Published online Dec 27, 2021. doi: 10.4240/wjgs.v13.i12.1673
Peer-review started: May 28, 2021
First decision: June 26, 2021
Revised: July 7, 2021
Accepted: November 3, 2021
Article in press: November 3, 2021
Published online: December 27, 2021
Processing time: 209 Days and 6.7 Hours
The Partington-Rochelle pancreaticojejunostomy (PJ) is an essential management option for patients with chronic pancreatitis (CP) associated with intractable pain and a dilated pancreatic duct (PD). Wide ductotomy and long PJ (L-PJ) have been advocated as the standard of care to ensure full PD decompression. However, the role of short PJ (S-PJ) in a uniformly dilated PD has not yet been evaluated.
To evaluate the possible advantages and disadvantages of S-PJ and L-PJ and to interpret the perspective of S-PJ in the treatment of CP.
A retrospective review of prospectively collected cohort data was conducted on surgically treated CP patients subjected to side-to-side PJ. The length of the PJ was adapted to anatomical alterations in PD. A comparison was made of S-PJ (< 50 mm) for uniformly dilated PD and L-PJ (50-100 mm) in the setting of multiple PD strictures, calcifications and dilatations. We hypothesized that S-PJ and L-PJ ensure comparable clinical outcomes. The primary outcomes were pain relief and quality of life (QOL); the secondary outcomes were perioperative characteristics, body weight, patients’ satisfaction with treatment, and readmission rate due to CP.
Overall, 91 patients underwent side-to-side PJ for CP, including S-PJ in 46 patients and L-PJ in 45 patients. S-PJ resulted in better perioperative outcomes: Significantly shorter operative time (107.5 min vs 134 min), lower need for intraoperative (0% vs 15.6%) and total (2.2% vs 31.1%) blood transfusions, and lower rate of perioperative complications (6.5% vs 17.8%). We noted no significant difference in pain relief, improvement in QOL, body weight gain, patients’ satisfaction with surgical treatment, or readmission rate due to CP.
Based on our data, in the setting of a uniformly dilated PD, S-PJ provides adequate decompression of the PD. As the clinical outcomes following S-PJ are not inferior to those of L-PJ, S-PJ should be preferred as a surgical option in the case of a uniformly dilated PD.
Core Tip: Pancreaticojejunostomy (PJ) is an essential management option in patients with chronic pancreatitis associated with intractable pain and a dilated pancreatic duct (PD). Our retrospective study demonstrated that in the setting of a uniformly dilated PD, short PJ provides adequate decompression of the PD. As the clinical outcomes following short PJ are not inferior to those of long PJ, short PJ should be preferred as a surgical option in the case of a uniformly dilated PD. The use of short PJ is beneficial to patients due to shorter operating time, lower need for blood transfusion and lower rate of surgical complications.
- Citation: Murruste M, Kirsimägi Ü, Kase K, Veršinina T, Talving P, Lepner U. ‘Short’ pancreaticojejunostomy might be a valid option for treatment of chronic pancreatitis in many cases. World J Gastrointest Surg 2021; 13(12): 1673-1684
- URL: https://www.wjgnet.com/1948-9366/full/v13/i12/1673.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i12.1673
Chronic pancreatitis (CP) is a benign chronic inflammatory disease of the pancreatic gland, which is characterized by irreversible morphologic changes resulting in progressive scarring and atrophy of the pancreatic tissue, ductal strictures and dilatations, calcifications, impairment of exocrine and endocrine functions, and chronic pain[1]. The main indication for surgical treatment is chronic intractable pain, but in up to one third of cases pain is combined with local complications[2]. Previous systematic reviews have noted that surgery remains the best option for the management of pain in these settings[3,4]. Although there are several controversies in the surgical treatment of CP, the basic options are: Drainage operations, most commonly decompression of the pancreatic duct (PD) through side-to-side pancreaticojejunostomy (PJ), resection of the chronically inflamed, painful and functionally impaired pancreatic mass (‘pseudotumor’), and in some cases, a combination of these approaches[4].
The indication for decompressive PJ is enlargement of the PD without pancreatic pseudotumor[5]. Various surgical drainage procedures have been employed during more than 60 years of the history of drainage operations. The Partington-Rochelle modification is the most widely used method owing to its safety and feasibility. Although there are dozens of reports on the surgical technique, morbidity, mortality and clinical effects of this modification on PJ, no comparative studies are available on the impact of the anastomotic length of PJ on the outcome of surgical treatment, especially regarding pain relief and quality of life (QOL). It has often been emphasized that the ‘standard’ Partington-Rochelle PJ has to achieve complete drainage of the Wirsung duct along the whole pancreas and has to be at least 10 cm long[6-9]. However, Partington and Rochelle[10] have stated in their original paper that ‘sacculations of the PD should be opened if possible, but a uniformly dilated duct need not be opened so extensively’. Thus, the accepted ‘standard’ anastomosis and the recommendations given by Partington and Rochelle[10] are somewhat contradictory.
Since the launch of our program of surgical treatment for CP at Tartu University Hospital in 1997, we have applied the basic treatment principle of the ‘large duct disease’: The goal of PD drainage has to be full decompression of the PD. However, the ways to achieve this can be variable, since the anatomical changes in the PD are variable. Therefore, a large, even total, opening of the PD using a long PJ (L-PJ) is reasonable and wholly justified in cases of multiple PD strictures, calcifications and dilatations. However, there is a large subgroup of patients whose situation is different; instead, they have a quite homogeneously dilated PD and significant strictures or calcifications only in a single region. In these cases, effective decompression of the PD can be achieved through its limited opening in the affected region, followed by a relatively short anastomosis. Additional opening of an almost uniformly dilated PD can hardly be beneficial.
The above considerations served as the basis for defining the indications for the use of short PJ (S-PJ) or L-PJ, depending on local anatomical changes in PD.
In this study, we report comparative data regarding the two above described groups. The aim was to evaluate the possible advantages and disadvantages of S-PJ and L-PJ and to interpret the perspective of S-PJ in the treatment of CP.
Following approval of the Research Ethics Committee of the University of Tartu, all consecutive adult patients (≥ 18 years of age) who were suffering from CP and were subjected to side-to-side PJ were reviewed within this single-center, retrospective study of prospectively collected data, comparing the outcomes following S-PJ and L-PJ.
We hypothesized that S-PJ and L-PJ ensure comparable clinical outcomes. The primary outcomes were pain relief and QOL, the secondary outcomes were perioperative characteristics, body weight, patients’ satisfaction with treatment, and readmission rate due to CP.
Data on the patients’ demographics and co-morbidities according to Charlson’s comorbidity index[11], CP associated data, and data of pancreatic function, as well as the characteristics of pain and QOL were recorded at baseline. CP associated data included duration and etiology of CP, number of hospital admissions due to CP (from onset of chronic pain) and local changes in the pancreatic gland (PD diameter, calcifications, pseudocysts). These data were obtained from routine CT scan in all cases; further information was obtained and recorded during surgery.
For assessment of pancreatic exocrine insufficiency (PEI), we introduced a set of five simple signs (weight loss, diarrhea, steatorrhea, flatulence and foul-smelling stool) that the patients assessed in a questionnaire. PEI was defined as the presence of two or more of the above-mentioned symptoms or as the need for supplementary treatment with pancreatic enzymes. Additionally, we recorded patients’ loss of body weight during one year before surgical treatment and body mass index (BMI) as possible markers for PEI. Pancreatic endocrine function was evaluated by the presence of diabetes mellitus.
Choice of the surgical method (S-PJ or L-PJ) was based on the anatomical characteristics of PD. Patients with a uniformly dilated PD and significant strictures or calcifications in only a single location of the duct were treated using S-PJ. For patients with multiple PD strictures, calcifications and dilatations, L-PJ was performed. S-PJ was defined as the anastomosis with a length of 30 up to 50 mm; in the case of L-PJ, the length of the anastomosis was 50 mm or more (up to 100 mm).
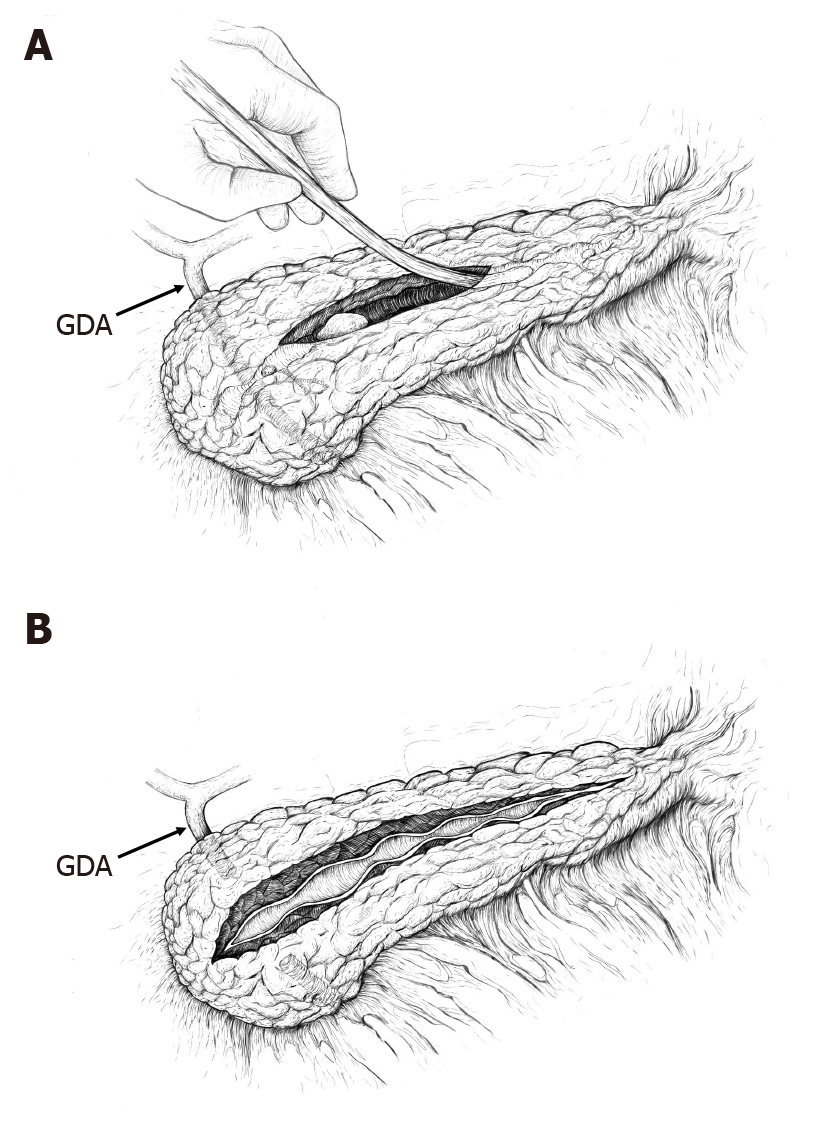
As a standardized approach, the dilated PD was opened distal to strictures or calcifications, usually in the region of the pancreatic body, after which ductotomy was extended proximally to overcome the stricture and/or to remove calcifications. The initial length of the ductotomy was usually 35-40 mm. All discovered calcifications were removed with graspers. This was followed by testing the adequacy of the drainage of the entire PD. For this, we used a 3 mm (9 Fr) metallic probe and a length of 100 mm of successful probing (proximal and distal duct together) was judged sufficient to ensure free outflow of pancreatic juice (Figure 1). If probing was successful (there were no more strictures or calcifications), a single-layer continuous PJ anastomosis with slowly absorbable suture material (4-0 polydiaxanone) was performed, involving a small portion of the transected parenchyma.
If probing was unsuccessful due to multiple PD strictures, initial ductotomy was extended beyond the last detected stricture. All calcifications were removed with graspers, and when necessary, additional ductotomy was carried out. The total length of L-PJ was dependent on the number and location of strictures and was somewhat variable (50 mm to 100 mm). However, the basic principle was the same: ductotomy has to be long enough to ensure complete decompression of the PD, which was tested by probing.
The recorded characteristics of the surgical treatment of CP were as follows: Duration of operation, intraoperative and total need for PRC (packed red cells) transfusion, morbidity, mortality and length of hospital stay. For assessment of morbidity, the Clavien-Dindo classification and comprehensive complication index (CCI) were used[12,13].
We evaluated the clinical effects of the two types of PJ by comparing the preoperative and 1-year follow-up data for both groups: QOL, intensity of chronic pancreatic pain, pain-associated role limitations, changes in pain treatment, BMI, hospital admissions due to CP, and patients’ satisfaction with surgical treatment.
Data on the QOL and characteristics of pain before and after surgery were obtained from the questionnaires completed by the patients. QOL was evaluated using the RAND 36-item Short Form Health Survey (SF-36, RAND Corporation)[14]. For assess
To highlight the surgical effect of pain treatment, we made a comparative analysis of preoperative and 1-year follow-up use of pain medications. The patients were divided into three groups: Opioid users, users of non-opioid painkillers, and patients without the need for any pain medications.
The magnitude of the effect of surgical treatment on the exacerbations of CP requiring hospital admission was calculated as the number of admissions per patient year (PY). Preoperative PY was calculated as the period from the first admission due to CP to the time of surgery. These data were compared with the data of admissions during follow-up.
For evaluation of the patients’ satisfaction with surgical treatment, we used Likert’s five-level scale (from 1 — not satisfied at all, to 5 — very much satisfied, and from 1 — much worse, to 5 — much better, as appropriate)[17]. We asked all patients to evaluate satisfaction with the results of surgical treatment in general, changes in pain characteristics after surgical treatment, and changes in QOL after surgery.
All collected data were entered in a computerized database (Microsoft Access 2016, Microsoft Inc., WA, United States). The main characteristics are presented as means with SD, or medians with the interquartile range as appropriate. Comparisons between the groups were made using the following tests: Fisher’s exact test in the case of percentages, unpaired t-test in the case of samples’ means for independent groups, paired t-test in the case of samples’ means when the samples included the same subjects, the Mann-Whitney test in the case of medians for non-parametric unpaired data groups, and Fisher’s exact test with the 95%CI in the case of PY. The software package Statistica version 13.3 (TIBCO Software, CA, United States) was employed for statistical calculations.
Between 10/1997 and 12/2020, 91 patients underwent side-to-side PJ: S-PJ in 46 patients and L-PJ in 45 patients.
A comparison of the preoperative data in these two groups revealed some anatomical and clinical differences (Table 1). The most important anatomical characteristic of the L-PJ group was the presence of multiple strictures or calcifications in the PD: the outflow of pancreatic juice was compromised in several locations, which was decisive for carrying out L-PJ.
| Characteristics | S-PJ (n = 46) | L-PJ (n = 45) | P value |
| Preoperative data | |||
| Age (yr) | 52.6 ± 9.7 | 45.6 ± 7.6 | < 0.001 |
| Male (%) | 73.9 | 88.9 | 0.116 |
| Co-morbidity (Charlson’s index) | 2 (1-3) | 1 (1-3) | 0.066 |
| Disabled persons (%) | 45.6 | 73.3 | 0.013 |
| Chronic pancreatitis | |||
| Alcoholic etiology (%) | 82.6 | 95.6 | 0.096 |
| Time from onset of pain (mo) | 18 (6-36) | 24 (10-36) | 0.420 |
| N0 of admissions due to CP | 4 (2-5) | 5 (3-7) | 0.002 |
| Rate of admissions per PY1 | 1.8 (1.5-2.1) | 2.0 (1.8-2.3) | 0.240 |
| Anatomical changes in CP | |||
| PD diameter (mm) | 6 (5-7) | 8 (7-9) | 0.002 |
| Pancreatic calcifications (%) | 58.7 | 77.8 | 0.082 |
| Pseudocysts (%) | 58.7 | 53.3 | 0.760 |
| Pancreatic endo- and exocrine function | |||
| DM (%) | 28.3 | 28.9 | 0.999 |
| BMI (kg/m2) | 23.6 ± 5.0 | 22.3 ± 3.3 | 0.161 |
| Loss of body weight (kg)2 | 9 (6-12) | 9 (5-17) | 0.366 |
| ≥ 2 symptoms of PEI (%) | 47.8 | 73.3 | 0.022 |
| Characteristics of surgery | |||
| Length of anastomosis (mm) | 40 (35-45) | 65 (60-70) | < 0.0001 |
| Duration of surgery (min) | 107.5 (85.0-139.0) | 134.0 (110.0-155.0) | 0.006 |
| IO PRC transfusion (%) | 0 | 15.6 | 0.011 |
| PRC transfusion in total (%) | 2.2 | 31.1 | 0.001 |
| Length of stay (d) | 8.5 (8.0-11.0) | 9.0 (8.0-11.0) | 0.668 |
| Morbidity (%) | 6.5 | 17.8 | 0.182 |
| CCI3 | 26.6 (20.9-29.6) | 20.9 (20.9-34.6) | 0.919 |
| Mortality (%) | 0 | 0 | |
Patients in the L-PJ group, compared to those in the S-PJ group, were significantly younger (45.6 years vs 52.6 years), had more previous admissions due to CP (5 vs 4), and had a larger main PD (8.0 mm vs 6.0 mm); the proportion of disabled persons was higher (73.3% vs 45.7%), as well as the proportion of patients with ≥ 2 symptoms of PEI (73.3% vs 47.8%). Also, the proportion of patients with alcoholic etiology (95.6% vs 82.6%) and pancreatic calcifications (77.8% vs 58.7%) was higher in this group, but these differences were statistically nonsignificant.
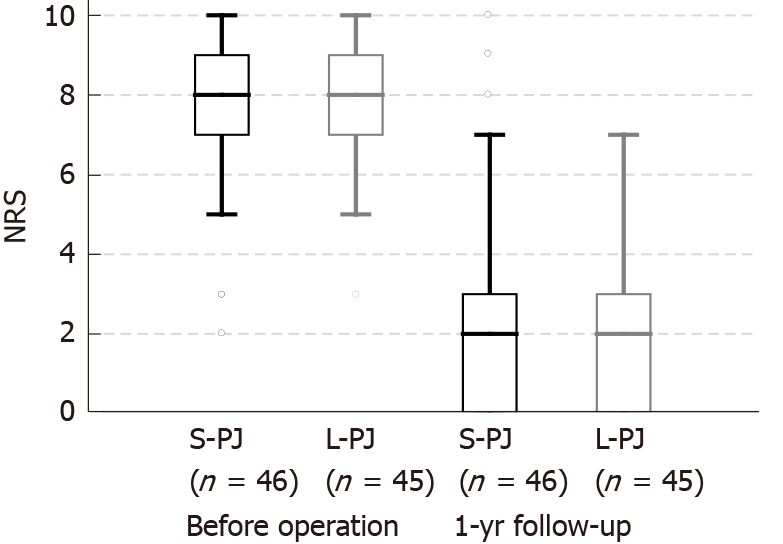
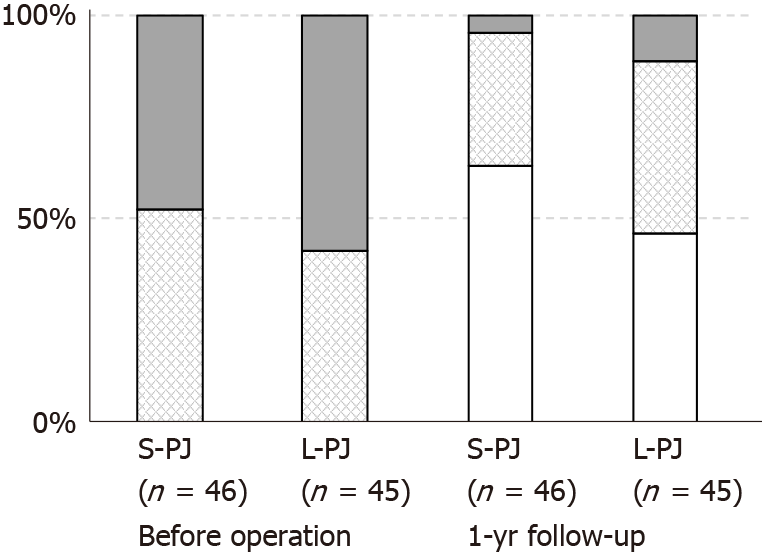
There were no differences between the groups regarding patients’ gender, time from onset of chronic pain, endocrine insufficiency, BMI, loss of body weight or proportion of patients with pancreatic pseudocysts. Pain characteristics (NRS and PDI) did not differ between the groups before surgery (Figures 2 and 3). Approximately half of the patients required pain treatment with opioids (45.7% in the S-PJ group and 57.8% in the L-PJ group, Figure 4). The preoperative characteristics of QOL were similar for both groups (Figure 5).
The indications for surgical treatment were chronic intractable pain in 79 cases (86.8%) and complications of CP associated with intraductal hypertension in 12 cases (13.2%). There were no differences in the indications between the groups.
Assessment of the surgical characteristics of PJ revealed significantly shorter operating time (107.5 min vs 134.0 min), lower need for intraoperative PRC transfusion (0% vs 15.6%), as well as for total PRC transfusion in the perioperative period (2.2% vs 31.1%) in the S-PJ group (Table 1).
In addition, morbidity was lower in the S-PJ group (6.5% vs 17.8%), but this difference was statistically nonsignificant. The total number of complications was 11; most of them were mild according to the Clavien-Dindo classification (grades I-II). There were only three grade III complications: in the S-PJ group there was one case of peripancreatic fluid collection (grade IIIa), which was percutaneously drained. In the L-PJ group there were two cases of postoperative intra-abdominal hemorrhage (associated with pancreatic ductotomy) both of which required relaparotomy (grade IIIb). Use of CCI for evaluation of severity of complicated cases revealed no difference between the groups: median CCI was 26.6 for the S-PJ group and 20.9 for the L-PJ group. Perioperative mortality was zero in both groups.
There was no difference in the median length of hospital stay between the groups (8.5 d for S-PJ and 9.0 d for L-PJ).
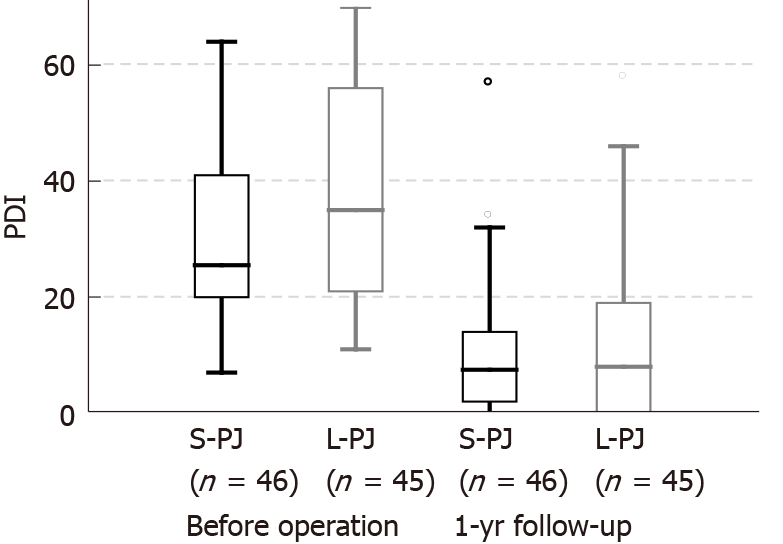
All clinical effects were assessed before surgery and one year after surgery. Pain assessment revealed significant pain reduction in both study groups without differences between them. Median NRS decrease was 6 points (8 to 2) in both groups (Figure 2). Analogously, a significant decrease in the median PDI was seen in both groups, without a significant difference between them: 18.0 points (25.5 to 7.5) in the S-PJ group and 27.0 points (35.0 to 8.0) in the L-PJ group (Figure 3). Complete or partial pain relief was then 84.8% and 88.9%, respectively.
Pain relief was correlated with marked changes in pain treatment: when before surgery all patients needed some kind of pain treatment, then one year after surgery almost two thirds of the patients in the S-PJ group (63.0%) and almost half of the patients in the L-PJ group (46.7%) did not need any pain treatment (Figure 4). The proportion of patients with the occasional need for opioids was 4.4% (two patients) in the S-PJ group and 11.1% (5 patients) in the L-PJ group; the difference between the groups was nonsignificant.
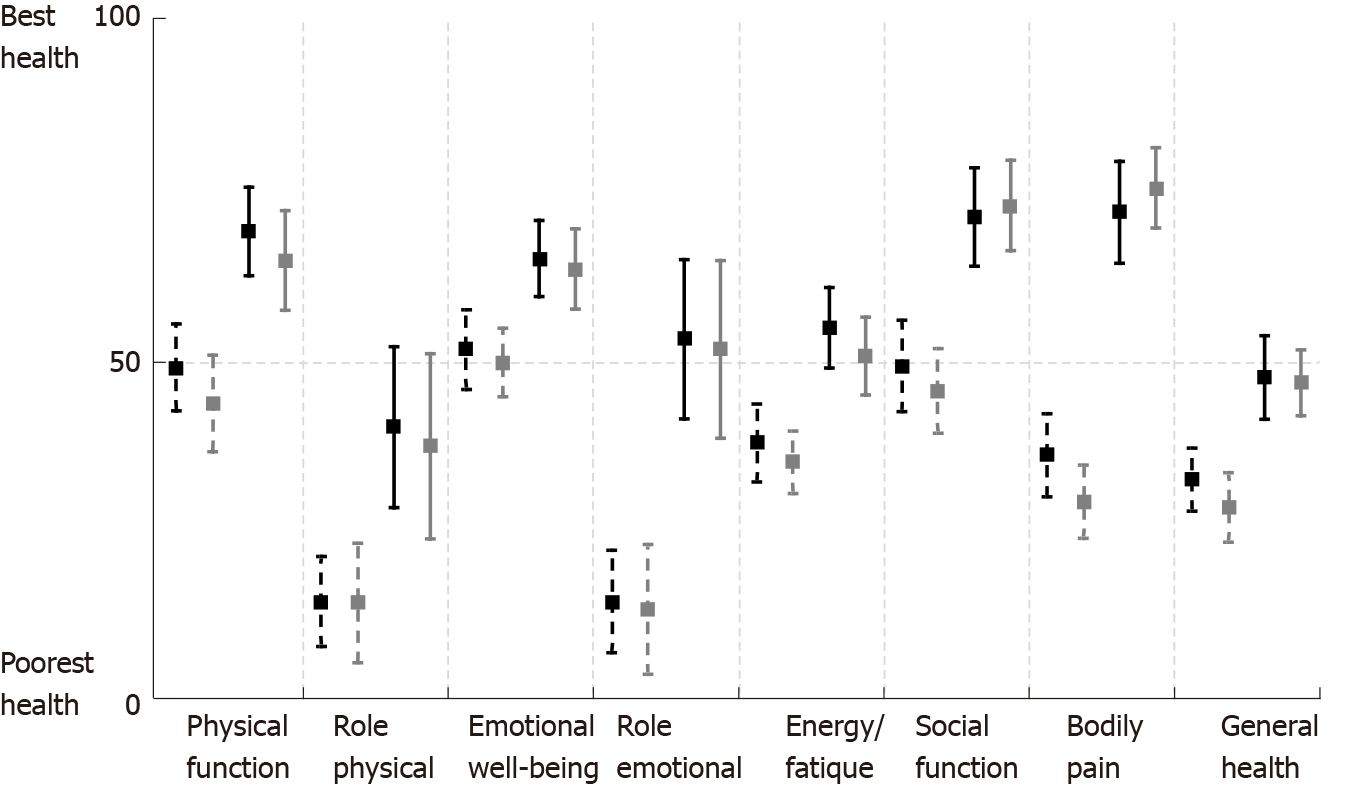
Changes in QOL were measured using the RAND SF-36 scale. All eight assessed aspects of QOL showed significant improvement in both study groups, with the most notable positive effect regarding the impact of pain on QOL and role limitations due to emotional problems (Figure 5).
Patients’ BMI increased during the first year after surgery in most cases: 75.6% in the S-PJ group and 55.8% in the L-PJ group. However, despite the high proportion of patients with weight gain, the average increase in BMI was modest, being only 1.1 and 0.4 kg/m2, respectively.
PJ showed high effectiveness in preventing new hospital admissions due to exacerbations or complications of CP in both groups. There were 1.8 (S-PJ group) and 2.0 (L-PJ group) hospital admissions because of CP per PY before surgery, which dropped to 0.1 admissions per PY in both groups after surgery.
Patients’ general satisfaction with the results of the surgical treatment of CP according to the Likert 5-point scale (1 — not satisfied at all, to 5 — very much satisfied) was very high: 4.7 in the S-PJ group and 4.9 in the L-PJ group. Changes in chronic abdominal pain were rated as much less intense, at 4.9 points compared to the baseline in both groups (1 — much more intense, to 5 — much less intense).
This retrospective study provides comparative data on aspects of the surgical treatment of CP and the clinical effects of surgery, using either S-PJ or traditional L-PJ. The S-PJ was applied in cases of an almost uniformly dilated PD and L-PJ was applied in cases with multiple ductal changes: strictures, dilatations and calcifications. According to our study, S-PJ showed better perioperative results: shorter operating time, lower need for PRC transfusion and lower rate of perioperative complications. We observed no significant difference in the clinical results regarding pain relief, improvement in QOL, weight gain, patients’ satisfaction with surgical treatment, and decrease in the rate of postoperative hospital admissions per PY due to CP.
Thus, the main outcome of our study is that for patients with a uniformly dilated PD and strictures or calcifications in a single region, S-PJ shows better operative characteristics, while the subsequent clinical effects are not inferior to those of L-PJ.
Assessment of the preoperative data showed that our study groups were similar regarding the patients’ main complaints (intensity of pain, time from onset of pain, pain medications) and QOL. At the same time, the groups were dissimilar regarding some other important aspects. The L-PJ group was characterized by a higher rate of alcoholic CP, and the patients in this group had more admissions due to CP in the history of the disease. Several studies (Hao et al[18], Dancour et al[19], and Miyake et al[20]) have shown that alcoholic CP is associated with a more aggressive disease course and a higher rate of complications compared to other etiologies. In support of these findings, the patients in the L-PJ group had more pronounced local changes in the pancreatic gland: multiple ductal changes (strictures, dilatations and calcifications) and a larger diameter of PD.
According to the predominant statement, ‘standard’ PJ necessitates the full-length anastomosis with total opening of the PD. Indeed, the obvious advantage of this approach is easy clearance of the entire PD of calcifications and full decompression of the duct[21,22]. However, variable suggestions concerning the length of PJ have been proposed. Bradley[23] stated in his review, that the length of the PJ should be at least 6 cm to gain long-term success in pain treatment; Yeo et al[24] reported having attempted to obtain a minimum of 8 cm ductotomy; Prinz et al[25] suggested that ductotomy should be carried out to within 1 cm of the ampulla of Vater and to within 1 cm of the tip of the pancreatic tail on the left side[23-25]. Regarding the extent of ductotomy, the pioneers of the method, Partington and Rochelle[10], stated in 1960: ‘uniformly dilated duct need not be opened extensively’, ‘PD split should continue somewhat right to mesenteric vessels’ and ‘it is rarely necessary to split distal portion in the tail’[10]. Some authors admit that the extent of the ductal incision does not have a fixed length; rather, ductotomy has to ensure full PD decompression. Thus, instead of the widely accepted ‘standard’, there exist slightly different practices and up to the present no comparative data have been available on the effectiveness of the shorter or longer PJ.
Despite the obvious advantages, total ductotomy has also some disadvantages and surgical risks. Unroofing of the PD is especially challenging in the region of the pancreatic head: The gastroduodenal artery (GDA) is usually located in the proximal 1.5-3 cm of the pancreatic head and has to be suture ligated superiorly and inferiorly in front of the ductotomy (Figure 1). Nevertheless, despite ligation of the GDA, the pancreatic head is still very well vascularized and ductotomy in this region is associated with a considerable risk of bleeding. Therefore, some surgeons have suggested performing partial resection of the pancreatic head in this situation (as described by Frey) as a less risky procedure compared to ductotomy[26,27].
One of the options to avoid wide ductotomy is to replace it with intraoperative instrumental exploration of the PD. We used intraoperative probing and in case we found additional calcifications or strictures, further ductotomy was performed. An alternative would be endoscopic visualization of the PD, which has been pioneered mainly by laparoscopic surgeons. Kurian and Gagner[28] used a choledochoscope for visualization of PD and Fogarty catheters for ductal clearance of calcifications; Tantia et al[29] used a 30° laparoscope to visualize the lumen of the PD and cleared the unopened part of the pancreatic head of calcifications using graspers — a procedure which the authors called ‘pancreaticodochoscopy’. Bhandarwar et al[30] suggested using a 5 mm zero-degree laparoscope to confirm ductal clearance beyond the ductotomy, while Sahoo and Kumar[31] used a cystoscope for this purpose[30,31].
The value of ductotomy in the region of the pancreatic tail is also debatable: in the splenic hilum PD is not well accessible and is narrowing anyway, so the effect of the extensive distal PD incision (up to within 1 cm of the tip of the pancreatic tail) for allowing better pancreatic juice drainage can be quite modest. Considering the above mentioned aspects, several surgeons have abandoned opening the PD in the region of the pancreatic tail (e.g., Sahoo and Kumar[31], Ceppa and Pappas[32]) and have replaced it with intraoperative exploration of the PD.
According to our study, avoiding total ductotomy provided significant benefits in terms of operating time, need for PRC transfusion, and morbidity. However, the rate of severe complications was low in both groups: only two patients in the L-PJ group needed relaparotomy due to postoperative hemorrhage, both cases being due to ductotomy in the region of the pancreatic head.
The clinical effects of the two types of PJ were evaluated one year after surgery. Both surgical options, S-PJ in the treatment of patients with a uniformly dilated PD and L-PJ in the treatment of patients with multiple ductal changes (strictures, dilatations and calcifications), were effective in resolving the main clinical problems without significant differences in the results.
The proportion of patients with pain relief was comparable to that reported in previous studies (D’Haese et al[33], Tian et al[34]). Interestingly, despite the fact that 4.4% (S-PJ) and 11.1% (L-PJ) of the patients occasionally used opioids, they rated (according to the Likert 5-point scale) abdominal pain as much less intense compared to the baseline. Some patients reported that ‘they were used to take opioids even in the case of mild pain because of effectiveness of this medication’. Patients’ general satisfaction with the results of the surgical treatment of CP was high, being on average 4.7 in the S-PJ group and 4.9 in the L-PJ group (Likert scale).
Significant improvement in QOL was evident in all eight aspects of the SF-36 tool. The most marked changes were seen in pain associated QOL and in role limitations because of emotional problems. The importance of pain in predicting QOL is well known[35]. Hence, a greater than 30-point improvement in pain associated QOL was to be expected.
One of the anticipated effects of the surgical treatment of CP is prevention of new admissions due to pain and exacerbations or complications of CP[10,36]. In this study, the effectiveness of surgical treatment in preventing new admissions was higher than 95%: there were 1.8 (in the S-PJ group) and 2.0 (in the L-PJ group) hospital admissions because of CP per PY before surgery; after surgery this indicator dropped to 0.1 admissions per PY in both groups. This effect cannot be underestimated, as it translates into a decrease of the health care burden for patients with CP. Hall et al[37] found in their systematic review that most treatment costs for patients with CP are associated with pain management. Hence effective surgical pain treatment leads to a considerable economic effect.
This study has some limitations. Firstly, as the choice of the surgical method was based on the anatomical characteristics of the PD, the study groups were dissimilar. Secondly, lack of randomization: it would be important to randomly compare patients with a uniformly dilated PD, using either S-PJ or L-PJ. Thirdly, as surgeons specialized in pancreatic surgery operated on all enrolled patients, the obtained results (zero mortality and relatively low morbidity) may not be generalizable to outcomes at hospitals that have less expertise. It has been shown that centralization of pancreatic surgery is important and its beneficial effect is associated in particular with better short-term results after surgery[38].
Based on our data, in the setting of a uniformly dilated PD, S-PJ provides adequate decompression of PD. As the clinical outcomes following S-PJ are not inferior to those of L-PJ, S-PJ should be preferred as a surgical option in the case of a uniformly dilated PD.
The Partington-Rochelle pancreaticojejunostomy (PJ) is an essential management option in patients with chronic pancreatitis (CP) associated with intractable pain and a dilated pancreatic duct (PD). Wide ductotomy and long PJ (L-PJ) have been advocated as the standard of care to ensure full PD decompression. Nevertheless, the role of short PJ (S-PJ) in uniformly dilated PD has not yet been evaluated.
The aim of this study was to evaluate the possible advantages and disadvantages of S-PJ and L-PJ and to interpret the perspective of S-PJ in the treatment of CP.
We hypothesized that S-PJ and L-PJ ensure comparable clinical outcomes. The primary outcomes were pain relief and quality of life, secondary outcomes were perioperative characteristics, body weight, patients’ satisfaction with treatment, and readmissions rate due to CP.
A retrospective review of prospectively collected cohort data was conducted on surgically treated CP patients subjected to side-to-side PJ. The length of PJ adapted to anatomical alterations in PD: A S-PJ (< 50 mm) in uniformly dilated PD, and a L-PJ (50-100 mm), in the setting of multiple PD strictures, calcifications and dilatation were compared.
S-PJ resulted in improved perioperative outcomes: significantly shorter operative time (107.5 min vs 134 min), lower need for intraoperative (0% vs 15.6%) and total (2.2% vs 31.1%) blood transfusions, and lower rate of perioperative complications (6.5% vs 17.8%). We noted no significant difference in pain relief, improvement in quality of life, body weight gain, patients’ satisfaction with surgical treatment, and readmission rate due to CP.
Based on our data, in the setting of a uniformly dilated PD, the S-PJ provides adequate decompression of the PD. As the clinical outcomes following S-PJ are not inferior to those of L-PJ, S-PJ should be preferred as a surgical option in a uniformly dilated PD.
It would be important to compare randomly selected patients with uniformly dilated PD using either S-PJ or L-PJ.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Estonia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhao CF S-Editor: Gao CC L-Editor: Webster JR P-Editor: Gao CC
| 1. | Majumder S, Chari ST. Chronic pancreatitis. Lancet. 2016;387:1957-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 254] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 2. | Murruste M, Kirsimägi Ü, Kase K, Saar S, Talving P. Long-term survival, risk factors and causes of mortality in surgically treated chronic pancreatitis. Pancreatology. 2021;21:714-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 3. | Jawad ZAR, Kyriakides C, Pai M, Wadsworth C, Westaby D, Vlavianos P, Jiao LR. Surgery remains the best option for the management of pain in patients with chronic pancreatitis: A systematic review and meta-analysis. Asian J Surg. 2017;40:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Kleeff J, Stöß C, Mayerle J, Stecher L, Maak M, Simon P, Nitsche U, Friess H. Evidence-Based Surgical Treatments for Chronic Pancreatitis. Dtsch Arztebl Int. 2016;113:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Strobel O, Büchler MW, Werner J. Surgical therapy of chronic pancreatitis: indications, techniques and results. Int J Surg. 2009;7:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Andren-Sandberg A, Hafström A. Partington–Rochelle: when to drain the pancreatic duct and why. Dig Surg. 1996;13:109-112. |
| 7. | Friess H, Berberat PO, Wirtz M, Büchler MW. Surgical treatment and long-term follow-up in chronic pancreatitis. Eur J Gastroenterol Hepatol. 2002;14:971-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Wani NA, Parray FQ, Wani MA. Is any surgical procedure ideal for chronic pancreatitis? Int J Surg. 2007;5:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Parekh D, Natarajan S. Surgical Management of Chronic Pancreatitis. Indian J Surg. 2015;77:453-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Partington PF, Rochelle RE. Modified Puestow procedure for retrograde drainage of the pancreatic duct. Ann Surg. 1960;152:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 217] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 38304] [Article Influence: 1008.0] [Reference Citation Analysis (0)] |
| 12. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24842] [Article Influence: 1183.0] [Reference Citation Analysis (0)] |
| 13. | Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1297] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 14. | RAND Corporation. 36-Item Short Form Survey (SF-36). [cited May 10, 2021]. In: RAND Corporation [Internet]. Available from: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html. |
| 15. | Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006;15 Suppl 1:S17-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 772] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 16. | Chibnall JT, Tait RC. The Pain Disability Index: factor structure and normative data. Arch Phys Med Rehabil. 1994;75:1082-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 245] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Likert R. A Technique for the Measurement of Attitudes. Arch Psychol. 1932;140:1-55. |
| 18. | Hao L, Wang LS, Liu Y, Wang T, Guo HL, Pan J, Wang D, Bi YW, Ji JT, Xin L, Du TT, Lin JH, Zhang D, Zeng XP, Zou WB, Chen H, Xie T, Li BR, Liao Z, Cong ZJ, Xu ZL, Li ZS, Hu LH. The different course of alcoholic and idiopathic chronic pancreatitis: A long-term study of 2,037 patients. PLoS One. 2018;13:e0198365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Dancour A, Lévy P, Milan C, Bernades P. [Natural history of non-alcoholic chronic pancreatitis. Study of 37 cases and comparison with 319 cases of alcoholic chronic pancreatitis]. Gastroenterol Clin Biol. 1993;17:915-924. [PubMed] |
| 20. | Miyake H, Harada H, Ochi K, Kunichika K, Tanaka J, Kimura I. Prognosis and prognostic factors in chronic pancreatitis. Dig Dis Sci. 1989;34:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Sudo T, Murakami Y, Uemura K, Hashimoto Y, Kondo N, Nakagawa N, Sueda T. Short- and long-term results of lateral pancreaticojejunostomy for chronic pancreatitis: a retrospective Japanese single-center study. J Hepatobiliary Pancreat Sci. 2014;21:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Greenlee HB, Prinz RA, Aranha GV. Long-term results of side-to-side pancreaticojejunostomy. World J Surg. 1990;14:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Bradley EL 3rd. Long-term results of pancreatojejunostomy in patients with chronic pancreatitis. Am J Surg. 1987;153:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 140] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Yeo JC, Kennedy EP, Lillemoe KD. Roux-en-Y Lateral Pancreaticojejunostomy for Chronic Pancreatitis. In: Lillemoe K, Jarnagin W. Hepatobiliary and Pancreatic Surgery. Philadelphia Lippincott Williams & Wilkins, 2013: 137-146. |
| 25. | Prinz RA, Gaffud M, Edwards M. Pancreatic duct drainage procedures. In: Beger HG, Matsuno S, Cameron JL. Diseases of the Pancreas. Berlin Springer-Verlag, 2008: 387. |
| 26. | Sakorafas GH, Sarr MG. Tricks in the technique of lateral pancreaticojejunostomy. Eur J Surg. 2000;166:498-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 27. | Ho HS, Frey CF. The Frey procedure: local resection of pancreatic head combined with lateral pancreaticojejunostomy. Arch Surg. 2001;136:1353-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Kurian MS, Gagner M. Laparoscopic side-to-side pancreaticojejunostomy (Partington-Rochelle) for chronic pancreatitis. J Hepatobiliary Pancreat Surg. 1999;6:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Tantia O, Jindal MK, Khanna S, Sen B. Laparoscopic lateral pancreaticojejunostomy: our experience of 17 cases. Surg Endosc. 2004;18:1054-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Bhandarwar A, Arora E, Gajbhiye R, Gandhi S, Patel C, Wagh A, Kothari P, Jadhav S. Laparoscopic lateral pancreaticojejunostomy: an evolution to endostapled technique. Surg Endosc. 2019;33:1749-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Sahoo MR, Kumar A. Laparoscopic longitudinal pancreaticojejunostomy using cystoscope and endoscopic basket for clearance of head and tail stones. Surg Endosc. 2014;28:2499-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Ceppa EP, Pappas TN. Modified puestow lateral pancreaticojejunostomy. J Gastrointest Surg. 2009;13:1004-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | D'Haese JG, Ceyhan GO, Demir IE, Tieftrunk E, Friess H. Treatment options in painful chronic pancreatitis: a systematic review. HPB (Oxford). 2014;16:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Tian X, Ma Y, Gao H, Zhuang Y, Yang Y. Surgical options for control of abdominal pain in chronic pancreatitis patients. J Pain Res. 2019;12:2331-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Machicado JD, Amann ST, Anderson MA, Abberbock J, Sherman S, Conwell DL, Cote GA, Singh VK, Lewis MD, Alkaade S, Sandhu BS, Guda NM, Muniraj T, Tang G, Baillie J, Brand RE, Gardner TB, Gelrud A, Forsmark CE, Banks PA, Slivka A, Wilcox CM, Whitcomb DC, Yadav D. Quality of Life in Chronic Pancreatitis is Determined by Constant Pain, Disability/Unemployment, Current Smoking, and Associated Co-Morbidities. Am J Gastroenterol. 2017;112:633-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 36. | Diener MK, Hüttner FJ, Kieser M, Knebel P, Dörr-Harim C, Distler M, Grützmann R, Wittel UA, Schirren R, Hau HM, Kleespies A, Heidecke CD, Tomazic A, Halloran CM, Wilhelm TJ, Bahra M, Beckurts T, Börner T, Glanemann M, Steger U, Treitschke F, Staib L, Thelen K, Bruckner T, Mihaljevic AL, Werner J, Ulrich A, Hackert T, Büchler MW; ChroPac Trial Group. Partial pancreatoduodenectomy vs duodenum-preserving pancreatic head resection in chronic pancreatitis: the multicentre, randomised, controlled, double-blind ChroPac trial. Lancet. 2017;390:1027-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 37. | Hall TC, Garcea G, Webb MA, Al-Leswas D, Metcalfe MS, Dennison AR. The socio-economic impact of chronic pancreatitis: a systematic review. J Eval Clin Pract. 2014;20:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Ahola R, Sand J, Laukkarinen J. Centralization of Pancreatic Surgery Improves Results: Review. Scand J Surg. 2020;109:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |