Published online Dec 27, 2021. doi: 10.4240/wjgs.v13.i12.1708
Peer-review started: June 28, 2021
First decision: July 27, 2021
Revised: August 20, 2021
Accepted: November 3, 2021
Article in press: November 3, 2021
Published online: December 27, 2021
Processing time: 178 Days and 14 Hours
Ghrelin is an adipokine that plays an important role in energy balance. Expression of ghrelin and ghrelin receptor has been investigated in different tissues and tumors. Studies regarding expression of ghrelin and ghrelin receptor in colorectal tumors are scarce and no data on expression of ghrelin and its receptor in colorectal adenomas has been published. Ghrelin and ghrelin receptor were highly expressed in colon carcinoma cells while expression was decreased in less differentiated tumors, presuming that ghrelin might be important in early phases of tumorigenesis.
To investigate the expression of ghrelin and ghrelin receptor in human colorectal adenomas and adjacent colorectal tissue.
In this prospective study (conducted from June 2015 until May 2019) we included 92 patients (64 male and 28 female) who underwent polypectomy for colorectal adenomas in the Department of Gastroenterology and Hepatology, “Sestre milosrdnice” Clinical Hospital Center in Zagreb, Croatia. After endoscopic removal of colorectal adenoma, an additional sample of colon mucosa in the proximity of the adenoma was collected for pathohistological analysis. Adenomas were graded according to the stage of dysplasia, and ghrelin and ghrelin receptor expression were determined immunohistochemically in both adenoma and adjacent colon tissue using the polyclonal antibody for ghrelin (ab150514, ABCAM Inc, Cambridge, United States) and ghrelin receptor (ab48285, ABCAM Inc, Cambridge, United States). Categorical and nominal variables were described through frequencies and proportions and the difference between specific groups were analyzed with Fisher’s and Fisher-Freeman-Halton’s method respectively. Spearman's rank correlation coefficient was determined for correlation of expression of ghrelin and ghrelin receptor in adenoma and adjacent colon tissue with the grade of adenoma dysplasia.
Among 92 patients with colorectal adenoma 43 had adenomas with high-grade dysplasia (46.7%). High expression of ghrelin was 7 times more common in high-grade adenoma compared to low-grade adenomas (13.95% to 2.04%, P = 0.048), while the expression of ghrelin in adjacent colon tissue was low. We found no correlation between ghrelin receptor expression in adenoma and adjacent colon tissue and the grade of colorectal adenoma dysplasia. The most significant correlation was found between ghrelin and ghrelin receptor expression in adenomas with high-grade dysplasia (rho = 0.519, P < 0.001).
Ghrelin and ghrelin receptor are expressed in colorectal adenoma and adjacent tissue with ghrelin expression being more pronounced in high grade dysplasia as a possible consequence of increased local synthesis.
Core Tip: Colorectal adenomas are benign, but premalignant lesions of the large intestine, as dysplasia may progress over time and result in the occurrence of colorectal carcinoma. The risk of progression is increased in adenomas with high-grade dysplasia. There are several risk factors for adenomas with high-grade dysplasia, of which energy imbalance and metabolic syndrome are increasing in importance because of their rising prevalence. Ghrelin is an adipokine important in energy balance and its expression was investigated in different tumors and tissues. With this prospective observational study we gained new insight on the expression and role of ghrelin and ghrelin receptor in colorectal adenomas.
- Citation: Stojsavljevic-Shapeski S, Virovic-Jukic L, Tomas D, Duvnjak M, Tomasic V, Hrabar D, Kralj D, Budimir I, Barsic N, Ljubicic N. Expression of adipokine ghrelin and ghrelin receptor in human colorectal adenoma and correlation with the grade of dysplasia. World J Gastrointest Surg 2021; 13(12): 1708-1720
- URL: https://www.wjgnet.com/1948-9366/full/v13/i12/1708.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i12.1708
Ghrelin is an adipokine, an endogenous ligand of growth hormone (GH) secretagogue receptor (GHS-R), which was first isolated in 1999 by Kojima et al[1] from rat gastric cells. Ghrelin stimulates the release of GH through activation of its receptors and for some time it was though that its main and only function was the regulation of energy and appetite[1]. However, ghrelin stimulates the release of other pituitary hormones, influences gastric motility and secretion of gastric acid, modulates pancreatic endocrine function and influences glucose metabolism, insulin resistance and cell proliferation[2]. Apart from its production by gastric cells, it is expressed and produced in almost all tissues of the gastrointestinal tract and body in general[3,4]. Serum concentrations of total ghrelin were found to be lower in obese individuals on the account of decreased levels of deacylated ghrelin, while acylated ghrelin levels were mostly constant[5]. Ghrelin receptor was found to be highly expressed in adipose tissue where its activation induced the differentiation and proliferation of adipocyes and decreased their apoptosis which is mediated through MAP/PIP 3/Akt pathway[6]. Since ghrelin influences the release of GH and is a regulator of the GH/insulin like GH (IGF)-1 pathway, it has been also linked to tumor progression[7]. Gastric carcinoma cells exposed to ghrelin showed increased migratory and invasion abilities while their apoptosis was reduced[8]. This was shown to be also mediated through the PI3K/Akt pathway[8].
Ghrelin receptor expression varies among different types of tumors. Two types of ghrelin receptor forms have been described, type GHS-R1a and GHS-R1b, with GHS-R1a recognized as predominant and therefore responsible for ghrelin activity[9]. However, one study found GHS-R1b more expressed in tumor cells with advancing colorectal carcinoma stage while GHS-R1a expression was decreased[10]. Ghrelin has been investigated in different tumor tissues and although not all results concurred, most were consistent in tumor expression of ghrelin and in favor of its proliferative and anti-apoptotic role[11-16].
Colorectal adenomas are premalignant lesions that are differentiated among other characteristics on the grade of dysplasia in high and low-grade dysplasia adenomas[17]. With time, progression of dysplasia leads to a well-known adenoma-carcinoma sequence. Various risk factors have been associated with high-grade dysplasia adenoma, including genetic predisposition, inflammatory bowel diseases, age, male sex, smoking, poor dietary habits, obesity and metabolic syndrome[18,19]. Since metabolic syndrome is experiencing a worldwide epidemic-like rise in incidence, its clinical consequences such as tumors, with colorectal adenomas and carcinomas among others, are also experiencing a dramatic rise[20,21]. Although the influence of insulin resistance and hyperinsulinemia in colorectal carcinoma formation and progression has been well established, the role of adipokines connected to the metabolic syndrome such as ghrelin has still not been completely clarified[22]. Researching the published data regarding influence of ghrelin and its receptor in colorectal carcinoma and colorectal adenoma progression, we realized that there is a need for further insight on this subject. Current data are not sufficient for complete understanding of all ghrelin effects, and there are missing data from large cohort studies, tissue expression, genetic and plasma level studies which was also emphasized in a recently published review on ghrelin role in gastrointestinal tract tumors[23]. In this study we aimed to investigate the expression of ghrelin and ghrelin receptor in colorectal adenoma and adjacent healthy tissue, and to our knowledge this is the first study dealing with this issue. New information on this subject could influence the current recommendations for colorectal adenoma and carcinoma screening, giving more attention to patients burdened with metabolic syndrome features as well as influence postpolypectomy surveillance guidelines. Current guidelines rely on conventional adenoma characteristics such as number, size, histology and presence of dysplasia, but the burden imposed on patients and health services by surveillance colonoscopies encourages research of novel genomic and immunohistochemical markers for identifying risk of metachronous polyp development[24]. Understanding the complex involvement of adipokines in the pathways responsible in adenoma to carcinoma progression could influence potential management strategies[25]. Ghrelin as an important adipokine is in this respect still insufficiently investigated and further studies are needed.
In this prospective observational study we included 92 patients who underwent endoscopic polypectomy for colorectal adenoma at the Department of Gastroenterology, “Sestre milosrdnice” University Hospital Center in Zagreb, Croatia. The participants were included in the study in the period from June 2015 until May 2019. All participants were prior to recruitment informed of the nature of the study and gave their informed consent for participation. Exclusion criteria were an active or prior malignant disease, history of inflammatory bowel disease or any abdominal surgical procedure, prior removal of colorectal adenoma and a lack of informed consent.
All patients underwent a total colonoscopy with the removal of colorectal adenoma or adenomas. During the procedure, an additional biopsy of adjacent, “healthy” tissue was taken 5 cm proximally or distally from the removed adenoma. In cases where more adenomas were removed, only the largest adenoma and the tissue adjacent to it were used in further immunohistochemical analysis. Adenoma sample and the adjacent tissue sample underwent pathohistological analysis for dysplasia that was graded either high or low, and immunohistochemical analysis for expression of ghrelin and ghrelin receptor. We used tissue fixation technique with solution of 40 g/L formaldehyde (10% neutral buffered formalin) and the samples were embedded in paraffin blocks and cut into 5 μm slices. A power analysis was done in a pilot study to determine the number of participants needed to reach statistical significance.
For immunohistochemical analysis we used a polyclonal antibody for the ghrelin receptor (ab150514, ABCAM Inc, Cambrige, United States) and a polyclonal antibody for ghrelin (ab48285 ABCAM Inc, Cambrige, United States), both in concentrations of 5 mg/mL. The analysis for both antibodies was performed on a Dako Autostainer automated slide processing system (Dako, Copenhagen, Denmark) by EnVision FLEX-PTL method. The results of the immunohistochemical analysis were expressed semi-quantitatively by determination of the immunohistochemical staining index (ISI), taking in account the intensity of the reaction (IR) and the percentage of the immunoreactive cells (PC). Two experienced pathologists independently performed the interpretation of the IR and the percentage of immunoreactive cells. In cases of discordant results a third pathologist was consulted to reach an agreement. Intensity of the staining was classified as 0 for no reaction, 1 for a poor cytoplasmic reaction, 2 for a moderate one and 3 for an intense cytoplasmic reaction. The percentage of immunoreactive cells was classified as 0 for no reaction, 1 for reaction in ≤ 33 percent of cells, 2 for reaction in more that 33 percent and ≤ 66 percent, and 3 for a reaction in more that 66 percent of cells. Each sample was in that way assigned a grade for the percentage of immunoreactiove cells and a grade for the intensity of staining. ISI was determined as a multiplication of the IR and the percentage of reactive cells. We distinguished two groups of specimens: those with the ISI value of 9, which represents the strong reaction and the group with ISI values less than 9 representing no, poor or slight reaction.
Categorical and nominal variables were described through frequencies and proportions and the difference between specific groups were analyzed with Fisher’s and Fisher-Freeman-Halton’s method respectively. Spearman’s rank correlation coefficient was determined for correlation of expression of ghrelin and ghrelin receptor in adenoma and adjacent colon tissue with the grade of adenoma dysplasia. P values less than 0.05 were considered significant and in the analysis we used the licensed program support IBM SPSS Statistics, version 25.0 (https://www.ibm.com/analytics/spss-statistics-software).
From 123 screened, 92 patients were included in the study (due to later drop out), 64 male (69.9%) and 28 female (30.4%). The youngest patient was 29 and the oldest 83 years old, age median was 66. Forty-nine patients (53.3%) had a low-grade dysplasia adenoma and 43 patients (46.7%) high-grade dysplasia adenoma. Adenomas were categorized according to size in larger than 5 mm and smaller than 5 mm, and adenomas larger than 5 mm were according to type categorized in sessile, subpeduncular, peduncular and flat. The descriptive statistics regarding the localization, size and type of adenomas is presented in Table 1.
| n | % | |
| Adenoma < 5 mm in ascending colon | ||
| Not found | 65 | 70.7 |
| Found | 27 | 29.3 |
| Adenoma > 5 mm in ascending colon | ||
| Not found | 62 | 67.4 |
| Found | 30 | 32.6 |
| Type of adenoma > 5 mm in ascending colon | ||
| Sessile | 24 | 54.6 |
| Peduncular | 3 | 6.8 |
| Subpeduncular | 7 | 15.9 |
| Flat | 10 | 22.7 |
| Adenoma < 5 mm in transverse and descending colon | ||
| Not found | 75 | 81.5 |
| Found | 17 | 18.5 |
| Adenoma > 5 mm in transverse and descending colon | ||
| Not found | 69 | 75.0 |
| Found | 23 | 25.0 |
| Type of adenoma > 5 mm in transverse and descending colon | ||
| Sessile | 15 | 51.7 |
| Peduncular | 7 | 24.1 |
| Subpeduncular | 4 | 13.8 |
| Flat | 3 | 10.4 |
| Adenoma < 5 mm in sigmoid colon | ||
| Not found | 68 | 73.9 |
| Found | 24 | 26.1 |
| Adenoma > 5 mm in sigmoid colon | ||
| Not found | 43 | 46.7 |
| Found | 49 | 53.3 |
| Type of adenoma > 5 mm in sigmoid colon | ||
| Sessile | 18 | 31.1 |
| Peduncular | 26 | 44.8 |
| Subpeduncular | 12 | 20.7 |
| Flat | 2 | 3.4 |
| Adenoma < 5 mm in rectum | ||
| Not found | 75 | 81.5 |
| Found | 17 | 18.5 |
| Adenoma > 5 mm in rectum | ||
| Not found | 73 | 79.3 |
| Found | 19 | 20.7 |
| Type of adenoma > 5 mm in rectum | ||
| Sessile | 14 | 73.7 |
| Peduncular | 4 | 21.1 |
| Subpeduncular | 1 | 5.2 |
| Flat | 0 | 0.0 |
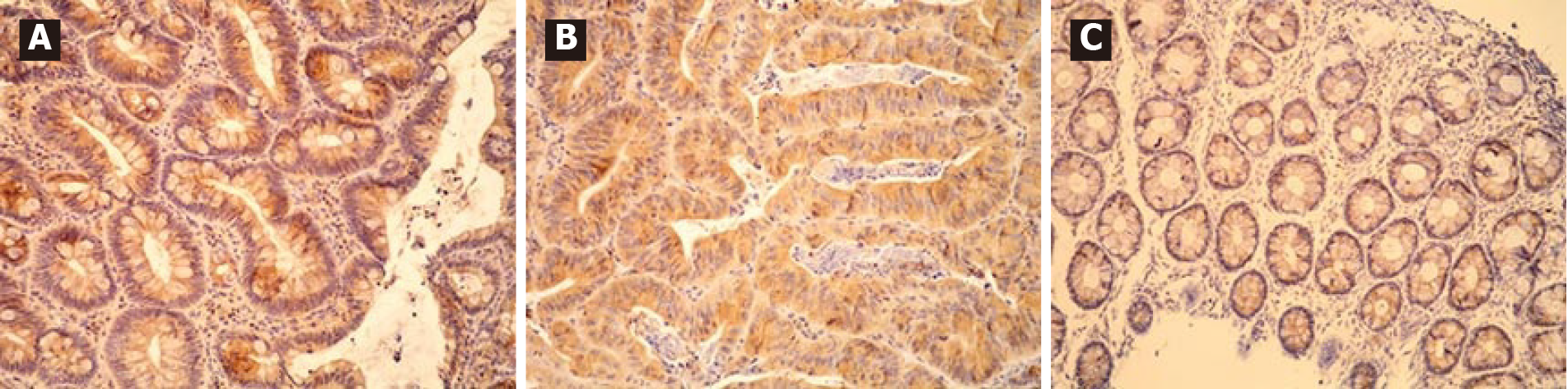
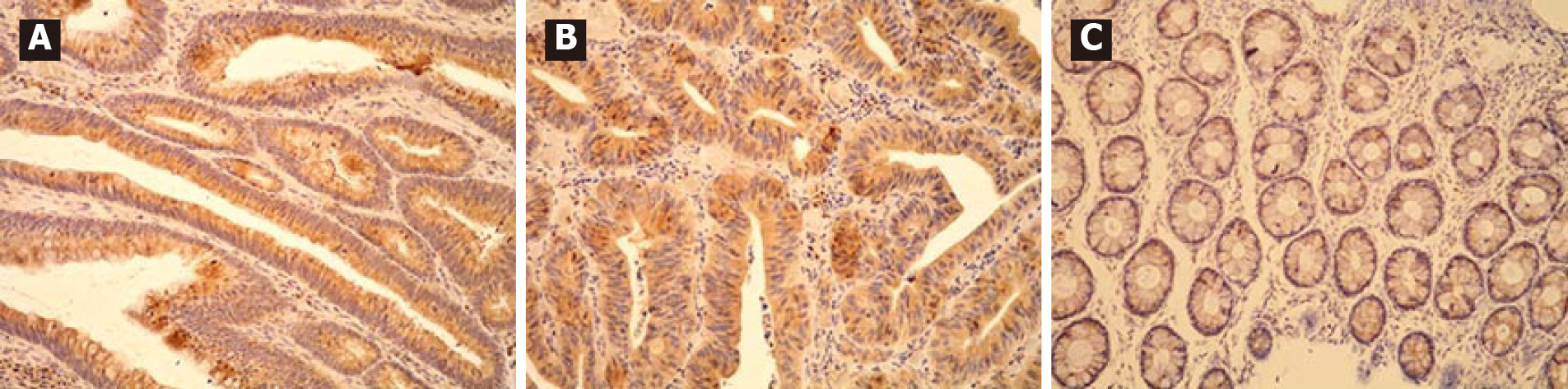
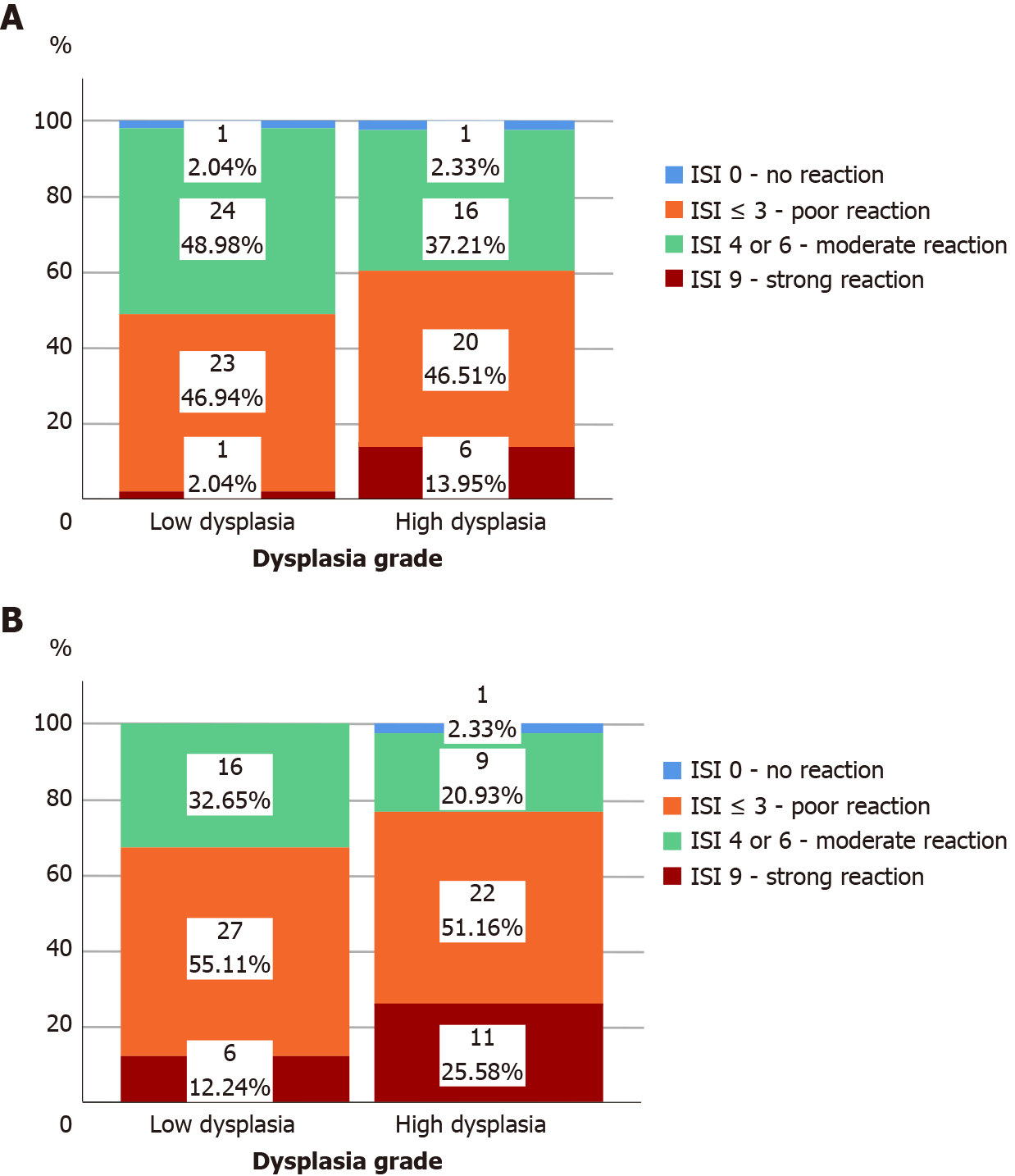
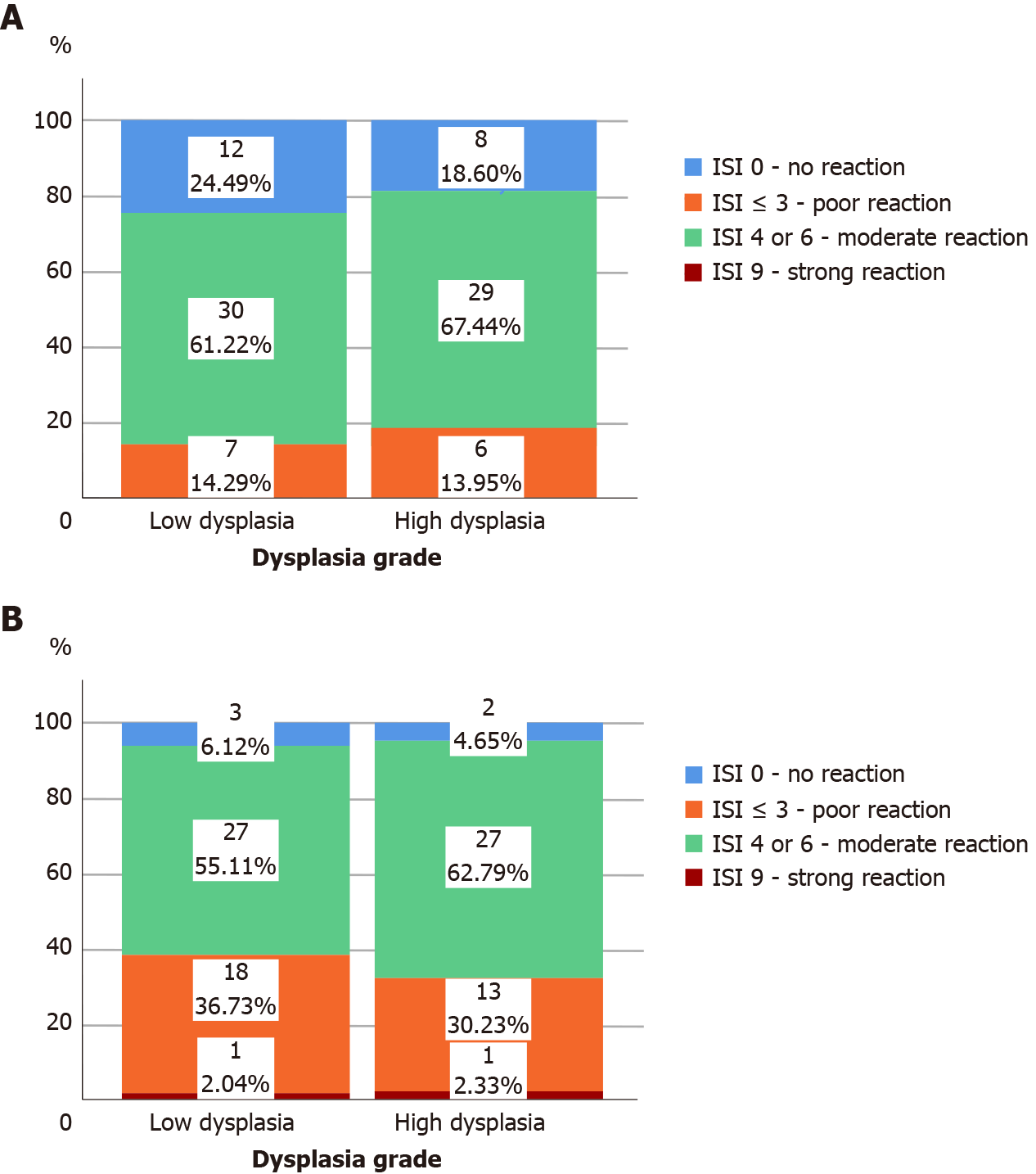
All adenomas as well as adjacent tissue were immunohistochemically stained to evaluate ghrelin and ghrelin receptor expression. Figure 1 shows different intensities of immunohistochemical staining for ghrelin in adenoma and adjacent tissue (Figure 1A-C). Figure 2 shows different intensities of immunohistochemical staining for ghrelin receptor in adenoma and adjacent tissue (Figure 2A-C). Figure 3 shows the statistical distribution of ISI values for ghrelin and ghrelin receptor among adenomas depending on dysplasia grade, and Figure 4 the statistical distribution of ISI values for ghrelin and ghrelin receptor in adjacent tissue (Figures 3 and 4).
We found that ghrelin was in different intensities expressed in 98.8% of all adenomas, and 79.3% of adjacent tissue samples, while ghrelin receptor was expressed in 98.9% of adenoma and 94.6% of adjacent tissue samples.
In Table 2 we showed the correlation of immunohistochemical expression of ghrelin and ghrelin receptor based on two groups of ISI values in adenoma and adjacent tissue to the stage of adenoma dysplasia (Table 2). In adenomas with high-grade dysplasia strong expression of ghrelin was 7 times more frequent than in adenomas with low-grade dysplasia (P = 0.048). We found no correlation between immunohistochemical expression of ghrelin receptor in adenoma and adjacent tissue to the stage of adenoma dysplasia (P > 0.05).
| Dysplasia grade | P value | ||||
| Low dysplasia | High dysplasia | ||||
| n | % | n | % | ||
| ISI for ghrelin in adenoma | 0.048a | ||||
| ISI < 9 | 48 | 98.0 | 37 | 86.0 | |
| ISI 9-strong reaction | 1 | 2.0 | 6 | 14.0 | |
| ISI for ghrelin in adjacent tissue | 1.000 | ||||
| ISI 0 < 6 | 42 | 85.7 | 37 | 86.0 | |
| ISI 6-moderate reaction | 7 | 14.3 | 6 | 14.0 | |
| ISI for ghrelin receptor in adenoma | 0.114 | ||||
| ISI < 9 | 43 | 87.8 | 32 | 74.4 | |
| ISI 9-strong reaction | 6 | 12.2 | 11 | 25.6 | |
| ISI for ghrelin receptor in adjacent tissue | 0.664 | ||||
| ISI < 9 | 30 | 61.2 | 29 | 67.4 | |
| ISI 9-strong reaction | 19 | 38.8 | 14 | 32.6 | |
The results of Spearman's rank correlation coefficient (rho) analysis for correlation between immunohistochemical expression (value of ISI index) of ghrelin and ghrelin receptor in adenoma (and adjacent colon tissue) and grade of adenoma dysplasia are shown in Table 3.
| ISI for ghrelin in adenoma | ISI for ghrelin in adjacent tissue | ISI for ghrelin receptor in adenoma | ISI for ghrelin receptor in adjacent tissue | |
| Low grade dysplasia | ||||
| ISI for ghrelin in adenoma | ||||
| Rho | 1.000 | 0.173 | -0.108 | -0.096 |
| P value | 0.235 | 0.459 | 0.511 | |
| n | 49 | 49 | 49 | 49 |
| ISI for ghrelin in adjacent tissue | ||||
| Rho | 0.173 | 1.000 | 0.159 | 0.367 |
| P value | 0.235 | 0.276 | 0.009a | |
| n | 49 | 49 | 49 | 49 |
| ISI for ghrelin receptor in adenoma | ||||
| Rho | -0.108 | 0.159 | 1.000 | 0.576 |
| P value | 0.459 | 0.276 | < 0.001b | |
| n | 49 | 49 | 49 | 49 |
| ISI for ghrelin receptor in adjacent tissue | ||||
| Rho | -0.096 | 0.367 | 0.576 | 1.000 |
| P value | 0.511 | 0.009 | 0.000 | |
| n | 49 | 49 | 49 | 49 |
| High grade dysplasia | ||||
| ISI for ghrelin in adenoma | ||||
| Rho | 1.000 | 0.347 | 0.519 | 0.077 |
| P value | 0.023d | < 0.001c | 0.622 | |
| n | 43 | 43 | 43 | 43 |
| ISI for ghrelin in adjacent tissue | ||||
| Rho | 0.347 | 1.000 | 0.230 | 0.409 |
| P value | 0.023d | 0.138 | 0.007e | |
| n | 43 | 43 | 43 | 43 |
| ISI for ghrelin receptor in adenoma | ||||
| Rho | 0.519 | 0.230 | 1.000 | 0.467 |
| P value | < 0.001c | 0.138 | 0.002f | |
| n | 43 | 43 | 43 | 43 |
| ISI for ghrelin receptor in adjacent tissue | ||||
| Rho | 0.077 | 0.409 | 0.467 | 1.000 |
| P value | 0.622 | 0.007e | 0.002f | |
| n | 43 | 43 | 43 | 43 |
In adenomas with high-grade dysplasia there is a positive correlation between immunohistochemical expression of ghrelin in adenoma and the immunohistochemical expression of ghrelin receptor in adenoma (rho = 0.519; P < 0.001) and expression of ghrelin in adjacent tissue (rho = 0.467; P = 0.002). In adenomas with low-grade dysplasia we have not found a positive correlation between immunohistochemical expression of ghrelin and the ghrelin receptor but we found a positive correlation between expression of ghrelin receptor in adenoma and the expression of ghrelin receptor in adjacent tissue (rho = 0.567; P < 0.001). Regardless of the stage of adenoma dysplasia in adjacent colon tissue we found a positive correlation between expression of ghrelin and ghrelin receptor (rho = 0.367; P = 0.009 in low dysplasia group and rho = 0.409; P = 0.002 for high-grade dysplasia group respectively). For interpretation of this correlation it is important to note that regardless of the dysplasia grade in adenoma we have not found in any obtained sample of adjacent colon tissue a high expression of ghrelin, and in more than 75% of adjacent tissue samples ISI index was ≤ 3 which marked poor to none ghrelin expression.
To our knowledge there have been no studies regarding the expression of ghrelin and ghrelin receptor in human colorectal adenomas. We wanted to investigate the expression of ghrelin and ghrelin receptor in colorectal adenoma and in adenoma adjacent normal colorectal tissue. In our study we found that in adenomas ghrelin was in different intensity expressed in 98.8% of samples and ghrelin receptor in 98.9% respectively. In adjacent tissue ghrelin was in different intensity expressed in 79.3% of samples and ghrelin receptor in 94.6% respectively. Although ghrelin and ghrelin receptor are expressed in adenomas with low and high-grade dysplasia, in high-grade dysplasia there is a stronger expression of ghrelin, which could suggest that adenomas with high grade dysplasia produce locally more ghrelin. Waseem et al[10] in their study on 110 patients with colorectal carcinoma found that tumors cells as well as normal cells express ghrelin and ghrelin receptor, but the cells of well and moderately differentiated tumors produce more ghrelin in comparison with normal large intestine cells. The intensity of the immunohistochemical reaction for ghrelin was graded 0 to 4 and well differentiated tumors had a 1.92 ± 0.4 higher expression of ghrelin than normal cells, and moderately differentiated tumors had 2.25 ± 0.5 higher ghrelin expression than normal cells[10]. Interestingly, they also found that as the tumor cells lose its potential to differentiate, they also lose their ability to express ghrelin and ghrelin receptor (P < 0.05)[10]. Their results imply that ghrelin and ghrelin receptor could have a role in early tumor progression and that their importance is lost in poorly differentiated tumors. Ghrelin in an in vitro study acted proliferative on normal large intestine cells and tumor cells since it promoted the shift from G1 to S cell phase and influenced cell cycle progression (P < 0.05)[26]. This was mediated through activation of the adenylate cyclase independent epidermal growth factor receptor (EGFR) trans-activation and PI3K-Akt phosphorylation. Both these pathways converge to stimulate MAPK, ERK 1/2 signaling[26]. A genomic study on intra-tumor heterogeneity analyzing clonal origins and subclonal composition of adenomas and colorectal tumors detected several signaling pathways important in colorectal cancer evolution[27]. Accumulation of mutations in the PI3K-Akt pathway was found, among others, to be of vital importance[27]. A study assessing the expression of EGFR in normal colon tissue and colorectal adenoma tissue found that adenomas with high-grade dysplasia and tubule-villous features overexpress EGFR, while only 10 percent of adenomas with low-grade dysplasia expressed EGFR[28]. Another in vitro study found that ghrelin acts proliferative on colorectal carcinoma cells activating Ras, PI3K, Akt and mTOR signaling pathway[29]. Study on gastric adenocarcinoma and normal gastric cells found that gastric cells express ghrelin but adenocarcinoma cells lose its potential to express ghrelin[30]. Although we are moving away from the alimentary system, well differentiated breast tumors have a great potential for expression of ghrelin while less differentiated ones lose this ability[31]. In patients with serous ovarian tumors expression of ghrelin was increased in malignant compared to benign tumors[13].
We have not found a significant difference in ghrelin receptor expression between high and low-grade adenomas or adjacent normal colorectal tissue. Although our results point out that, based on ISI values, strong expression of ghrelin receptor was two times more frequent in adenomas with high grade dysplasia than in low grade dysplasia, it was not significant. A study by Liu et al[9], found that ghrelin and its receptor are markedly expressed in colorectal tumors and cell lines. They also report that after ghrelin receptor activation the probable mechanism of downstream regulation is through inhibiting phosphatase and tensin homolog, activating Akt and inhibiting p53[9]. In their mouse model, the expression of ghrelin receptor significantly correlated with colorectal cancer cell growth and tumor burden[9]. Similar results were reported in a mouse model of endometrial carcinoma[32]. Although our results don’t concur with the previous studies we could hypothesize that the expression and importance of ghrelin receptor is more pronounced further down the dysplasia progression pathway. Ghrelin receptor role in colorectal adenoma dysplasia progression should be investigated in further studies.
Our results showed a positive correlation between immunohistochemical expression of ghrelin and ghrelin receptor in adjacent normal colorectal tissue independently of the fact whether the corresponding removed adenoma had high or low-grade dysplasia (P = 0.009 for low grade dysplasia, P = 0.023 for high grade dysplasia). We have to emphasize that in adjacent tissue samples we didn’t find a great intensity of ghrelin expression, and in more than 75% of those samples ISI index was ≤ 3 which marked poor to none ghrelin expression. Our results didn’t show a positive correlation between ghrelin and ghrelin receptor in adenomas with low-grade dysplasia (P < 0.05). Since similar studies concerning ghrelin and ghrelin receptor expression in adenoma with low and high-grade dysplasia as well as adjacent tissue are lacking we cannot compare our results with other studies, but are looking forward to future studies. The lack of our study is that the immunohistochemical staining used in our study did not differentiate the two types of ghrelin receptor (types GHS-R1a and GHS-R1b) in colorectal adenoma and adjacent tissue so this could be a subject for new studies. Although this was a relatively simple study our strongest point is that we are the first to address ghrelin and ghrelin receptor expression in colorectal adenomas since there has been no published data on this issue.
Our results point out to the conclusion that although ghrelin and ghrelin receptor are expressed in normal and adenoma tissue, in high-grade adenomas there is a higher expression of ghrelin due to its higher production, which promotes further proliferation.
Our study shows that ghrelin and ghrelin receptor are expressed in colorectal adenomas and adjacent tissue. We found that ghrelin expression was more pronounced in adenomas with high-grade dysplasia compared to those with low-grade dysplasia and that here is a positive correlation between ghrelin and ghrelin receptor expression in colorectal adenomas with high-grade dysplasia. Our results indicate the important role of ghrelin in dysplasia progression. Further studies on expression of specific ghrelin receptor types in colorectal adenomas are needed to ensure better understanding of the role of ghrelin receptors in promotion of cell proliferation and malignant transformation.
Ghrelin is an adipokine that influences energy expenditure and appetite, modulates gastric motility, secretion of gastric acid, pancreatic endocrine function and has an important role in glucose metabolism, insulin resistance and metabolic syndrome. Metabolic syndrome is one of the known risk factors for colorectal carcinoma development, and both diseases have had a significant rise in prevalence. Colorectal adenomas are premalignant lesions that can with time progress to colorectal carcinoma, and have also been linked to metabolic syndrome. Ghrelin, as one of the links between metabolic syndrome and tumor progression, has been investigated in several tissues and tumors but current data are not sufficient for complete understanding of all ghrelin effects.
Researching the published data regarding influence of ghrelin and its receptor in colorectal carcinoma and colorectal adenoma progression, we realized that there is a need for further insight on the subject since data on this topic is lacking. Current guidelines on colorectal adenoma and carcinoma screening and postpolypectomy surveillance do not focus on the presence of metabolic syndrome or any of its components. Obtaining more insight into the link between metabolic syndrome and colorectal adenoma and carcinoma occurrence could possibly in future influence new guidelines.
We aimed to investigate the expression of ghrelin and ghrelin receptor in colorectal adenomas and adjacent colorectal tissue to give a new perspective on this problem.
We conducted a prospective study (from June 2015 until May 2019) that included 92 patients who underwent polypectomy for colorectal adenomas in the Department of Gastroenterology and Hepatology, “Sestre milosrdnice” Clinical Hospital Center in Zagreb, Croatia. An additional sample of colon mucosa was collected in the proximity of the removed colorectal adenoma for further pathohistological analysis. Adenomas were graded according to the stage of dysplasia, and ghrelin and ghrelin receptor expression were determined immunohistochemically in both adenoma and adjacent colon tissue using the polyclonal antibody for ghrelin and ghrelin receptor.
High expression of ghrelin was 7 times more common in high-grade adenoma compared to low-grade adenomas (13.95% to 2.04%, P = 0.048), while the expression of ghrelin in adjacent colon tissue was low. We found no correlation between ghrelin receptor expression in adenoma and adjacent colon tissue and the grade of colorectal adenoma dysplasia. The most significant correlation was found between ghrelin and ghrelin receptor expression in adenomas with high-grade dysplasia (rho = 0.519, P < 0.001).
Our study is the first to show that ghrelin and ghrelin receptor are expressed in colorectal adenomas and adjacent tissue. We found that ghrelin expression was more pronounced in adenomas with high-grade dysplasia compared to those with low-grade dysplasia. The results of this study underline the importance of ghrelin in progression of dysplasia in colorectal adenoma but there is a need for further studies to determine the expression of different subtypes of ghrelin receptors in colorectal adenomas and exact ghrelin receptors role.
Ghrelin and metabolic syndrome role in general need to be adequately investigated in colorectal adenoma progression since we are experiencing an epidemic of colorectal carcinoma intertwined with an epidemic of obesity. We believe that obtaining more insight into this problem could help us to better understand the dysplasia progression pathways, influence the surveillance programs and guidelines, and in that way ensure early recognition of patients in greater risk for colorectal carcinoma development.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abdalla MMI, Mori H, Tan X S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5961] [Cited by in RCA: 5889] [Article Influence: 226.5] [Reference Citation Analysis (0)] |
| 2. | Gahete MD, Rincón-Fernández D, Villa-Osaba A, Hormaechea-Agulla D, Ibáñez-Costa A, Martínez-Fuentes AJ, Gracia-Navarro F, Castaño JP, Luque RM. Ghrelin gene products, receptors, and GOAT enzyme: biological and pathophysiological insight. J Endocrinol. 2014;220:R1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Ghelardoni S, Carnicelli V, Frascarelli S, Ronca-Testoni S, Zucchi R. Ghrelin tissue distribution: comparison between gene and protein expression. J Endocrinol Invest. 2006;29:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 758] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 5. | Barazzoni R, Zanetti M, Nagliati C, Cattin MR, Ferreira C, Giuricin M, Palmisano S, Edalucci E, Dore F, Guarnieri G, de Manzini N. Gastric bypass does not normalize obesity-related changes in ghrelin profile and leads to higher acylated ghrelin fraction. Obesity (Silver Spring). 2013;21:718-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Kim MS, Yoon CY, Jang PG, Park YJ, Shin CS, Park HS, Ryu JW, Pak YK, Park JY, Lee KU, Kim SY, Lee HK, Kim YB, Park KS. The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:2291-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Bustin SA, Jenkins PJ. The growth hormone-insulin-like growth factor-I axis and colorectal cancer. Trends Mol Med. 2001;7:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Li H, Zhang X, Feng L. Ghrelin Regulates Cyclooxygenase-2 Expression and Promotes Gastric Cancer Cell Progression. Comput Math Methods Med. 2021;2021:5576808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Liu A, Huang C, Xu J, Cai X. Lentivirus-mediated shRNA interference of ghrelin receptor blocks proliferation in the colorectal cancer cells. Cancer Med. 2016;5:2417-2426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Waseem T, Javaid-Ur-Rehman, Ahmad F, Azam M, Qureshi MA. Role of ghrelin axis in colorectal cancer: a novel association. Peptides. 2008;29:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Volante M, Allìa E, Gugliotta P, Funaro A, Broglio F, Deghenghi R, Muccioli G, Ghigo E, Papotti M. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocrinol Metab. 2002;87:1300-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 178] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Dagli AF, Aydin S, Karaoglu A, Akpolat N, Ozercan IH, Ozercan MR. Ghrelin expression in normal kidney tissue and renal carcinomas. Pathol Res Pract. 2009;205:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Nurkalem C, Celik H, Dagli F, Gurates B, Kavak B, Dogan Z, Baykus Y, Aydin S. Ghrelin and obestatin expression in serous ovarian tumours. Gynecol Endocrinol. 2012;28:941-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Dagli AF, Aydin S, Kocdor H, Gurates B, Sahin I, Catak Z, Ozercan MR, Ozercan IH. Ghrelin expression of endometrium hyperplasia and endometrioid carcinoma. Gynecol Endocrinol. 2011;27:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Nikolopoulos D, Theocharis S, Kouraklis G. Ghrelin's role on gastrointestinal tract cancer. Surg Oncol. 2010;19:e2-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Andrusiewicz M, Komarowska H, Skibińska I, Chmielewska M, Jaskuła-Świtek M, Liebert W, Waśko R, Kotwicka M. Expression of ghrelin and ghrelin functional receptor GHSR1a in human pituitary adenomas. Pol Arch Intern Med. 2017;127:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Konishi F, Morson BC. Pathology of colorectal adenomas: a colonoscopic survey. J Clin Pathol. 1982;35:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 243] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Anderson JC, Calderwood AH, Christensen BC, Robinson CM, Amos CI, Butterly L. Smoking and Other Risk Factors in Individuals With Synchronous Conventional High-Risk Adenomas and Clinically Significant Serrated Polyps. Am J Gastroenterol. 2018;113:1828-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Peipins LA, Sandler RS. Epidemiology of colorectal adenomas. Epidemiol Rev. 1994;16:273-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1964] [Cited by in RCA: 2417] [Article Influence: 345.3] [Reference Citation Analysis (0)] |
| 21. | Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402-2411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 958] [Cited by in RCA: 885] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 22. | Guraya SY. Association of type 2 diabetes mellitus and the risk of colorectal cancer: A meta-analysis and systematic review. World J Gastroenterol. 2015;21:6026-6031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (2)] |
| 23. | Spiridon IA, Ciobanu DGA, Giușcă SE, Căruntu ID. Ghrelin and its role in gastrointestinal tract tumors (Review). Mol Med Rep. 2021;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Johnstone MS, Lynch G, Park J, McSorley S, Edwards J. Novel Methods of Risk Stratifying Patients for Metachronous, Pre-Malignant Colorectal Polyps: A Systematic Review. Crit Rev Oncol Hematol. 2021;164:103421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Khan AA. Exploring polyps to colon carcinoma voyage: can blocking the crossroad halt the sequence? J Cancer Res Clin Oncol. 2021;147:2199-2207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Waseem T, Duxbury M, Ashley SW, Robinson MK. Ghrelin promotes intestinal epithelial cell proliferation through PI3K/Akt pathway and EGFR trans-activation both converging to ERK 1/2 phosphorylation. Peptides. 2014;52:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Wu H, Zhang XY, Hu Z, Hou Q, Zhang H, Li Y, Li S, Yue J, Jiang Z, Weissman SM, Pan X, Ju BG, Wu S. Evolution and heterogeneity of non-hereditary colorectal cancer revealed by single-cell exome sequencing. Oncogene. 2017;36:2857-2867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Williet N, Petcu CA, Rinaldi L, Cottier M, Del Tedesco E, Clavel L, Dumas O, Jarlot C, Bouarioua N, Roblin X, Peoc'h M, Phelip JM. The level of epidermal growth factor receptors expression is correlated with the advancement of colorectal adenoma: validation of a surface biomarker. Oncotarget. 2017;8:16507-16517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Lien GS, Lin CH, Yang YL, Wu MS, Chen BC. Ghrelin induces colon cancer cell proliferation through the GHS-R, Ras, PI3K, Akt, and mTOR signaling pathways. Eur J Pharmacol. 2016;776:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Mottershead M, Karteris E, Barclay JY, Suortamo S, Newbold M, Randeva H, Nwokolo CU. Immunohistochemical and quantitative mRNA assessment of ghrelin expression in gastric and oesophageal adenocarcinoma. J Clin Pathol. 2007;60:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Cassoni P, Papotti M, Ghè C, Catapano F, Sapino A, Graziani A, Deghenghi R, Reissmann T, Ghigo E, Muccioli G. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab. 2001;86:1738-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Fung JN, Jeffery PL, Lee JD, Seim I, Roche D, Obermair A, Chopin LK, Chen C. Silencing of ghrelin receptor expression inhibits endometrial cancer cell growth in vitro and in vivo. Am J Physiol Endocrinol Metab. 2013;305:E305-E313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |