Published online Feb 27, 2021. doi: 10.4240/wjgs.v13.i2.153
Peer-review started: November 16, 2020
First decision: December 20, 2020
Revised: December 26, 2020
Accepted: January 14, 2021
Article in press: January 14, 2021
Published online: February 27, 2021
Processing time: 79 Days and 23.4 Hours
Preoperative portal vein embolization (PVE) is a widely used strategy to enable major hepatectomy in patients with insufficient liver remnant. PVE induces hypertrophy of the future liver remnant (FLR) and a shift of the functional reserve to the FLR. However, whether the increase of the FLR volume (FLRV) corresponds to the functional transition after PVE remains unclear.
To investigate the sequential relationship between the increase in FLRV and functional transition after preoperative PVE using 3-dimensional (3D) computed tomography (CT) and 99mTc-galactosyl-human serum albumin (99mTc-GSA) single-photon emission computed tomography (SPECT) fusion images.
Thirty-three patients who underwent major hepatectomy following PVE at the Department of Gastroenterological Surgery I, Hokkaido University Hospital between October 2013 and March 2018 were enrolled. Three-phase dynamic multidetector CT and 99mTc-GSA SPECT scintigraphy were performed at pre-PVE, and at 1 and 2 wk after PVE; 3D 99mTc-GSA SPECT CT-fused images were constructed from the Digital Imaging and Communications in Medicine data using 3D image analysis system. Functional FLRV (FFLRV) was defined as the total liver volume × (FLR volume counts/total liver volume counts) on the 3D 99mTc-GSA SPECT CT-fused images. The calculated FFLRV was compared with FLRV.
FFLRV increased by a significantly larger extent than FLRV at 1 and 2 wk after PVE (P < 0.01). The increase in FFLRV and FLRV was 55.1% ± 41.6% and 26.7% ± 17.8% (P < 0.001), respectively, at 1 wk after PVE, and 64.2% ± 33.3% and 36.8% ± 18.9% (P < 0.001), respectively, at 2 wk after PVE. In 3 of the 33 patients, FFLRV levels decreased below FLRV at 2 wk. One of the three patients showed rapidly progressive fatty changes in FLR. The biopsy at 4 wk after PVE showed macro- and micro-vesicular steatosis of more than 40%, which improved to 10%. Radical resection was performed at 13 wk after PVE. The patient recovered uneventfully without any symptoms of pos-toperative liver failure.
The functional transition lagged behind the increase in FLRV after PVE in some cases. Evaluating both volume and function is needed to determine the optimal timing of hepatectomy after PVE.
Core Tip: Preoperative portal vein embolization (PVE) induces hypertrophy of the future liver remnant (FLR) and a shift of the functional reserve to the FLR. However, whether the increase in FLR volume (FLRV) corresponds to the functional transition after PVE remains unclear. We investigated the sequential relationship between the increase in the FLRV and the functional transition after preoperative PVE. The functional transition lagged behind the increase in FLRV after PVE in 3 of the 33 cases. Evaluating both volume and function is needed to determine the optimal timing of hepatectomy after PVE.
- Citation: Tsuruga Y, Kamiyama T, Kamachi H, Orimo T, Shimada S, Nagatsu A, Asahi Y, Sakamoto Y, Kakisaka T, Taketomi A. Functional transition: Inconsistently parallel to the increase in future liver remnant volume after preoperative portal vein embolization. World J Gastrointest Surg 2021; 13(2): 153-163
- URL: https://www.wjgnet.com/1948-9366/full/v13/i2/153.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i2.153
Liver resection is often the only option for long-term survival of patients with primary or secondary liver cancer. The mortality rate after major liver resection has been reported to be 2.0%-6.8%, which is mainly due to postoperative liver failure[1-3]. Small future liver remnant volume (FLRV) is a predictor of perioperative morbidity and mortality[4]. For patients with a normal liver, a remnant volume of more than 20%-40% of the total liver or standardized liver volumes has been proposed as the threshold of surgical safety[5-9].
Preoperative portal vein embolization (PVE) is widely used to enable major liver resection with insufficient liver remnant[10-13]. PVE induces hypertrophy of the future liver remnant (FLR) and a shift of the functional reserve to FLR[14,15]. However, whether the increase of the FLRV corresponds to the functional transition after PVE remains unclear.
99mTc-galactosyl-human serum albumin (99mTc-GSA) was developed as a liver scintigraphy agent that binds to the asialoglycoprotein receptor on hepatocytes[16].
In the present study, we investigated the sequential relationship between the increase in FLRV and the functional transition after preoperative PVE using 3D CT/99mTc-GSA SPECT fusion imaging to develop a strategy for preventing fatal liver failure after major hepatectomy.
We retrospectively analyzed the data of 33 patients who underwent major hepatectomy following PVE at the Department of Gastroenterological Surgery I, Hokkaido University Hospital between October 2013 and March 2018. This study was reviewed and approved by the Institutional Review Board of Hokkaido University Hospital for Clinical Research (approval number: 018-0263), which waived the need for written informed consent due to the retrospective design of the study. The baseline characteristics of the patients are shown in Table 1. At our institution, preoperative PVE is generally performed for patients with a PHRR of > 60%[19]. All patients underwent a three-phase dynamic multidetector CT scan and 99mTc-GSA scintigraphy at pre-PVE, and at 1 and 2 wk after PVE.
| Characteristics | n |
| Age (yr), mean (range) | 67.7 (40-80) |
| Men/women | 20/13 |
| HBsAg positivity, n (%) | 3 (9.0) |
| HCV positivity, n (%) | 2 (6.1) |
| Diagnosis, n (%) | |
| Hilar cholangiocarcinoma | 20 (60.6) |
| Hepatoma | 6 (18.2) |
| Gallbladder cancer | 3 (9.1) |
| Intrahepatic cholangiocarcinoma | 2 (6.1) |
| Metastatic tumor | 2 (6.1) |
| Child-Pugh score (5/6/7/8), n | 23/7/1/2 |
| Child-Pugh classification (A/B), n | 30/3 |
| ICGR15 (%), mean (range) | 10.1 (1.5-33.2) |
| Preoperative biliary drainage, n (%) | 17 (51.5) |
| Initial resection ratio (%), mean ± SD | 64.5 ± 5.48 |
| Initial CT volume of FLR (mL), mean ± SD | 410.9 ± 79.4 |
| Average time between PVE and operation, d (range) | 30.0 (15-94) |
| Type of hepatectomy, n (%) | |
| Right hepatectomy + caudal lobectomy | 19 (57.6) |
| Right hepatectomy | 9 (27.3) |
| Left trisectionectomy + caudal lobectomy | 4 (12.1) |
| Left hepatectomy + caudal lobectomy | 1 (3.0) |
| Biliary reconstruction, n (%) | 24 (72.7) |
The ipsilateral approach was routinely used, with the contralateral approach reserved for patients for whom the ipsilateral approach was judged to be unsuitable. Ethanol is used as the embolizing agent at our institution[20]. The intrahepatic portal vein was punctured under sonographic guidance. A guidewire was inserted into the portal vein through the needle, followed by the introduction of a 5.5-French sheath introducer. Balloon occlusion was performed, and contrast material was injected until the targeted portal branches were enhanced. The balloon was then deflated, and an equal amount (equivalent to the previously injected contrast material) of 0.5% lidocaine was injected. Finally, balloon occlusion was repeated, and an equal amount of ethanol (equivalent to the previously injected contrast material or 0.5% lidocaine) was injected. The balloon was deflated after 5 min, and the complete embolization of targeted vessels was determined by test portography through a manual injection of contrast medium. Subsequently, for incomplete embolization, the ethanol injections were repeated in the same way. Finally, the 5.5-French sheath was extracted by packing the puncture tract with gelatin sponge torpedoes.
An algorithm (Hokkaido University Algorithm) incorporating the indocyanine green retention at 15 min and FLRV is generally used to determine the nature of sectionectomy required, e.g., bisectionectomy[19]. The timing of the operation after PVE was generally determined by a PHRR decrease of less than 60% at least two weeks after PVE.
Three-phase dynamic CT scan was performed with a 320-row multidetector device (Aquilion ONE; Toshiba Medical Systems Co., Otawara, Japan). The obtained Digital Imaging and Communications in Medicine (DICOM) data were imported to the 3D image analysis system (Volume Analyzer SYNAPSE VINCENT; Fuji Film Medical, Tokyo, Japan)[21]. Three-dimensional images were reconstructed from the DICOM data.
99mTc-GSA scintigraphy was performed separately from CT. Dynamic scanning was initially performed using a large-field view gamma camera (E.CAM; Siemens, Tokyo, Japan) in an anterior view, equipped with a low-energy high-resolution collimator, with the patient in a supine position after a bolus intravenous injection of 185 MBq of 99mTc-GSA. Dynamic planar images were obtained for 30 min by 147 serial frames (60 × 1 s, 87 × 20 s), with a matrix size of 128 × 128. Hepatic SPECT images were acquired after the dynamic study. The DICOM data obtained from SPECT were also imported to the SYNAPSE VINCENT and subsequently fused with the 3D CT images. Functional FLRV (FFLRV) was calculated using the following formula: FFLRV (mL) = [(total liver volume counts - resection volume counts)/total liver volume counts) × total liver volume] (mL).
Hounsfield unit attenuation values of the FLR and spleen at 10 regions of interest were obtained using unenhanced CT, and the average values were calculated. The regions were selected while taking care to avoid the vessels. The ratio of liver to spleen attenuation was then calculated.
The statistical analyses were performed using EZR software, version 1.36 (Saitama Medical Center, Jichi Medical University, Saitama, Japan)[22]. The Wilcoxon signed rank test was used for comparing FFLRV and FLRV.
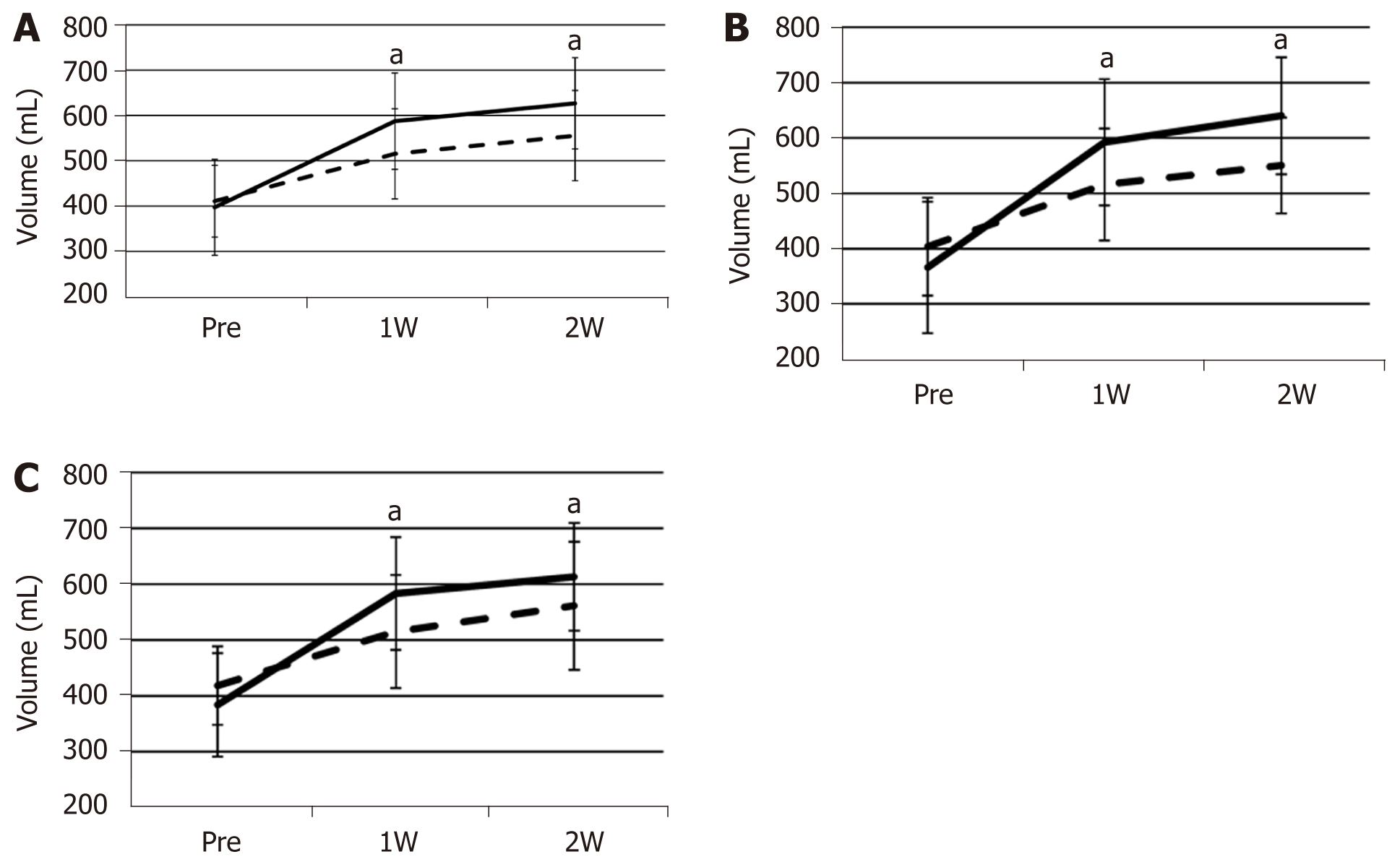
FFLRV increased by a significantly larger extent than FLRV at 1 and 2 wk after PVE (Figure 1A). The FFLRV and FLRV were 55.1% ± 41.6% and 26.7% ± 17.8% (P < 0.001), respectively, at 1 wk after PVE, and 64.2% ± 33.3% and 36.8% ± 18.9% (P < 0.001), respectively, at 2 wk after PVE. We also compared FLRV and FFLRV between the groups with and without preoperative biliary drainage. However, no significant differences were observed between these two groups (Table 2). The similar tendency of the sequential increase in FLRV and FFLRV after PVE was observed in these two groups (Figure 1B and C).
| Group with preoperative biliary | Group without preoperative biliary | P value | |
| ICGR15 (%), mean ± SD | 9.09 ± 5.0 | 11.2 ± 7.2 | 0.28 |
| Initial resection ratio (%), mean ± SD | 64.6 ± 6.36 | 64.4 ± 4.57 | 0.692 |
| Initial FLRV (mL), mean ± SD | 403.8 ± 88.6 | 418.4 ± 70.3 | 0.402 |
| Initial FFLRV (mL), mean ± SD | 366.4 ± 118.7 | 384.0 ± 92.7 | 0.601 |
| FLRV at 1 wk (mL), mean ± SD | 515.7 ± 100.9 | 515.0 ± 101.1 | 0.914 |
| FFLRV at 1 wk (mL), mean ± SD | 592 ± 114.0 | 583.0 ± 101.0 | 0.986 |
| FLRV at 2 wk (mL), mean ± SD | 550.1 ± 86.6 | 561.1 ± 114.5 | 0.958 |
| FFLRV at 2 wk (mL), mean ± SD | 640.0 ± 105.8 | 613.0 ± 96.6 | 0.382 |
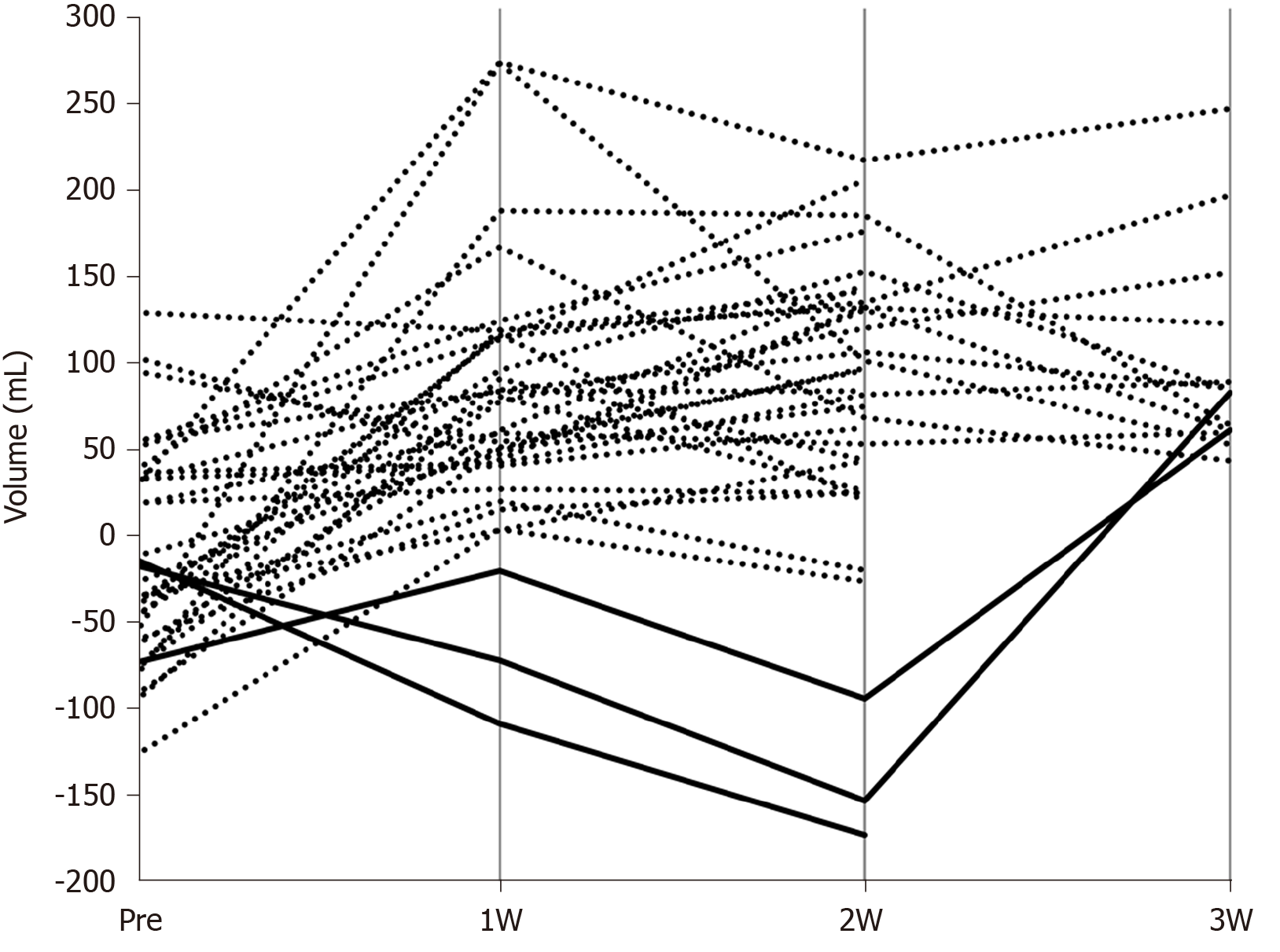
We calculated the difference by subtracting the FLRV values from FFLRV values of all patients before and after PVE (Figure 2). The results after PVE were almost entirely positive, while in 3 patients, the FFLRV became lower than the FLRV at 2 wk after PVE. FFLRV caught up with FLRV at 3 wk after PVE in 2 of these 3 patients. Subsequently, all the patients were classified into two groups: FLRV superior group included those in whom FFLRV decreased below the FLRV at 2 wk after PVE and the FFLRV superior group included the remaining patients. No significant differences were observed when the background factors were compared between these two groups (Table 3).
| FFLRV superior group (n = 30) | FLRV superior group (n = 3) | |
| Age (yr), mean ± SD | 67.4 ± 9.1 | 69.7 ± 9.5 |
| Gender | ||
| Male, n (%) | 18 (60.0) | 2 (66.7) |
| Female, n (%) | 12 (40.0) | 1 (33.3) |
| Body mass index (kg/m2), mean ± SD | 21.8 ± 3.5 | 23.4 ± 7.0 |
| Comorbidity (n) | Diabetes (5), hypertension (3), dyslipidemia (1), COPD (1), alcoholism (1), angina (1), lacunar infarction (1) | Diabetes (1), fatty liver (1), alcoholism (1) |
| HBV/HCV infection (n) | 3/1 | 0/1 |
| Child-Pugh score, mean ± SD | 5.4 ± 0.9 | 5.7 ± 0.6 |
| ICGR15 (%), mean ± SD | 10.4 ± 6.3 | 7.2 ± 2.0 |
| Initial resection ratio (%), mean ± SD | 64.3 ± 5.6 | 66.8 ± 4.4 |
| Initial FLRV (mL), mean ± SD | 405.4 ± 79.0 | 466.3 ± 73.2 |
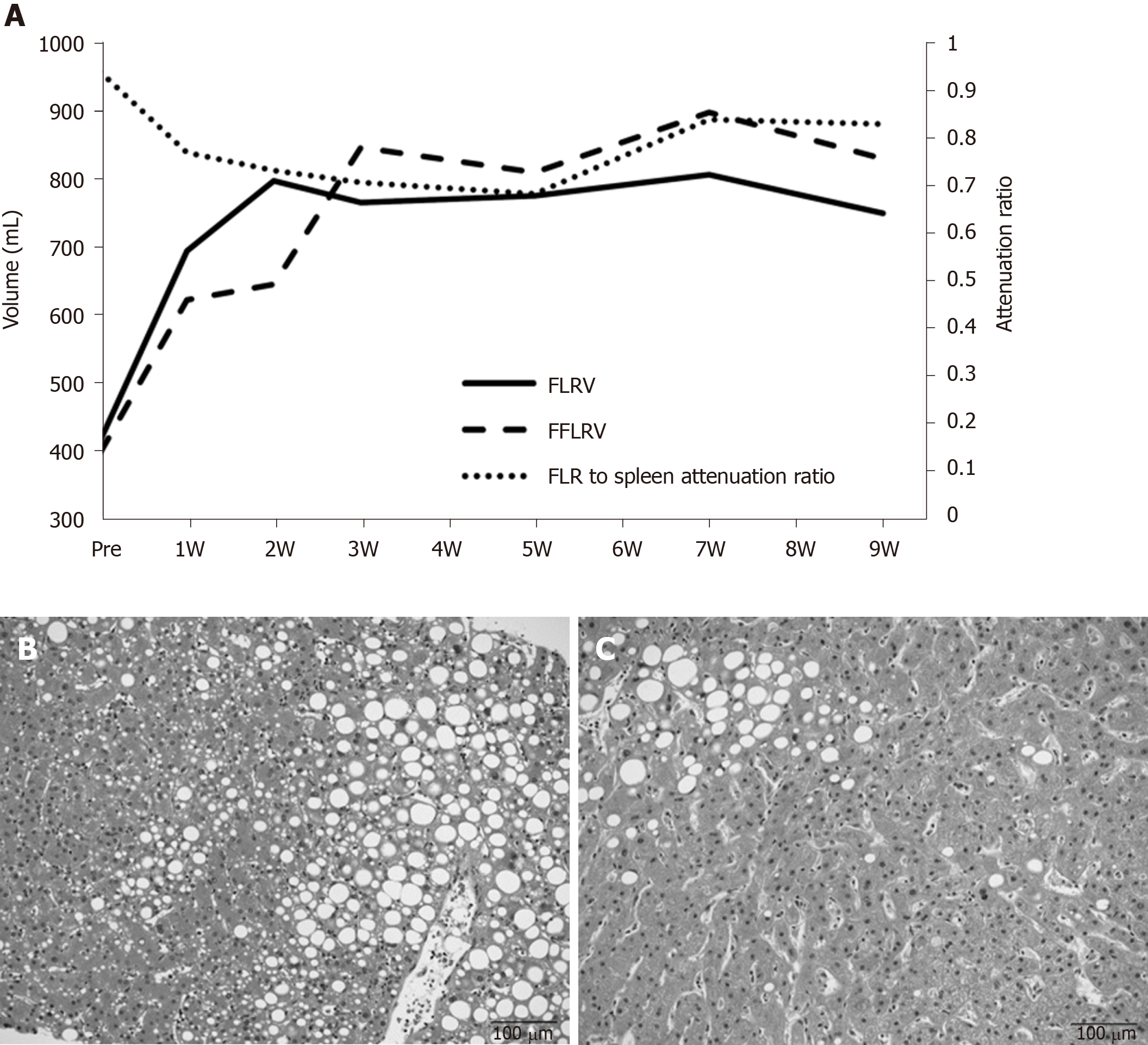
A 77-year-old woman was diagnosed with hilar cholangiocarcinoma. Right hepatectomy and caudal lobectomy were planned. PVE was performed because the initial resection ratio was 71.3%. FLRV increased from 414 mL to 796 mL at two weeks after PVE; however, the change in function lagged behind the increase in FLRV for 3 wk (Figure 3A). The decrease in the FLR to spleen CT attenuation ratio at up to 5 wk after PVE indicated fatty changes in the FLR. Biopsy at 4 wk after PVE showed macro- and micro-vesicular steatosis of more than 40% (Figure 3B). FLRV on CT was sufficient to proceed with resection, although we postponed the operation until the FLR to spleen CT attenuation ratio recovered. Pioglitazone was administered for the treatment of nonalcoholic fatty liver disease. The CT attenuation ratio recovered at 7 wk after PVE, and radical liver resection was successfully performed at 13 wk. Intraoperative biopsy showed that the steatosis had improved to approximately 10% (Figure 3C). The patient recovered uneventfully without any symptoms of postoperative liver failure.
In the present study investigating the sequential relationship between the increase in FLRV and the functional changes after preoperative PVE, we found that in almost all cases, the increase in FLRV lagged behind that of the FFLRV. However, several cases of marked lagging of the FFLRV behind the increase in FLRV within the first 2 wk were observed.
Embolized lobe atrophy and FLR hypertrophy occur after PVE[12]. A meta-analysis reported the mean relative rate of hypertrophy of FLR to be 43.1%[23]. In this study, the increase in FLRV at 2 wk was 36.8% ± 18.9%, which is roughly in accordance with the previously published mean value. The increase in FFLRV at 2 wk (64.2% ± 33.3%) was greater than that of the FLRV. Beppu et al[14] also reported the marked increase in FLR after PVE using 99mTc-GSA scintigraphy SPECT-CT fusion data. They reported that the percentage increase in FLRV after PVE was greater than that of the non-tumorous remnant liver volume. Moreover, the increased portal flow, heat shock protein (HSP), ATP (adenosine triphosphate) concentrations, and DNA synthesis in the non-embolized liver after PVE may be related to the mechanism of hyper-function of the FLR[24]. Functional transition evidently occurs after PVE because of the hypofunction of the embolized lobe and hyperfunction of the FLR.
Moreover, as for the sequential relationship between FLRV increases and FFLRV, 3 patients experienced a marked decrease in FFLRV to levels below the FLRV at 2 wk after PVE. While these patients did not share obvious similarities, rapidly progressive fatty changes in FLR were observed after PVE in 1 patient. Tsai et al[25] reported the first case of acute non-alcoholic fatty change in the liver after PVE, suggesting a relationship between hemodynamic changes after PVE and fatty changes. A time-dependent fatty change in FLR was proven by biopsy in our study. There is a possible hypothesis on the mechanism underlying these fatty changes. Miyake et al[26] reported that HSP70 Levels after right PVE were 2- to 4-fold higher in the non-embolized lobe than in the embolized lobe in 4 of 5 patients[24,26]. The patient who did not experience this marked increase ultimately died of hepatic failure after extended right hepatic lobectomy. Archer et al[27] also showed that the decrease in HSP72 by siHSP72 Leads to lipid accumulation in the primary mouse hepatocytes. Thus, inhibition of HSP70 induction in the FLR after PVE may lead to fatty changes in the FLR and delay functional transition.
In contrast, it was demonstrated that liver steatosis quantified with gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid -enhanced liver MRI was associated with an impaired growth of FLR after portal vein occlusion in clinical cases[28], and steatotic rats fed with a methionine-choline-deficient diet demonstrated impaired liver generation and less FLR function compared to control rats after portal vein ligation[29].
Increase in FLRV has generally only been used to determine the timing of hepatectomy after PVE; however, the risk of postoperative liver failure remains in some cases due to the functional transition lagging behind the increase in FLRV after PVE. However, in most cases, the increase in FFLRV is larger than that of FLRV after PVE. Therefore, both FLRV and FFLRV must be carefully considered when hepatectomy is performed. Furthermore, besides CT volumetry, evaluation of function with GSA is required.
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) enables rapid and extensive hypertrophy of the remnant liver[30]. However, higher morbidity and mortality rates have been reported compared to the conventional methods of volume enhancement[31]. Olthof et al[32] reported that the increase in the function of the FLR was less than that of the volume after stage 1. Calculation of FFLRV using 3D CT and 99mTc-GSA SPECT fusion images may contribute to the improvement of the safety of ALPPS.
One of the limitations of this study is that only one of three patients who experienced a marked decrease in FFLRV to levels below the FLRV showed fatty changes in FLR after PVE. There could be multiple causes underlying the lagging of FFLRV behind the increase of FLRV. A large prospective study is needed to elucidate the underlying mechanism of the functional transition delay and fatty changes in FLR. Additionally, the effect of functional transition delay in clinical results, including postoperative liver failure, should be investigated in future studies.
Although in most cases, the increase in FLRV lagged behind that of the FFLRV after PVE, the functional transition lagged behind the increase in FLRV in some cases. Evaluation of both volume and function by CT volumetry and 99mTc-GSA is required to determine the optimal timing of hepatectomy after PVE.
Preoperative portal vein embolization (PVE) is a widely used strategy to enable major hepatectomy in patients with insufficient liver remnant. The timing of hepatectomy after PVE has been usually determined from future liver remnant volume (FLRV) based on computed tomography (CT) volumetry.
PVE induces hypertrophy of the future liver remnant (FLR) and a shift of the functional reserve to the FLR. However, whether the increase in FLRV corresponds to the functional transition after PVE remains uncertain.
The present study investigated the sequential relationship between the increase in the FLRV and functional transition after preoperative PVE.
Thirty-three patients who underwent major hepatectomy following PVE were enrolled in this retrospective study. Functional FLRV (FFLRV) was defined as the total liver volume × (FLR volume counts / total liver volume counts) on the 3-dimensional 99mTc-galactosyl-human serum albumin (99mTc-GSA) single-photon emission CT CT-fused images. The calculated FFLRV was compared with FLRV.
FFLRV increased by a significantly larger extent than FLRV at 1 and 2 wk after PVE (P < 0.01); however, in 3 of the 33 patients, FFLRV levels decreased below FLRV at 2 wk. One of the three patients showed rapidly progressive fatty changes in FLR.
The results indicate that functional transition lagged behind the increase in FLRV after PVE in some cases. The evaluation of both volume and function by CT volumetry and 99mTc-GSA are needed to determine the optimal timing of hepatectomy after PVE for preventing fatal liver failure after major hepatectomy.
Further research is needed to elucidate the underlying mechanism of the functional transition delay and fatty changes in FLR.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li HL S-Editor: Gao CC L-Editor: A P-Editor: Zhang YL
| 1. | Reissfelder C, Rahbari NN, Koch M, Kofler B, Sutedja N, Elbers H, Büchler MW, Weitz J. Postoperative course and clinical significance of biochemical blood tests following hepatic resection. Br J Surg. 2011;98:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 798] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 3. | Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM, Vauthey JN. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-62; discussion 862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 516] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 4. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1148] [Cited by in RCA: 1076] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 5. | Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, Curley SA, Vauthey JN. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 363] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 6. | Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 339] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF, Caridi J. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 489] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 8. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ; Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER). The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 10. | Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 310] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Giraudo G, Greget M, Oussoultzoglou E, Rosso E, Bachellier P, Jaeck D. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single institution experience. Surgery. 2008;143:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 403] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 13. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 473] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 14. | Beppu T, Hayashi H, Okabe H, Masuda T, Mima K, Otao R, Chikamoto A, Doi K, Ishiko T, Takamori H, Yoshida M, Shiraishi S, Yamashita Y, Baba H. Liver functional volumetry for portal vein embolization using a newly developed 99mTc-galactosyl human serum albumin scintigraphy SPECT-computed tomography fusion system. J Gastroenterol. 2011;46:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Nanashima A, Tobinaga S, Abo T, Sumida Y, Araki M, Hayashi H, Sakamoto I, Kudo T, Takeshita H, Hidaka S, Sawai T, Hatano K, Nagayasu T. Relationship of hepatic functional parameters with changes of functional liver volume using technetium-99m galactosyl serum albumin scintigraphy in patients undergoing preoperative portal vein embolization: a follow-up report. J Surg Res. 2010;164:e235-e242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Ha-Kawa SK, Tanaka Y, Hasebe S, Kuniyasu Y, Koizumi K, Ishii Y, Yamamoto K, Kashiwagi T, Ito A, Kudo M, Ikekubo K, Tsuda T, Murase K. Compartmental analysis of asialoglycoprotein receptor scintigraphy for quantitative measurement of liver function: a multicentre study. Eur J Nucl Med. 1997;24:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Akaki S, Okumura Y, Sasai N, Sato S, Tsunoda M, Kuroda M, Kanazawa S, Hiraki Y. Hepatectomy simulation discrepancy between radionuclide receptor imaging and CT volumetry: influence of decreased unilateral portal venous flow. Ann Nucl Med. 2003;17:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Tsuruga Y, Kamiyama T, Kamachi H, Shimada S, Wakayama K, Orimo T, Kakisaka T, Yokoo H, Taketomi A. Significance of functional hepatic resection rate calculated using 3D CT/(99m)Tc-galactosyl human serum albumin single-photon emission computed tomography fusion imaging. World J Gastroenterol. 2016;22:4373-4379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, Taniguchi M, Shimamura T, Matsushita M, Todo S. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Sakuhara Y, Abo D, Hasegawa Y, Shimizu T, Kamiyama T, Hirano S, Fukumori D, Kawamura T, Ito YM, Tha KK, Shirato H, Terae S. Preoperative percutaneous transhepatic portal vein embolization with ethanol injection. AJR Am J Roentgenol. 2012;198:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Ohshima S. Volume analyzer SYNAPSE VINCENT for liver analysis. J Hepatobiliary Pancreat Sci. 2014;21:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13207] [Article Influence: 1100.6] [Reference Citation Analysis (0)] |
| 23. | Isfordink CJ, Samim M, Braat MNGJA, Almalki AM, Hagendoorn J, Borel Rinkes IHM, Molenaar IQ. Portal vein ligation versus portal vein embolization for induction of hypertrophy of the future liver remnant: A systematic review and meta-analysis. Surg Oncol. 2017;26:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Tashiro S. Mechanism of liver regeneration after liver resection and portal vein embolization (ligation) is different? J Hepatobiliary Pancreat Surg. 2009;16:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Tsai CY, Nojiri M, Yokoyama Y, Ebata T, Mizuno T, Nagino M. Uneven acute non-alcoholic fatty change of the liver after percutaneous transhepatic portal vein embolization in a patient with hilar cholangiocarcinoma - a case report. BMC Gastroenterol. 2017;17:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Miyake H, Fujii M, Sasaki K, Ando T, Ikeyama S, Iwata T, Rokutan K, Tashiro S. Heat shock protein 70 induction in hepatocytes after right portal vein embolization. Hepatogastroenterology. 2003;50:2084-2087. [PubMed] |
| 27. | Archer AE, Rogers RS, Von Schulze AT, Wheatley JL, Morris EM, McCoin CS, Thyfault JP, Geiger PC. Heat shock protein 72 regulates hepatic lipid accumulation. Am J Physiol Regul Integr Comp Physiol. 2018;315:R696-R707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Barth BK, Fischer MA, Kambakamba P, Lesurtel M, Reiner CS. Liver-fat and liver-function indices derived from Gd-EOB-DTPA-enhanced liver MRI for prediction of future liver remnant growth after portal vein occlusion. Eur J Radiol. 2016;85:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Hsiao IT, Lin KJ, Chang SI, Yen TC, Chen TC, Yeh TS. Impaired liver regeneration of steatotic rats after portal vein ligation: a particular emphasis on (99m)Tc-DISIDA scintigraphy and adiponectin signaling. J Hepatol. 2010;52:540-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 931] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 31. | Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel M, Abdalla EK, Hernandez-Alejandro R, Jovine E, Machado M, Malago M, Robles-Campos R, Petrowsky H, Santibanes ED, Clavien PA. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann Surg. 2015;262:780-5; discussion 785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 32. | Olthof PB, Tomassini F, Huespe PE, Truant S, Pruvot FR, Troisi RI, Castro C, Schadde E, Axelsson R, Sparrelid E, Bennink RJ, Adam R, van Gulik TM, de Santibanes E. Hepatobiliary scintigraphy to evaluate liver function in associating liver partition and portal vein ligation for staged hepatectomy: Liver volume overestimates liver function. Surgery. 2017;162:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |