Published online Mar 27, 2021. doi: 10.4240/wjgs.v13.i3.267
Peer-review started: October 9, 2020
First decision: December 8, 2020
Revised: December 13, 2020
Accepted: January 15, 2021
Article in press: January 15, 2021
Published online: March 27, 2021
Processing time: 157 Days and 19.9 Hours
Neoadjuvant therapy (NAT) is becoming increasingly important in locally advanced rectal cancer. Hence, such research has become a problem.
To evaluate the downstaging effect of NAT, its impact on postoperative complications and its prognosis with different medical regimens.
Seventy-seven cases from Shanghai Ruijin Hospital affiliated with Shanghai Jiaotong University School of Medicine were retrospectively collected and divided into the neoadjuvant radiochemotherapy (NRCT) group and the neoadjuvant chemotherapy (NCT) group. The differences between the two groups in tumor regression, postoperative complications, rectal function, disease-free survival, and overall survival were compared using the χ2 test and Kaplan-Meier analysis.
Baseline data showed no statistical differences between the two groups, whereas the NRCT group had a higher rate of T4 (30/55 vs 5/22, P < 0.05) than the NCT groups. Twelve cases were evaluated as complete responders, and 15 cases were evaluated as tumor regression grade 0. Except for the reduction rate of T stage (NRCT 37/55 vs NCT 9/22, P < 0.05), there was no difference in effectiveness between the two groups. Preoperative radiation was not a risk factor for poor reaction or anastomotic leakage. No significant difference in postoperative complications and disease-free survival between the two groups was observed, although the NRCT group might have better long-term overall survival.
NAT can cause tumor downstaging preoperatively or even complete remission of the primary tumor. Radiochemotherapy could lead to better T downstaging and promising overall survival without more complications.
Core Tip: Neoadjuvant therapy can cause tumor downstaging preoperatively or even complete remission of the primary tumor. Radiochemotherapy had better T downstaging as well as promising overall survival without major complications. This may help clinicians realize the indispensability of preoperative radiation.
- Citation: Li WC, Zhao JK, Feng WQ, Miao YM, Xu ZF, Xu ZQ, Gao H, Sun J, Zheng MH, Zong YP, Lu AG. Retrospective research of neoadjuvant therapy on tumor-downstaging, post-operative complications, and prognosis in locally advanced rectal cancer. World J Gastrointest Surg 2021; 13(3): 267-278
- URL: https://www.wjgnet.com/1948-9366/full/v13/i3/267.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i3.267
After Macfarlane et al[1] and Heald et al[2] promoted the concept of total mesorectal excision (TME), the local recurrence rate of locally advanced rectal cancer (LARC) fell below 30%. The Stockholm I and Stockholm II trials[3-6] showed that preoperative radiation could reduce this rate to less than 15%. The side effects of neoadjuvant therapy (NAT) must not be neglected. Radiation can directly destroy normal rectal tissue[7-10], and chemotherapy always causes systematic side effects.
Therefore, this study assessed the efficacy of NAT in LARC and retrospectively explored its impact on postoperative complications and prognosis.
From January 1, 2016 to January 31, 2019, 1497 patients from Shanghai Ruijin Hospital affiliated with Shanghai Jiaotong University School of Medicine were diagnosed with rectal tumors. Seventy-seven patients met the inclusion criteria and were followed up for 2 years. Patient characteristics included the following: patient age between 18 to 75 years old, tumor location at the anal edge ≤ 15 cm according to endoscopy, histopathologically confirmed adenocarcinoma, tumor staging T3/T4 or N+ with magnetic resonance imaging (MRI), and willingly accepted and finished NAT. All included patients underwent laparoscopic radical rectal cancer surgery. Patients unable to finish NAT, undergoing an emergency operation, with severe organic comorbidities, and with coexisting other malignant tumors were excluded. Patients were divided into the neoadjuvant chemotherapy (NCT) group and the neoadjuvant radiochemotherapy (NRCT) group according to the regimen they received.
The NAT regimen was planned by our multiple discipline team and carried out with the full understanding of patients and their families. NRCT comprised radiation (50 Gy in 25 fractions) with simultaneous capecitabine (1000 mg/m2) plus chemotherapy (Capeox or mFolfox6 for 1-2 cycles). The NCT was Capeox or mFolfox6 for four cycles. The interval time between surgery and NAT was at least 6 wk after NRCT or 2 wk after NCT. Then radical TME of rectal cancer was carried out (Dixon, Miles, or Hartmann procedure).
Patients were divided into the NCT group and NRCT group to compare the baseline data, tumor-related data, operation-related data, and postoperative complication-related data. The tumor downstaging evaluation was based on the Response Evaluation Criteria in Solid Tumors (RECIST) standard[11] according to MRI and tumor regression grade (TRG) from the American Joint Committee on Cancer, 7th edition according to pathology. Postoperative complications included anastomotic leakage (AL), incision complications, and stoma complications.
The Wexner Continence Grading Scale was used to evaluate postoperative rectal function. The time of distal or local recurrence was recorded to assess disease-free survival (DFS). Overall survival (OS) was also compared between groups.
All data were analyzed, described, and processed by SPSS 23. P < 0.05 was considered statistically significant. The Mann-Whitney U or Wilcoxon test was used to compare the two groups, and the χ2 test was used for comparison of categorical variables. Multivariable analysis was used to reveal the potentially influential factors of the NAT effect and postoperative complications. Kaplan-Meier analysis was used to describe and compare survival, and log-rank and Breslow tests were used to confirm the statistical significance.
A total of 77 cases of LARC were included. General data are shown in Table 1. The average interval between radiotherapy and surgery was 61 ± 16 d (Table 1). The general data were analyzed and compared, as shown in Table 2. No significant difference in general data was found between the two groups.
| Case counts | ||
| Gender: Male/Female | 62 | 15 |
| Diabetes mellitus: Yes/No | 11 | 66 |
| Hypertension: Yes/No | 19 | 58 |
| Preoperative radiation: Yes/No | 55 | 22 |
| NRCT | NCT | P value | |
| Median age in yr | 60 | 62 | 0.64 |
| Gender, n | 1.00 | ||
| Male | 44 | 18 | |
| Female | 11 | 4 | |
| Hypertension, n | 0.80 | ||
| Yes | 14 | 5 | |
| No | 41 | 17 | |
| Diabetes mellitus, n | 0.80 | ||
| Yes | 7 | 4 | |
| No | 48 | 18 |
There was only one significant difference in the preoperative characteristics (T stage) between the two groups before intervention (Table 3). A total of 43 cases showed retraction of the lower edge of the tumors by a median distance of 1.0 cm. Forty-one patients had T downstaging after treatment, and fifty-five patients had N downstaging. According to the RECIST standard, 12 cases were classified as complete response (CR). For TRG according to pathology, 15 cases were classified as TRG-0 (Table 4). Regarding the efficacy of NAT between the two groups (Table 5), there was only a significant difference in T downstaging, with no significant difference in the CR ratio or TRG-0 ratio.
| NRCT | NCT | P value | |
| Pre-operative median distance of tumor lower edge in cm | 5.0 | 6.0 | 0.37 |
| T3 | 25 | 17 | 0.01 |
| T4 | 30 | 5 | |
| Pre-operative N stage, n | |||
| N0 | 5 | 2 | 1.00 |
| N+ | 50 | 20 |
| Neoadjuvant therapy | n |
| RECIST, n | |
| CR | 12 |
| PR | 45 |
| SD | 20 |
| PD | 0 |
| TRG, n | |
| 0 | 15 |
| 1 | 12 |
| 2 | 23 |
| 3 | 27 |
| NRCT | NCT | P value | |
| Retraction of lower edge, n | 0.72 | ||
| Yes | 30 | 9 | |
| No | 25 | 13 | |
| Median retraction distance of lower edge in cm | 1.0 | 1.0 | 0.97 |
| T-downstaging, n | 0.03 | ||
| Yes | 37 | 9 | |
| No | 18 | 13 | |
| N-downstaging, n | 0.50 | ||
| Yes | 37 | 13 | |
| No | 18 | 9 | |
| CR, n | 0.71 | ||
| Yes | 20 | 7 | |
| No | 35 | 15 | |
| TRG-0, n | 1.00 | ||
| Yes | 11 | 4 | |
| No | 44 | 18 |
All 77 patients received surgical treatment, including Dixon (n = 56), Hartmann (n = 4), and Miles (n = 17) procedures. The organ preservation rate was 77.9%. The median operation duration was 167 min, with a median blood loss of 60 mL. Reoperation was performed in four cases caused by stoma obstruction (1 case), stoma ischemia (2 cases), and stoma bleeding (1 case). Among the 56 patients who underwent Dixon surgery, 48 (85.7%) underwent ileostomy. The average distance of the anastomosis from the anal margin was 5.4 ± 1.6 cm. Comparing the operation-related data between the two groups (Table 6), there were no significant differences in organ-preserving rate, intraoperative bleeding volume, or operation time (P > 0.05). In all cases receiving the Dixon operation, there was no difference in the ratio of ileostomy, but the distance from anastomosis to the anus in the NRCT group was lower than that in the NCT group.
Among all of the cases included, the median postoperative hospital stay was 8 d, the median postoperative time of consuming a liquid diet was 3 d, and 16 cases had postoperative complications. Among the 56 Dixon cases, 6 (10.7%) had AL. No incision-related complications and two (2/48, 4.2%) stoma-related complications were observed. Among the four Hartmann cases, one had stoma ischemia, and one had stoma obstruction. No incision complications were observed. Of the 17 Miles cases, 4 (23.5%) had incision-related complications, and no stoma-related complications occurred.
The data on perioperative management and complications were compared between the two groups (Table 7). There was no significant difference in the postoperative hospital stay, time of open fluid diet, reoperation rate, or occurrence of complications between the two groups (P > 0.05).
| NRCT | NCT | P value | |
| Median hospitalization time in d | 7 | 8 | 0.75 |
| Liquid diet time in d | 3 | 2 | 0.96 |
| Re-operation, n | 0.47 | ||
| Yes | 4 | 0 | |
| No | 51 | 22 | |
| Complications, n | 0.33 | ||
| Yes | 13 | 3 | |
| No | 42 | 19 | |
| Anastomotic leakage1, n | 0.56 | ||
| Yes | 5 | 1 | |
| No | 31 | 19 | |
| Incision complications, n | 0.47 | ||
| Yes | 4 | 0 | |
| No | 51 | 22 |
TRG-0 or 1 was used as the strain variable. Possibly related factors were selected as independent variables including sex, age, comorbidity, radiotherapy, pre-T stage, and tumor lower edge. The results of logistic regression analysis showed that radiation was not a risk factor, while male sex (odds ratio [OR] = 0.251, 95% confidence interval [CI]: 0.080-0.788; P = 0.02) and age < 60 years (OR = 0.306, 95%CI: 0.101-0.932; P = 0.04) were protective factors for TRG 0 or 1 (Tables 8 and 9).
| Factor | Variable | Assignment |
| Gender | X1 | M = 1, F = 0 |
| Age | X2 | ≤ 60 yr = 1, > 60 yr = 0 |
| Hypertension | X3 | N = 1, Y = 0 |
| Diabetes mellitus | X4 | N = 1, Y = 0 |
| Pre-operative radiation | X5 | N = 1, Y = 0 |
| Pre-T stage | X6 | T3 = 1, T4 = 0 |
| Tumor lower edge | X7 | ≤ 5 cm = 1, > 5 cm = 0 |
| TRG-0 or 1 | Y | Y = 1, N = 0 |
| Variable | β | Wals value, χ2 | P value | OR | 95%CI | ||
| Lower limit | Upper limit | ||||||
| Gender | -1.381 | 5.609 | 0.02 | 0.251 | 0.08 | 0.788 | |
| Age | -1.183 | 4.34 | 0.04 | 0.306 | 0.101 | 0.932 | |
| Hypertension | 0.725 | 1.245 | 0.26 | 2.065 | 0.578 | 7.385 | |
| Diabetes mellitus | 0.527 | 0.622 | 0.43 | 1.694 | 0.457 | 6.273 | |
| Pre-operative radiation | -0.601 | 1.012 | 0.31 | 0.548 | 0.17 | 1.768 | |
| Pre-T stage | 0.526 | 0.967 | 0.33 | 1.692 | 0.593 | 4.827 | |
| Tumor lower edge | 0.073 | 0.021 | 0.89 | 1.075 | 0.401 | 2.884 | |
AL occurrence was selected as the strain variable. Possible risk factors were selected as independent variables including age, sex, comorbidities, preoperative radiation administration, anastomotic site location, and TRG-0 or TRG-1 (Table 10). Preoperative radiation (OR = 0.177, 95%CI: 0.014-2.173; P = 0.18) was not a risk factor for AL (Table 11).
| Factor | Variable | Assignment |
| Gender | X1 | M = 1, F = 0 |
| Age | X2 | ≤ 60 yr = 1, > 60 yr = 0 |
| Comorbidities | X3 | N = 1, Y = 0 |
| Radiation | X4 | N = 1, Y = 0 |
| Stoma | X5 | N = 1, Y = 0 |
| TRG-0 or 1 | X6 | N = 1, Y = 0 |
| Anastomosis site location | X7 | ≤ 5.4 cm = 1, > 5.4 cm = 0 |
| Anastomotic leakage | Y | Y = 1, N = 0 |
| Variable | β | Wals value, χ2 | P value | OR | 95%CI | |
| Lower limit | Upper limit | |||||
| Gender | -0.777 | 0.971 | 0.32 | 0.46 | 0.098 | 2.156 |
| Age | -0.349 | 0.136 | 0.71 | 0.706 | 0.11 | 4.514 |
| Comorbidity | 0.146 | 0.024 | 0.88 | 1.157 | 0.185 | 7.239 |
| Radiation | -1.732 | 1.832 | 0.18 | 0.177 | 0.014 | 2.173 |
| Stoma | 1.176 | 0.917 | 0.34 | 3.241 | 0.292 | 35.961 |
| TRG-0 or 1 | -1.198 | 1.564 | 0.21 | 0.302 | 0.046 | 1.973 |
| Anastomosis site | -1.46 | 2.386 | 0.12 | 0.232 | 0.036 | 1.481 |
The median follow-up time was 26 mo, with three cases that were lost to follow-up. Forty-one patients finished the Wexner scale, while thirty-six patients did not finish the Hartmann or Miles procedure (n = 22), died (n = 8), had a temporary to permanent stoma (n = 5), or were lost to follow-up (n = 1). The median score of the Wexner scale was 3 (0-14), with no significant difference between the NRCT group and NCT group (P = 0.26).
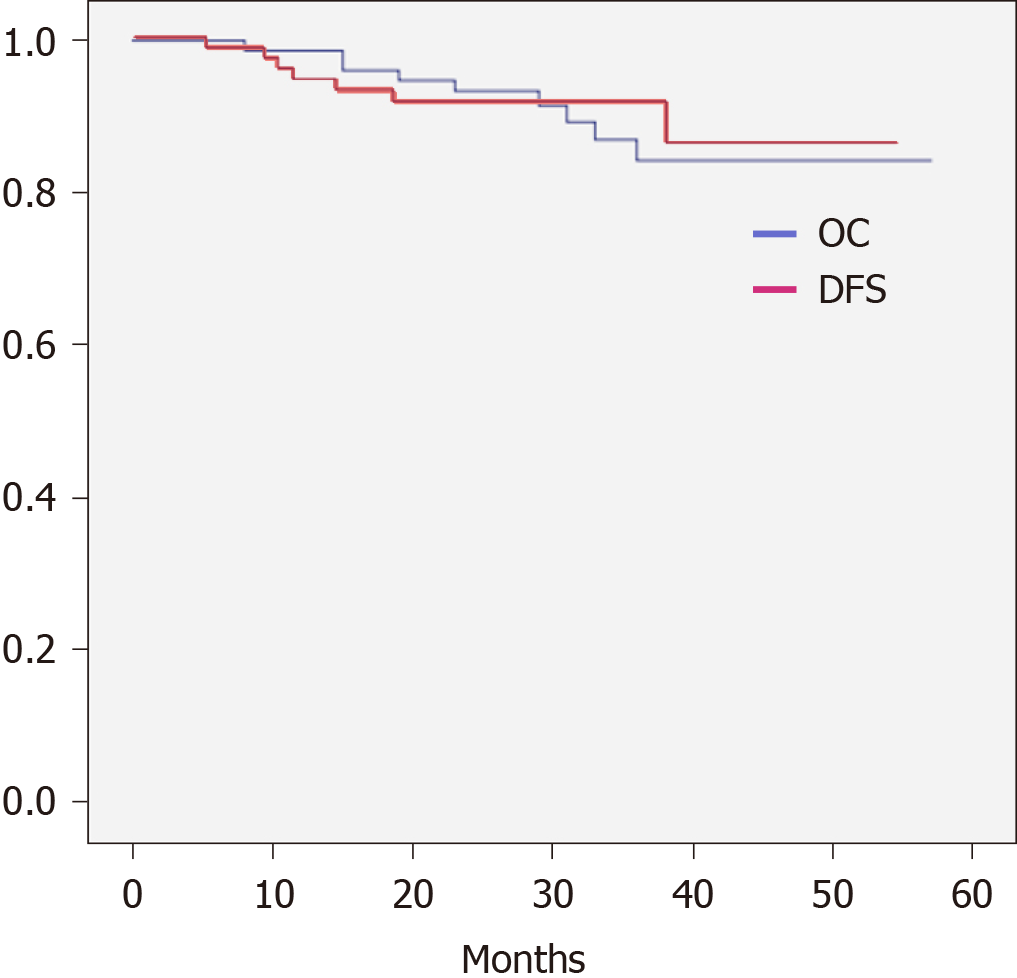
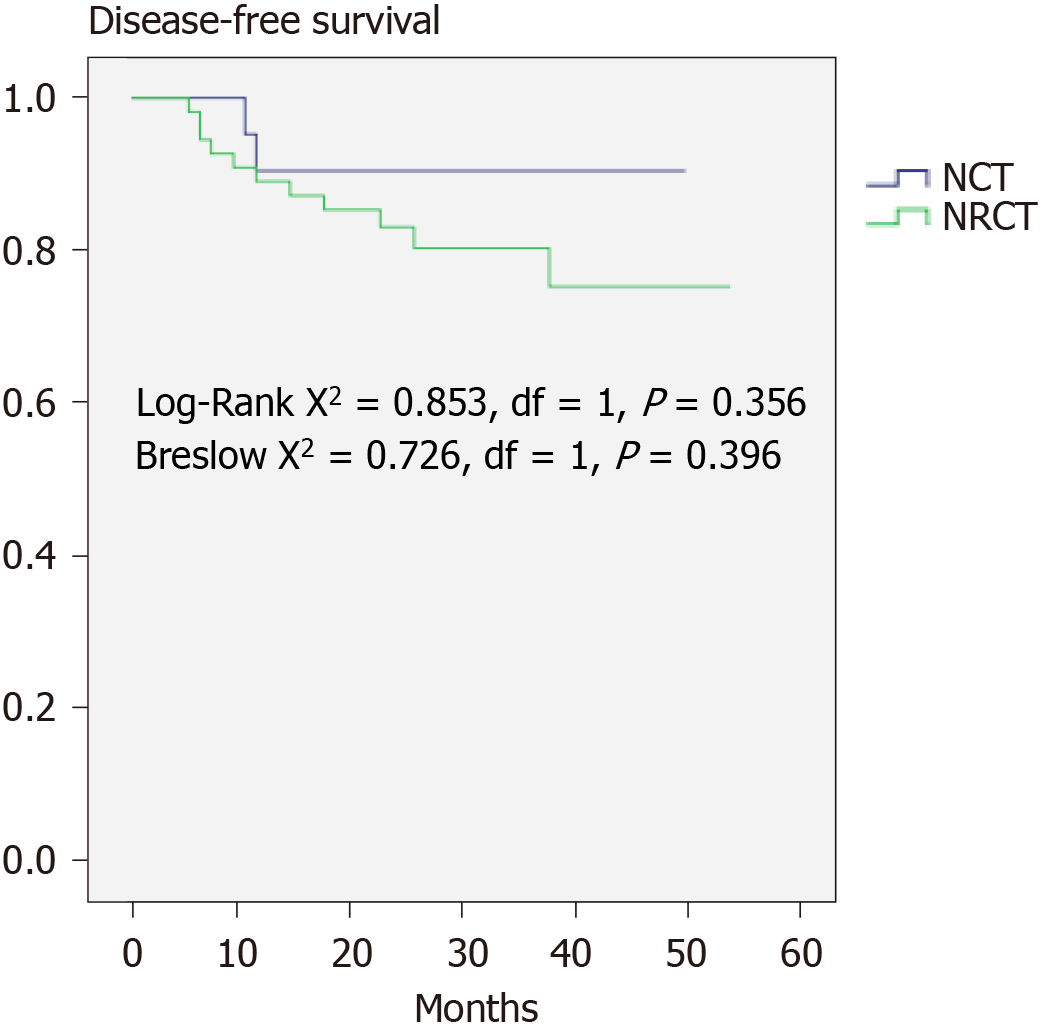
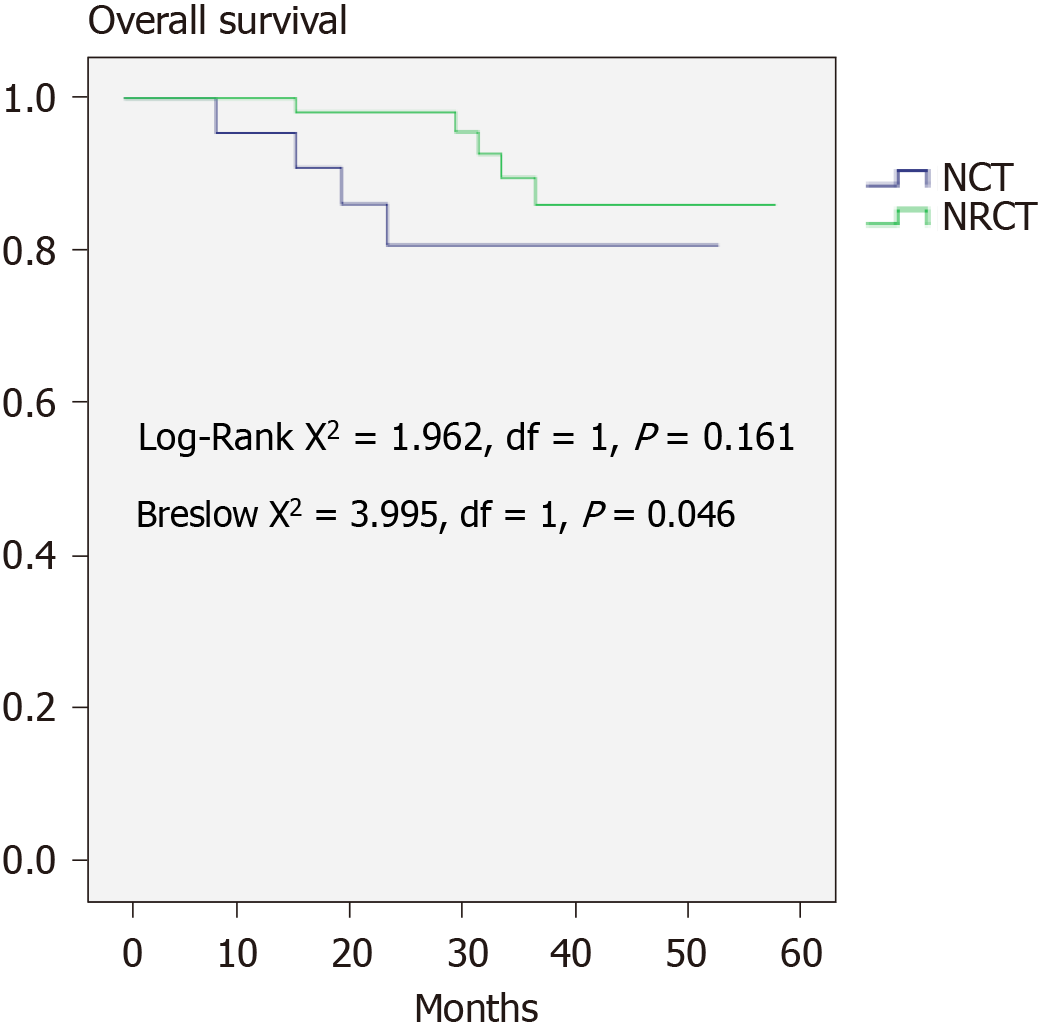
The 2-year DFS (91.7%) and OS (93.4%) are shown in Figure 1. The DFS between the NRCT group and NCT group showed no significant difference (83.1% vs 90.5%, P > 0.05), whereas the NRCT group had significantly better OS (98.2% vs 80.7%; Breslow test P = 0.046) (Figures 2 and 3).
NAT has a beneficial effect on tumor downstaging. In this study, preoperative radiation promised a better local tumor reduction rate and OS without increasing operation difficulty and complications or causing worse rectal function.
The EXPERT[12] trial showed that chemoradiotherapy had a superior pathological complete remission (PCR) rate compared with chemotherapy alone. Additionally, the FORWARC[13] trial showed the same results. In our study, the effect of NAT was not significantly different between the NCT and NRCT groups except for the T-downstaging rate. To some extent, it revealed the superior effect of the local tumor downstaging effect of preoperative radiation treatment.
Although female sex and age > 60 years were related to better tumor reaction, as shown in Tables 8 and 9, according to multivariate analysis, we failed to draw the same conclusion for preoperative radiation. Some experts have tried to find some clinical predictors of NAT response. Jung et al[14] postulated that apparent diffusion coefficient parameters in MRI could somehow relate to tumor reduction volume. Qiu et al[15] showed a poor response in patients with poor differentiation and T4 staging together.
Studies have previously shown the destruction of local rectal mucosa after radiation[7-10]. Fibrosis is the unfortunate result of previous radiation, causing more difficulties during operation. However, operation duration or blood loss volume showed no difference between groups, in agreement with the results of many studies performed in China[16,17].
Overall, NAT means more dysfunctioning stoma. No significant difference was shown in this study between the groups. However, two patients had nonclosure stomas due to unfinished systemic therapy or other unclear personal reasons. Andrew revealed a nonclosure rate of 14.5% and was concerned about its physical and psychological impact[18].
Park et al[19] revealed that NAT increased the incidence of AL (hazard ratio [HR] = 6.284; 95%CI: 2.829-13.961; P < 0.001); another report showed the same findings (OR = 3.05, 95%CI: 1.26-7.37; P = 0.01)[20]. However, some studies have shown opposite results. Rahbari et al[21] conducted a meta-analysis and concluded that the incidence of AL did not increase (HR = 0.96; 95%CI: 0.58-1.60; P = 0.87). Parc et al[22] also found that there was no significant difference in the incidence of AL between groups (P = 0.25). Although no difference in AL was observed in the study, a high proportion of dysfunctioning stoma could be overlooked, as it could conceal grade A AL from clinical observation. AL is also related to many other factors[23-25].
Incisional complications are mostly a concern for patients after the Miles procedure. El-Gazzaz et al[26] carried out a multivariate analysis and found that preoperative radiation increased the risk of perineal incision infection (OR = 1.66, 95%CI: 1.10-2.48); Musters et al[27] reached the same conclusion (OR = 1.74; 95%CI: 1.29-2.34).
Rectal function did not show any difference in this study according to the Wexner scale. However, six patients suffered from temporary-to-permanent conversion of their stoma for different reasons. Rosa et al[28] found that most patients could retain relatively good rectal function after NAT. Ghiselli et al[29] observed that female and elderly patients might suffer from worse sphincter function after NAT.
NAT in LARC showed better DFS according to the EORTC 22921 study[30], while its superiority in OS failed to be observed even with a longer follow-up duration. The concept of total NAT has been raised in recent years. However, randomized clinical evidence for selective preoperative radiation is still lacking. This study did not find any difference in DFS between the groups. The Breslow test confirmed better OS in the NRCT group, implying the advantage of NRCT.
Despite its retrospective nature, one limitation of the study was the small sample size. Some of the differences in complications or survival data may not arise with such a small sample. The side effects of NAT were not taken in to account in the research, which may have directly affected patient compliance and survival.
NAT can cause tumor downstaging preoperatively or even complete remission of the primary tumor. Radiochemotherapy had better T downstaging as well as promising OS without major complications. This may help clinicians realize the indispensability of preoperative radiation.
Neoadjuvant therapy (NAT) is becoming the standard way to treat locally advanced rectal cancer (LARC). Radiation has been an important part of NAT. More research on preoperative radiation is warranted.
To explore what kind of impact preoperative radiation has on tumor downstaging, postoperative complications, and survival in LARC. To provide more evidence for choosing a NAT regimen.
To compare the downstaging effect, postoperative complications, and prognosis between two different NAT regimens: The combination of radiation and chemotherapy and chemotherapy alone.
We retrospectively collected and analyzed the data of the two different regimens of NAT. The χ2 test was used to compare the downstaging effect, postoperative complications, etc. Kaplan-Meier analysis was used to describe and compare survival.
The study found that the primary tumor regression effect was better with the combination of radiation and chemotherapy than chemotherapy alone. This agrees with many previous articles. There were no significant differences in postoperative complications between the two groups, while overall survival was better in the radiochemotherapy group. However, no article comparing survival in LARC with or without radiation before surgery has been carried out. This waits to be confirmed by further studies.
This study tried to compare two different NAT regimens in LARC. Preoperative radiation may contribute to radical surgery in LARC and improve the prognosis as well.
A prospective study comparing postoperative complications and survival in NAT with or without preoperative radiation waits to be carried out.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kasztelan-Szczerbinska B S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL
| 1. | MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1220] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 2. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 1914] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 3. | Stockholm Rectal Cancer Study Group. Short-term preoperative radiotherapy for adenocarcinoma of the rectum. An interim analysis of a randomized multicenter trial. Am J Clin Oncol. 1987;10:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Stockholm Rectal Cancer Study Group. Preoperative short-term radiation therapy in operable rectal carcinoma. A prospective randomized trial. Cancer. 1990;66:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Cedermark B, Johansson H, Rutqvist LE, Wilking N. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer. 1995;75:2269-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | study on preoperative radiotherapy in rectal carcinoma. Stockholm Colorectal Cancer Study Group. Ann Surg Oncol. 1996;3:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 170] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Ehrenpreis ED, Marsh RW, Jr WS. Radiation Therapy for Pelvic Malignancy and its Consequences. Springer New York; 2015: 79-81. [DOI] [Full Text] |
| 8. | Yeoh EK, Russo A, Botten R, Fraser R, Roos D, Penniment M, Borg M, Sun WM. Acute effects of therapeutic irradiation for prostatic carcinoma on anorectal function. Gut. 1998;43:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Lim JF, Tjandra JJ, Hiscock R, Chao MW, Gibbs P. Preoperative chemoradiation for rectal cancer causes prolonged pudendal nerve terminal motor latency. Dis Colon Rectum. 2006;49:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Da Silva GM, Berho M, Wexner SD, Efron J, Weiss EG, Nogueras JJ, Vernava AM 3rd, Connor JT, Gervaz P. Histologic analysis of the irradiated anal sphincter. Dis Colon Rectum. 2003;46:1492-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21598] [Article Influence: 1349.9] [Reference Citation Analysis (1)] |
| 12. | Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, Tait D, Massey A, Tebbutt NC, Chau I. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 13. | Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol. 2016;34:3300-3307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 14. | Jung SH, Heo SH, Kim JW, Jeong YY, Shin SS, Soung MG, Kim HR, Kang HK. Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J Magn Reson Imaging. 2012;35:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Qiu HZ, Wu B, Xiao Y, Lin GL. Combination of differentiation and T stage can predict unresponsiveness to neoadjuvant therapy for rectal cancer. Colorectal Dis. 2011;13:1353-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Yu ZQ, Zhang C, Gao XH, Zuo ZG, Liu QZ, Dou WL, Xu XW, Fu CG. [Impacts of preoperative radiochemotherapy on operation and postoperative complications in patients with mid-low rectal carcinomas]. Zhonghua Weichang Waike Zazhi. 2012;15:332-335. [PubMed] |
| 17. | Hu JJ, Zhang XM, Zhou ZX, Liang JW, Zhou HT, Liu FH, Hou HR. Impact of neoadjuvant chemoradiotherapy on short-term outcomes of laparoscopic surgery for rectal cancer. Zhonghuo Zhongliu Linchuang Yu Kangfu Zazhi. 2013;20. [DOI] [Full Text] |
| 18. | Chiu A, Chan HT, Brown CJ, Raval MJ, Phang PT. Failing to reverse a diverting stoma after lower anterior resection of rectal cancer. Am J Surg. 2014;207:708-11; discussion 711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, Bae BN, Son GM, Lee SI, Kang H. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 20. | Qin Q, Ma T, Deng Y, Zheng J, Zhou Z, Wang H, Wang L, Wang J. Impact of Preoperative Radiotherapy on Anastomotic Leakage and Stenosis After Rectal Cancer Resection: Post Hoc Analysis of a Randomized Controlled Trial. Dis Colon Rectum. 2016;59:934-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 21. | Rahbari NN, Elbers H, Askoxylakis V, Motschall E, Bork U, Büchler MW, Weitz J, Koch M. Neoadjuvant radiotherapy for rectal cancer: meta-analysis of randomized controlled trials. Ann Surg Oncol. 2013;20:4169-4182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Parc Y, Zutshi M, Zalinski S, Ruppert R, Fürst A, Fazio VW. Preoperative radiotherapy is associated with worse functional results after coloanal anastomosis for rectal cancer. Dis Colon Rectum. 2009;52:2004-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Riss S, Mittlböck M, Riss K, Chitsabesan P, Stift A. Intraoperative complications have a negative impact on postoperative outcomes after rectal cancer surgery. Int J Surg. 2014;12:833-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Trifunović B, Kršić J, Bezmarević M, Grbović D, Zeljković D, Nešković B, Soldatović I, Prelević R, Mirković D. Relationship between of short-course preoperative radiotherapy and serum albumin level and postoperative complications in rectal cancer surgery. Vojnosanit Pregl. 2015;72:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Shekarriz H, Eigenwald J, Shekarriz B, Upadhyay J, Shekarriz J, Zoubie D, Wedel T, Wittenburg H. Anastomotic leak in colorectal surgery: are 75 % preventable? Int J Colorectal Dis. 2015;30:1525-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | El-Gazzaz G, Kiran RP, Lavery I. Wound complications in rectal cancer patients undergoing primary closure of the perineal wound after abdominoperineal resection. Dis Colon Rectum. 2009;52:1962-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Musters GD, Buskens CJ, Bemelman WA, Tanis PJ. Perineal wound healing after abdominoperineal resection for rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum. 2014;57:1129-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Rosa C, Caravatta L, Di Tommaso M, Ursini1 LA, Allajbej A, Zecca IA, Di Nicola M, Genovesi D. EP-1492: Bowel and anal sphincter function after neoadjuvant chemoradiotherapy in rectal cancer patients. Radiother Oncol. 2018;127:S809-S810. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Ghiselli R, Ortenzi M, Cardinali L, Skrami E, Gesuita R, Guerrieri M. Functional outcomes after TEM in patients with complete clinical response after neoadjuvant chemoradiotherapy. Surg Endosc. 2017;31:2997-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M, Marchal D, Van Laethem JL, Klein V, Giralt J, Clavère P, Glanzmann C, Cellier P, Collette L; EORTC Radiation Oncology Group. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 554] [Article Influence: 50.4] [Reference Citation Analysis (0)] |