, the conventional tests performed for TB have distinct limitations. The detection rate of acid fast bacillus smear is quite low[9]. TB culture is very specific and can also guide regarding drug resistance but its processing period is quite long (6 wk to 6 mo)[9]. Therefore, the practical utility of TB culture is quite limited. The sensitivity and specificity of HPE to detect TB is also not very high as has been reconfirmed in the present study. Therefore, in the last two decades, a lot of studies recommended that PCR should be employed routinely to detect TB[8,11,20,21]. However, in the literature, most of the studies utilized HPE[15-19,22,23] and very few studies used PCR for testing[11]. PCR has been shown to be significantly more effective in detecting TB in anal fistulas[5,9,11] and this is corroborated by the present study.
GeneXpert has the added advantage that it diagnoses Rifampicin-sensitivity at the same time[10]. Ironically, the detection rate of GeneXpert was significantly lower as compared to PCR (Table 1). The exact reason for this could not be ascertained. However, the sensitivity of GeneXpert in detecting TB in extrapulmonary TB has been shown to be quite variable with sensitivities ranging from 25.0% to 95.1%[10,24,25]. Therefore, the present study reinforces the superiority of PCR over other existing tests and makes a strong case for recommending PCR routinely in all fistula patients for detecting TB especially in countries where TB is endemic[5].
One major limitation of PCR is that it cannot differentiate between live and dead bacilli[5]. Due to this, PCR can potentially over-diagnose TB in fistula patients[5]. Therefore, it is recommended that a positive TB report in a fistula patient should always be correlated with the clinical findings before initiating treatment[5,11]. A positive PCR report in a patient with a complex fistula or with a high suspicion (non-healing of fistula, occurrence of new abscesses during treatment or delayed recurrences) would make a strong case for starting anti-TB treatment.
Another issue in the management was that an initial negative report did not rule out TB[8]. Therefore, it was imperative to send repeated samples in patients with a high level of suspicion. In the present study, 18/64 samples (17 pus and one tissue) sent again in postoperative period in patients with high suspicion of TB tested positive for TB on PCR (Table 1). Thus, in 18/113 (15.9%) patients, the diagnosis of TB would have been missed if repeat samples had not been sent. Therefore, it is logical to send repeated samples in patients with high levels of suspicion (non-healing of fistula, occurrence of new abscesses during treatment or delayed recurrences) especially in regions where TB is endemic.
An important point perceived by previous studies[5,16,22] as well as highlighted by the present study was that anti-TB therapy should be initiated as soon as possible after fistula surgery[5]. The chances of cure were highest when TB was diagnosed and anti-TB therapy was started either before the fistula surgery or within a span of six (preferably three) weeks of the operation. If anti-TB therapy was initiated more than six weeks after operation, then the chances of non-healing of the fistula or its recurrence were quite high. The possible reason could be that once the untreated TB bacilli have impaired the healing of the internal opening during the first six weeks after surgery, then the tracts get epithelialized. After that, a full anti-TB treatment may eradicate TB bacilli but the fistula was likely to remain unhealed due to patent “epithelialized” internal opening[5]. This further strengthens the recommendation of sending repeat samples in patients with high levels of suspicion of having anorectal TB.
The fistulas associated with TB were significantly more complex than fistulas without TB infection (69% vs 44.3% respectively, P < 0.00001) (Table 4) (Figures 1 and 2). As per the SJUH classification also, the presence of higher grade fistulas (III, IV and V) was also significantly higher in the TB fistula group as compared to the non-TB fistula group (TB-84.1% vs non-TB-71.1%, P = 0.0038) (Table 3). Most parameters characteristic of complex fistulas like multiple tracts, associated abscess, horseshoe tracts and recurrent fistulas were also significantly higher in the TB group than the non-TB group (Table 3). Several previous studies have also documented that TB fistulas are associated with higher proportion of multiple tracts[15-17,19,22] and recurrent fistulas[11,15-19,23]. There are several plausible reasons for this. First, the diagnosis of TB in fistulas is difficult and is frequently missed due to which these fistulas keep recurring. This leads to fistulas becoming complex as fistulas keep spreading after each recurrence and multiple surgeries may result in sphincter damage. Second, the long duration of anti-TB therapy leads to treatment non-compliance by the patient. This leads not only to recurrence of fistula but also to emergence of multidrug-resistant TB strains[5]. Third, unlike pyogenic infections, the TB infection and abscess formation progress gradually and are not very painful (“cold abscess”). Due to this, there is a tendency on the patient’s part to ignore the disease, which keeps on spreading and, by the time the patient reports to the physician, the fistula is already quite complex[5].
The study has a few limitations. TB culture is the most specific test available and is the gold-standard test for diagnosis of TB. Therefore, corroborating all TB positive patients with TB culture would have imparted additional value to the study. However, due to the long time required for culture (6 wk to 6 mo), this test could not be done. The risk of recurrence of fistula would have increased significantly if the detection and subsequent treatment of TB was delayed for such a long time. Moreover, it would have incurred additional cost.
The rate of TB positive cases diagnosed with PCR is higher in the present study as compared to a previous study[5]. However, the difference is not statically significant. In the present study, 12.4% (129/1034) of PCR (pus plus tissue) samples tested positive for TB whereas in the previous study[5], 10.3% (47/456) of PCR (pus plus tissue) samples tested positive for TB (P = 0.25, not significant, Fisher’s exact test). The detection rate of TB with HPE was also similar in the present study (1.5%-3/197) and the previous study (1.1%-2/181)[5] (P = 1.0, not significant, Fisher’s exact test). So, the increase in TB detection rate by PCR and HPE in the present study is not significantly different from the previous published study and seems to be a random variation.
TB is associated with more complex fistulas but timely diagnosis and treatment leads to similar overall success rates as for non-TB fistulas. Failure to detect TB in time increases the risk of recurrence. Anti-TB therapy started more than 6 wk after surgery increases the risk of recurrence. In patients with initial negative test result for TB but with high levels of suspicion (non-healing of fistula, occurrence of new abscesses during treatment or delayed recurrences), additional repeat samples may be needed to detect TB. Pus is more sensitive than tissue samples to detect TB. PCR is more sensitive than histopathology and GeneXpert to detect TB in anal fistulas. Therefore, PCR should be done in every fistula patient especially in TB endemic regions. As PCR cannot distinguish dead from viable mycobacteria, correlation with the clinical picture is mandatory.
The management of anal fistulas is quite complicated. The association of tuberculosis (TB) with anal fistulas can make its treatment even more difficult. The main challenge is timely detection of TB in anal fistulas and its proper management.
There is little data available on diagnosis and management of TB in anal fistulas.
To test the detection rate of TB by commonly used tests like histopathology, polymerase chain-reaction (PCR) and GeneXpert.
Three most commonly utilized tests, PCR, GeneXpert and histopathology were performed to detect TB in pus and tissue (fistula tract wall or lining) samples in anal fistula patients. The results were then compared and analyzed.
In 1336 tissue and pus samples tested in 776 anal fistula patients, it was found that PCR was significantly more sensitive than histopathology and GeneXpert to detect TB. Pus was significantly more sensitive than tissue samples to detect TB. TB fistula had a significantly higher proportion of complex fistulas.
PCR is the most sensitive method to detect TB in anal fistulas. Though TB is associated with complex fistulas but timely diagnosis and treatment led to a high success rate in these fistulas.
More tests need to be developed which can detect TB rapidly with high sensitivity as well as specificity.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society of Colon Rectum Surgeons; Endoscopic and Laparoscopic Surgeons of Asia; and American Society of Gastrointestinal Endoscopic Surgeons.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ikeda T S-Editor: Zhang L L-Editor: A P-Editor: Ma YJ
| 1. | Addison NV. Abdominal tuberculosis--a disease revived. Ann R Coll Surg Engl. 1983;65:105-111. [PubMed] |
| 2. | Ibn Majdoub Hassani K, Ait Laalim S, Toughrai I, Mazaz K. Perianal tuberculosis: a case report and a review of the literature. Case Rep Infect Dis. 2012;2012:852763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Neto I, Siá O, Lopes E, Macacari R, Watté H, Souza R, Rolim A, Robles L. Perianal tuberculosis: A rare disease of late diagnosis. J Col. 2014;34:124-127. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Mathew S. Anal tuberculosis: report of a case and review of literature. Int J Surg. 2008;6:e36-e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Garg P, Garg M, Das BR, Khadapkar R, Menon GR. Perianal Tuberculosis: Lessons Learned in 57 Patients From 743 Samples of Histopathology and Polymerase Chain Reaction and a Systematic Review of Literature. Dis Colon Rectum. 2019;62:1390-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Wijekoon NS, Samarasekera DN. The value of routine histopathological analysis in patients with fistula in-ano. Colorectal Dis. 2010;12:94-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Bokhari I, Shah SS, Inamullah, Mehmood Z, Ali SU, Khan A. Tubercular fistula-in-ano. J Coll Physicians Surg Pak. 2008;18:401-403. [PubMed] |
| 8. | Park DY, Kim JY, Choi KU, Lee JS, Lee CH, Sol MY, Suh KS. Comparison of polymerase chain reaction with histopathologic features for diagnosis of tuberculosis in formalin-fixed, paraffin-embedded histologic specimens. Arch Pathol Lab Med. 2003;127:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Garg P. Comparison of histopathology and real-time polymerase chain reaction (RT-PCR) for detection of Mycobacterium tuberculosis in fistula-in-ano. Int J Colorectal Dis. 2017;32:1033-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Sachdeva K, Shrivastava T. CBNAAT: A Boon for Early Diagnosis of Tuberculosis-Head and Neck. Indian J Otolaryngol Head Neck Surg. 2018;70:572-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Shan YS, Yan JJ, Sy ED, Jin YT, Lee JC. Nested polymerase chain reaction in the diagnosis of negative Ziehl-Neelsen stained Mycobacterium tuberculosis fistula-in-ano: report of four cases. Dis Colon Rectum. 2002;45:1685-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Garg P. Nontuberculous mycobacteria in fistula-in-ano: A new finding and its implications. Int J Mycobacteriol. 2016;5:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000;20:623-35; discussion 635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Garg P. Assessing validity of existing fistula-in-ano classifications in a cohort of 848 operated and MRI-assessed anal fistula patients - Cohort study. Ann Med Surg (Lond). 2020;59:122-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Shukla HS, Gupta SC, Singh G, Singh PA. Tubercular fistula in ano. Br J Surg. 1988;75:38-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Kraemer M, Gill SS, Seow-Choen F. Tuberculous anal sepsis: report of clinical features in 20 cases. Dis Colon Rectum. 2000;43:1589-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Sultan S, Azria F, Bauer P, Abdelnour M, Atienza P. Anoperineal tuberculosis: diagnostic and management considerations in seven cases. Dis Colon Rectum. 2002;45:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Stupart D, Goldberg P, Levy A, Govender D. Tuberculous anal fistulas--prevalence and clinical features in an endemic area. S Afr J Surg. 2009;47:116-118. [PubMed] |
| 19. | Moujahid M, Tajdine MT, Achour A, Janati IM. [Anoperineal tuberculosis: 40 cases]. Gastroenterol Clin Biol. 2010;34:98-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Song H, Lee H, Choi G, Shin J. Cutaneous nontuberculous mycobacterial infection: a clinicopathological study of 7 cases. Am J Dermatopathol. 2009;31:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Shrestha NK, Tuohy MJ, Hall GS, Reischl U, Gordon SM, Procop GW. Detection and differentiation of Mycobacterium tuberculosis and nontuberculous mycobacterial isolates by real-time PCR. J Clin Microbiol. 2003;41:5121-5126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Tai WC, Hu TH, Lee CH, Chen HH, Huang CC, Chuah SK. Ano-perianal tuberculosis: 15 years of clinical experiences in Southern Taiwan. Colorectal Dis. 2010;12:e114-e120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Sahu M, Mishra JK, Sharma A, Fatmi U. A prospective study on tubercular fistula in ano and its management. J Col. 2017;37:211-215. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Maynard-Smith L, Larke N, Peters JA, Lawn SD. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect Dis. 2014;14:709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Lawn SD, Zumla AI. Diagnosis of extrapulmonary tuberculosis using the Xpert(®) MTB/RIF assay. Expert Rev Anti Infect Ther. 2012;10:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |