Published online Mar 27, 2021. doi: 10.4240/wjgs.v13.i3.256
Peer-review started: November 3, 2020
First decision: December 20, 2020
Revised: December 23, 2020
Accepted: January 28, 2021
Article in press: January 28, 2021
Published online: March 27, 2021
Processing time: 134 Days and 23.2 Hours
There have been different reports on mortality of sepsis; however, few focus on the prognosis of patients with sepsis after surgery.
To study the clinical features and prognostic predictors in patients with sepsis after gastrointestinal tumor surgery in intensive care unit (ICU).
We retrospectively screened patients who underwent gastrointestinal tumor surgery at Peking University Cancer Hospital from January 2015 to December 2019. Among them, 181 patients who were diagnosed with sepsis in ICU were included in our study. Survival was analysed by the Kaplan-Meier method. Univariate and multivariate adjusted analyses were performed to identify predictors of prognosis.
The 90-d all-cause mortality rate was 11.1% in our study. Univariate analysis showed that body mass index (BMI), shock within 48 h after ICU admission, leukocyte count, lymphocyte to neutrophil ratio, international normalized ratio, creatinine, procalcitonin, lactic acid, oxygenation index, and sequential organ failure assessment (SOFA) score within 24 h after ICU admission might be all significantly associated with the prognosis of sepsis after gastrointestinal tumor surgery. In multiple analysis, we found that BMI ≤ 20 kg/m2, lactic acid after ICU admission, and SOFA score within 24 h after ICU admission might be independent risk predictors of the prognosis of sepsis after gastrointestinal tumor surgery. Compared with SOFA score, SOFA score combined with BMI and lactic acid might have higher predictive ability (area under the receiver operating characteristic curve, 0.859; 95% confidence interval, 0.789-0.929).
Lactic acid and SOFA score within 24 h after ICU admission are independent risk predictors of the prognosis of sepsis after gastrointestinal tumor surgery. SOFA score combined with BMI and lactic acid might have good predictive value.
Core Tip: There have been different reports on mortality of sepsis, but few focus on the prognosis of patients with sepsis after surgery. The purpose of this study was to investigate the prognostic factors of patients with sepsis who were admitted to intensive care unit (ICU) after gastrointestinal surgery. This study retrospectively screened patients who underwent the gastrointestinal tumor surgery at the Peking University Cancer Hospital from January 2015 to December 2019. Among them, 181 patients who were diagnosed with sepsis in ICU were enrolled in our study. In multiple analysis, we found that body mass index ≤ 20 kg/m2, lactic acid after ICU admission, and sequential organ failure assessment (SOFA) score within 24 h after ICU admission might be independent risk predictors of the prognosis of sepsis after gastrointestinal tumor surgery in ICU. Compared with SOFA score, SOFA score combined with body mass index and lactic acid might have higher predictive ability (area under the receiver operating characteristic curve, 0.859; 95% confidence interval, 0.789-0.929).
- Citation: Chen RX, Wu ZQ, Li ZY, Wang HZ, Ji JF. Prognostic predictors in patients with sepsis after gastrointestinal tumor surgery: A retrospective study. World J Gastrointest Surg 2021; 13(3): 256-266
- URL: https://www.wjgnet.com/1948-9366/full/v13/i3/256.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i3.256
Sepsis is a worldwide problem, and it is estimated that there are 31.5 million sepsis patients in the world every year, causing about 5.3 million deaths each year[1]. It is associated with a high mortality and can be caused by any type of infection. Pathogenic microorganisms include bacteria, fungi, viruses, and parasites. Since previous definition of sepsis (infection plus systemic inflammatory response syndrome) is too sensitive, its new definition is life-threatening organ dysfunction resulting from the host's dysfunctional response to infection. Organ dysfunction is characterized by the sequential organ failure assessment (SOFA) score of not less than two points[2].
Early identification of infection, control of infection source, proper use of antibiotics, and rapid resuscitation of critical patients are the cornerstone of abdominal infection management[3-6]. There are many factors affecting the prognosis of sepsis. It has been reported that the prognosis of sepsis is related to lactic acid, interleukin-6, procalcitonin (PCT), C-reactive protein, and heart-fatty acid binding protein[7-11]. However, as described by definition, sepsis is a syndrome with extreme heterogeneity. In the past, there were many reports of sepsis mortality; however, few focused on the prognosis of patients with sepsis after gastrointestinal surgery. The purpose of this cohort study was to explore the prognostic predictors of sepsis patients admitted to intensive care unit after gastrointestinal tumor surgery.
From January 2015 to December 2019, a total of 1636 patients were admitted to the intensive care unit (ICU) after elective and emergency surgery at the Gastrointestinal Cancer Center of Peking University Cancer Hospital. According to the new definition of sepsis, 181 patients diagnosed with sepsis were included in this cohort study. The exclusion criteria were: (1) Patients were admitted to ICU for other reasons or did not have sepsis during the ICU stay; and (2) Patients’ sepsis occurred out of the ICU stay. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Peking University Cancer Hospital and informed consent was obtained from all the patients or their next of kin.
We followed the guidelines of sepsis treatment strategy[5,6]. The clinical data and laboratory tests of the patients were collected as follows: Age, body mass index (BMI), underlying diseases, length of the first operation, culture and sensitivity tests, antibiotics used, whether shock occurred within 48 h after ICU admission, leukocyte count, lymphocyte to neutrophil ratio, international standardized ratio (INR), activated partial thromboplastin time (APTT), albumin, creatinine, cardiac troponin I (TNI), PCT, lactic acid, oxygenation index (PaO2/FiO2) after ICU admission, and SOFA score. Unless otherwise stated, the first test after ICU admission was used for analysis. They were followed in a clinic or by telephone for 90 d.
Continuous variables are statistically described as the mean ± SD. Non-continuous variables are described as medians [quartile 1 (Q1), quartile 3 (Q3)]. Counting variables are described as numerical values (percentages). The survival rate was calculated by the Kaplan-Meier method, and the log-rank test was used for univariate analysis. Multivariate adjustment analysis was performed using Cox regression and forward LR method. The predictive ability of the factors was assessed using the area under the receiver operating characteristic (AUROC) curve. Statistical analyses were performed using SPSS version 24.0 and P values less than 0.05 (two-tailed) were considered significant.
According to the new definition of sepsis, a total of 181 patients were diagnosed with sepsis, of whom 86 were diagnosed with septic shock within 48 h after ICU admission. The most common postoperative infection for gastrointestinal tumor was abdominal infection. There were 13 cases with abdominal or gastrointestinal bleeding, 16 with deep vein thrombosis, 1 with cerebral infarction, and 1 with myocardial infarction. The baseline characteristics of the patients are shown in Table 1.
| Baseline characteristic | n (%) |
| Age, median (Q1, Q3) | 65 (59.71) |
| Sex | |
| Male | 145 (80.1) |
| Female | 36 (19.9) |
| BMI, mean (SD), kg/m2 | 23.5 (0.3) |
| Tumor type | |
| Gastric cancer | 91 (50.3) |
| Colorectal cancer | 84 (46.4) |
| Other abdominal tumors | 6 (3.3) |
| Coexisting condition1 | |
| Hypertension | 64 (35.4) |
| Diabetes | 32 (17.7) |
| Coronary heart disease | 17 (9.4) |
| Chronic obstructive pulmonary disease | 11 (6.1) |
| Arrhythmia | 9 (5.0) |
| Chronic renal insufficiency | 2 (1.1) |
| Location of infection2 | |
| Abdominal infection | 134 (74.0) |
| Enterogenous infection | 12 (6.6) |
| Intrathoracic infection | 17 (9.4) |
| Pulmonary infection | 31 (17.1) |
| Skin and soft tissue infection | 6 (3.3) |
| Surgical wound infection | 4 (2.2) |
| Central line-associated bloodstream infection | 3 (1.7) |
| Urinary tract infection | 2 (1.1) |
| Length of first operation, median (Q1, Q3), min | 195 (140, 246) |
Univariate analysis is shown in Table 2. All the sepsis patients were followed for 90 d; 20 patients died (19 died of sepsis related organ failure and 1 died of hemorrhagic shock), and the 90-d all-cause mortality rate was 11.1%. Univariate analysis showed that there were statistically significant differences in BMI, shock within 48 h after ICU admission, leukocyte count, lymphocyte to neutrophil ratio, INR, creatinine, PCT, lactic acid, oxygenation index after ICU admission, and SOFA score within 24 h after ICU admission. Especially, the P values of shock within 48 h after ICU admission, INR, creatinine, lactic acid, oxygenation index, and SOFA score within 24 h after ICU admission were all less than 0.01.
| Parameter | n (%) | Survival rate at 90-d | P value |
| Age, yr | 0.840 | ||
| ≤ 65 | 96 (53.0) | 0.885 | |
| > 65 | 85 (47.0) | 0.894 | |
| Sex | 0.254 | ||
| Male | 145 (80.1) | 0.876 | |
| Female | 36 (19.9) | 0.944 | |
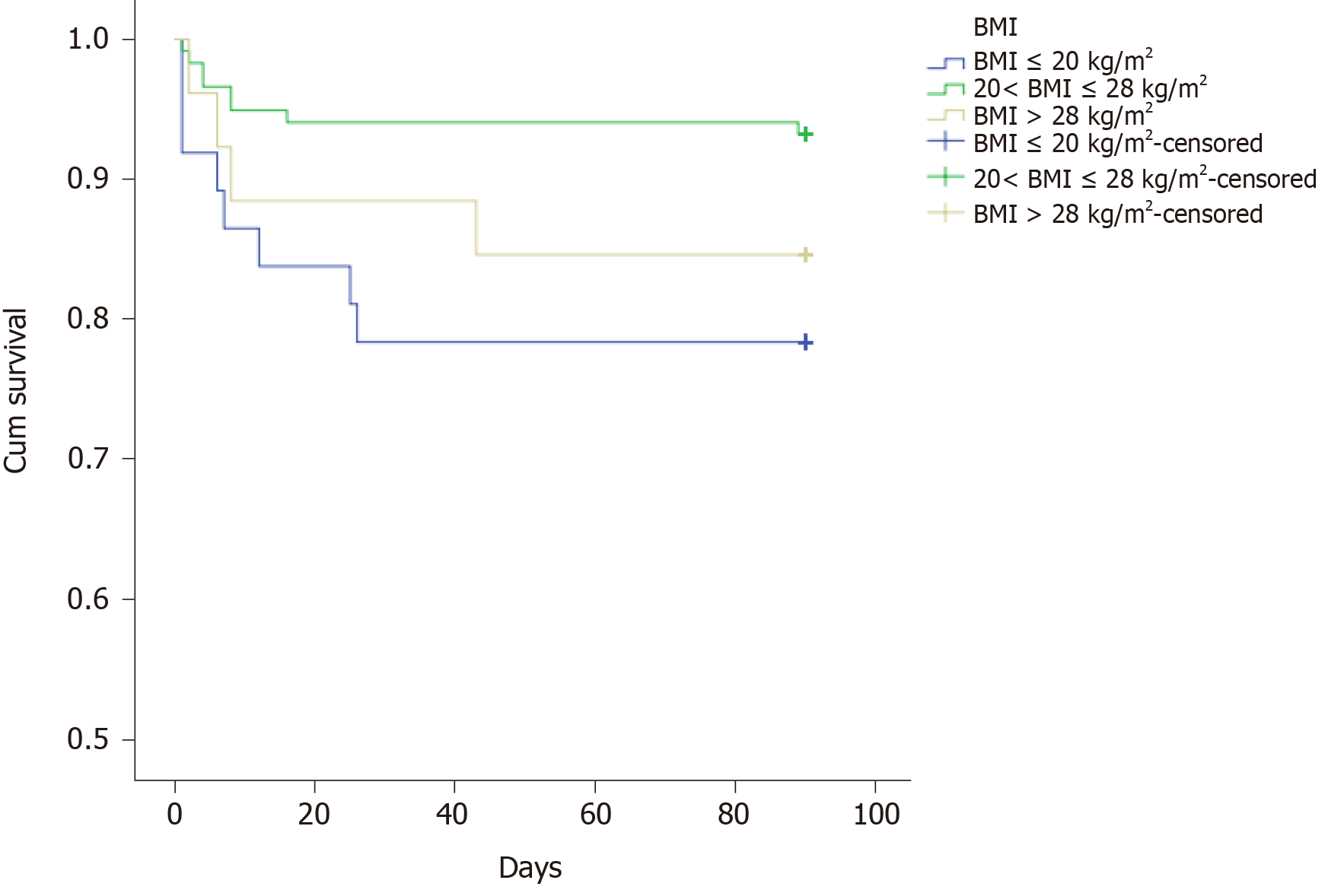
| BMI, kg/m2 | 0.028 | ||
| ≤ 20 | 37 (20.4) | 0.784 | |
| 20 < BMI ≤ 28 | 118 (65.2) | 0.932 | |
| > 28 | 26 (14.4) | 0.846 | |
| Length of first operation, min | 0.361 | ||
| ≤ 240 | 129 (71.3) | 0.876 | |
| > 240 | 52 (28.7) | 0.923 | |
| Empirical anti infection evaluation | 0.729 | ||
| Sensitive | 132 (72.9) | 0.894 | |
| Resistance | 18 (10.0) | 0.833 | |
| No pathogen detected | 31 (17.1) | 0.903 | |
| Shock within 48 h after ICU admission | 0.001 | ||
| No | 95 (52.5) | 0.979 | |
| Yes | 86 (47.5) | 0.791 | |
| Leukocyte count, 109/L | 0.010 | ||
| ≤ 4 | 31 (17.1) | 0.774 | |
| 4 < WBC ≤ 12 | 77 (42.6) | 0.963 | |
| > 12 | 73 (40.3) | 0.863 | |
| Lymphocyte to neutrophil ratio | 0.035 | ||
| ≤ 0.15 | 148 (81.8) | 0.912 | |
| > 0.15 | 33 (18.2) | 0.788 | |
| International standardized ratio | 0.001 | ||
| ≤ 1.5 | 127 (70.2) | 0.937 | |
| > 1.5 | 54 (29.8) | 0.778 | |
| Activated partial thromboplastin time, s | 0.064 | ||
| ≤ 50 | 138 (76.2) | 0.913 | |
| > 50 | 43 (23.8) | 0.814 | |
| Albumin, g/L | 0.058 | ||
| ≤ 30 | 99 (54.7) | 0.848 | |
| > 30 | 82 (45.3) | 0.939 | |
| Creatinine, μmol/L | 0.001 | ||
| ≤ 120 | 150 (82.9) | 0.927 | |
| > 120 | 31 (17.1) | 0.710 | |
| Cardiac troponin I, ng/mL | 0.063 | ||
| ≤ 0.05 | 138 (76.2) | 0.913 | |
| > 0.05 | 43 (23.8) | 0.814 | |
| Procalcitonin, ng/mL | 0.011 | ||
| ≤ 5 | 93 (51.4) | 0.946 | |
| > 5 | 88 (48.6) | 0.830 | |
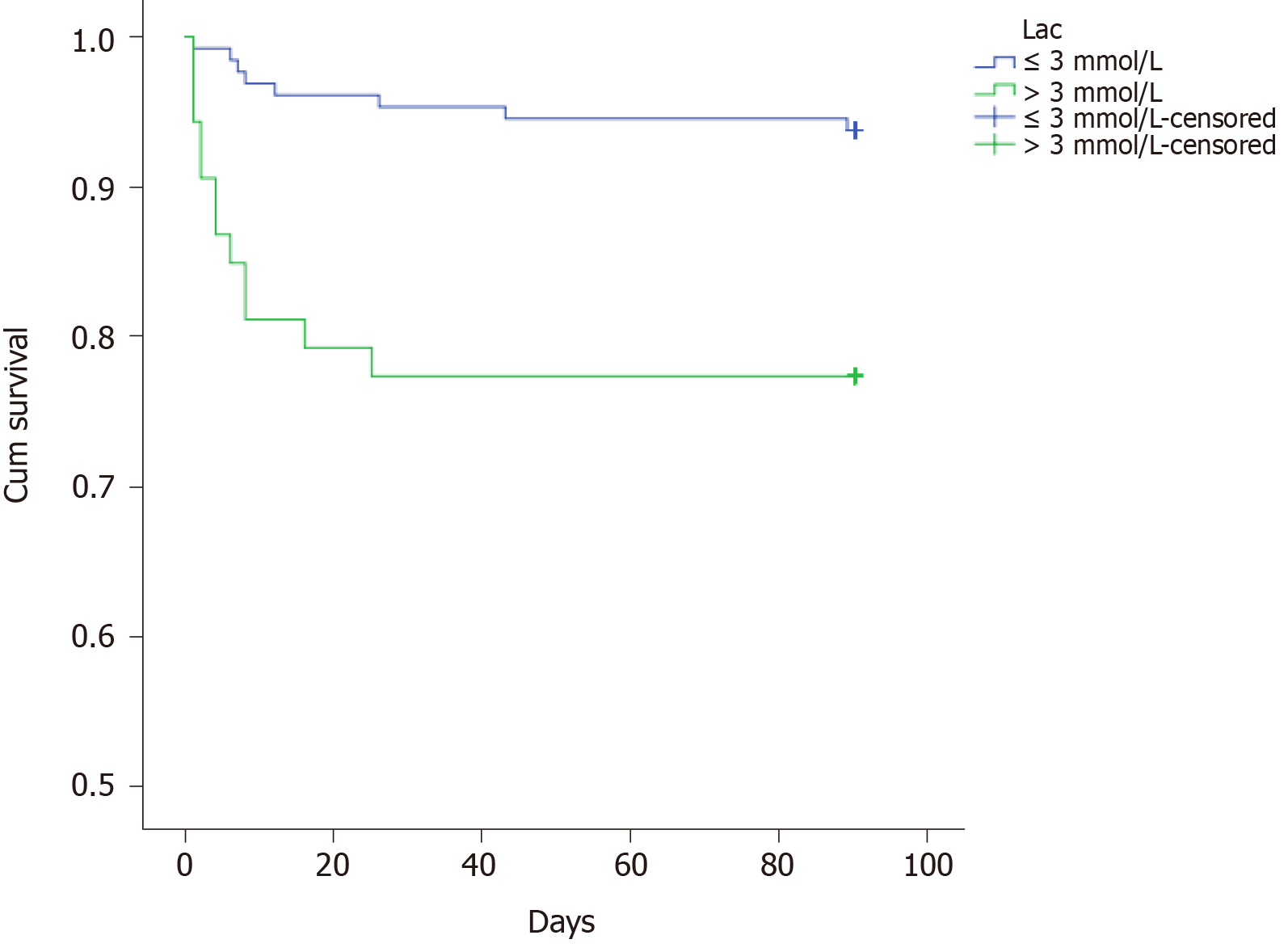
| Lactic acid, mmol/L | 0.001 | ||
| ≤3 | 128 (70.7) | 0.938 | |
| > 3 | 53 (29.3) | 0.774 | |
| Oxygenation index, mmHg | 0.003 | ||
| ≤ 200 | 97 (53.6) | 0.825 | |
| > 200 | 84 (46.4) | 0.964 | |
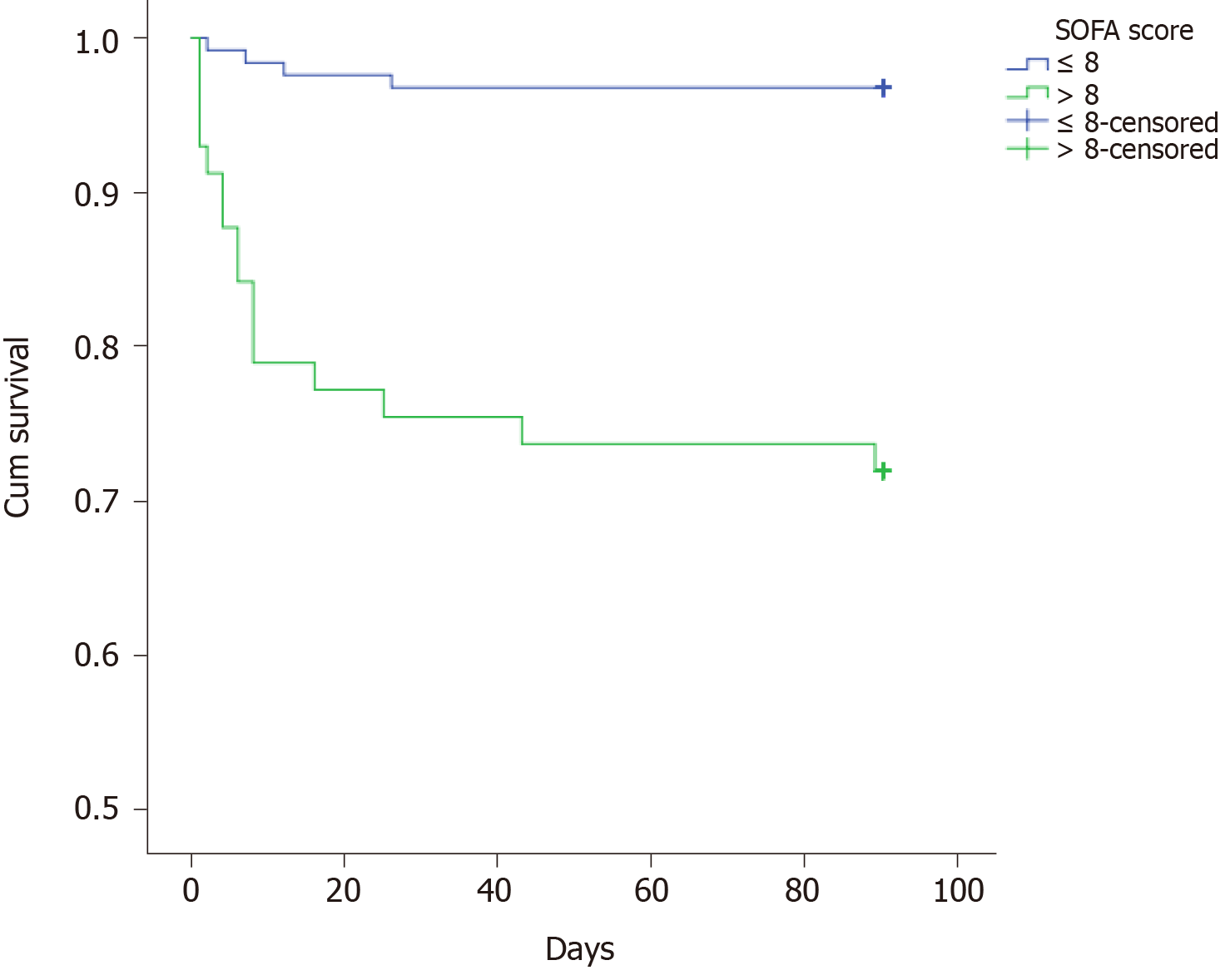
| SOFA score | 0.001 | ||
| ≤ 8 | 124 (68.5) | 0.968 | |
| > 8 | 57 (31.5) | 0.719 |
The multiple analysis is presented in Table 3. Those factors with a P value less than 0.05 were enrolled in the Cox regression analysis. The results showed that BMI ≤ 20 kg/m2, lactic acid after ICU admission, and SOFA score within 24 h after ICU admission might be independent prognostic predictors. However, there was no significant difference between those with 20 < BMI ≤ 28 kg/m2 and BMI > 28 kg/m2. The survival curves of these three predictors are shown in Figures 1-3.
| Factor | RR | 95%CI | P value | |
| Lower limit | Upper limit | |||
| BMI (Ref) | 0.011 | |||
| BMI (1) | 1.778 | 0.532 | 5.942 | 0.350 |
| BMI (2) | 0.377 | 0.113 | 1.262 | 0.114 |
| Lactic acid | 2.950 | 1.168 | 7.450 | 0.022 |
| SOFA score | 8.359 | 2.741 | 25.496 | 0.001 |
BMI had a mild ability to predict mortality of these patients (AUROC, 0.569); lactic acid had a mild ability to predict mortality (AUROC, 0.673); SOFA score had a modest ability to predict mortality (AUROC, 0.773). Compared with SOFA score, SOFA score combined with BMI and lactic acid might have higher predictive ability (AUROC, 0.859; 95% confidence interval, 0.789-0.929). The ROC curve of the SOFA score combined with BMI and lactic acid is shown in Figure 4.
Sepsis is one of the most common causes of death in critically ill patients. Until now, there have been few studies on postoperative sepsis. In this study, postoperative sepsis after gastrointestinal tumor surgery was investigated. The mortality rate was lower than that of sepsis reported in the literature[12], which might be related to the fact that the most common source of infection in our patients was abdominal infection. Hence, by a multidisciplinary team, we could control the infection source actively through minimally invasive drainage or surgical debridement. There are many factors that might influence the prognosis in patients with sepsis. In our study, 181 patients with sepsis admitted to intensive care unit after gastrointestinal tumor surgery were analyzed retrospectively and we found that BMI, lactic acid after ICU admission, and SOFA score within 24 h after ICU admission were independent prognostic predictors.
First, we found that patients with BMI ≤ 20 kg/m2 had a worse prognosis than those with 20 < BMI ≤ 28 kg/m2 and BMI > 28 kg/m2, so we guessed that BMI ≤ 20 kg/m2 might be a risk predictor. However, the number of patients in this study was limited. The relationship between BMI and the prognosis of sepsis had been widely reported, but the results remained controversial[13,14]. Papadimitriou-Olivgeris et al[15] found that the mortality of obese patients with sepsis increased significantly. Nevertheless, one recent meta-analysis divided sepsis patients into three groups: Overweight (25 < BMI ≤ 30 kg/m2), obesity (30 < BMI ≤ 40 kg/m2), and morbid obesity (BMI > 40 kg/m2). The results showed that the death risk of overweight patients with sepsis was reduced, while obesity and morbid obesity patients with sepsis did not increase the death risk. The reason for this controversy might be linked to the distribution of adipose tissue. It was pointed out that the visceral fat (VAT) accumulation detected by CT scan was a risk factor for poor prognosis of sepsis. Sepsis patients with a high ratio of visceral fat area to the subcutaneous fat area had an increased risk of death and organ damage[16]. In the future, more detailed and rigorous studies should aim to elucidate the relationship between sepsis and BMI.
Generally speaking, when the energy of the tissue could not be satisfied by aerobic respiration, the tissue could not get enough oxygen or could not deal with oxygen fast enough, the concentration of lactic acid would rise. Hence, sepsis and septic shock guidelines used lactic acid as an indicator of tissue hypoperfusion and as a target for fluid resuscitation[5,6]. Many studies have shown that lactic acid was an independent risk factor for sepsis prognosis[17-19]. In our study, it was further confirmed that lactic acid > 3 mmol/L after ICU admission was an independent risk predictor of patients with sepsis after gastrointestinal tumor surgery.
There have been many scoring systems for evaluating the severity of critical patients, such as SOFA score and acute physiology and chronic health evaluation II score[20-22]. Several studies confirmed that the SOFA score was an independent risk predictor of the prognosis of patients with sepsis[23,24]. In our study, we found that the SOFA score within 24 h after ICU admission was statistically significant in the univariate and multivariate analysis. Compared with SOFA score, SOFA score combined with BMI and lactic acid might have better predictive value.
The limitations of this study should be referred. First, this study is a retrospective cohort study and the subjects of this study are sepsis patients admitted to ICU after gastrointestinal tumor surgery. Whether the results can be extended to all sepsis patients remains to be confirmed. Second, patients with sepsis in the general wards were not included in this study, and most of these patients improved in our hospital. Therefore, the mortality of patients with sepsis after gastrointestinal tumor surgery might be overestimated in our study. In the future, we will design prospective studies to elucidate it. Third, there were several missing data, especially brain natriuretic peptide, echocardiography, etc. Thus, we could not accurately evaluate their impact on the prognosis of sepsis patients. Finally, the sample size of this study was limited. Many factors were significantly different in univariate analysis, but not in multivariate analysis. We hope that there will be more large-scale studies in the future to confirm these results.
Lactic acid and SOFA score within 24 h after ICU admission are independent risk predictors of the prognosis of sepsis after gastrointestinal tumor surgery. SOFA score combined with BMI and lactic acid might have good predictive value.
There have been different reports on mortality of sepsis, but few focus on the prognosis of patients with sepsis after surgery.
To explore the prognostic predictors in patients with sepsis after gastrointestinal tumor surgery.
We studied the clinical features and prognostic predictors in patients with sepsis after gastrointestinal tumor surgery in intensive care unit (ICU).
We retrospectively screened patients who underwent gastrointestinal tumor surgery at Peking University Cancer Hospital from January 2015 to December 2019. Among them, 181 patients who were diagnosed with sepsis in ICU were included in our study. Survival was analysed by the Kaplan-Meier method. Univariate and multivariate adjusted analyses were performed to identify predictors of prognosis.
The 90-d all-cause mortality rate was 11.1% in our study. In multiple analysis, we found that body mass index (BMI) ≤ 20 kg/m2, lactic acid after ICU admission, and sequential organ failure assessment (SOFA) score within 24 h after ICU admission might be independent risk predictors of the prognosis of sepsis after gastrointestinal tumor surgery. Compared with SOFA score, SOFA score combined with BMI and lactic acid might have higher predictive ability (area under the receiver operating characteristic curve, 0.859; 95% confidence interval, 0.789-0.929).
Lactic acid and SOFA score within 24 h after ICU admission are independent risk predictors of the prognosis of sepsis after gastrointestinal tumor surgery. SOFA score combined with BMI and lactic acid might have good predictive value.
More large-scale studies are needed in the future to confirm these results.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mishra TS S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Li JH
| 1. | Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1639] [Cited by in RCA: 2312] [Article Influence: 256.9] [Reference Citation Analysis (0)] |
| 2. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17171] [Article Influence: 1907.9] [Reference Citation Analysis (2)] |
| 3. | Sawyer RG, Claridge JA, Nathens AB, Rotstein OD, Duane TM, Evans HL, Cook CH, O'Neill PJ, Mazuski JE, Askari R, Wilson MA, Napolitano LM, Namias N, Miller PR, Dellinger EP, Watson CM, Coimbra R, Dent DL, Lowry SF, Cocanour CS, West MA, Banton KL, Cheadle WG, Lipsett PA, Guidry CA, Popovsky K; STOP-IT Trial Investigators. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;372:1996-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 486] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 4. | Sartelli M, Catena F, Abu-Zidan FM, Ansaloni L, Biffl WL, Boermeester MA, Ceresoli M, Chiara O, Coccolini F, De Waele JJ, Di Saverio S, Eckmann C, Fraga GP, Giannella M, Girardis M, Griffiths EA, Kashuk J, Kirkpatrick AW, Khokha V, Kluger Y, Labricciosa FM, Leppaniemi A, Maier RV, May AK, Malangoni M, Martin-Loeches I, Mazuski J, Montravers P, Peitzman A, Pereira BM, Reis T, Sakakushev B, Sganga G, Soreide K, Sugrue M, Ulrych J, Vincent JL, Viale P, Moore EE. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1784] [Cited by in RCA: 1986] [Article Influence: 248.3] [Reference Citation Analysis (1)] |
| 6. | Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44:925-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 687] [Article Influence: 98.1] [Reference Citation Analysis (0)] |
| 7. | Fraunberger P, Wang Y, Holler E, Parhofer KG, Nagel D, Walli AK, Seidel D. Prognostic value of interleukin 6, procalcitonin, and C-reactive protein levels in intensive care unit patients during first increase of fever. Shock. 2006;26:10-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Yanaral TU, Idin IK, Uzman S, Toptas M,Bican G. The prognostic value of procalcitonin and C-reactive protein in critically ill patients A comparison with APACHE II and SOFA scores: 12AP5-2. Eur J Anaesth. 2010;1068:27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Tanrıverdi H, Tor MM, Kart L, Altın R, Atalay F, SumbSümbüloğlu V. Prognostic value of serum procalcitonin and C-reactive protein levels in critically ill patients who developed ventilator-associated pneumonia. Ann Thorac Med. 2015;10:137-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Ríos-Toro JJ, Márquez-Coello M, García-Álvarez JM, Martín-Aspas A, Rivera-Fernández R, Sáez de Benito A, Girón-González JA. Soluble membrane receptors, interleukin 6, procalcitonin and C reactive protein as prognostic markers in patients with severe sepsis and septic shock. PLoS One. 2017;12:e0175254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Ryoo SM, Lee J, Lee YS, Lee JH, Lim KS, Huh JW, Hong SB, Lim CM, Koh Y, Kim WY. Lactate Level Versus Lactate Clearance for Predicting Mortality in Patients With Septic Shock Defined by Sepsis-3. Crit Care Med. 2018;46:e489-e495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 12. | Herrán-Monge R, Muriel-Bombín A, García-García MM, Merino-García PA, Martínez-Barrios M, Andaluz D, Ballesteros JC, Domínguez-Berrot AM, Moradillo-Gonzalez S, Macías S, Álvarez-Martínez B, Fernández-Calavia MJ, Tarancón C, Villar J, Blanco J. Epidemiology and Changes in Mortality of Sepsis After the Implementation of Surviving Sepsis Campaign Guidelines. J Intensive Care Med. 2019;34:740-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Trivedi V, Bavishi C, Jean R. Impact of obesity on sepsis mortality: A systematic review. J Crit Care. 2015;30:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Wang S, Liu X, Chen Q, Liu C, Huang C, Fang X. The role of increased body mass index in outcomes of sepsis: a systematic review and meta-analysis. BMC Anesthesiol. 2017;17:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Papadimitriou-Olivgeris M, Aretha D, Zotou A, Koutsileou K, Zbouki A, Lefkaditi A, Sklavou C, Marangos M, Fligou F. The Role of Obesity in Sepsis Outcome among Critically Ill Patients: A Retrospective Cohort Analysis. Biomed Res Int. 2016;2016:5941279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Pisitsak C, Lee JG, Boyd JH, Coxson HO, Russell JA, Walley KR. Increased Ratio of Visceral to Subcutaneous Adipose Tissue in Septic Patients Is Associated With Adverse Outcome. Crit Care Med. 2016;44:1966-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Malmir J, Bolvardi E, Aghaee MA. Serum lactate is a useful predictor of death in severe sepsis and septic shock. Rev Clin Med. 2014;1:97-104. [DOI] [Full Text] |
| 18. | Lokhandwala S, Andersen LW, Nair S, Patel P, Cocchi MN, Donnino MW. Absolute lactate value vs relative reduction as a predictor of mortality in severe sepsis and septic shock. J Crit Care. 2017;37:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Siddiqui I, Jafri L, Abbas Q, Raheem A, Haque AU. Relationship of Serum Procalcitonin, C-reactive Protein, and Lactic Acid to Organ Failure and Outcome in Critically Ill Pediatric Population. Indian J Crit Care Med. 2018;22:91-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Ho KM. Combining sequential organ failure assessment (SOFA) score with acute physiology and chronic health evaluation (APACHE) II score to predict hospital mortality of critically ill patients. Anaesth Intensive Care. 2007;35:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, Pilcher DV; Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE). Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA. 2017;317:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 796] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 22. | Probst L, Schalk E, Liebregts T, Zeremski V, Tzalavras A, von Bergwelt-Baildon M, Hesse N, Prinz J, Vehreschild JJ, Shimabukuro-Vornhagen A, Eichenauer DA, Garcia Borrega J, Kochanek M, Böll B; Working Party on Intensive Care Medicine in Hematologic and Oncologic Patients (iCHOP) of the German Society of Hematology and Medical Oncology (DGHO). Prognostic accuracy of SOFA, qSOFA and SIRS criteria in hematological cancer patients: a retrospective multicenter study. J Intensive Care. 2019;7:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Ho KM, Lan NS. Combining quick Sequential Organ Failure Assessment with plasma lactate concentration is comparable to standard Sequential Organ Failure Assessment score in predicting mortality of patients with and without suspected infection. J Crit Care. 2017;38:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Liu Z, Meng Z, Li Y, Zhao J, Wu S, Gou S, Wu H. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with Sepsis. Scand J Trauma Resusc Emerg Med. 2019;27:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |