Published online Dec 27, 2021. doi: 10.4240/wjgs.v13.i12.1628
Peer-review started: March 17, 2021
First decision: May 4, 2021
Revised: May 17, 2021
Accepted: November 24, 2021
Article in press: November 24, 2021
Published online: December 27, 2021
Processing time: 281 Days and 11.1 Hours
With the increasing use of extended-criteria donor organs, the interest around T-tubes in liver transplantation (LT) was restored whilst concerns regarding T-tube-related complications persist.
To describe insertion and removal protocols implemented at our institution to safely use pediatric rubber 5-French T-tubes and subsequent outcomes in a consecutive series of adult patients.
Data of consecutive adult LT patients from brain-dead donors, treated from March 2017 to December 2019, were collected (i.e., biliary complications, adverse events, treatment after T-Tube removal). Patients with upfront hepatico-jejunostomy, endoscopically removed T-tubes, those who died or received retransplantation before T-tube removal were excluded.
Seventy-two patients were included in this study; T-tubes were removed 158 d (median; IQR 128-206 d) after LT. In four (5.6%) patients accidental T-tube removal occurred requiring monitoring only; in 68 (94.4%) patients Nelaton drain insertion was performed according to our protocol, resulting in 18 (25%) patients with a biliary output, subsequently removed after 2 d (median; IQR 1-4 d). Three (4%) patients required endoscopic retrograde cholangiopancreatography (ERCP) due to persistent Nelaton drain output. Three (4%) patients developed suspected biliary peritonitis, requiring ERCP with sphincterotomy and nasobiliary drain insertion (only one revealing contrast extravasation); no patient required percutaneous drainage or emergency surgery.
The use of pediatric rubber 5-French T-tubes in LT proved safe in our series after insertion and removal procedure refinements.
Core Tip: The use of small caliber T-tubes and a peculiar insertion technique minimize the size of the choledochotomy and reduce the chance of T-tube related adverse events; a careful T-tube removal procedure with the insertion of a temporary Nelaton drain mitigates the risk of uncontrolled biliary fistula and the need for emergency procedures.
- Citation: Spoletini G, Bianco G, Franco A, Frongillo F, Nure E, Giovinazzo F, Galiandro F, Tringali A, Perri V, Costamagna G, Avolio AW, Agnes S. Pediatric T-tube in adult liver transplantation: Technical refinements of insertion and removal. World J Gastrointest Surg 2021; 13(12): 1628-1637
- URL: https://www.wjgnet.com/1948-9366/full/v13/i12/1628.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i12.1628
Duct-to-duct biliary anastomosis is the most common technique of biliary reconstruction in deceased-donor liver transplantation (LT). In the past, T-tubes have been routinely used during biliary anastomoses in LT because it provides easy access to the biliary tree, and maintains lower pressure inside the biliary system. Furthermore, quality and quantity of bile production can be monitored and the occurrence of anastomotic strictures and early bile leaks can be reduced. However, in the 90’s, many centers stopped using T-tubes based on growing evidence of safe duct-to-duct biliary reconstruction without biliary splinting, and randomized trials demonstrated non-inferior results without the use of T-tubes[1,2]. In addition, concerns regarding the risk of biliary fistulae, major adverse events following T-tube removal and reduced cost-effectiveness were reported[3,4]. Since nowadays more marginal organs are accepted (e.g., steatotic livers, liver donation after cardiac death, and reconditioned grafts) the usefulness of T-tubes in LT is newly discussed. Two single-center randomized trials showed improved results using T-tubes; in particular, a reduced incidence and severity of biliary complications and anastomotic strictures were reported[5,6]. The risk of biliary peritonitis after T-tube removal seems to be attenuated using rubber instead of silicone-coated T-tubes. No consensus around the benefits of T-tube use in LT has been achieved yet, even though evidences were reassessed with several meta-analyses[7-10].
Our center continued to use T-tubes in LT, and the insertion technique, removal protocol and device types were continuously modified and updated over the years with the intent to minimize the risk of T-tube-related adverse events. In particular, a pediatric T-tube in adult patients was implemented to minimize the size of the necessary choledochothomy and possibly post-removal complications. In this study, we describe insertion and removal protocols implemented at our institution for the safe use of pediatric rubber 5-French T-tubes and subsequent outcomes in a consecutive series of adult patients.
Consecutive adult patients who underwent LT, between March 2017 and December 2019, after the introduction of a pediatric 5-French rubber T-tube at our unit, were included in this retrospective analysis; enrollment was limited to December 2019 to allow a minimum follow-up of 6 mo.
Perioperative and follow-up data were collected including: recipients, donors and intraoperative assessment, early bile leaks (defined as bile leakage from surgical drains or wounds within 30 d from LT), accidental T-tube removal, endoscopic and interventional radiology procedures on the bile ducts, time to T-tube removal, biliary fistulae after T-tube removal, episodes of biliary peritonitis and need for surgical intervention. Biliary fistulae were divided in uncontrolled and controlled depending whether the patients developed biliary peritonitis or not.
The primary objective of our study was to investigate the safety profile of T-tube insertion and removal techniques in LT recipients defined as the incidence of T-tube removal related biliary complications. We analyzed the incidence of biliary complications after T-tube removal in patients who received a duct-to-duct biliary anastomosis over a 5-French pediatric rubber T-tube, which was subsequently removed without instrumental aid [e.g., endoscopic retrograde cholangiopancreatography (ERCP)-assisted or during surgery]. In particular, we focused on the need for endoscopic, interventional radiology or surgical treatment after T-tube removal. Even patients with an accidental T-tube removal were included in our analysis to evaluate whether our insertion technique is protective against bile leaks in such circumstance. Patients who had the T-tube removed during endoscopic interventions on the bile ducts were excluded and summarized separately. Patients with an up-front bilio-enteric anastomosis, those who died or received retransplantation before T-tube removal were excluded.
The study protocol was approved by the Ethics Committee of the Fondazione Policlinico Universitario Agostino Gemelli IRCCS (protocol No. 3796).
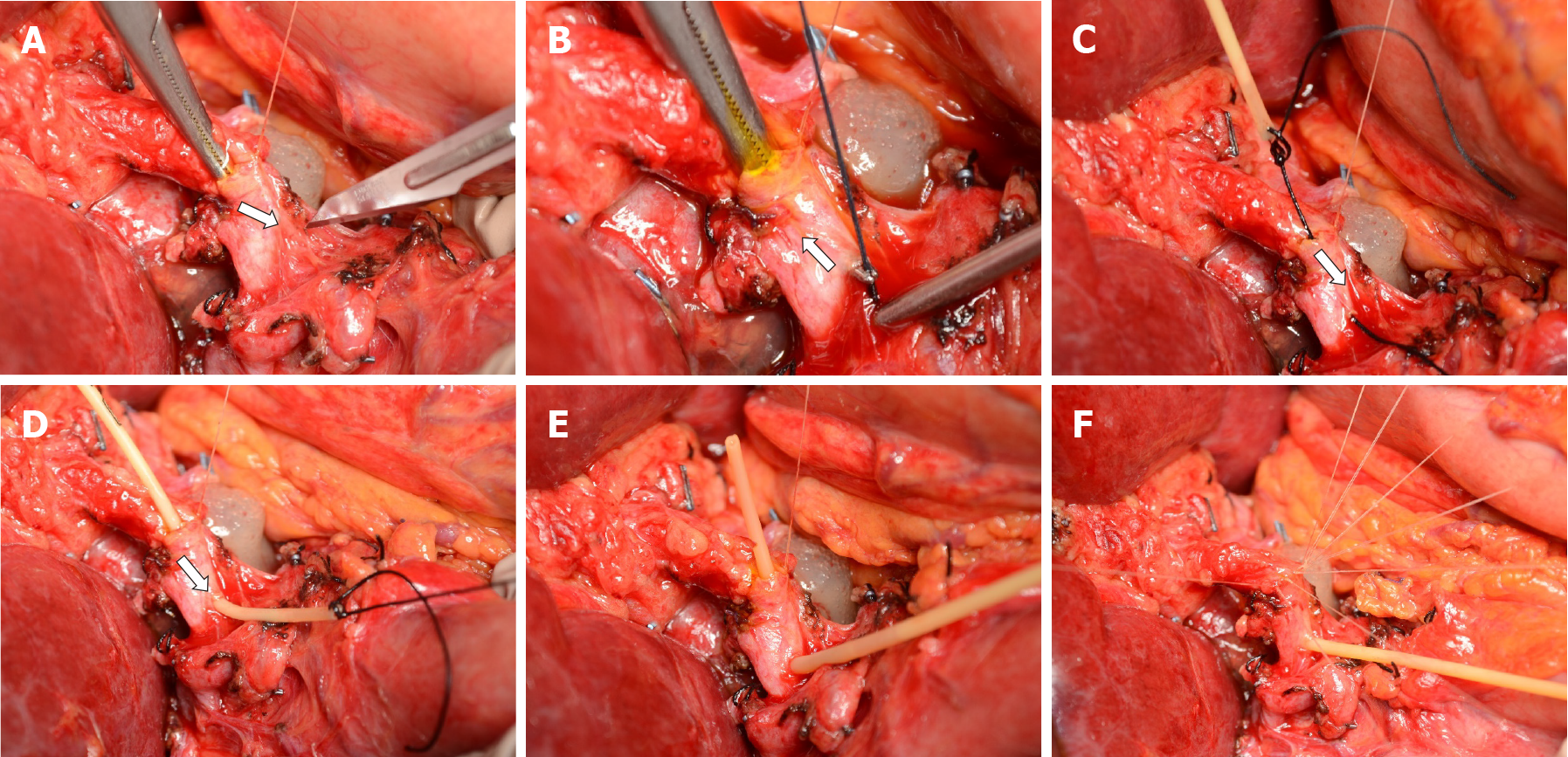
The policy of our hospital foresees routine placement of T-tubes in all liver transplants except for bilio-enteric anastomoses. Our standard biliary reconstruction technique is an end-to-end duct-to-duct anastomosis with interrupted Vicryl 6/0 stitches and extraluminal knots. Recipient’s and graft bile duct ends are trimmed short enough to obtain a straight, non-redundant bile duct to avoid kinking after minimizing liver cephalad and duodenum caudal retraction. Firstly, the posterior half of the anastomosis is sewn with interrupted stitches. Then, a T-tube is inserted through a small choledochotomy approximately 2 cm caudal to the anastomosis. In March 2017, we adopted pediatric 5-French rubber T-tubes (Bard Medical, GA) as our standard device. Main steps of T-tube insertion are shown in Figure 1. The smallest right-angle dissector in our DDLT set (6¼ inches Mixter forceps, Aesculap, DE) is advanced inside the recipient bile duct through the open anterior half of the anastomosis; the tip of the instrument is pushed against the anterior wall of the choledochus; the resulting bulge on the choledochus is incised with a no. 11 scalpel (Figure 1A). This allows creating a choledochotomy < 2 mm in size which is necessary to advance the tip of the right-angle dissector. Grabbing and pulling the T-tube is avoided as this would require opening the jaws of the right-angle and inevitably expand the size of the choledochotomy. Instead, a 3/0 silk tie is pulled through the choledochotomy (Figure 1B) and stitched to the horizontal end of the T-tube (Figure 1C). Only then the silk tie is completely pulled through, which allows the T-tube to slide through the choledochotomy to obtain a perfect fit of the T-tube (Figure 1D). The lower branch of the vertical portion of the T-tube usually self-allocates inside the distal choledochus (Figure 1E) while the upper branch is placed with forceps. The anterior wall of the anastomosis is completed (Figure 1F) with interrupted stitches and the T-tube is externalized through the right upper quadrant, cranial to the transverse skin incision. A video of the insertion technique is provided separately (see supplementary material).
During the first week after LT, bile output is collected daily for quantitative and qualitative assessment to aid postoperative patient management. Before discharge, a T-tube cholangiogram is performed and, in absence of biliary anomalies, the T-tube is capped and secured underneath a wound dressing, which is kept in place for approximately 3 mo. T-tube cholangiograms are performed on demand. In order to insure adequate T-tube management after discharge at home, patients receive care instructions. During outpatient visits, T-tubes are evaluated by dedicated transplant staff.
T-tube removal is planned approximately 90 d after LT as an in-patient procedure. Directly after admitting the patients, history and examination are taken and transplant records are reviewed in search of details that could predict a higher risk of biliary complications from T-tube removal (e.g., discrepancy in ducts caliber graft > recipient, complex arterial reconstruction, arterial thrombosis). Patients without evidence of increased risk proceed directly with the removal after the administration of a smooth-muscle relaxant and antibiotic prophylaxis. Patients with increased risk of biliary complications receive a T-tube cholangiogram to detail biliary anatomy and to anticipate critical anatomical conditions to insure safe T-tube removal. If the cholangiogram reveals anatomical problems, the bile duct is prophylactically stented during ERCP or the removal proceeds under fluoroscopic guidance and a stent is inserted on demand. An abdominal ultrasound is also performed in high-risk patients to acquire baseline information before the T-tube is removed.
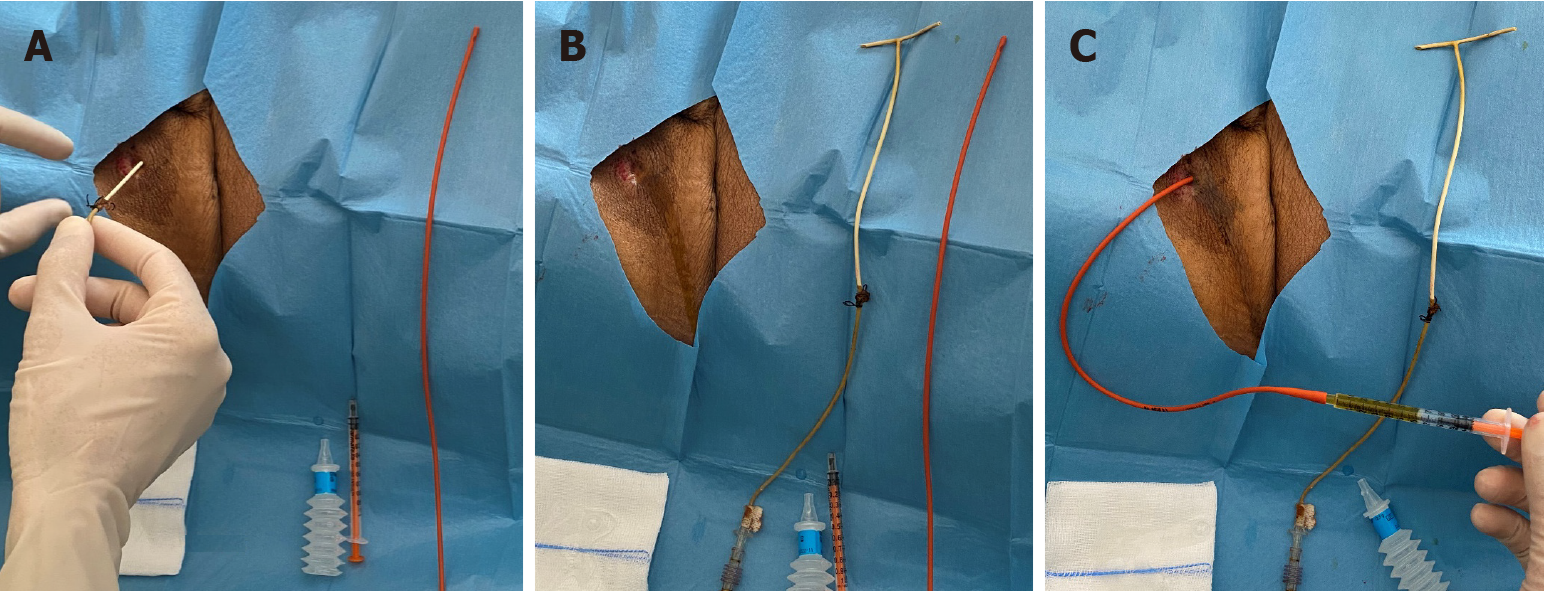
Figure 2 shows a bedside, standard removal procedure. After informed consent, the patient’s abdomen is prepped and draped and local anesthetic is administered around the T-tube exit site. The T-tube is removed and the length between the exit site and the end of the T-tube is measured (Figure 2A and B). Through the exit site of the T-tube, a flexible 8-French Nelaton drain (Teleflex Medical, PA) is advanced 2-3 cm shorter than the length measured on the T-tube with the aim to reach the space near the choledochotomy without entering it accidentally. The drain is secured to the skin with a stitch and connected to a drain bag. The insertion of a Nelaton drain aims at draining possible bile leaks from the choledochotomy. An abdominal ultrasound is performed in search of intrabdominal fluid collections when clinically indicated. The following day, the Nelaton drain is removed, if no bile leak was present, or retracted by approximately 2 cm in case of bile output. The same process is repeated on the following day until the drain is clear of bile and the patient can be discharged.
During the study period, 96 patients received 97 Liver transplants in our center: 92 had an end-to-end duct-to-duct anastomosis over a 5 French T-tube and 5 received an up-front hepaticojejunostomy. Seven patients (five with biliary complications from ischemic cholangiopathy) required endoscopic intervention and removal of the T-tube together with endoscopic biliary stenting: One patient with a bile leak on day 28 post-LT required hepaticojejunostomy after failed endoscopic management attempts due to bile duct end necrosis; four patients had non-anastomotic biliary strictures, and two had biliary stones. After applying the exclusion criteria, 72 patients were included in the study. The characteristics of the study population are reported in Table 1.
| Variable | Study population n = 72, median (IQR)/n |
| Male sex | 61 (84.7%) |
| Recipient age (yr) | 57 (50-61) |
| Body mass index (kg/m2) | 27 (23-29) |
| Underlying liver disease | |
| Hepatitis C virus | 21 (29.2%) |
| Hepatitis B virus | 7 (9.7%) |
| Alcohol-related liver disease | 25 (34.7%) |
| Primary biliary cirrhosis | 2 (2.8%) |
| Polycystic liver disease | 2 (2.8%) |
| Acute liver failure | 4 (5.6%) |
| Other | 11 (15.3%) |
| HCC | 41 (56.9%) |
| MELD score | 17 (12-22) |
| Donor age (yr) | 62 (45-73) |
| Use of temporary porto-caval shunt | 29 (40.3%) |
| Use of veno-venous bypass | 6 (8.3%) |
| Total ischemia time (min) | 435 (390-488) |
Accidental T-tube removal occurred in four (5.6%) cases, on days 11, 13, 87 and 122 post-LT, respectively, and none required active treatment (abdominal ultrasound showing no signs of bile leak).
T-tube cholangiograms were performed in all patients before capping the T-tube. Removal of the T-tube took place as an in-patient procedure, after a median of 158 d (IQR 128-206 d) post-LT. Twenty-five (34.7%) patients received a T-tube cholangiogram before removal, because of deranged liver function tests (n = 14) followed by a history of complex anatomy or complications with the hepatic artery (n = 11). There were no cases of incomplete T-tube removal (i.e., no retained broken pieces after extraction). In 68 (94.6%) cases it was possible to insert a Nelaton drain through the exit site of the T-tube. Of these, 18 (25%) patients had a biliary output from the Nelaton drain, which was removed after a median of 2 d (IQR 1-4 d) from insertion. Table 2 summarizes management details of the study population. Three (4%) patients had persistent output from the Nelaton drain despite the progressive retraction on a daily basis, as described in the protocol. Therefore, the patients underwent ERCP with endoscopic sphincterotomy and temporary naso-biliary drain (n = 1), endoscopic stenting (n = 1), while one patient required hepatico-jejunostomy due to a tight bile duct stenosis. Three (4%) patients without output from the Nelaton drain developed symptoms of biliary peritonitis, and underwent ERCP. Of note, ERCP revealed contrast extravasation from the bile duct only in one patient; nevertheless, all three patients received sphincterotomy and temporary naso-biliary drain insertion, which was removed after symptom resolution. Altogether, biliary fistula after T-tube removal occurred in 6 patients (4% controlled and 4% uncontrolled fistula respectively). T-tube-related events are shown in Table 3.
| Variable | Study population n = 72, median (IQR)/n |
| Time to removal of T-tube (d) | 158 (128-206) |
| T-tube cholangiogram before removal | 25 (35%) |
| Nelaton drain successful insertion | 68 (94%) |
| Nelaton drain with bile output | 18 (25%) |
| Time to removal of Nelaton drain | 2 (2-4) |
| Active treatment required | |
| ERCP | 6 (8%) |
| Hepatico-jejunostomy | 1 (1%) |
| Emergency surgery | 0 |
| Events | First-line treatment | Definitive treatment | |
| Accidental T-tube removal | 4 | 4 monitoring | |
| Post T-tube removal bile leak | |||
| Controlled fistula (through Nelaton drain) | 18 | 15 onitoring | - |
| 3 ERCP | 2 stent; 1 HJ | ||
| Biliary peritonitis | 3 suspected | 3 ERCP (1 confirmed) | 3 NBD |
No patient required percutaneous drainage of bile collections or emergency surgery after T-tube removal. No patient death occurred or was related to T-tube removal.
Biliary complications from T-tube use are within the most feared and disappointing events after LT. Minimizing the risk of such complications is pivotal for maintaining positive outcomes in LT recipients. With this intent, we refined our insertion technique and removal protocol. Suspected biliary peritonitis after T-tube removal occurred only in 4% of our patients. Of these, only one patient had confirmed contrast extravasation at the time of ERCP, and, therefore, the remaining two had probably a self-limiting bile leak. Remarkably, no patient developed biliary peritonitis or bile collections requiring emergency interventional radiology procedures or surgery. Compared with a systematic review, reporting the incidence of biliary peritonitis of 5% to 33% after T-tube removal, our experience demonstrates that the attention to the insertion and removal technique is essential to preserve LT recipients’ safety[11]. As shown in clinical and animal studies, rubber have been preferred over silicone T-tubes in general surgery owing to their ability to induce a stronger fibrogenic reaction forming a pseudo-channel that conveys the bile externally after the tube is removed[12]. Keeping the choledochotomy small using a pediatric 5-French T-tube supposedly increases chances that the surrounding tissues can fold the hole in the choledochus after the T-tube is removed; this may explain our low incidence of uncontrolled post-removal biliary fistula. Furthermore, developed fistulae seemed smaller, requiring endoscopic intervention only in a few cases and emergency surgery in no cases. In addition, no bile leak from choledochotomy occurred during cholangiogram in controls, probably owing to the exact fit achieved keeping the choledochotomy the size of the T-tube.
Biliary peritonitis is a surgical emergency that can lead to sepsis and multiorgan failure, especially in the vulnerable LT population[13]. By inserting a Nelaton drain through the T-tube exit site, our removal protocol minimizes consequences of bile leaks. The placement of a straight drain by interventional radiologists, with the aim to reduce the occurrence of biliary peritonitis, has been already described in 1998[14]. Similarly, we use a Nelaton drain, which is inserted at the bedside as soon as the T-tube is removed. Some patients with biliary output from the Nelaton drain (controlled biliary fistula) could be considered as potentially saved from developing biliary peritonitis (uncontrolled fistula).
Several alternatives to conventional T-tube placement have been described. A tunneled retroperitoneal route has been proposed with the rationale to support T-tube tract development allowing to control fistula after removal[15]. Intraductal stent placement has been adopted to overcome the side effects of external T-tubes in 20 patients with a bile duct caliber < 5 mm. The downside with this approach is the need for endoscopic removal 4 to 6 mo after placement and the reported complication rate was still significant in 4 patients[16]. A randomized clinical trial is ongoing in which custom-made 2 cm-segment of an 8 French T-tube are inserted in the biliary duct without suture fixation, which is removed via ERCP and sphincterotomy 4 to 6 mo after LT[17]. Resorbable internal biliary stents for LT have been tested in vitro and have been already employed to treat refractory anastomotic strictures in LT recipients[18,19]. In a matched case–control study, a transcystic straight drain has been used with improved results compared with T-tube placement; however, the technique is not applicable in bile ducts with a low cystic duct confluence [20].
Concerns over the lack of fibrous tract formation around the T-tube have been addressed by delaying the time of removal (transplant recipients might have impaired healing processes due to the steroidal treatment and ascites formation). On the other hand, a higher rate of biliary stricture have been observed, supposedly because of the prolonged permanence of the T-tube inside the bile duct and increased risk of microbial contamination[21].
In our center, we maintained our T-tube policy, which is a limitation of our study as we have no comparative group (no-T-tube group). Moreover, our LT recipients are inevitably subjected to an extra hospital admission to remove the T-tube. The need to hospitalize patients for the removal procedure can also cause delays due to limited bed capacities, as shown in our time to T-tube removal, which is often longer than 3 mo. A relevant proportion of patients had the T-Tube removed during interventional ERCP for biliary complications mainly related to ischemic cholangiopathy, if T-tubes aggravate such complications remains unclear. The long-lasting presence of a foreign body could contaminate ischemic bile ducts and contribute to damage[22].
We managed to mitigate the risk of complications related to T-tube insertion and removal, which partly explains the reason why we did not change our policy. Altogether, we demonstrated safety, and our outcomes are not inferior to those reported in literature. With our study, we provide insight on LT management of patients who received a T-tube, which we believe could be of interest in the contemporary context of increased donor risk and regained attention around the use of T-tubes.
The use of T-tube in liver transplantation (LT) remains controversial despite being the objective of randomized trials and meta-analyses. Since the 90’s many centers stopped using T-tubes in LT. More recently, the increasing use of extended-criteria organs has revived the interest around the usefulness of T-tube in LT.
In our center, we maintained our T-tube policy refining the T-tube insertion and removal techniques continuously. Since March 2017, we have adopted a pediatric rubber 5-French T-tube for splinting the biliary duct-to-duct anastomosis in adult LT recipients.
To describe the insertion and removal protocols implemented at our institution for the safe use of pediatric rubber 5-French T-tubes and the subsequent outcomes in a consecutive series of adult patients.
We retrospectively analyzed data of consecutive adult LT recipients from brain-dead-donors, treated from March 2017 to December 2019, regarding biliary complications, adverse events, and treatment required after T-tube removal. Patients with upfront hepatico-jejunostomy, endoscopically removed T-tubes, those who died or received retransplantation before T-tube removal were excluded.
Out of 72 patients who had the T-tube removed, 68 (94.4%) had per-protocol Nelaton drain insertion through the T-tube exit site. Of these, biliary output was observed in 18 (25%) patients. The Nelaton drain was removed after 2 d (median; IQR 1-4 d). Three (4%) patients required endoscopic retrograde cholangiopancreatography (ERCP) due to persistent Nelaton drain biliary output. Three (4%) patients developed suspected biliary peritonitis, requiring ERCP with sphincterotomy and nasobiliary drain insertion (only one revealing contrast extravasation). No patients required percutaneous drainage of bile collections or emergency surgery after T-tube removal. In four (5.6%) patients accidental T-tube removal occurred, none requiring active treatment. There was no mortality associated with T-tube removal.
In our series of adult LT recipients, the use of a pediatric T-tube was safe with insertion and removal technique refinements, resulting in minor morbidity and no mortality after T-tube removal.
With the increasing use of extended-criteria donor grafts in LT, the use of T-tubes is regaining interest, regarding bile output and quality measure and for bile duct protection purposes to reduce the risk of stenosis and leaks. In this perspective, refined insertion and removal techniques are pivotal to ensure low morbidity associated with the use of the T-tube.
We would like to thank Franziska M Lohmeyer, PhD, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, for her support revising our manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Transplantation
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Perisetti A S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Vougas V, Rela M, Gane E, Muiesan P, Melendez HV, Williams R, Heaton ND. A prospective randomised trial of bile duct reconstruction at liver transplantation: T tube or no T tube? Transpl Int. 1996;9:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Scatton O, Meunier B, Cherqui D, Boillot O, Sauvanet A, Boudjema K, Launois B, Fagniez PL, Belghiti J, Wolff P, Houssin D, Soubrane O. Randomized trial of choledochocholedochostomy with or without a T tube in orthotopic liver transplantation. Ann Surg. 2001;233:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 182] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Shimoda M, Saab S, Morrisey M, Ghobrial RM, Farmer DG, Chen P, Han SH, Bedford RA, Goldstein LI, Martin P, Busuttil RW. A cost-effectiveness analysis of biliary anastomosis with or without T-tube after orthotopic liver transplantation. Am J Transplant. 2001;1:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Amador A, Charco R, Martí J, Navasa M, Rimola A, Calatayud D, Rodriguez-Laiz G, Ferrer J, Romero J, Ginesta C, Fondevila C, Fuster J, García-Valdecasas JC. Clinical trial on the cost-effectiveness of T-tube use in an established deceased donor liver transplantation program. Clin Transplant. 2007;21:548-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Weiss S, Schmidt SC, Ulrich F, Pascher A, Schumacher G, Stockmann M, Puhl G, Guckelberger O, Neumann UP, Pratschke J, Neuhaus P. Biliary reconstruction using a side-to-side choledochocholedochostomy with or without T-tube in deceased donor liver transplantation: a prospective randomized trial. Ann Surg. 2009;250:766-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | López-Andújar R, Orón EM, Carregnato AF, Suárez FV, Herraiz AM, Rodríguez FS, Carbó JJ, Ibars EP, Sos JE, Suárez AR, Castillo MP, Pallardó JM, De Juan Burgueño M. T-tube or no T-tube in cadaveric orthotopic liver transplantation: the eternal dilemma: results of a prospective and randomized clinical trial. Ann Surg. 2013;258:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Sotiropoulos GC, Sgourakis G, Radtke A, Molmenti EP, Goumas K, Mylona S, Fouzas I, Karaliotas C, Lang H. Orthotopic liver transplantation: T-tube or not T-tube? Transplantation. 2009;87:1672-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Riediger C, Müller MW, Michalski CW, Hüser N, Schuster T, Kleeff J, Friess H. T-Tube or no T-tube in the reconstruction of the biliary tract during orthotopic liver transplantation: systematic review and meta-analysis. Liver Transpl. 2010;16:705-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Paes-Barbosa FC, Massarollo PC, Bernardo WM, Ferreira FG, Barbosa FK, Raslan M, Szutan LA. Systematic review and meta-analysis of biliary reconstruction techniques in orthotopic deceased donor liver transplantation. J Hepatobiliary Pancreat Sci. 2011;18:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Sun N, Zhang J, Li X, Zhang C, Zhou X. Biliary tract reconstruction with or without T-tube in orthotopic liver transplantation: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2015;9:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 12. | Koivusalo A, Eskelinen M, Wolff H, Talva M, Mäkisalo H. Development of T-tube tracts in piglets: effect of insertion method and material of T-tubes. Res Exp Med (Berl). 1997;197:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Avolio AW, Franco A, Schlegel A, Lai Q, Meli S, Burra P, Patrono D, Ravaioli M, Bassi D, Ferla F, Pagano D, Violi P, Camagni S, Dondossola D, Montalti R, Alrawashdeh W, Vitale A, Teofili L, Spoletini G, Magistri P, Bongini M, Rossi M, Mazzaferro V, Di Benedetto F, Hammond J, Vivarelli M, Agnes S, Colledan M, Carraro A, Cescon M, De Carlis L, Caccamo L, Gruttadauria S, Muiesan P, Cillo U, Romagnoli R, De Simone P. Development and Validation of a Comprehensive Model to Estimate Early Allograft Failure Among Patients Requiring Early Liver Retransplant. JAMA Surg. 2020;155:e204095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Goodwin SC, Bittner CA, Patel MC, Noronha MA, Chao K, Sayre JW. Technique for reduction of bile peritonitis after T-tube removal in liver transplant patients. J Vasc Interv Radiol. 1998;9:986-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Navez J, Mohkam K, Darnis B, Cazauran JB, Ducerf C, Mabrut JY. Biliary Duct-to-Duct Reconstruction with a Tunneled Retroperitoneal T-Tube During Liver Transplantation: a Novel Approach to Decrease Biliary Leaks After T-Tube Removal. J Gastrointest Surg. 2017;21:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Tranchart H, Zalinski S, Sepulveda A, Chirica M, Prat F, Soubrane O, Scatton O. Removable intraductal stenting in duct-to-duct biliary reconstruction in liver transplantation. Transpl Int. 2012;25:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Goumard C, Cachanado M, Herrero A, Rousseau G, Dondero F, Compagnon P, Boleslawski E, Mabrut JY, Salamé E, Soubrane O, Simon T, Scatton O. Biliary reconstruction with or without an intraductal removable stent in liver transplantation: study protocol for a randomized controlled trial. Trials. 2015;16:598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Battistel M, Senzolo M, Ferrarese A, Lupi A, Cillo U, Boccagni P, Zanus G, Stramare R, Quaia E, Burra P, Barbiero G. Biodegradable Biliary Stents for Percutaneous Treatment of Post-liver Transplantation Refractory Benign Biliary Anastomotic Strictures. Cardiovasc Intervent Radiol. 2020;43:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Girard E, Chagnon G, Moreau-Gaudry A, Letoublon C, Favier D, Dejean S, Trilling B, Nottelet B. Evaluation of a biodegradable PLA-PEG-PLA internal biliary stent for liver transplantation: in vitro degradation and mechanical properties. J Biomed Mater Res B Appl Biomater. 2021;109:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Panaro F, Glaise A, Miggino M, Bouyabrine H, Carabalona J, Gallix B, Navarro F. Rubber transcystic drainage reduces the post-removal biliary complications in liver transplantation: a matched case-control study. Langenbecks Arch Surg. 2013;398:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Lattanzi B, Lai Q, Guglielmo N, Giannelli V, Merli M, Giusto M, Melandro F, Ginanni Corradini S, Mennini G, Berloco PB, Rossi M. Graft macrosteatosis and time of T-tube removal as risk factors for biliary strictures after liver transplantation. Clin Transplant. 2013;27:E332-E338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Pravisani R, De Simone P, Patrono D, Lauterio A, Cescon M, Gringeri E, Colledan M, Di Benedetto F, di Francesco F, Antonelli B, Manzia TM, Carraro A, Vivarelli M, Regalia E, Vennarecci G, Guglielmo N, Cesaretti M, Avolio AW, Valentini MF, Lai Q, Baccarani U. An Italian survey on the use of T-tube in liver transplantation: old habits die hard! Updates Surg. 2021;73:1381-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |