Published online Mar 27, 2021. doi: 10.4240/wjgs.v13.i3.222
Peer-review started: December 30, 2020
First decision: January 18, 2021
Revised: January 23, 2021
Accepted: March 10, 2021
Article in press: March 10, 2021
Published online: March 27, 2021
Processing time: 77 Days and 22.4 Hours
There is ample clinical evidence suggesting that the presence of large axial or paraesophageal hernias may lead to iron deficiency anemia. So-called Cameron lesions, as well as other small mucosa erosions, in the sliding area of these diaphragmatic hernias lead to invisible chronic blood loss and consequently to iron depletion. While the spectrum of symptoms in these patients is large, anemia is often not the only indication and typically not the primary indication for surgical correction of diaphragmatic hernias. Drug treatment with proton pump inhibitors and iron substitution can alleviate anemia, but this is not always successful. To exclude other possible bleeding sources in the gastrointestinal tract, a comprehensive diagnostic program is necessary and reviewed in this manuscript. Additionally, we discuss controversies in the surgical management of paraesophageal hernias.
Core Tip: Large axial or paraesophageal hernias may cause iron deficiency anemia, but the detailed mechanisms, necessary diagnostic procedures and therapeutic possibilities are not completely clear and have not been standardized. This review summarizes the knowledge regarding these aspects in an often-neglected cause of anemia, especially in older patients.
- Citation: Dietrich CG, Hübner D, Heise JW. Paraesophageal hernia and iron deficiency anemia: Mechanisms, diagnostics and therapy. World J Gastrointest Surg 2021; 13(3): 222-230
- URL: https://www.wjgnet.com/1948-9366/full/v13/i3/222.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i3.222
Iron deficiency anemia without acute bleeding signs is a common disease, especially in older patients[1]. Possible causes are iron malabsorption or subclinical chronic iron loss via occult bleeding. In older patients, chronic blood loss is the dominant cause[1], leading to depletion of iron stores. In many cases, small, barely macroscopically visible mucosal lesions lead to iron deficiency anemia via chronic erythrocyte loss over a very long period of time.
As early as 1929, 1933, and 1949 and in a large case series in 1967, a clinical connection between large hiatal hernias and iron deficiency anemia was established[2-5]. However, since a causal relationship is difficult to prove clinically, this pathogenesis for iron deficiency anemia has been repeatedly disputed[6]. In recent years, however, it has become increasingly clear that larger diaphragmatic hernias, resulting from intermittent incarceration and recurrent sliding movement in the diaphragmatic orifice, can lead to small mucosal lesions that can cause chronic iron deficiency anemia. Some of these lesions are visible during gastroscopy and then labeled Cameron lesions after their initial descriptor[7]. However, at the patient level, it remains in individual cases to assess the significance of these lesions in causing iron deficiency anemia, and there are still many cases of iron deficiency anemia where the importance of a hiatal hernia in the pathogenesis is not noticed in the workup[8]. Consequently, even comprehensive examinations of the gastrointestinal tract with exclusion of further bleeding lesions are not always sufficient. Clear evidence of the significance of these lesions and thus the significance of these hiatal hernias for the development of iron deficiency anemia often cannot be provided prospectively.
This review summarizes the data on the clinical significance of larger diaphragmatic hernias in the development and preservation of chronic iron deficiency anemia. It also provides a review of diagnostic tests required to confirm the significance of hiatal hernias for iron deficiency anemia. Finally, treatment options, including surgical procedures, are discussed.
For more than 50 years[4,5], numerous case reports and case series have indicated that larger hernias, especially paraesophageal hiatal hernias, are involved in the pathogenesis of chronic iron deficiency anemia. According to many studies, women are significantly more frequently affected[9-11]. A total of 6%-7% of patients with iron deficiency anemia show large hiatal hernias[12,13], indicating at least their partial involvement in causing anemia. In a study with an unselected cohort of patients with iron deficiency anemia, large hiatal hernias were claimed to be responsible for approximately 15% of cases of iron deficiency, especially in older patients[1]. Conversely, the prevalence of iron deficiency anemia in all patients with hiatal hernias is reported to be 8%-42%[14], with simple sliding hernias accounting for approximately 11% and paraesophageal hernias accounting for 30% in the first large case series[5]. However, this high prevalence of iron deficiency anemia is, depending on the study, also generated by the occasional presence of esophagitis in hiatal hernias[15].
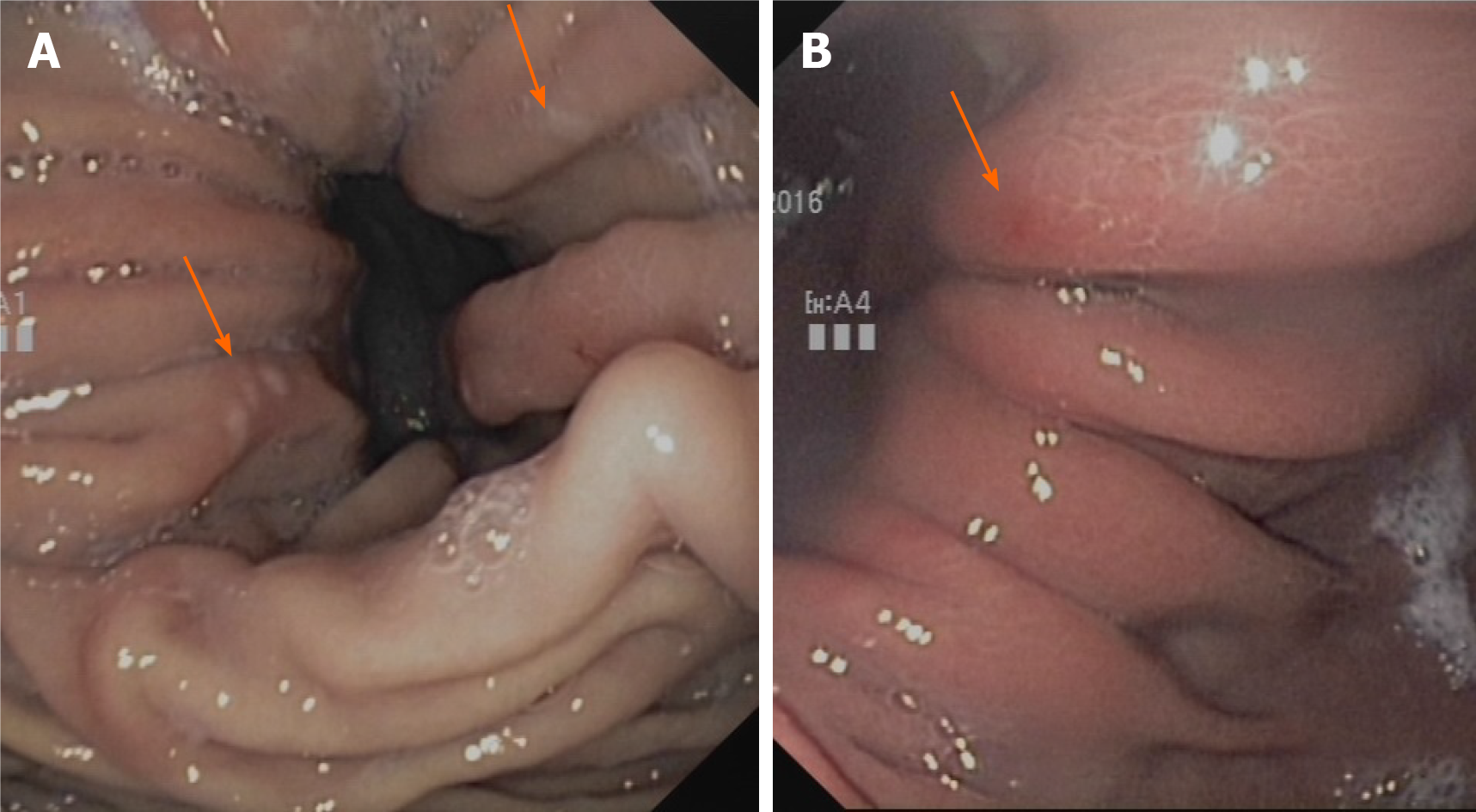
In the hiatal hernia itself, the so-called Cameron lesions are repeatedly referred to as macroscopic correlates of bleeding activity[16] (Figure 1). These lesions are often overlooked or misinterpreted on gastroscopy, hindering a clear estimate of their prevalence[17,18]. According to different studies, they are present in 5%-50%[7,19,20] of all hernias, occurring mainly in larger hernias (> 5 cm)[21]. Although there are case series reporting an association of Cameron lesions with overt bleeding[22], most studies indicate that the absence of such typical bleeding lesions does not exclude iron deficiency anemia and that there is no correlation with the extent of anemia or visible bleeding signs[9,10,20]. Therefore, hiatal hernias without such lesions must also be considered possible triggers of iron deficiency anemia. Anticoagulation therapy can increase the bleeding tendency even in cases of slight mucosal lesions. However, there have been no studies on the significance of oral anticoagulants in triggering iron deficiency in hiatal hernia patients. Our own clinical experience indicates that inhibition of platelet aggregation with ASA may play a major role in triggering chronic blood loss rather than inhibition of plasmatic coagulation with phenprocoumon or direct anticoagulants (own unpublished results).
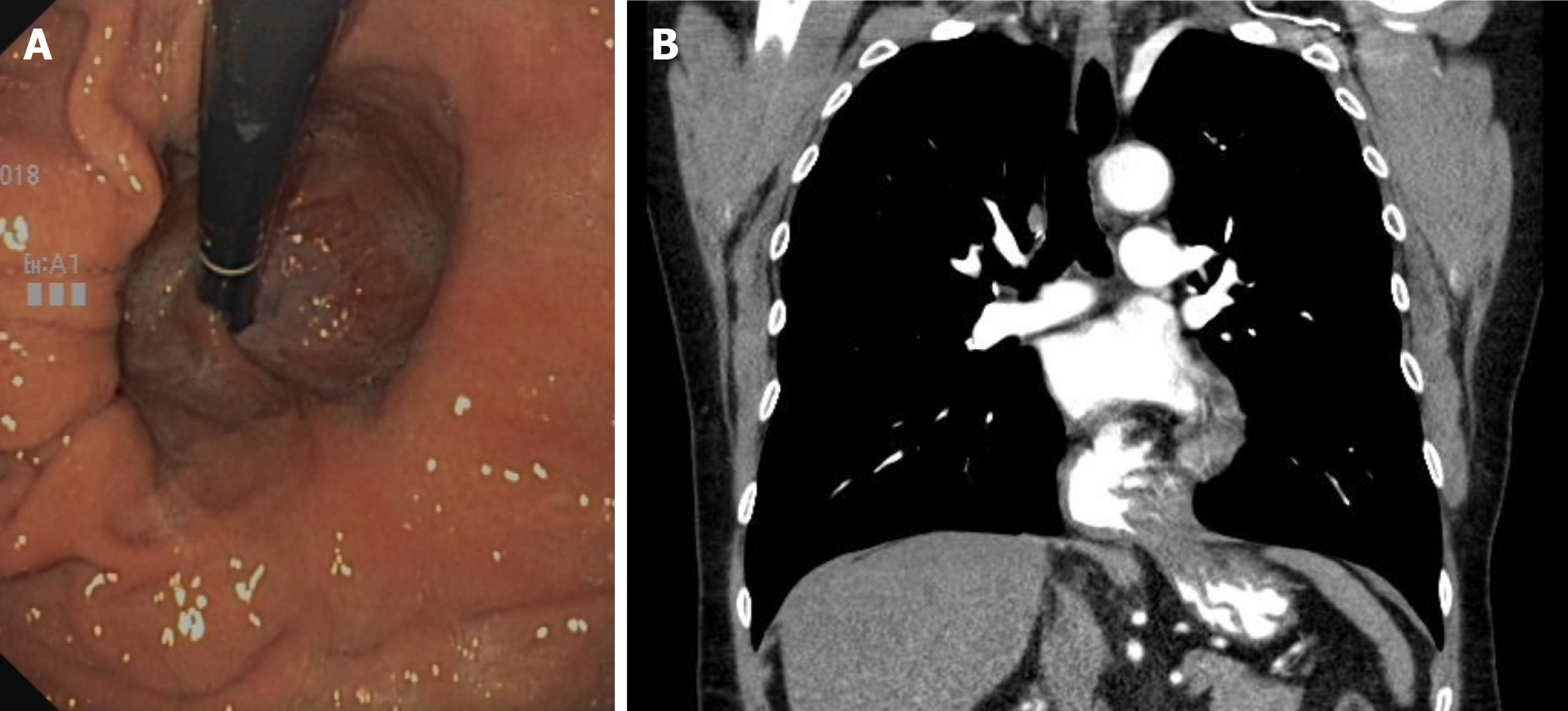
Case series suggest that the size and complexity of the diaphragmatic hernia correlates with the frequency and extent of anemia[5]. In general, a small axial hiatal hernia (type I, smaller than 3 cm) can probably be considered unproblematic in the development of anemia. However, larger sliding hernias, initially gastroscopically estimated as purely axial (type I), can lead to the presence of small lesions with chronic blood loss due to the recurrent sliding movement through the hernial orifice. In addition, a first-pass gastroscopic size and type estimation is not always reliable, and it is sometimes unclear whether a paraesophageal portion (type II or III) and thus a combined hernia is present (Figure 2). Depending on the clinical situation, additional diagnostic procedures may be useful (see “DIAGNOSTICS” section).
In cases of more complex hernias, surgical correction can lead to a cessation of iron deficiency anemia[11,23]. Studies with H2 antagonists more than 20 years ago[12] showed that gastric acid can also play an important role in hiatal hernias. The only prospective controlled study involved 21 patients and compared surgery and proton pump inhibitors (PPI) therapy (10 patients) to PPI therapy alone (11 patients). There were no differences in hemoglobin increase at the 1-year follow-up[9], so PPI therapy seemed to be equivalent to surgery. However, these data are not in line with the results of large retrospective studies. In a study with 96 analyzed surgical patients, a large proportion of whom had been treated preoperatively without success with PPI and iron substitution, there was a significant reduction in the need for PPI postoperatively without the addition of iron and a simultaneous resolution of anemia[23]. Obviously, only in a few cases is inhibition of gastric acid secretion sufficient to prevent chronic blood loss. For this reason, the diagnosis and the therapy that should be administered remain a highly individual decision for all patients and should be carefully reached jointly among the patient, the gastroenterologist and a surgeon experienced in this clinical picture, based on careful exclusion diagnostics of the entire gastrointestinal tract.
The initial history of the patient should take into account red flags for tumours such as recent unintentional weight loss or localized pain. To assess the significance of iron deficiency anemia, comprehensive laboratory diagnostics are important first. Ferritin should not be assessed as the sole marker of iron deficiency because ferritin is an acute phase marker and can therefore be falsely elevated in inflammatory conditions. Laboratory diagnostics assessing iron deficiency anemia should therefore always include the determination of C-reactive protein, transferrin saturation and reticulocytes. In individual cases, determination of the soluble transferrin receptor can also be helpful[14,24]. If there are no clear clinical signs of bleeding, only patients with laboratory-proven iron deficiency should be referred to an appropriate diagnostic procedure (Table 1).
| Methods | The specific content |
| Laboratory parameters | Hemoglobin, MCV, ferritin, iron, transferrin, transferrin saturation, soluble transferrin receptor, reticulocytes, vitamin B12, folic acid, IgA transglutaminase and total IgA. Iron absorption test. Determination of reticulocytes after iron administrationiFOBT |
| Endoscopy | Gastroscopy (push-Enteroscopy if necessary). Colonoscopy (including insight into terminal Ileum). Video capsule endoscopy. (enteroscopy only with abnormal capsule findings) |
| Radiology | Barium swallow. CT of thoracoabdominal junction with oral contrast medium (if necessary whole abdominal CT with intravenous contrast medium). Magnetic resonance imaging of the intestine only for certain symptoms |
| Functional investigations | 24-h pH-metry, urea breath test, High resolution manometry |
| Optional (only with appropriate symptoms) | Bronchoscopy, thoracic CT. Ear, nose, and throat examination up to panendoscopy of the nasal cavity |
Chronic iron deficiency anemia in elderly patients requires a comprehensive (bidirectional) endoscopic examination of the entire gastrointestinal tract[24]. First, an immunological Fecal Occult Blood Test can be considered for documenting chronic blood loss gastrointestinally. However, the value of this test is low in this case (intermittent bleeding activity is possible).
The basis of endoscopic diagnostics is the performance of gastroscopy and colonoscopy. Gastroscopy should be performed at least up to the band of Treitz and may include routine small bowel and gastric biopsies, to test for celiac disease or Helicobacter pylori, although this is controversial[24]. Testing for celiac disease or Helicobacter pylori can also be done in a non-invasive manner, as the American Gastroenterological Association recommends[24]. In addition, an iron absorption test may be necessary (with reticulocyte determination a few days later) to rule out malabsorption. A colonoscopy should always be performed up to the terminal ileum. A conventional barium swallow may be helpful in the assessment of hiatal hernia because functional imaging with contrast medium filling often measures the hernia to be significantly larger than in the mere visual assessment from gastroscopy[9].
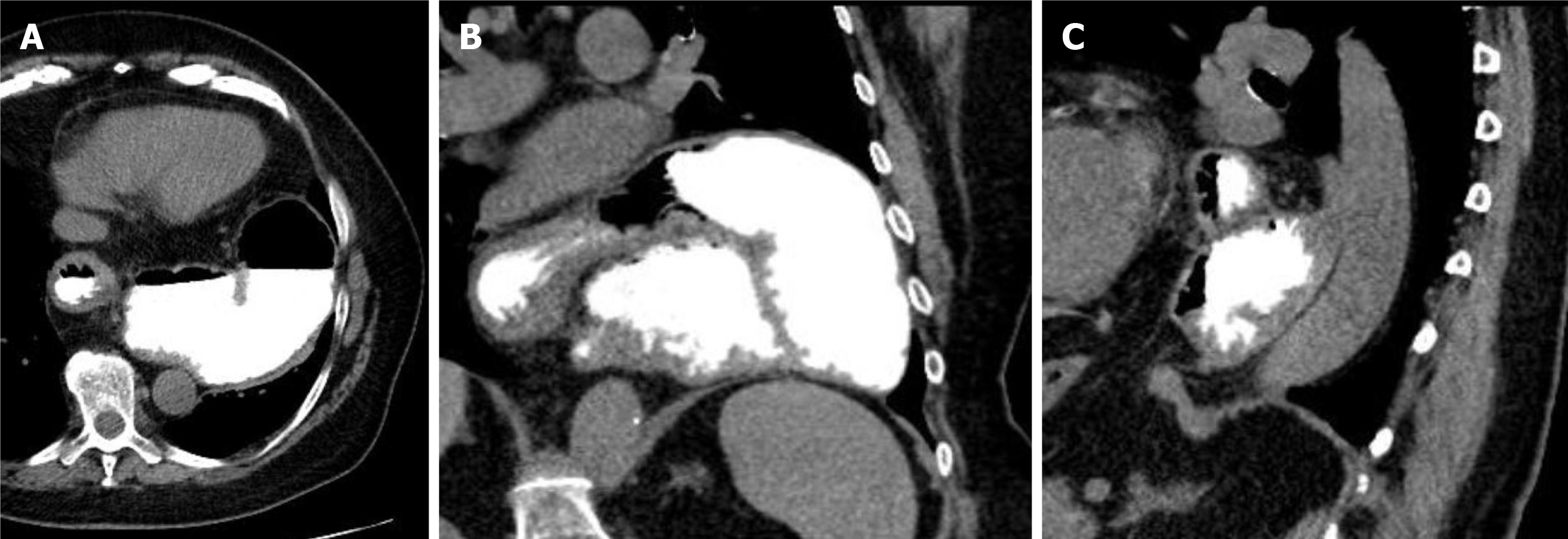
The most reliable imaging of the topography of a hiatal hernia is provided by computed tomography of the lower thorax and the upper abdomen. This can be performed natively; in cases of small or complexly shaped large hiatal hernias, oral administration of a reduced amount of contrast medium (1-2 beakers) is advantageous. This is the best way to image the three-dimensional extension of a paraesophageal hernia retrocardially with demarcation of the position, nature and size of the diaphragmatic passage. This examination thus not only provides information for an even greater understanding of the individual pathomechanism but also provides the surgeon with the most important information for precise risk assessment and planning of the intervention. If the entire abdomen is additionally visualized with intravenous contrast medium, this procedure can also help to further exclude other sources of bleeding (Figure 3).
There are different recommendations for the assessment of the small intestine. Due to the distribution of possible sources of bleeding in the small intestine, in some cases, a simple push enteroscopy of the small intestine may be appropriate as the best measure to exclude relevant bleeding lesions in the small intestine[25]. However, video capsule endoscopy is generally considered the standard for small bowel examination[26] and should be performed for a clear exclusion of bleeding sources other than the hiatal hernia[24,27]. Single- or double-balloon enteroscopy (or spiral enteroscopy) is not necessary if the capsule findings are inconspicuous. The importance of magnetic resonance imaging in special small bowel technology for the detection of bleeding sources can be considered rather low following the introduction of the video capsule[28]. However, with appropriate water filling, this examination leads to high sensitivity in the detection of stenoses, inflammatory mucosal sections or tumors in the small intestine and is not affected by anatomical peculiarities that may occasionally limit the sensitivity of endoscopic examinations, including capsule endoscopy. In the presence of certain clinical symptoms (e.g., postprandial abdominal pain), magnetic resonance imaging may therefore be the diagnostic imaging technique of choice even if chronic bleeding lesions are suspected.
Investigations of the nasopharynx, trachea and lungs are only necessary in the event of corresponding clinical symptoms or depending on the patient's medical history. Clinically inapparent bleeding from these areas is extremely rare.
To plan the operation and to exclude manifested motility disorders, which can lead to considerable swallowing difficulties postoperatively, it is recommended to perform 24-h pH-metry and high-resolution manometry preoperatively. Both the quantification of relevant acid reflux in pH measurement and the determination of inefficient esophageal motility (low distal contractility integral DCI) can influence the surgical strategy for hernia repair in individual cases (see also the following part of the manuscript).
The decision to surgically correct a paraesophageal hiatal hernia or an upside-down stomach is frequently made because of dysphagia and/or postprandial thoracic pain[29], not because of anemia. In quite a few cases, the thoracic stomach occupying the space above the diaphragm competes with the lung volume, leading to noticeable dyspnea[30] or compression of the left atrium or a pulmonary vein[31]. The question of whether a more or less pronounced paraesophageal hiatal hernia actually needs to be corrected surgically always depends on the individual symptoms, patient conditions and possible improvements after surgery[32]. Extensive paraesophageal hernias are often found incidentally in the course of an otherwise indicated thoracic computed tomography examination in elderly patients, where the clinical relevance is at least unclear. Of course, surgery does not necessarily have to be performed in such cases to prevent any unnecessary risk for the patient. However, the patient and his or her relatives should be appropriately informed about the possible symptoms and risks of the newly diagnosed paraesophageal hernia. However, if significant dysphagia, dyspnea and/or anemia exists and is not explained by other factors, surgery should be considered, and the risks of the intervention should be related to the patient's individual risk factors, such as age and cardiopulmonary conditions[32].
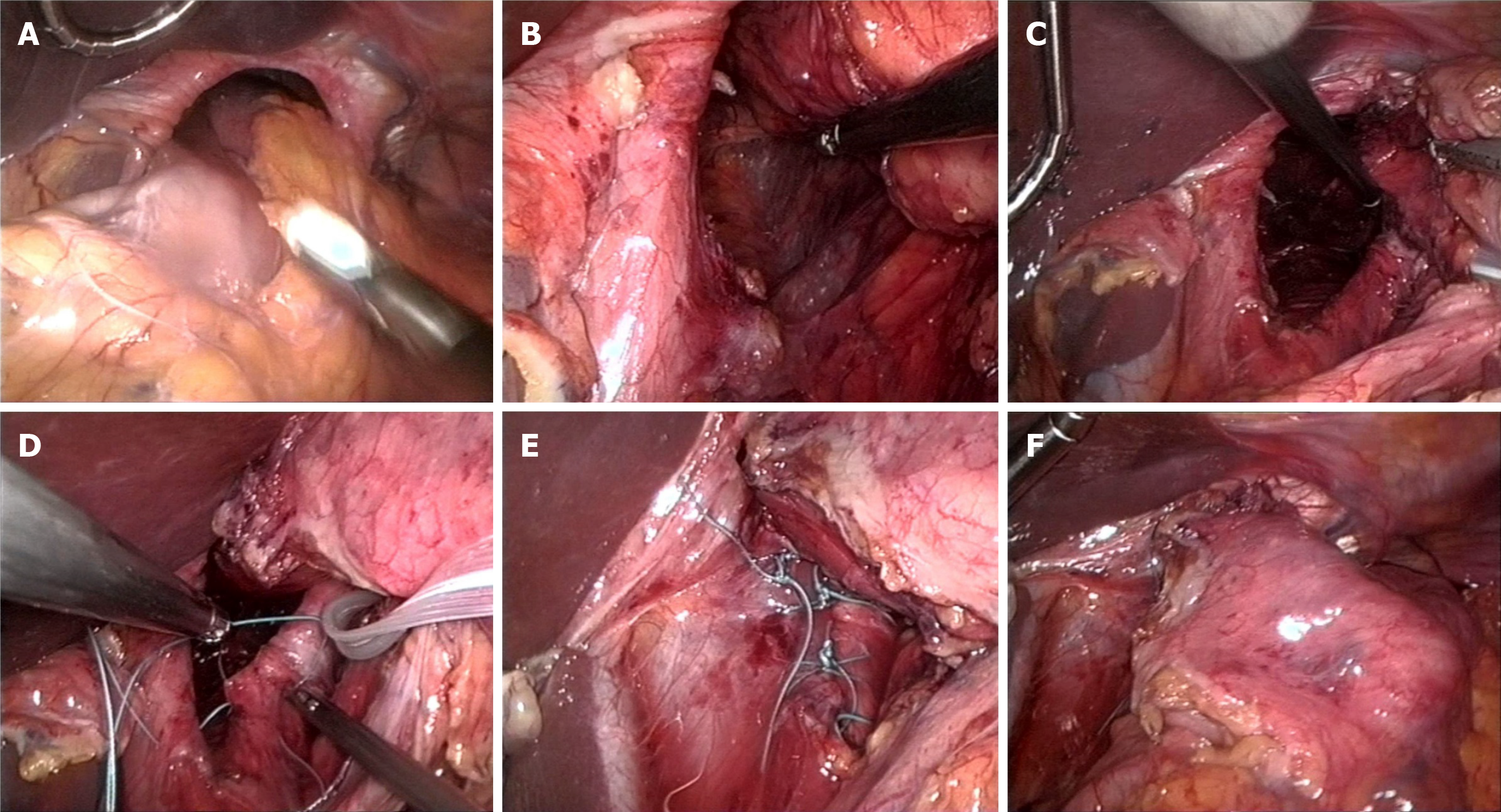
Currently, the procedure itself is almost entirely minimally invasive[33]. The laparoscopic approach in the posterior upper abdomen, moving transabdominally into the mediastinum, is much easier than the open approach. Conversion from laparoscopic to open repair is more complex, and often, a clear view of the operation field is lacking. In a beach-chair position, a capnoperitoneum is generated, and then a total of five 10 mm trocars are inserted, the first approximately 4-5 cm supraumbilical, the other 4 distributed evenly in a fan shape in the upper abdomen. From the right side, the left lobe of the liver is lifted with a suitable retractor so that the wide open hiatus esophagei can be seen. First, the stomach, which is often completely located in the thorax, is manipulated back into the abdomen with grasping forceps and pedicles as far as possible. Pulling the proximal stomach slightly downward to the left side, the minor omentum is opened, from which the edge of the right diaphragm is reached. Here, it is necessary to penetrate backwards into the mediastinum, partly with ultrasound scissors but mostly with blunt dissection, very close to the muscle structure. This must be achieved in a completely avascular layer, as, particularly, the large vessels near the stomach in the small gastric curve must be avoided at all costs. In this way, the anterior surface of the aorta can be reached. Here, we atraumatically dissect in an avascular layer to the left behind the esophagogastric junction. The entrance to the posterior mediastinum is then reached by pulling the stomach slightly downwards to the right and dissecting again close to the left diaphragm towards the aortic surface previously reached from the right (Figure 4).
Next, a plastic strap can be inserted from the right side and combined with clips in front of the stomach, forming a loop for further complete atraumatic manipulation of the organs, including the stomach and the esophagus, with both vagal trunks. In this way, the anterior part of the intrathoracic hernial sac can be dissected with the ultrasonic knife, and the esophagus can be released into the mediastinum. The posterior part of the hernial sac is then dissected completely to expose the dorsal parts of the diaphragm. This complete hernial sac dissection seems to be an important factor in minimizing recurrence[34], whereas the complete removal of the hernial sack from the thoracic cavity not only seems to be unnecessary but can also incur risks of bleeding and injury to the pleura. After 360-degree dissection at the hiatal level, the stomach must lie completely tension-free in the abdomen, and the esophagus should be circularly free. For dorsal closure of the wide diaphragmatic gap, we use 4 to 6 double-stitched nonabsorbable sutures. We advise against the use of mesh since it does not provide any clinical advantage[35] but can instead cause serious complications such as erosion into and stenosis of the esophagus[36]. Fundoplication has been controversially discussed in the context of the correction of paraesophageal hernia[37]. In our opinion, partial fundoplication (Toupet) might be helpful for patients with relevant acid reflux but is not necessary for anchoring the stomach below the diaphragm. Given the voluminous hernial sac collar, fundoplication is not always technically feasible at the level of the cardia. However, the most important factor in reducing the high risk of recurrence (up to 40%[35]) is careful gastrophrenicopexy, preferably by means of two double-stitched sutures each on the right and left side between the cardia and His's angle and the corresponding diaphragmatic edges. After removal of the holding loop, the procedure is finished. The patient can begin drink in the evening, and after checking the position and tightness of the stomach by swallowing contrast medium, the patient can start eating the next day, initially with soft food.
The etiologic significance of a large hiatal hernia in iron deficiency anemia can be regarded as basically certain. It remains unclear why many patients develop anemia, while others with similar anatomical or clinical conditions of hiatal hernia do not. The role of platelet aggregation, oral anticoagulation or other risk factors in this process has not been sufficiently investigated.
Study data also suggest that not all patients benefit equally from surgery. In one study, an analysis of surgical results showed that in particular, women and patients < 70 years of age experienced resolution of anemia after surgery, while postoperative acid blockade with H2 antagonists or PPIs or the preoperative presence of Cameron lesions played no role[10]. This suggests further minimal bleeding sources in some, particularly male and elderly patients, for whom the bleeding may be more distally located in the gastrointestinal tract.
The indication for surgical hernia reduction is always easy to establish when there are clearly attributable symptoms such as dysphagia. In our opinion, existing iron deficiency anemia alone can be an indication for surgical hernia correction, especially in the following situations: (1) The morphological characteristics of the hiatal hernia (size, paraesophageal portion, etc.) suggest a causal role of the hernia in the anemia (Cameron lesions, however, are not obligatory); (2) PPI therapy and iron substitution cannot control the anemia or are not feasible in the long run; (3) Other sources of bleeding have been largely excluded; and (4) No functional deterioration is expected after surgery.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morozov S S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Vannella L, Aloe Spiriti MA, Di Giulio E, Lahner E, Corleto VD, Monarca B, Delle Fave G, Annibale B. Upper and lower gastrointestinal causes of iron deficiency anemia in elderly compared with adult outpatients. Minerva Gastroenterol Dietol. 2010;56:397-404. [PubMed] |
| 2. | Cabot RC, Painter FM. Case 15022: Treatment in an Obscure Gastric Case. N Engl J Med. 1929;200:88-92. [DOI] [Full Text] |
| 3. | Bock AV, Dulin JW, Brooke PA. Diaphragmatic Hernia and Secondary Anemia; Ten Cases. N Engl J Med. 1933;209:615-625. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Schwartz SO, Blumenthal SA. Diaphragmatic hiatus hernia with sever iron-deficient anemia. Am J Med. 1949;7:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Windsor CW, Collis JL. Anaemia and hiatus hernia: experience in 450 patients. Thorax. 1967;22:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Sayer JM, Long RG. A perspective on iron deficiency anaemia. Gut. 1993;34:1297-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Cameron AJ, Higgins JA. Linear gastric erosion. A lesion associated with large diaphragmatic hernia and chronic blood loss anemia. Gastroenterology. 1986;91:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 74] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Skipworth RJ, Staerkle RF, Leibman S, Smith GS. Transfusion-Dependent Anaemia: An Overlooked Complication of Paraoesophageal Hernias. Int Sch Res Notices. 2014;2014:479240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Panzuto F, Di Giulio E, Capurso G, Baccini F, D'Ambra G, Delle Fave G, Annibale B. Large hiatal hernia in patients with iron deficiency anaemia: a prospective study on prevalence and treatment. Aliment Pharmacol Ther. 2004;19:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Carrott PW, Markar SR, Hong J, Kuppusamy MK, Koehler RP, Low DE. Iron-deficiency anemia is a common presenting issue with giant paraesophageal hernia and resolves following repair. J Gastrointest Surg. 2013;17:858-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Cheverie JN, Lam J, Neki K, Broderick RC, Lee AM, Matsuzaki T, Cubas R, Sandler BJ, Jacobsen GR, Fuchs KH, Horgan S. Paraesophageal hernia repair: a curative consideration for chronic anemia? Surg Endosc. 2020;34:2243-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Moskovitz M, Fadden R, Min T, Jansma D, Gavaler J. Large hiatal hernias, anemia, and linear gastric erosion: studies of etiology and medical therapy. Am J Gastroenterol. 1992;87:622-626. [PubMed] |
| 13. | Annibale B, Capurso G, Chistolini A, D'Ambra G, DiGiulio E, Monarca B, DelleFave G. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am J Med. 2001;111:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Stein J, Connor S, Virgin G, Ong DE, Pereyra L. Anemia and iron deficiency in gastrointestinal and liver conditions. World J Gastroenterol. 2016;22:7908-7925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (6)] |
| 15. | Ruhl CE, Everhart JE. Relationship of iron-deficiency anemia with esophagitis and hiatal hernia: hospital findings from a prospective, population-based study. Am J Gastroenterol. 2001;96:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kimer N, Schmidt PN, Krag A. Cameron lesions: an often overlooked cause of iron deficiency anaemia in patients with large hiatal hernias. BMJ Case Rep. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Zullo A, Manta R, De Francesco V, Fiorini G, Lahner E, Vaira D, Annibale B. Cameron lesions: A still overlooked diagnosis. Case report and systematic review of literature. Clin Res Hepatol Gastroenterol. 2018;42:604-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Mehershahi S, Jog A, Ronderos DM, Shaikh D, Ihimoyan A. Cameron Ulcers: Rare Case of Overt Upper Gastrointestinal Bleed in a Patient with Alcohol Use Disorder. Cureus. 2020;12:e7644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 19. | Weston AP. Hiatal hernia with cameron ulcers and erosions. Gastrointest Endosc Clin N Am. 1996;6:671-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Pauwelyn KA, Verhamme M. Large hiatal hernia and iron deficiency anaemia: clinico-endoscopical findings. Acta Clin Belg. 2005;60:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Maganty K, Smith RL. Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;77:214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Yakut M, Kabaçam G, Öztürk A, Soykan I. Clinical characteristics and evaluation of patients with large hiatal hernia and Cameron lesions. South Med J. 2011;104:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Addo A, Broda A, Reza Zahiri H, Brooks IM, Park A. Resolution of anemia and improved quality of life following laparoscopic hiatal hernia repair. Surg Endosc. 2020;34:3072-3078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Ko CW, Siddique SM, Patel A, Harris A, Sultan S, Altayar O, Falck-Ytter Y. AGA Clinical Practice Guidelines on the Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology. 2020;159:1085-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 25. | De Leusse A, Landi B, Edery J, Burtin P, Lecomte T, Seksik P, Bloch F, Jian R, Cellier C. Video capsule endoscopy for investigation of obscure gastrointestinal bleeding: feasibility, results, and interobserver agreement. Endoscopy. 2005;37:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Gerson LB, Fidler JL, Cave DR, Leighton JA. ACG Clinical Guideline: Diagnosis and Management of Small Bowel Bleeding. Am J Gastroenterol. 2015;110:1265-87; quiz 1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 448] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 27. | Annibale B, Capurso G, Baccini F, Lahner E, D'Ambra G, Di Giulio E, Delle Fave G. Role of small bowel investigation in iron deficiency anaemia after negative endoscopic/histologic evaluation of the upper and lower gastrointestinal tract. Dig Liver Dis. 2003;35:784-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Wiarda BM, Heine DG, Mensink P, Stolk M, Dees J, Hazenberg HJ, Stoker J, Kuipers EJ. Comparison of magnetic resonance enteroclysis and capsule endoscopy with balloon-assisted enteroscopy in patients with obscure gastrointestinal bleeding. Endoscopy. 2012;44:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Maziak DE, Todd TR, Pearson FG. Massive hiatus hernia: evaluation and surgical management. J Thorac Cardiovasc Surg. 1998;115:53-60; discussion 61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 159] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Carrott PW, Hong J, Kuppusamy M, Kirtland S, Koehler RP, Low DE. Repair of giant paraesophageal hernias routinely produces improvement in respiratory function. J Thorac Cardiovasc Surg. 2012;143:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Naoum C, Falk GL, Ng AC, Lu T, Ridley L, Ing AJ, Kritharides L, Yiannikas J. Left atrial compression and the mechanism of exercise impairment in patients with a large hiatal hernia. J Am Coll Cardiol. 2011;58:1624-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Carrott PW, Hong J, Kuppusamy M, Koehler RP, Low DE. Clinical ramifications of giant paraesophageal hernias are underappreciated: making the case for routine surgical repair. Ann Thorac Surg. 2012;94:421-6; discussion 426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Hayden JD, Jamieson GG. Effect on iron deficiency anemia of laparoscopic repair of large paraesophageal hernias. Dis Esophagus. 2005;18:329-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Watson DI, Davies N, Devitt PG, Jamieson GG. Importance of dissection of the hernial sac in laparoscopic surgery for large hiatal hernias. Arch Surg. 1999;134:1069-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Watson DI, Thompson SK, Devitt PG, Aly A, Irvine T, Woods SD, Gan S, Game PA, Jamieson GG. Five Year Follow-up of a Randomized Controlled Trial of Laparoscopic Repair of Very Large Hiatus Hernia With Sutures Versus Absorbable Versus Nonabsorbable Mesh. Ann Surg. 2020;272:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 36. | Tatum RP, Shalhub S, Oelschlager BK, Pellegrini CA. Complications of PTFE mesh at the diaphragmatic hiatus. J Gastrointest Surg. 2008;12:953-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Andolfi C, Plana A, Furno S, Fisichella PM. Paraesophageal Hernia and Reflux Prevention: Is One Fundoplication Better than the Other? World J Surg. 2017;41:2573-2582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |