INTRODUCTION
The phenomenon of weight regain (WR) is a frequent issue in bariatric surgery and has been reported in many studies analyzing obesity recurrence, its related comorbidities and worsening of health-related quality of life[1-3]. The background of WR remains unknown and associated with high initial body mass index (BMI), insufficient lifestyle modification and lack of patient adherence to psychological support[2,4]. Many obese patients rarely change their eating habits and remain sedentary after surgery[1]. The bariatric procedures, independently from the WR phenomenon, could also be responsible for protein malnutrition, iron deficiency anemia, vitamin A deficiency, megaloblastic anemia and dumping syndrome[3].
Despite the complexity of this issue in modern medicine, there is still no consensus on the definition of WR[5]. Luckily, many options are available today ranging from behavioral interventions, drugs approved for weight loss (WL) to endoscopic procedures and revision surgery to overcome some of the factors contributing to WR[6]. It is very important to stress that all bariatric surgery treatments are temporary, and patients should be re-educated. Patient selection through a multidisciplinary approach is essential and a psychologic and/or psychiatric follow-up is necessary before and after treatment, regardless of the type of bariatric revision[7].
Furthermore, primary bariatric procedures are increasing rapidly. As stated in the recent global registry review provided from an international association[8], around 400000 of those interventions are performed annually, among which laparoscopic sleeve gastrectomy (LSG) and Roux-en-Y gastric bypass (RYGB) are the most frequent (46% and 38%, respectively)[8,9]. Nevertheless, not all patients undergoing primary bariatric interventions are able to maintain postoperative WL. In a recent prospective, long-term study of obese patients undergoing RYGB after 12 years of follow-up, 93% of them maintained a 10% WL from baseline, 70% maintained a 20% WL, while only 40% were able to maintain a 30% WL[10-12]. According to the same source, revision bariatric surgery in the United States accounts for 15.4% of all bariatric interventions, which is more than triple than in 2011[9]. Besides, up to one third of all patients undergoing LSG or RYGB will experience suboptimal WL and/or significant long-term WR, underscoring the chronic recurrent natural course of obesity and leading to an increased treatment risk and cost, especially when revision surgery is proposed without a specific personalized approach. In comparison with the primary bariatric surgery, both LSG and RYGB show inferior clinical outcomes in terms of morbidity and weight reduction due to an increased technical complexity and anatomical alterations[13,14]. Surgical revision is applied in a traditional manner in 3%–13% of cases, with a 15%-50% of adverse events, a more than double mortality rate compared to primary procedures and high medical costs[15]. A less invasive endoluminal approach, if safe and effective, could be a reasonable option offering a more favorable risk profile in these patients. Endoscopic revision is not only recommended for the WR treatment but also for the management of its complications, such as the dumping syndrome[16].
In this review, we discuss the indication, selection and feasibility of different endoscopic techniques that could be used in the treatment of WR after primary bariatric surgery.
ENDOSCOPIC OPTIONS FOR REVISION OF RYGB
Factors leading to WR after RYGB include dilation of the gastrojejunal anastomosis (GJA), mechanical dehiscence of the staples and patient-related factors like physical inactivity, psychiatric comorbidities and patient adherence to diet. According to the main United States bariatric society[17], the incidence of revisional bariatric surgery rapidly increased in the last decade, from 6% in 2011 to more than 15% in 2018. The management of revisional surgery following RYGB is not standardized yet[18]. Gastric banding revision, conversion to a distal RYGB with creation of a new ileal anastomosis and biliopancreatic diversion/duodenal switch revision represent the possible management options, together with novel endoscopic procedures, such as suturing and plication, e.g., transoral outlet reduction (TORe) or Revision Obesity Surgery Endoluminal (ROSE) and some other endoluminal procedures [e.g., sclerotherapy, mucosal cryoablation, argon plasma coagulation (APC)].
Sclerotherapy
This type of injection therapy consists of an intramuscular sodium morrhuate application close to the GJA in order to narrow the anastomosis creating a circumferential edema[19]. The endoscopic procedure is performed using a needle catheter to inject 5% sodium morrhuate solution in 2 mL aliquots around the GJA. Vomiting, pain and early satiety are the main reported symptoms in the first 2 mo. A follow-up upper endoscopy is performed after this period to assess the size of GJA, and if needed the same intervention is repeated until a diameter of 10 mm is achieved[20].
The first results showed WL in 75% of the patients at 2 mo follow-up[21]. Another study of Spaulding et al[22] analyzed 32 obese subjects with a dilated GJA undergoing sclerotherapy, showing a monthly WL rate of almost 0.4 kg. Furthermore, around 56% of patients reported WL, one third maintained the same initial weight, while around 10% presented WR. The largest series included a retrospective analysis of 231 subjects undergoing one or more sessions of sclerotherapy and showed that those receiving two or three sessions reached higher rates of weight stabilization than the single session group (90% vs 60% at 12 mo; P = 0.003). The average WL at 6 mo from the previous sclerotherapy session was 10 lbs for the entire cohort, representing 18% of the weight regained after RYGB. A subset of 32% of patients of the same cohort had higher WL at 6 mo (26 lbs). Predictors of a favorable outcome were greater WR and higher number of sclerotherapy procedures with low complication rate[23]. A prospective comparative study from Jirapinyo et al[24] analyzed 43 RYGB patients with WR comparing endoscopic suturing (9/43) vs sclerotherapy (34/43). Many parameters, such as ghrelin level, BMI, GJA diameter and eating behavior were analyzed. Endoscopic suturing technique showed a significant WL, reduction of outlet diameter and eating behavior improvement compared to the sclerotherapy group. The most relevant point highlighted by this study was the direct correlation between the post-procedural GJA size and WL, establishing the outlet reduction as a significant predictor of WL.
Cryoablation
This novel endoscopic GJA reduction technique employs a cryoablation balloon to apply a circumferential ablation of the superficial mucosal layer by a cryogen, inducing fibrosis with a subsequent reduction of the GJA size and gastric pouch volume. A retrospective study at two university hospitals was performed on subjects with WR after RYGB[25]. Pouch length > 4 cm and/or outlet size > 15 mm were considered as inclusion criteria for cryoablation. Patients were extensively informed about APC vs cryoablation procedures and about the new indication of cryoablation[26], which was performed in the caudocranial direction starting from the GJA. In the outlet, ablations were applied circumferentially and clockwise, overlapping the consecutive ablation sites for about 20%-40%. Concerning the pouch, only the greater curvature was ablated. Technical success rate for the outlet ablation was almost 90%, while for the pouch ablation was 93%. At 8 wk follow-up, the GJA size decreased from 24 to 17 mm (P < 0.001), the pouch size decreased from 5 to 4 cm (P < 0.05) and a total body WL (TBWL) of 8.1% was achieved. In the short term this new approach appears to be safe, effective and feasible for the reduction of the GJA and the pouch, deserving to be analyzed in association with suturing and plication techniques in the future.
APC
APC represents one of the simplest endoscopic techniques for treatment of WR after RYGB. The first case of APC was made up of three separate sessions, every 6 wk[27]. A 2.0 L/min flow rate and a 70 W power were applied on each session. After a 45 d follow-up a 10 mm narrow stoma was observed, experiencing slight resistance while advancing the endoscope. Repeated radiological examinations showed a transient hold up of liquid contrast and a delay of solid contrast. The subject lost around 14 kg in 10 wk and 30 kg after 1 year (weight 67 kg, BMI 29). At 1 year follow-up endoscopy, a 10 mm stable outlet was detected.
APC settings could be different according to processor type, catheter shape and technique of application. However, a non-contact technique with 1.0 L/min and 50-80 W appeared to be quite effective[19]. Patients usually undergo procedures every 8–12 wk as required, until an optimal 8–10 mm outlet size and an effective WL are achieved[28].
In order to propose the optimal APC settings for GJA thermoablation, a single-center retrospective study analyzed 217 RYGB patients treated by APC[29] for WR. Low-dose (45-55 W) vs high-dose (70-80 W) APC were compared: 53.5% patients underwent low-dose APC sessions (2.4 sessions/patient), and 46.5% patients underwent high-dose APC (1.4 sessions/patient). At 6 mo follow-up, the low- and high-dose groups reported 7.3% and 8.1% TBWL, respectively (P = 0.41). At 1 year, the low- and high-dose groups reported 5.1% and almost 10% TBWL, respectively (P = 0.008). The key point of this study reveals that the high-dose APC appears to be a valid predictor of a greater WL at 1 year follow-up.
Furthermore, a multicenter (eight obesity centers, one in the United States and seven in Brazil) retrospective study was conducted on 558 subjects undergoing APC for WR after RYGB[30]. The mean WL was considered statistically significant, being 6.5, 7.7 and 8.3 kg at 6, 12 and 24 mo, respectively (P < 0.0001). At a 1 and 2 year follow-up the group with BMI < 30 kg/m2 had a greater TBWL than the group with BMI ≥ 30 kg/m2.
Finally, a randomized controlled study was performed on patients with WR, comparing APC vs multidisciplinary approach only[31]. Two groups counting a total of 42 patients were analyzed (22 APC and 20 controls). At a 14 mo follow-up with a crossover at 6 mo, satiety and WL were significantly improved in the APC group and after crossover. A significant WL (9.73 vs + 1.38) in the APC group was observed as well as the reduction of the outlet size (P < 0.001), early satiation (P < 0.001) and improvement of quality of life (P = 0.04). However, concerning the total mean WL along the whole follow-up period almost the same WL was observed in both groups.
In terms of WL, early satiation and quality of life improvement, the management of the GJA with APC appears to be safe and effective. Many positive results in treatment of WR after RYGB gives APC the chance to be used as a dual therapy together with other restrictive endoscopic procedures.
Full-thickness suturing TORe
The introduction of full-thickness suturing technique has made an important breakthrough in the endoscopic treatment of obesity. A special role of this novel endoscopic technology, named suturing TORe (S-TORe), is reserved for the treatment of WR after RYGB. Many case reports, case series, prospective studies, systematic reviews and meta-analyses are establishing this procedure as the most frequent and commonly used.
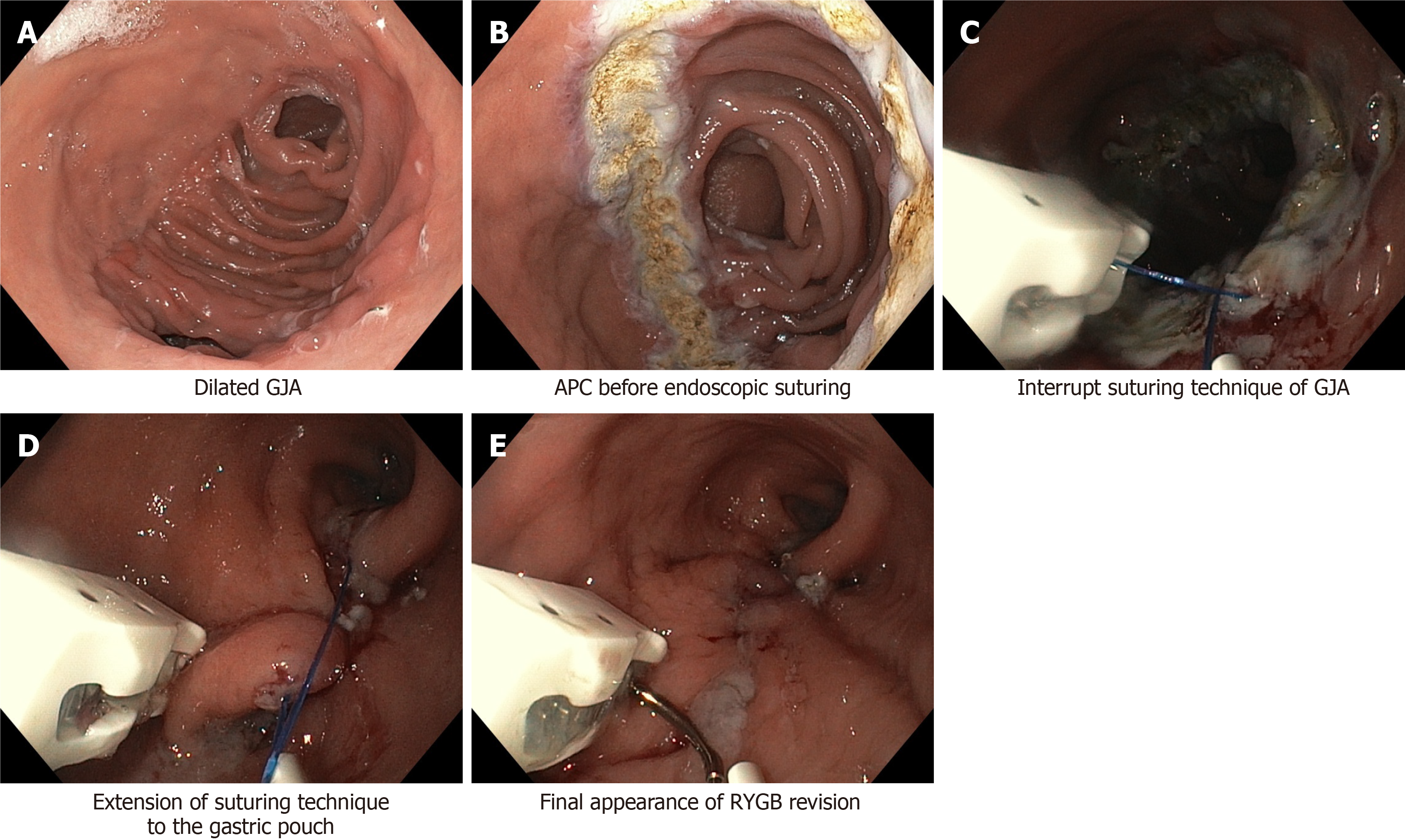
Full-thickness tissue acquisition and suturing has been improved by a new OverStitch device (Apollo Endosurgery, Austin, TX) made up of an over-the-scope single or double-channel suturing device with a curved needle driver and a catheter type tissue screw (Helix) to ensure sequential full-thickness bites (Figure 1), using a non-absorbable 2.0 polypropylene filament to provide simple running stitches or more complex suture patterns (vest-over-pants or purse-string stitches)[32,33]. The suture reloading is performed during the procedure, without removing the device out of patient.
Figure 1 Suturing transoral outlet reduction.
A: Dilated gastrojejunal anastomosis (GJA); B: Argon plasma coagulation (APC) before endoscopic suturing; C: Interrupt suturing technique of GJA; D: Extension of suturing technique to the gastric pouch; E: Final appearance of Roux-en-Y gastric bypass (RYGB) revision.
One of the main characteristics of this procedure is the possibility of its combination with APC[34] and other potentially GJA restrictive techniques, such as endoscopic submucosal dissection TORe[35]. The electrocautery injury inducing the subsequent mucosal scarring process plays a key role in GJA reduction. An enlarged GJA diameter has been demonstrated to be a significant risk factor for WR after RYGB[11], and therefore the measurement of the GJA and gastric pouch is mandatory before performing S-TORe.
The efficacy of S-TORe was highlighted in a multicenter randomized study that provided level I evidence that TORe reduces WR following RYGB[36]. Patients undergoing TORe showed a statistically significant WL from baseline (3.5%) than sham controls (0.4%). Patients undergoing TORe achieved a higher rate of WL or weight stabilization compared to controls (96% vs 78%, respectively; P < 0.019).
Some medium-term follow-up studies[37] showed safety, efficacy and durability of S-TORe in treatment of WR following RYGB[32,38]. In a study of Thompson et al[36], considering 331 RYGB subjects undergoing 342 TORe procedures, patients experienced 8.5%, 6.9% and 8.8% TBWL at 1, 3 and 5 years, respectively, with follow-up rates of 83.3%, 81.8% and 82.9%, respectively. Around 76%, 18%, 4% and 2% of all TORe procedures, were performed by single purse-string, interrupted, double purse-string and running suture patterns, respectively, with 9 ± 4 stitches per GJA on average. Reinforcement suturing of the pouch was performed with 3 ± 2 stitches on average in 57.3% of cases[32].
Another retrospective study analyzed 70 patients with WR after RYGB. On the day of S-TORe procedure, the average weight was 116 kg and BMI 42. The study showed that WL and percentage of excess WL (EWL) at follow-up were: 10.7 kg and 18.5% at 6 mo, 8.5 kg and 14.9% at 1 year, 6.9 kg and 12.2% at 2 years, 5.3 kg and 8.7% at 3 years, 3.1 kg and 3.2% at 4 years and 3.9 kg and 7.0% at 5 years. Subjects undergoing a purse-string suturing pattern or presenting a greater reduction in GJA size showed more significant %EWL[38].
In recent years, better quality data have been published in this area. A systematic review and meta-analysis[39] included in a qualitative manner 32 papers, among which 26 analyzed endoscopic full-thickness (FT) suturing, showing the following results in terms of absolute WL, EWL and TBWL: at 3 mo 8.5 kg, 21.6%, 7.3%; at 6 mo 8.6 kg, 23.7%, 8.0% and at 1 year 7.6 kg, 16.9%, 6.6%, respectively. A subgroup analysis highlighted that all these outcomes were superior in patients undergoing FT suturing combined with APC (P < 0.0001). The same meta-analysis considered 15 S-TORe studies confirming that the FT suturing was effective in treatment of WR following RYGB and showing better results in terms of WL when APC was performed prior to suturing.
Another systematic review[40] analyzed 26 studies involving all endoscopic bariatric procedures for WR and their combinations (endoscopic OverStitch device and sclerotherapy, APC or mucosal ablation). Endoscopic suturing systems showed best post-procedural results in terms of initial WL at 1 year, which were not confirmed at 18 mo. A greater sustained WL with a peak EWL of 19.9% after 18 mo follow-up was reported in only one study utilizing sclerotherapy. The greatest sustained EWL (36.4%) at 18 mo has been achieved by the combination therapy. Endoscopic suturing systems showed a better performance in terms of technical success (91.8%) and recurrence rate of WR (5%) compared to sclerotherapy or APC (46.8% and 21.5%, respectively)[40].
A further systematic review and meta-analysis[18] on S-TORe following RYGB extracted 13 studies involving 850 patients. The absolute WL at 3, 6 and 12 mo was 6.1 kg, 10.2 kg and 7.1 kg, respectively. The percent TBWL at 3, 6 and 12 mo was 6.7, 11.3 and 8.6, respectively. Among reported adverse events, abdominal pain was the most frequent (11.4%). At 1 year follow-up a significant inverse correlation between post-S-TORe GJA size and WL was observed (-0.11, P < 0.001). This study confirms safety and feasibility of S-TORe in patients with WR following RYGB.
Finally, the latest systematic review and meta-analysis was performed to summarize the two most common techniques in terms of efficacy and safety: FT suturing plus mucosal APC (ft-TORe) and mucosal APC alone (APMC-TORe)[34]. Nine ft-TORe (n = 737) and seven APMC-TORe (n = 888) studies were considered. APMC-TORe was performed as a series of sessions (mean number from 1.2 to 3.0), while a single session was mostly performed in the ft-TORe group. At 3, 6 and 12 mo after ft-TORe the percentage of TBWL was 8.0%, 9.5% and 5.8%, while after APMC-TORe was 9.0%, 10.2% and 9.5%, respectively, with no difference at 3 and 6 mo in terms of WL (P > 0.05). Greater WL with APMC-TORe and numerical trends with ft-TORe correlated with a smaller GJA size after TORe and a greater modification in GJA size. The same meta-analysis demonstrated that significant and similar WL outcomes are provided by both procedures, with good and comparable results in terms of safety. This study highlights the role of APMC-TORe, emphasizing the need for multiple endoscopic sessions as its main disadvantage over ft-TORe.
Full-thickness plicating TORe
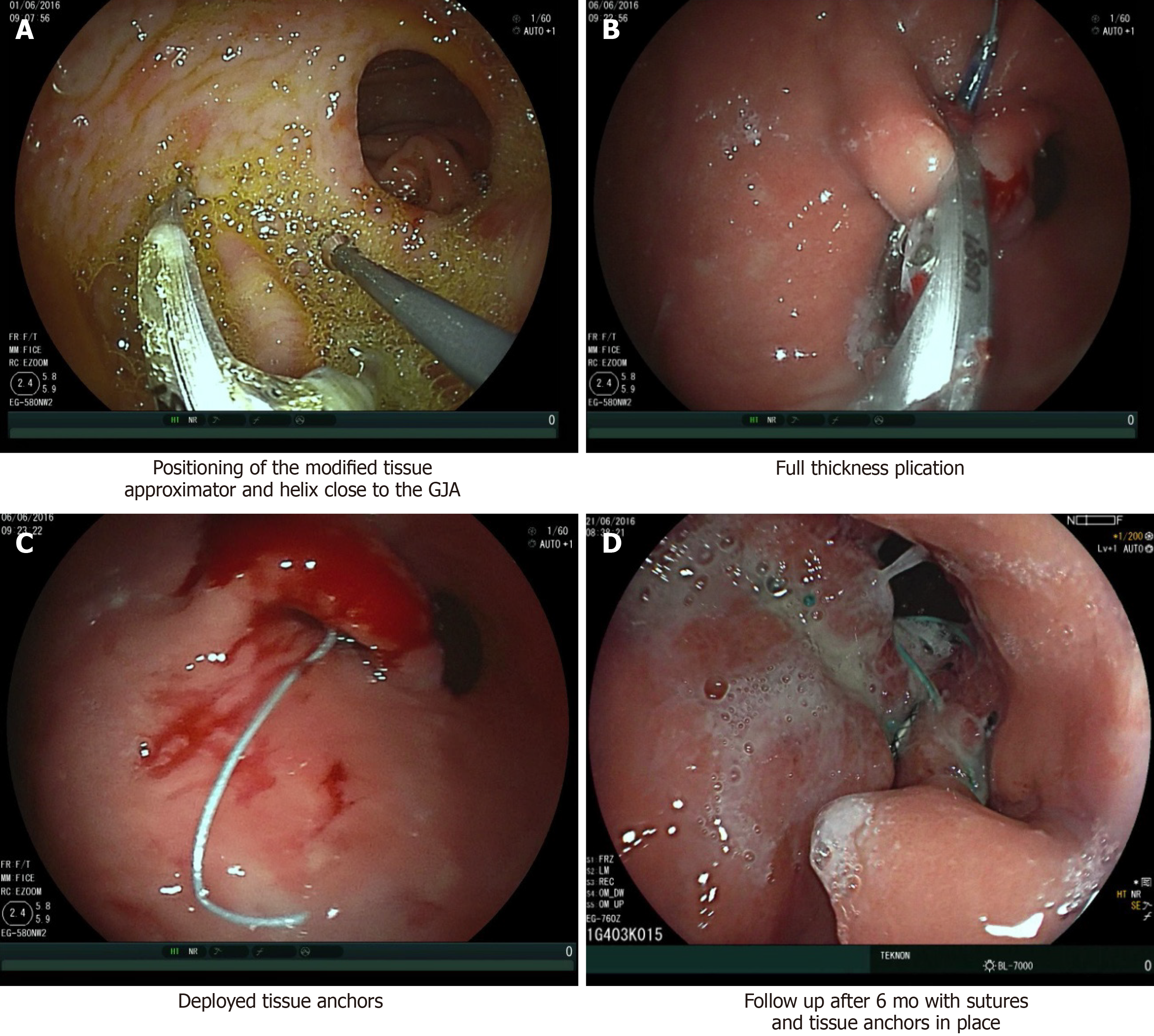
Another full-thickness technique proposed for WR following RYGB is the ROSE. This is the modified variant of the Primary Obesity Surgery Endoluminal procedure that uses the Incisionless Operating Platform (IOP; USGI, San Clemente, California). This technique is mostly focused on the management of enlarged pouch[41]. Full-thickness plications are placed by the IOP with the aim of reducing both pouch size and GJA diameter. A tissue approximator, a tissue grasper and a neonatal gastroscope are placed through the IOP. Tissue plication is performed by pulling the grasper into the approximator and aspirating the air to enlarge the plication surface. Then the needle deploys a pair of self-expanding tissue anchors, and the connecting suture is tightened (Figure 2).
Figure 2 Revision obesity surgery endoscopic (Courtesy of Dr. Roman Turró).
A: Positioning of the modified tissue approximator and helix close to the gastrojejunal anastomosis (GJA); B: Full thickness plication; C: Deployed tissue anchors; D: Follow up after 6 mo with sutures and tissue anchors in place.
A retrospective study analyzed the ROSE procedure’s outcome in 27 patients with WR following RYGB from 2008 to 2013[42]. Preoperative average pouch length and GJA size were 6.8 and 2.1 cm, respectively. On average, 4 stitches were placed. Postoperative pouch length and GJA size were 3.4 and 0.86 cm with 50% and 61% reduction, respectively. A control upper endoscopy at 3 and 12 mo was performed in 12 (46%) and 7 (28%) patients. The mean pouch length and GJA size were 5 cm (26.5% reduction) and 1.2 cm (42.9% reduction) at 3 mo and 6.14 cm (10.0% reduction) and 2.2 cm (4.7% increase) at 12 mo, respectively. The %EWL was 8.9, 9.3, 8.0, 6.7, -10.7, -13.5, -5.8, -4.5 at 3, 6, 12, 24, 36, 48, 60 and 72 mo, respectively. Although endoscopic plication achieved the expected reduction in the pouch and stoma diameter at 3 mo, the patients regained the preoperative diameter at 12 mo.
A prospective multicenter study analyzed a total of 116 consecutive subjects with WR following RYGB undergoing ROSE. The procedure was technically successful in 97% of patients, with GJA size and pouch length reduction of 50% and 44% on average, respectively. More than 30% of WR following RYGB had been lost at 6 mo after the ROSE procedure, while EWL was 18% on average. At 1 year follow-up the anchors were still in place and tissue folds were stable[43]. A further report concluded that those patients with a dilated GJA (> 12 mm) who had a post-repair diameter of < 10 mm (30% of 66 patients) had more than doubled the EWL compared with the remaining cohort (24% vs 10%; P = 0.03)[44].
Other superficial and full-thickness restrictive procedures
The development of endoscopic suturing technology has led to the safe placement of full-thickness sutures in the gastrointestinal tract, which has created the space for novel endoscopic gastric restrictive procedures[45]. Some of these procedures were used primarily for other indications, such as EndoCinch Suturing System (C.R. Bard, Inc., Murray Hill, NJ) for gastro-esophageal reflux disease[46] or over-the-scope clip (OTSC; Ovesco Endoscopy AG, Tubingen, Germany) for fistula closure[47]. However, the chance to be applied in RYGB revision resulted in their further modification and improvement. In comparison to the previously mentioned major restrictive procedures, these techniques are not widely described in the literature.
StomaphyX (Endo Gastric Solutions) is another device that appeared on the market in the last decade as a gastric restrictive procedure. The first study to test the efficacy and safety of this device was a randomized controlled trial performed in patients with WR following RYGB in 2014[48]. One of the endpoints of this study was to achieve a significant WL in at least 50% of patients compared to a sham group. The gastroscope was introduced through the StomaphyX sheath. A vacuum was used to pull a large gastric fold of the pouch into the device shaft. The stylet, completely located inside the shaft, was advanced through aspirated gastric tissue, and the first polypropylene fastener was deployed forming a plication. Without device removal, 4-6 plications per 3-4 rows (12-24 total) were placed from the most distal portion of the pouch to the GJA, in a circumferential way. The aim was to reduce the gastric pouch and the GJA diameter by at least 75% and 50%, respectively. Enrollment was interrupted earlier because preliminary results showed failure to meet the primary efficacy endpoint in at least 50% of study participants. However, at 3, 6 and 12 mo follow-up patients who successfully underwent this procedure had a significant WL and BMI reduction (P ≤ 0.05).
Despite the promising initial results of the EndoCinch Suturing System, the success of the procedure was limited by its inability to obtain deeper tissue plications and the necessity to extract the EndoCinch for suture reloading[49]. The device was then modified to allow deeper gastric plications and to avoid the device withdrawal for suture reloading (RESTORe Suturing System, Bard). Furthermore, the technique was adjusted with a sequence of running sutures to embed the greater curvature, similarly to gastric surgical imbrication. This procedure can be considered as a precursor to endoscopic sleeve gastroplasty (ESG)[49]. In a pilot study the aim was to show the feasibility and procedural safety of transoral gastric volume reduction (TRIM procedure) using the Restore Suturing System in patients with a BMI of 30-45 kg/m2. The TRIM procedure was successfully completed in all patients, with 4-8 plications per patient (6 on average)[50]. The mean EWL at 1 year was 27.7%[51]. The proportion of patients with an EWL of ≥ 20% or ≥ 30% was 57% and 50%, respectively. However, endoscopy at 1 year follow-up showed partial or complete dehiscence of plications in 13 patients.
The OTSC is made of super-elastic shape memory alloy (Nitinol) which re-takes its former unbent shape after the clip is released and thus exerts a constant compression on the tissue between the jaws of the clip. The material is biocompatible and can remain in the body even as a long-term implant, which represents at the same time its limitation for further removal or endoscopic re-intervention. In a series of 94 patients, the best clinical results were obtained by narrowing the GJA by placing two clips at opposite sites, reducing the outlet by more than 80%[52]. Between surgery and OTSC application, the mean BMI dropped from 45.8 to 32.8. At 3 mo follow-up, the mean BMI was 29.7. At 1 year follow-up, the mean BMI was 27.4. OTSC for revisional endoscopy after RYGB is reliable and effective in treating WR due to dilated pouch outlet with favorable short- and medium-term results. The different types of the OTSC could be applied for the endoscopic closure of traumatic wall lesions of the digestive tract, which could be helpful as a rescue therapy after unsuccessful TORe as well[19,53,54].
ENDOSCOPIC OPTIONS IN REVISION OF SLEEVE GASTRECTOMY
Sleeve gastroplasty reduces gastric volume by 75%-80%. Weight regain seems to be common in LSG after 3 years[55]. If the patient is prone to a continuous WR, sleeve dilation may be a contributing factor that could benefit from additional WL procedures, such as gastric sleeve volume reduction with endoluminal plication[56]. Advances in endoluminal endoscopy and other minimally invasive bariatric procedures have inspired innovative techniques and produced reliable suture tools for gastric volume reduction[57].
One of the first attempts in terms of endoscopic sleeve plication for revision of sleeve gastrectomy reported an M-shaped pattern to ensure adequate plication of the folds and prevention of a secondary internal lumen[58]. Approximately eight sutures were placed in an interrupted sequential stitch, creating the central length of the sleeve. A second layer of two sutures was added to further reduce the gastric body. This was confirmed by an upper gastrointestinal series 1 d later. The patient lost 20 lbs.
A detailed description of 54Fr IOP plication platform was reported in a case report by Jirapinyo et al[59]. The procedure was described as a sleeve-in-sleeve procedure, focusing on the placement of the plications in the gastric body using a belt-and-suspenders pattern. First, the distal belt plications were placed perpendicular to the greater curvature in the distal body. Then, two rows of suspender plications were placed parallel to the greater curvature in the midbody. These suspender plications served to shorten the length of the sleeve. Finally, proximal belt plications were placed perpendicular to the greater curvature in the proximal body. No direct plications were placed in the fundus. The patient did well postoperatively and achieved an overall WL of 8% and an EWL of 21%.
A recent multicenter retrospective study analyzed 34 patients with WR after sleeve gastrectomy who had undergone ESG for WL[60]. The technical success was 100%. At 1 year, 82.4% and 100% of patients achieved ≥ 10% TBWL and ≥ 25% EWL, respectively. Median %TBWL was 13.2% and 18.3% and %EWL was 51.9% and 69.9% at 6 mo and 1 year, respectively. The mean %TBWL was 14.2%, 19.3%, 17.5% and 20.4%, and the %EWL was 88.5%, 84.4%, 55.4% and 47.8% for the BMI categories of overweight and obesity class I, II and III at 1 year, respectively. No predictors of outcome were identified in the multivariable regression analysis. This study concludes that ESG appears to be safe and effective in the treatment of WR after sleeve gastrectomy.
However, the trend of implementation of revisional ESG (R-ESG) is increasing, and more prospectively collected data are arriving. In a multicenter study, nine centers with 82 patients who underwent R-ESG for WR after LSG were treated using the OverStitch device[61]. The general purpose of R-ESG was to reduce the volume of the dilated gastric sleeve and shorten its length. R-ESG was performed with full-thickness endoscopic 2-0 prolene sutures applied in various suture patterns (predominantly U-shaped) to overlap the anterior/greater curvature/posterior gastric wall and create a tubular, restricted sleeve along the lesser curvature of the stomach. In a per-protocol analysis, ≥ 10% TBWL was achieved by 72.5% of patients at 6 mo and 81.0% of patients at 12 mo; ≥ 15% TBWL was achieved by 43.5% patients at 6 mo and 52.4% patients at 12 mo. The authors concluded that R-ESG is a safe and effective means of facilitating WL in those with WR after LSG. Future studies of R-ESG should evaluate improvement in obesity-related comorbidities, such as diabetes mellitus, hypertension, obstructive sleep apnea, nonalcoholic fatty liver disease and gastroesophageal reflux disease.
Unlike the LSG, the physiopathological pathways inducing WL and metabolic changes following ESG are still not well investigated. As ESG becomes more and more popular among bariatric procedures, comparison of endoscopic therapies for revision of LSG and ESG is mandatory. Anatomically, the main difference between ESG and endoscopic therapy for revision of LSG is that LSG resects ghrelin producing cells, while ESG does not[62,63]. In the prospective pilot study, gastrointestinal hormone alterations following ESG and LSG were compared[64]. A significant decrease in leptin levels was observed at 6 mo after ESG. Insulin levels showed a decreasing trend, while insulin secretory pattern was improved. No change was observed in fasting ghrelin levels, glucagon-like peptide (GLP-1) and peptide Y-Y. However, peptide Y-Y, glucagon-like peptide and adiponectin levels were increased, while ghrelin and leptin levels were reduced significantly at 6 mo following LSG. At the same time, insulin levels were unchanged. At 6 mo, compared to ESG, a higher %TBWL (24.4 vs 13.3; P < 0.001) was obtained by LSG with a significant modification of peptide Y-Y, ghrelin and adiponectin levels. Changes in gut hormones followed different pathways between ESG and LSG. During WL a beneficial change in insulin secretion and a compensatory increase in ghrelin levels were promoted by ESG.
CONCLUSION
Management of WR following primary bariatric surgery is made of medical treatment and/or endoscopic or surgical revision and requires a multidisciplinary approach involving the surgeon (general and plastic), dietitian, endocrinologist, gastroenterologist, psychologist, psychiatrist and fitness trainer[3]. Endoscopic management offers several treatment options, ranging from less invasive approaches to the full-thickness endoscopic suture techniques. Traditionally, revisional surgery is an option in the setting of WR usually performed in patients who failed the medical treatment, but it is related to a significant postoperative morbidity and mortality[65]. Nowadays, revisional endoscopic bariatric therapy is a valid alternative for patients with WR unwilling to undergo surgical treatment again. All these options (surgery and endoscopy) should be considered in a multidisciplinary context[59], explaining and discussing with patients any possible advantage and disadvantage[66-68] in order to propose a “tailored therapy” for every single case.
Concerning the revisional endoscopic therapy, many aspects should be considered while managing patients with WR following primary bariatric surgery. Endobariatric techniques have different purposes according to the type of previously performed surgical procedure. That is to reduce the diameter of the GJA and pouch size in patients with prior RYGB, while reducing sleeve diameter in patients with prior LSG.
Concerning patients with RYGB, the first step preceding the endoscopic treatment is always the measurement of the GJA diameter and pouch. This data appears to be crucial in the decision of the type of restrictive technique, which can be individualized based on the patient’s anatomy. Endoscopic TORe of the GJA is the only bariatric revision procedure with level 1 evidence[45]. For pouch > 5 cm, plicating TORe should be considered when GJA is < 30 mm, with S-TORe being performed when GJA is ≥ 30 mm. For pouch ≤ 5 cm, both APC and S-TORe may be considered for GJA < 18 mm, with S-TORe being preferred when GJA is ≥ 18 mm[69]. These latest data, together with expert opinion, could represent a crucial moment in personalizing the endoscopic management to offer each patient the most adequate solution.
Considering endotherapy in patients with previous LSG, the first step is to delineate the exact anatomy of the gastric sleeve, assessing for the dilated areas to plan the suture distribution[61]. Despite the OverStitch device appearing in the most published data in literature, the plication technique is showing promising results. However, a high level of safety, feasibility and efficacy has been reported by both these procedures.
Performing restrictive endoscopic bariatric procedures requires advanced skills in therapeutic endoscopy, including hemostasis and perforation management, other than knowledge of each device feature and performance. For example, it is more difficult to assemble the single operating channel suturing device than the double channel device. No specific, standardized and recognized certification in ESG is currently available by the international scientific societies[45]. However, training in ESG can be obtained as part of a comprehensive endoscopic suturing program through society- or industry-sponsored courses. Similarly, credentialing in ESG is institution specific. Proctoring in initial cases is recommended, although not mandatory, especially for operators with already recognized skills on the OverStitch device. Currently, there is limited data on the learning curve for ESG[70,71]. Those ESG learning curve studies are firstly focused on the number of cases necessary to achieve efficiency and later, mastery. The most recent study defined efficiency “as the point on the learning curve where the operator was able to make procedural improvements to decrease procedure time.” Mastery was defined “as the point at which the procedure time became consistent by eliminating outliers in terms of operating time.” Following this analysis, 29–38 procedures were necessary to reach efficiency, while 55 procedures were needed to achieve mastery. Interestingly, the overall outcome of WL was not conditioned by the improvement in procedure time. The majority of endoscopists performing bariatric procedures are not familiar with endoscopic suturing techniques and devices, thus they need to acquire general skills on ESG while learning this complex procedure[45].
Nowadays another important issue is the global coronavirus disease 2019 (COVID-19) pandemic, which has left a strong impact on the management of obese patients, especially in the field of endoscopy, both primary and revisional. Many bariatric centers worldwide were transformed into COVID hospitals thus creating long lasting bariatric procedure waiting lists. Therefore, a position statement from the International Federation for the Surgery of Obesity and Metabolic Disorders was adopted on the practice of bariatric endoscopy during the COVID-19 pandemic[72] concluding that all elective bariatric endoscopy procedures should be delayed for more than 8 wk, both primary and revisional (e.g., TORe, ROSE and R-ESG). In other words, the impact of the COVID-19 pandemic will inevitably bring a worldwide extension of bariatric surgery/endoscopy waiting lists with all possible consequences, both health-related and economic.
In conclusion, the causes of WR following primary bariatric surgery are multifactorial and join both pre- and postoperative parameters. Nowadays, there are several ways of managing WR, but this is still a challenge for both patients and professionals involved in the multidisciplinary team. Scientific societies and organizations should go on collaborating to develop a personalized approach that meets the needs of each individual patient.