Published online Dec 27, 2021. doi: 10.4240/wjgs.v13.i12.1567
Peer-review started: May 29, 2021
First decision: June 23, 2021
Revised: July 2, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: December 27, 2021
Processing time: 208 Days and 12.7 Hours
Mucinous adenocarcinoma (MAC) is a unique clinicopathological subtype of colorectal cancer, which is characterized by extracellular mucinous components that comprise at least 50% of the tumor tissue. The clinical characteristics, molecular features, response to chemo-/radiotherapy, and prognosis of MAC are different from that of non-MAC (NMAC). MAC is more common in the proximal colon, with larger volume, higher T-stage, a higher proportion of positive lymph nodes, poorer tumor differentiation, and a higher proportion of peritoneal implants compared to NMAC. Although biopsy is the main diagnostic method for MAC, magnetic resonance imaging is superior in accuracy, especially for rectal carcinoma. The aberrant expression of mucins, including MUC1, MUC2 and MUC5AC, is a notable feature of MAC, which may be related to tumor invasion, metastasis, inhibition of apoptosis, and chemo-/radiotherapy resistance. The genetic origin of MAC is mainly related to BRAF mutation, microsatellite instability, and the CpG island methylator phenotype pathway. In addition, the poor prognosis of rectal MAC has been confirmed by various studies, and that of colonic MAC is still controversial. In this review, we summarize the epi
Core tip: Colorectal mucinous adenocarcinoma (MAC) is a unique clinicopathological subtype in colorectal cancer. MAC exhibits a higher frequency of microsatellite instability, higher CpG island methylator phenotype of high degree, higher frequency of BRAF and KRAS gene mutations, and lower frequency of TP53 mutations. One of the most important features of MAC is the aberrant expression of a large number of mucins, including MUC1, MUC2 and MUC5AC. We discuss the epidemiology, clinicopathological characteristics, molecular features, methods of diagnosis, and treatments of MAC in order to provide references for further fundamental and clinical research.
- Citation: Huang A, Yang Y, Shi JY, Li YK, Xu JX, Cheng Y, Gu J. Mucinous adenocarcinoma: A unique clinicopathological subtype in colorectal cancer. World J Gastrointest Surg 2021; 13(12): 1567-1583
- URL: https://www.wjgnet.com/1948-9366/full/v13/i12/1567.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i12.1567
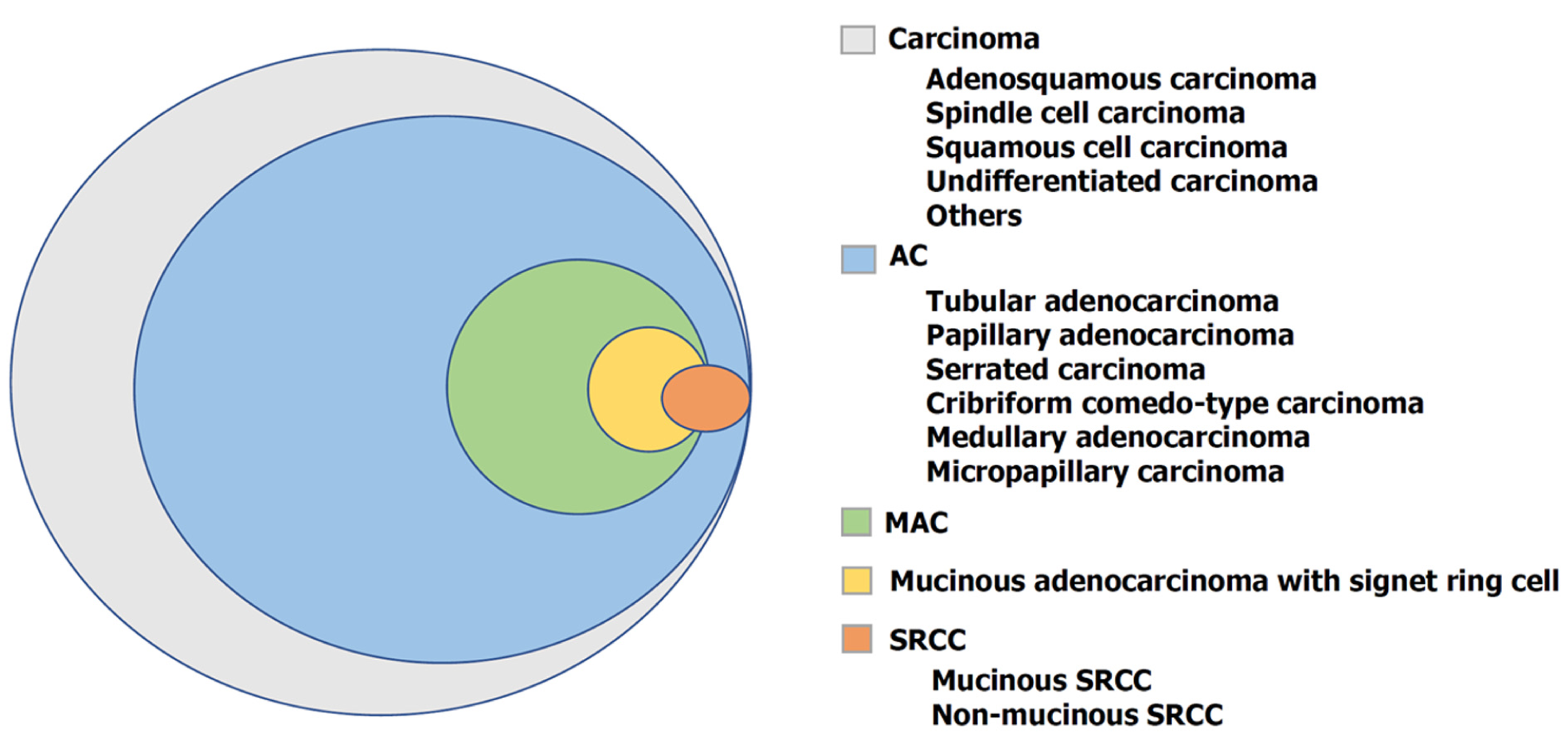
Colorectal cancer (CRC) has caused a great burden on global health. The World Health Organization (WHO) estimated > 1.9 million new CRC cases and 935 000 CRC-related deaths occurred in 2020, with 10% (third) and 9.4% (second) incidence and mortality rates, respectively, among all cancer types[1]. According to the WHO classification of tumors of the digestive system, the histological subtypes of CRC include adenocarcinoma, adenosquamous carcinoma, spindle cell carcinoma, squamous cell carcinoma, and undifferentiated carcinoma (Figure 1). Adenocarcinoma originating from epithelial cells of the colorectal mucosa accounts for more than 90% of CRC cases. Mucinous adenocarcinoma (MAC) is a unique subtype of adenocarcinoma characterized by more than 50% of the tumor tissue being extracellular mucinous com
Compared to non-MAC (NMAC), the clinicopathological characteristics, molecular features, response to chemo-/radiotherapy, and prognosis of MAC are evidently different. MAC are divided into two types based on the degree of histological structural differences: One type is the low-grade MAC, which originates from well-differentiated to moderately differentiated adenocarcinoma and papillary carcinoma, whereas the other type is the high-grade MAC, originated from poorly differentiated adenocarcinoma and signet ring cell carcinoma (SRCC)[4]. Currently, the prognosis of MAC remains controversial. Previous studies have suggested that colorectal MAC is associated with poor prognosis[5-8], while other studies reported no significant difference in prognosis between MAC and NMAC[9,10]. However, the poor prognosis of rectal MAC has been confirmed in most studies[11-13]. The clinicopathological characteristics of MAC suggest that it is a unique subtype of CRC.
Various studies have demonstrated regional differences in the occurrence of MAC in CRC. The occurrence of MAC in CRC was 6.9%[14], 8.9%[15], 8.17%[16] in China, 3.82%[17], 2.8%[18] in Japan, 11.6%[19], 10%[5] and 11%[20] in the USA, which ranged from 3.9% in Asia to 10%-13.6% in Europe and North America[21]. A large national cancer database study in the USA demonstrated that the distribution of histological subtypes of CRC among Caucasians, African Americans, and other races were similar[22]. However, another study reported that the occurrence of MAC in Chinese Americans with CRC (7.5%) was lower than that in Caucasians (9.3%) and African Americans (9.4%)[23]. This might be due to genetic differences between races as well as other factors (such as lifestyle and dietary habits). Studies on American[6] and German patients[24] found that MAC occurred in a higher proportion of women (MAC vs NMAC, 52.1% vs 48.6%, 47% vs 41%, respectively). In addition, a German study observed no difference in the age of patients with MAC and NMAC, whereas an American study observed that the proportion of MAC in patients aged > 65 years was higher. However, studies on Chinese patients reported no statistical difference in gender between patients with MAC and NMAC, and that MAC was more common in patients aged < 50 years[25].
Compared with that in NMAC, in MAC, the proportion of tumors occurring in the right hemicolon was higher (MAC vs NMAC, 35.0% vs 18.9% in China[25], 65.3% vs 46.2% in the USA[6], 51.0% vs 28.0% in Germany[24]), while the proportion of tumors in the rectum was lower (MAC vs NMAC, 41.0% vs 50.7% in China, 9.9% vs 17.7% in the USA, 27.0% vs 40.0% in Germany) in MAC. MAC was diagnosed with larger tumors, higher T stage, higher proportion of lymph node infiltration and peritoneal implantation, and poorer tumor differentiation compared to NMAC (Table 1)[6,24-27].
| China | USA | Germany | |||||||
| MAC (%) | NMAC (%) | P | MAC (%) | NMAC (%) | P | MAC (%) | NMAC (%) | P | |
| Age(yr) | 21.4% (< 50) | 11.3% (< 50) | 0.005 | 62.6% (> 65) | 56.3% (> 65) | < 0.001 | 67 (25-88) | 65 (15-96) | 0.037 |
| Gender | 0.603 | < 0.001 | 0.034 | ||||||
| Male | 58.1 | 55.4 | 47.9 | 51.4 | 52.8 | 58.5 | |||
| Female | 41.9 | 44.6 | 52.1 | 48.6 | 47.2 | 41.5 | |||
| Tumor location | < 0.001 | < 0.001 | < 0.001 | ||||||
| Right hemicolon | 35.0 | 18.9 | 65.3 | 46.2 | 51.5 | 27.5 | |||
| Left hemicolon | 23.9 | 30.4 | 24.8 | 36.2 | 18.9 | 29.8 | |||
| Rectum | 41.0 | 50.7 | 9.9 | 17.6 | 27.5 | 40.1 | |||
| Tumor size (cm) | < 0.001 | < 0.001 | - | ||||||
| ≤ 5 | 34.2 | 54.2 | 48.93 | 68.34 | - | - | |||
| > 5 | 65.8 | 45.8 | 51.07 | 31.66 | - | - | |||
| Primary tumor (T) | 0.001 | < 0.001 | < 0.001 | ||||||
| T1, T2 | 28.2 | 44.5 | 13.8 | 26.5 | 13.3 | 30.5 | |||
| T3, T4 | 71.8 | 55.4 | 86.2 | 73.5 | 86.7 | 69.5 | |||
| Regional lymph nodes (N) | < 0.001 | < 0.001 | 0.018 | ||||||
| N0 | 35.9 | 55.0 | 52.5 | 57.0 | 49.1 | 55.6 | |||
| N1, N2 | 64.0 | 45.0 | 47.5 | 43.0 | 50.9 | 44.4 | |||
| Distant metastasis (M) | 0.001 | 0.004 | < 0.001 | ||||||
| M0 | 56.4 | 72.4 | 84.7 | 85.8 | 75.5 | 78.5 | |||
| M1 | 43.6 | 27.6 | 15.3 | 14.2 | 24.5 | 21.5 | |||
| Stage | 0.001 | < 0.001 | < 0.001 | ||||||
| Ⅰ, Ⅱ | 28.2 | 44.5 | 21.5 | 31.2 | 44.8 | 52.0 | |||
| Ⅲ, Ⅳ | 71.8 | 55.5 | 78.5 | 68.8 | 55.2 | 48.0 | |||
| Histological grading | < 0.001 | < 0.001 | < 0.001 | ||||||
| G1, G2 | 82.9 | 89.8 | 76.4 | 80.1 | 55.2 | 69.6 | |||
| G3, G4 | 17.1 | 10.1 | 23.6 | 19.9 | 44.8 | 30.4 | |||
MAC exhibited a higher frequency of microsatellite instability (MSI) and BRAF and KRAS gene mutations, higher CpG island methylator phenotype of high degree (CIMP-H), and lower frequency of TP53 mutations[28]. Gene expression analysis illustrated that compared to NMAC, 317 genes were differentially regulated in MAC, of which 182 were upregulated and 135 were downregulated. These altered genes were primarily involved in O-glycan biosynthesis, keratin sulfate metabolism, lacto-series glycosphingolipid metabolism, histidine-glutamate-glutamine and proline metabolism, p38-MAPK pathway, coenzyme A biosynthesis, and 14-3-3 protein in cell cycle regulation[26]. Among them, O-glycan biosynthesis is associated with mucins synthesis. One of the most important features of MAC, the aberrant expression of several mucins, is associated with O-polysaccharide biosynthesis, including MUC1, MUC2, and MUC5AC[29].
Mucins are a class of high-molecular-weight epithelial glycoproteins with a high content of clustered oligosaccharides O-glycosidically linked to tandem repeat peptides rich in threonine, serine and proline[29]. They are differentially expressed by specialized epithelial cells on the mucosal surface in a specific way for organs and cells[30]. Mucins are classified as membrane-associated and secreted mucins. Secreted mucins are either gel-forming or non-gel-forming subtypes[31]. Under normal circumstances, mucins form a mucus barrier that protects the epithelial cells. In the process of tumorigenesis, aberrant expression of specific mucins may be related to tumor invasion, metastasis, apoptosis inhibition, and chemoradiotherapy resistance[32]. MUC1, MUC2 and MUC5AC are aberrantly expressed in colorectal MAC. MUC1 is a membrane-associated mucin, while MUC2 and MUC5AC are secreted gel-forming mucins[31].
MUC1 is expressed in almost all glandular epithelial cell membranes, making MUC1 overexpression one of the most common changes in cancers. During pathogen infection, upregulation of MUC1 expression in the mucosal barrier suppresses pathogen-mediated inflammation[33]. However, MUC1 expression is induced by inflammatory cytokines [tumor necrosis factor-α, interferon-γ, and interleukin (IL)-6], and abnormal activation of MUC1 may lead to chronic inflammation and cancers in the absence of IL-10 and corresponding anti-inflammatory responses[34]. MUC1 C-terminal transmembrane subunit (MUC1-C) can activate both the inhibitor of nuclear factor-κB (NF-κB) kinase-β (IKKβ) and the NF-κB family member RELA, while the activation of the IKKβ-NF-κB pathway is a likely mediator of inflammation-induced cancer progression[35,36]. Meanwhile, MUC1 can inhibit tumor cell apoptosis via the abnormal activation of NF-κB and Wnt/β-catenin signaling pathways, inhibition of the JNK1 signaling pathway, and formation of a physical barrier to prevent chemotherapeutic drugs from reaching tumor cells[32]. The resistance of MAC to chemoradiotherapy may be reversed by reducing the production of mucins or inhibiting their functions. Studies have been targeting MUC1 as a cancer vaccine for CRC, which reduces tumor burden and induces tumor regression in mouse models[37,38]. However, their application to patients with MAC requires further research.
MUC2 primarily exists in goblet cells of the colorectum, especially in the proximal colon, and is an important component of normal intestinal mucus, which acts as a physical barrier thereby limiting the damage to the epithelium by pathogens and weaken the activation of natural and acquired immune responses[39]. Feagins et al observed that the degree of ulcerative colitis was associated with reduction in MUC2 levels, while chronic inflammation associated with inflammatory bowel disease increased the risk of colon cancer[40]. MUC2 is strongly expressed in normal colon tissues (mean composite score ± standard error, 12 ± 0), and decreases sequentially in inflammation, hyperplastic polyps, and adenomas (11.4 ± 0.4, 9.7 ± 1.1, 7.4 ± 0.6, respectively), while in adenocarcinoma, the expression of MUC2 is significantly decreased (3.8 ± 0.9)[41]. Low levels of MUC2 are associated with poor overall survival (OS) [hazard ratio (HR) = 1.67, 95% confidence interval (CI): 1.43-1.94, P < 0.00001][42], which suggests that MUC2 can act as a tumor suppressor. However, compared to NMAC, MAC with no better prognosis overexpresses MUC2, which is inconsistent with the observation that MUC2 acts as a tumor suppressor. Gratchev et al[43] found that the strong expression of MUC2 in normal human goblet cells and human colorectal MAC tissues was related to ~50% of the average degree of methylation at the CpG site of each MUC2 promoter. MUC2 promoters in normal columnar cells and NMAC tissues that do not express MUC2 are methylated to nearly 100%. In this regard, MUC2 expression in carcinomas might reflect the origin of these tumors from cells that normally express MUC2, rather than a role for this mucin in the malignant process itself[34].
Another component of the mucus secreted by colorectal MAC is MUC5AC, which is usually secreted by tracheobronchial goblet cells, gastric epithelial cells, conjunctiva, and lacrimal gland cells, but is not expressed in the normal colonic mucosa[31,44]. Studies have shown that during adenoma-adenocarcinoma progression, the expression of MUC5AC is upregulated[41], which may be associated with tran
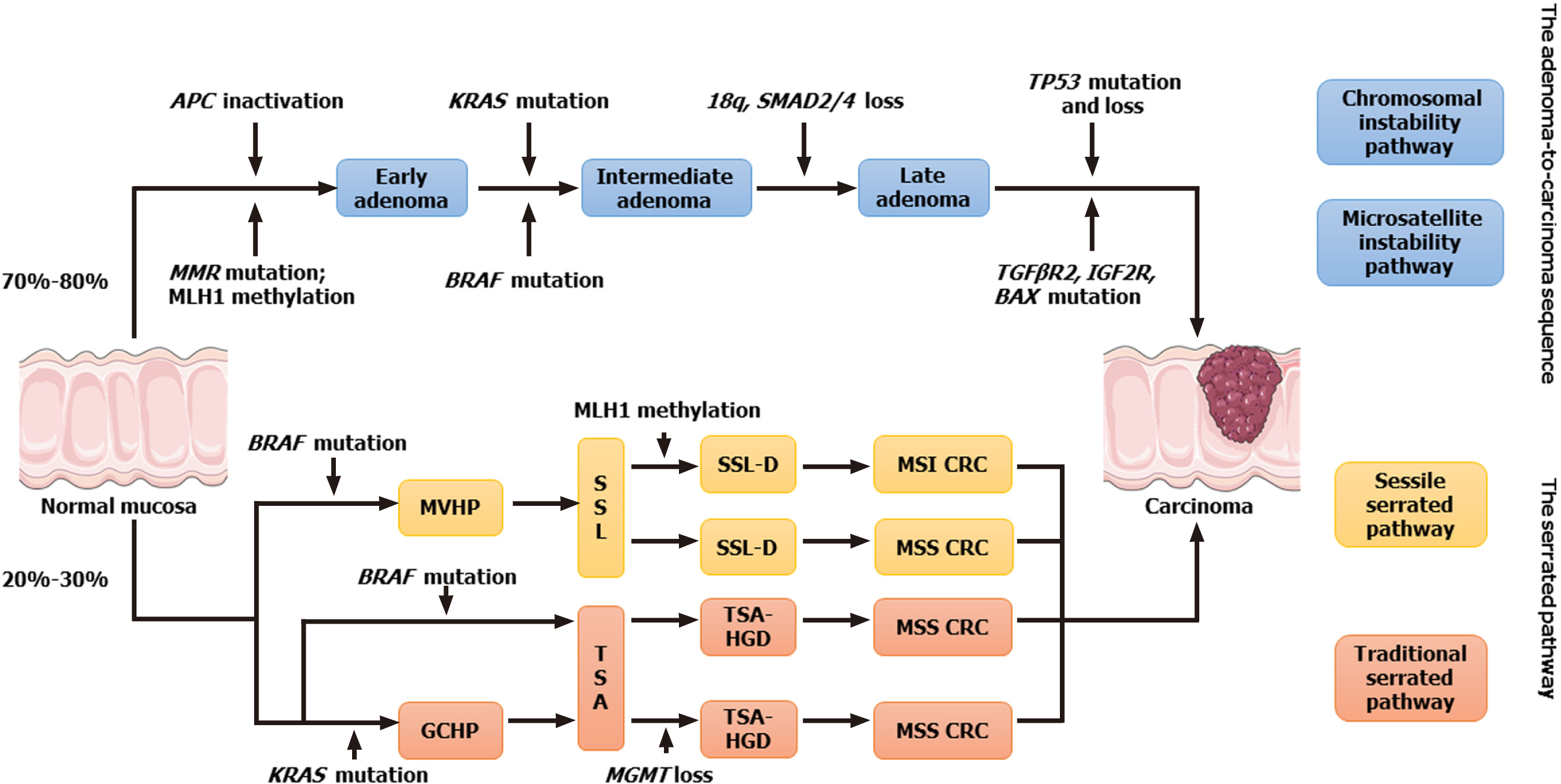
There are two main pathways for the occurrence of CRC (Figure 2)[51,52]: The conventional adenoma-carcinoma pathway, which accounts for 70%-80% of CRC cases. Usually mutations in APC, KRAS and TP53, account for 60%, 45% and 54% of cases, respectively. The other is the serrated pathway, which accounts for 20%-30% of CRC cases and usually has a high frequency of BRAF mutations (70%-100%), CIMP-H, and high MSI (MSI-H)[53-55]. A meta-analysis of 46 studies involving 17 746 patients demonstrated that MAC had higher KRAS [odds ratio (OR) = 1.46, 95%CI: 1.08-2.0, P = 0.014], BRAF (OR = 3.49, 95%CI: 2.50-4.87, P < 0.001), higher MSI (OR = 3.98, 95%CI: 3.30-4.79, P < 0.001), and CIMP-H (OR = 3.56, 95%CI: 2.85-4.43, P < 0.001), and lower p53 expression (OR = 0.46, 95%CI: 0.31-0.67; P < 0.001) compared to NMAC, which suggests that the genetic origin of MAC is primarily associated with the serrated pathway[56]. Some researchers have proposed that MAC can be divided into two subtypes. The first type, characterized by MSI, is mostly confined to the proximal colon, usually presents with loss of expression of hMLH1 and p27, and has a good prognosis. The second subtype, characterized by microsatellite stability, is more common in the distal colon and rectum, with normal expression of hMLH1 and p27, and a poor prognosis[57].
MSI is present in 15% of CRC cases[58], of which 3%[59] are present in Lynch syndrome, and 12% are sporadic cancers[60]. Currently, four pathogenic genes associated with Lynch syndrome have been characterized namely MSH2 plus EpCAM, MLH1, MSH6 and PMS2. Germline mutations in MLH1 and MSH2 account for most cases (60%-80%), with a limited number of Lynch syndrome cases with germline mutations in MSH6 and PMS2, and particularly rare germline EPCAM mutations that epigenetically inactivate MSH2[61]. Sporadic MSI CRC is primarily caused by acquired methylation in the promoter region of the MLH1 gene[60]. The association of BRAF mutations (usually V600E mutations) with MSI and CIMP-H has been well established[62]. BRAF mutations are extremely rare in Lynch syndrome[63], suggesting that MSI in MAC is primarily sporadic.
Currently, the diagnosis of MAC is primarily based on computed tomography (CT), magnetic resonance imaging (MRI), colorectal endoscopy, or postoperative pathological biopsy. Compared to NMAC and SRCC, CT of MAC shows more heterogeneous enhancement (MAC vs NMAC vs SRCC, 95.8% vs 54.1% vs 32.8%), larger attenuation area (greater than two thirds of the tumor tissue, 54.2% vs 5.9% vs 3.0%), and more calcification (17.9% vs 6.8% vs 3.0%)[64].
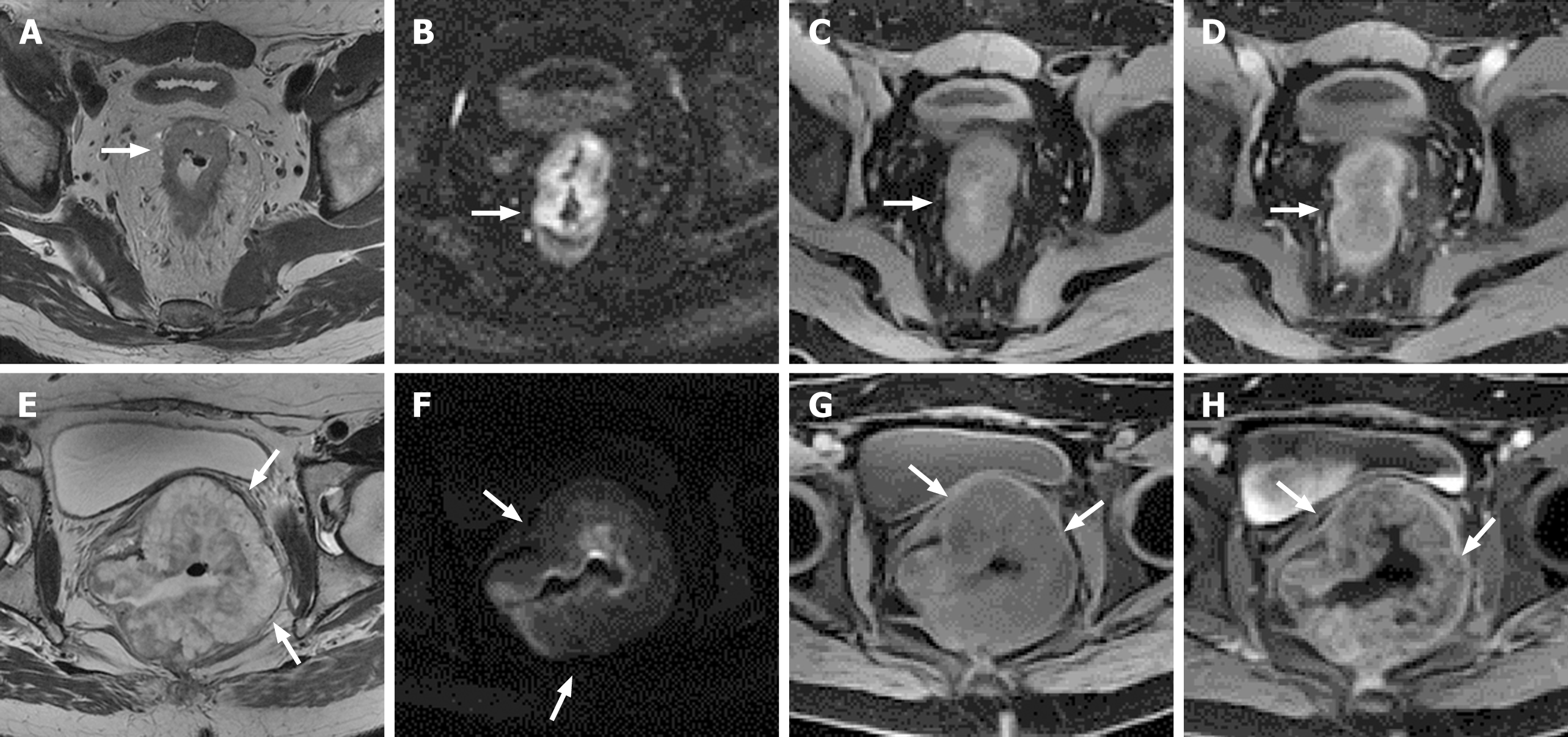
MRI can distinguish MAC from NMAC, which facilitates early diagnosis of MAC rather than relying on postoperative histopathological diagnosis. Since NMAC shows moderate signal intensity on T2-weighted imaging (T2WI), mucus displays low signal intensity on T1-weighted imaging, whereas T2WI shows high signal intensity (similar to or higher than that of the rectum fat signals) (Figure 3)[65]. MRI has an accuracy of 96%-97%, a sensitivity of 94%-100%, and a specificity of 95%-98% in diagnosing histological types of mucus[66]. Stanley et al believed that MRI was superior to preoperative biopsy for MAC diagnosis[67]. Before treatment, MRI diagnosed 60/330 (18%) mucinous rectal cancer cases, and initial biopsy diagnosed 15 (5%) (diagnostic OR = 4.67, P < 0.05) cases. The 60 patients who underwent surgery were ultimately confirmed to have mucinous tumors using histopathological analysis. MRI has great advantages not only in the diagnosis of MAC, but also in predicting the response of MAC to neoadjuvant therapy. Cao et al[68] used preoperative T2WI to clarify the mucus pool (high signal) and tumor solid components (medium signal), and classified MAC into two types: mixed type, where the mucus was rich in solid tumor components, and separated type, where the secretory mucus component was located outside the solid tumor, to predict the response of locally advanced rectal MAC to neoadjuvant therapy, since patients with mixed-type mucin pool showed a lower tumor response rate than those with separate type mucin pool following neoadjuvant chemotherapy (4.9% vs 25.5%, P = 0.002). However, using MRI to diagnose MAC can also produce false-positive results, possibly attributed to edema, congestion, abscess, or necrosis. False positives are especially important after treatment, as submucosal edema appears in the normal rectal wall after radiotherapy and chemotherapy[69]. More importantly, a few patients with CRC may form acellular mucin pools following adjuvant treatment, which is a manifestation of tumor response to treatment and is usually associated with a better prognosis[70,71]. However, due to the T2WI high signal on MRI, it is difficult to distinguish between persistent cell mucins (residual MAC tissue lacking response) and acellular mucin pools (therapeutic effect). There is currently no imaging technique to distinguish between the two[72], hence the comparison of MRI before and after treatment is particularly important.
Positron emission tomography (PET)/CT is an effective auxiliary test for patients with complicated conditions and cannot be clearly diagnosed by routine examination to determine the presence of distant metastases[73]. Although some studies have found no significant difference in the uptake of 18-fluorodeoxyglucose (FDG) between rectal MAC and NMAC in PET[74,75], it is not uncommon that MAC shows low uptake of 18-FDG on PET/CT and PET/MRI, and that the 18-FDG affinity of the tumor on a PET scan is inversely proportional to the total amount of mucins, which may lead to false-negative results[76].
Extracellular mucinous components > 50% are usually estimated by pathologists, while mucinous components vary in different pathological sections of the same tumor. In addition, Li et al[77] observed no significant difference in the distribution of mutations among the three adenocarcinoma subgroups with mucin characteristics (< 30%, 30%-50%, and > 50% mucinous components in tumor tissue)[77]. Furthermore, the more extracellular mucinous components of MAC tissue (50%-79%, 80%-89% and ≥ 90%), the worse the patient’s OS and recurrence-free survival[78]. These findings suggest that more objective and standardized histopathological analysis and molecular data are warranted to update the classification of MAC and adenocarcinoma with mucinous components.
The existing guidelines for the diagnosis and treatments of CRC are primarily based on TNM staging, biomarkers including BRAF, RAS, HER2 and microsatellite status[73], and do not make recommendations based on the characteristics of MAC. Differences in histopathology and molecular characteristics between MAC and NMAC influence their treatment and prognosis, therefore, establishing standards for the diagnosis and treatments of MAC is essential.
Studies on patients with stage II or III colon cancer receiving adjuvant chemotherapy after radical resection have reported no significant difference in OS (HR = 1.05, 95%CI: 1.02-1.08, P < 0.001) between patients with stage II NMAC and MAC[79,80], whereas in patients with stage III colon cancer, compared to NMAC, the OS (HR = 1.05, 95%CI: 1.02-1.08, P < 0.001)[79], cancer-specific survival (CSS) (5-year CSS rate: MAC vs NMAC, 72.7% vs 67.9%, P < 0.0001)[81] and disease-free survival (HR = 1.82, 95%CI: 1.03-3.23, P = 0.04)[82] of MAC were significantly decreased. Studies on patients with stage IV CRC receiving palliative chemotherapy illustrated that despite the different chemotherapy regimens used in these trials [5-fluorouracil (5-FU) with oxaliplatin and/or CPT-11[83], FOLFOX-4 regimen[84], CAP + oxaliplatin + bevacizumab with or without cetuximab[85], 5-FU-based first-line chemotherapy[12]], the median OS of patients with MAC was shorter than that of patients with NMAC (MAC vs NMAC, 14.0 mo vs 23.4 mo, 8.0 mo vs 18.0 mo, 13.1 mo vs 21.5 mo, 11.8 mo vs 17.9 mo, respectively). However, although patients with stage III and IV MAC have poor responses to adjuvant or palliative chemotherapy, current evidence shows that adjuvant chemotherapy can effectively improve the survival rate of patients with stage II and III MAC[79,81].
A meta-analysis that included eight comparative series on the association between mucinous histology and response to neoadjuvant chemoradiotherapy in rectal cancer reported that MAC had a reduced rate of pathological complete response (pCR) (OR = 0.078, 95%CI: 0.015-0.397, P = 0.002) and tumor downstaging (OR = 0.318, 95%CI: 0.185-0.547, P < 0.001) following neoadjuvant chemoradiotherapy with an increased rate of positive resection margins (OR = 5.018, 95%CI: 3.224-7.810, P < 0.001) and poor OS (OR = 1.526, 95%CI: 1.060-2.198, P = 0.023) following resection, which suggests mucinous histology of rectal MAC as a biomarker for poor prognosis after neoadjuvant chemoradiotherapy[86,87]. Approximately 30% of patients with rectal cancer who received neoadjuvant therapy can have a clinical complete response. At this time, a watch-and-wait strategy can be adopted to provide patients with the opportunity to preserve the rectum and avoid surgery[88]. Tan et al[87] discovered that patients with NMAC (21%) were more likely to achieve pCR (P < 0.001) than those diagnosed with MAC (14%); in patients who achieved pCR, those with MAC had a poorer survival, with a 3-year OS rate of 67.5%, while the 3-year OS of patients with NMAC was 93.8% (P < 0.001)[87]. Therefore, the watch-and-wait strategy should be used more cautiously in patients with MAC. For patients with rectal MAC, preoperative treatment (short-term preoperative radiotherapy and preoperative chemoradiotherapy) plus total mesorectal resection (TME)[89] or adjuvant chemotherapy after TME[90] can be used to narrow the survival gap between rectal MAC and NMAC.
The peritoneum is associated with treatment failure in patients with CRC. However, due to lack of clinical follow-up and available imaging technology, the diagnosis cannot be made in the early stages, resulting in an inaccurate assessment of the incidence of peritoneal metastasis. Sugarbaker[91] recommended a combination of cytoreductive surgery (CRS) to remove all visible peritoneal metastases and hyperthermic intraperitoneal chemotherapy (HIPEC) to remove minimal residual disease. Since the peritoneal metastatic rate of patients with colorectal MAC is higher than that of patients with NMAC[14], CRS combined with HIPEC is particularly important. Multiple studies have shown that the survival benefit of CRS and HIPEC in patients with peritoneal metastasis caused by CRC is better than that of systemic chemotherapy alone[92,93]. However, the results of a recent multicenter, randomized clinical trial showed that adding HIPEC to CRS did not benefit patients with peritoneal metastatic CRC (HR = 1.00, 95%CI: 0.63-1.58, P = 0.99), which resulted in more frequent postoperative late complications (CRS plus HIPEC group vs CRS group, 42% vs 32%, P = 0.083)[94]. Therefore, CRS alone should be the cornerstone of therapeutic strategies with curative intent for colorectal peritoneal metastases. CRS plus HIPEC should be selected after a careful and individualized assessment including Eastern Cooperative Oncology Group performance status scores, peritoneal cancer index, and previous chemotherapy lines. Klempner and Ryan[95] suggested that future studies of peritoneal cancer should be attentive to the rich translational opportunities that CRS can supply for multiple avenues of investigation.
Traditional chemotherapy usually targets rapidly proliferating cancer cells by interfering with cell division. However, it also nonspecifically targets healthy cells that divide rapidly, such as bone marrow and hair cells, resulting in recognized che
Drugs targeting mucins, one of the prominent features of MAC, are potential treatment strategies currently being investigated. Ahmad et al[37] found that the MUC1-C inhibitor, GO-203, could inhibit the growth of colon cancer cells in vitro and in nude mice, primarily by downregulating the expression of the TP53-inducible glycolysis and apoptosis regulator protein. In addition, since mucins are a class of O-glycosylated glycoproteins, the aberrant expression of O-glycan synthesis enzyme core 2 1,6 N-acetylglucosaminyltransferase (GCNT3/C2GnT-2) can lead to overexpression of mucins[102]. Therefore, targeting GCNT3 can inhibit mucin synthesis in MAC. At present, small-molecule GCNT3 inhibitors are under development[103].
The interaction of programmed cell death (PD)-1 on T cells and its interaction with its ligand, PD-L1, expressed on tumor cells and immune cells, including B cells, dendritic cells, and macrophages, plays an important role in immune checkpoint suppression[104]. The binding of PD-L1 on tumor cells to PD-1 on the surface of T cells inhibits T-cell-mediated antitumor immunity[105]. Immune checkpoint inhibitors have significantly improved the long-term outcomes of a few malignant tumors, such as melanoma, lung cancer, and renal cell carcinoma[106-108]. In MAC, the expression of PD-L1 in tumor cells and tumor-infiltrating immune cells is increased[109], which may be related to the high proportion of MSI-H in MAC. Studies have shown that compared to tumors with proficient mismatch repair (pMMR), tumors with deficient MMR (dMMR) highly express immune checkpoint proteins, including PD-1, PD-L1, and cytotoxic T-lymphocyte-associated protein (CTLA)-4[110]. MSI CRC has a higher tumor-infiltrating lymphocyte density and prominent Crohn’s-like lymphoid reaction than MSS CRC[111,112]. It has been previously believed that the increased levels of neoantigens produced by frameshift mutations also increase T cell infiltration in MSI CRC. Recent findings have supported this hypothesis, linking the number of frameshift mutations directly to the density of tumor-infiltrating lymphocytes[113]. Based on these observations, several clinical trials are studying the application of PD-1 immunotherapy in MSI CRC. Le et al[110] found that the efficacy of pembrolizumab in dMMR CRC was far better than that of pMMR CRC in terms of immune-related objective remission rate (40% vs 0%) and immune-related PFS rate within 20 wk (78% vs 11%)[110]. Therefore, pembrolizumab was the first drug that did not consider tumor types and only used biomarkers (dMMR/MSI-H) as treatment options based on overall response rates. Additional data also showed that nivolumab had benefits in advanced dMMR/MSI-H CRC where previous cytotoxic drugs had failed, with 31% of cases responding, and 69% of the overall disease control rate[114]. Therefore, the National Comprehensive Cancer Network guidelines have officially recommended pembrolizumab or nivolumab as second-line or third-line treatment for patients with MSI-H metastatic CRC since 2017[115]. Michael et al[116] reported that compared to anti-PD-1 monotherapy, nivolumab combined with ipilimumab had a higher response rate and better long-term clinical benefits, with controllable safety, and thus, should be considered as the first-line treatment for patients with metastatic dMMR/MSI-H CRC. The KEYNOTE-177 trial found that when pembrolizumab was used as the first-line treatment for metastatic dMMR/MSI-H CRC, patients had a significantly longer PFS (median, 16.5 vs 8.2 mo, HR = 0.60, 95%CI: 0.45-0.80, P = 0.0002) and fewer treatment-related adverse events (22% vs 66%) compared to those receiving chemotherapy[117]. Therefore, the US Food and Drug Administration approved pembrolizumab as a first-line treatment for unresectable or metastatic dMMR/MSI-H CRC in June 2020[118]. However, a subgroup analysis in the KEYNOTE-177 trial indicated that patients with metastatic dMMR/MSI-H CRC with KRAS or NRAS mutations could not benefit from pembrolizumab alone[117]. Whether adding chemotherapy or anti-CTLA-4 to PD-1 blockade could overcome this apparent resistance remains unknown.
The prognosis of patients with colorectal MAC remains controversial, which may be due to the higher TNM stage at the time of diagnosis. Studies have found that the 5-year OS rate of patients with MAC was lower than that of patients with NMAC, whereas no difference in prognosis was found when comparing patients with the same TNM stage[11,24,27]. However, other studies have indicated that in stage III colon cancer, patients with MAC have a poor 5-year CSS rate (MAC vs NMAC, 67.9% vs 72.7%)[81]. Catalano et al[119] believed that the controversy over the prognosis of colorectal MAC was caused by the poor prognosis of rectal MAC, while there was no significant difference between colonic MAC and NMAC. The authors also found, for patients with stage II and III colon cancer who underwent radical surgery, there was no significant difference in prognosis between MAC and NMAC. In addition, MAC is more likely to have nodal metastases, be diagnosed at an advanced stage, and have lower resectability of tumors in the rectum than the colon, thus leading to a poor prognosis of rectal MAC[119].
Studies have also demonstrated that higher age (> 65 years), tumor grades including moderately, poorly, and undifferentiated tumors, tumor location in the rectum, preoperative CEA level (> 5 ng/mL), higher pathological T or N stage, intestinal obstruction, and perineural infiltration were all significantly associated with poor OS in MAC[7,120]. A greater number of lymph nodes examined (no fewer than 12) significantly increased OS (HR = 0.601, 95%CI: 0.537-0.673, P < 0.001) and CSS (HR = 0.582, 95%CI: 0.511 to -0.664, P < 0.001) in patients with colorectal MAC[120]. BRAF mutations were significantly associated with CRC-specific mortality (multivariate HR = 1.64, 95%CI: 1.18-2.27, P = 0.003), while MSI-H was associated with a statistically significant reduction in CRC-specific mortality (multivariate HR = 0.28, 95%CI: 0.17-0.46, P < 0.001). Considering both MSI-H and BRAF, the 5-year CSS rates were 79%, 73%, 65%, and 46%, respectively, in MSI-H/BRAF-wild-type, MSI-H/BRAF-mutant, MSS/BRAF-wild-type, MSS/BRAF-mutant[121], suggesting that the prognosis of patients with MAC could be stratified according to the status of MSI-H combined with BRAF. Notably, in metastatic CRC, dMMR corresponds to a poorer prognosis compared with pMMR[122]. Immunotherapies, including anti-PD-1 and CTLA-4, emerged in recent years are promising treatment strategy.
Colorectal MAC is a unique clinicopathological subtype of CRC. This review comprehensively describes the clinicopathological characteristics, molecular features, diagnosis, treatment, and prognosis of colorectal MAC. One of the most notable features of MAC is the aberrant expression of multiple mucins, but the underlying mechanism remains unclear. The mucinous features of MAC suggest that it originates from cells expressing MUC2, with no clear understanding of the mechanism underlying mucus production by MAC against radiotherapy and chemotherapy. In the future, in-depth research is needed to clarify the role of mucus in MAC. Colorectal MAC has a higher frequency of KRAS, BRAF mutations, CIMP-H, and MSI-H, suggesting that the genetic origin of colorectal MAC is mainly related to the serrated pathway of CRC, namely the BRAF, MSI, and CIMP pathways, which also explains the high proportion of MSI-H in MAC. MSI-H indicates a better response to immunotherapy, which is hopeful for patients with MAC. The prognosis of patients with colorectal MAC remains controversial, which may be attributed to the poor prognosis of rectal MAC, while there is no significant difference in the prognosis of colonic MAC and NMAC.
In summary, MAC has various clinicopathological and molecular characteristics that differ from those of NMAC. Therefore, personalized diagnosis and treatment of MAC is beneficial. Further studies, such as targeted drugs for mucins, sensitization to chemoradiotherapy, and immunotherapy, are warranted to improve the prognosis of patients with MAC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ros J S-Editor: Liu M L-Editor: Kerr C P-Editor: Zhang YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64217] [Article Influence: 16054.3] [Reference Citation Analysis (174)] |
| 2. | Bosman FT, Carneiro F, Hruban RH, ND T. WHO Classification of Tumours of the Digestive System, 4th ed. Geneva: World Health Organization Classifcation of Tumours, 2010. |
| 3. | Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol. 2012;3:153-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 269] [Reference Citation Analysis (0)] |
| 4. | Onodera M, Nishigami T, Torii I, Sato A, Tao LH, Kataoka TR, Yoshikawa R, Tsujimura T. Comparison between colorectal low- and high-grade mucinous adenocarcinoma with MUC1 and MUC5AC. World J Gastrointest Oncol. 2009;1:69-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM, Rodriguez-Bigas MA, Cormier JN, Chang GJ. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19:2814-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 265] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 6. | Li ZP, Liu XY, Kao XM, Chen YT, Han SQ, Huang MX, Liu C, Tang XY, Chen YY, Xiang D, Huang YD, Lei ZJ, Chu XY. Clinicopathological characteristics and prognosis of colorectal mucinous adenocarcinoma and nonmucinous adenocarcinoma: a surveillance, epidemiology, and end results (SEER) population-based study. Ann Transl Med. 2020;8:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Park JS, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, Lee WY, Chun HK. Prognostic comparison between mucinous and nonmucinous adenocarcinoma in colorectal cancer. Medicine (Baltimore). 2015;94:e658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Khan M, Loree JM, Advani SM, Ning J, Li W, Pereira AAL, Lam M, Raghav K, Morris VK, Broaddus R, Maru D, Overman MJ, Kopetz S. Prognostic Implications of Mucinous Differentiation in Metastatic Colorectal Carcinoma Can Be Explained by Distinct Molecular and Clinicopathologic Characteristics. Clin Colorectal Cancer. 2018;17:e699-e709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Tarantino I, Hüttner FJ, Warschkow R, Schmied BM, Diener MK, Ulrich A. Prognostic Relevance of Mucinous Subtype in a Population-based Propensity Score Analysis of 40,083 Rectal Cancer Patients. Ann Surg Oncol. 2016;23:1576-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Xie L, Villeneuve PJ, Shaw A. Survival of patients diagnosed with either colorectal mucinous or non-mucinous adenocarcinoma: a population-based study in Canada. Int J Oncol. 2009;34:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Yamaguchi T, Taniguchi H, Fujita S, Sekine S, Yamamoto S, Akasu T, Kushima R, Tani T, Moriya Y, Shimoda T. Clinicopathological characteristics and prognostic factors of advanced colorectal mucinous adenocarcinoma. Histopathology. 2012;61:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Negri FV, Wotherspoon A, Cunningham D, Norman AR, Chong G, Ross PJ. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol. 2005;16:1305-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Sengul N, Wexner SD, Woodhouse S, Arrigain S, Xu M, Larach JA, Ahn BK, Weiss EG, Nogueras JJ, Berho M. Effects of radiotherapy on different histopathological types of rectal carcinoma. Colorectal Dis. 2006;8:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Song W, Wu SJ, He YL, Cai SR, Zhang CH, Zhang XH, Zhan WH. Clinicopathologic features and survival of patients with colorectal mucinous, signet-ring cell or non-mucinous adenocarcinoma: experience at an institution in southern China. Chin Med J (Engl). 2009;122:1486-1491. [PubMed] |
| 15. | You JF, Hsieh LL, Changchien CR, Chen JS, Chen JR, Chiang JM, Yeh CY, Hsieh PS, Fan CW, Liu CT, Tang R. Inverse effects of mucin on survival of matched hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer patients. Clin Cancer Res. 2006;12:4244-4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Gao P, Song YX, Xu YY, Sun Z, Sun JX, Xu HM, Wang ZN. Does the prognosis of colorectal mucinous carcinoma depend upon the primary tumour site? Histopathology. 2013;63:603-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Wang L, Hirano Y, Heng G, Ishii T, Kondo H, Hara K, Obara N, Asari M, Kato T, Yamaguchi S. Mucinous Adenocarcinoma as a High-risk Factor in Stage II Colorectal Cancer: A Propensity Score-matched Study from Japan. Anticancer Res. 2020;40:1651-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Kanda M, Oba K, Aoyama T, Kashiwabara K, Mayanagi S, Maeda H, Honda M, Hamada C, Sadahiro S, Sakamoto J, Saji S, Yoshikawa T; Japanese Foundation for Multidisciplinary Treatment of Cancer. Clinical Signatures of Mucinous and Poorly Differentiated Subtypes of Colorectal Adenocarcinomas by a Propensity Score Analysis of an Independent Patient Database from Three Phase III Trials. Dis Colon Rectum. 2018;61:461-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Kang H, O'Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 20. | Morikawa T, Kuchiba A, Qian ZR, Mino-Kenudson M, Hornick JL, Yamauchi M, Imamura Y, Liao X, Nishihara R, Meyerhardt JA, Fuchs CS, Ogino S. Prognostic significance and molecular associations of tumor growth pattern in colorectal cancer. Ann Surg Oncol. 2012;19:1944-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Hugen N, van Beek JJ, de Wilt JH, Nagtegaal ID. Insight into mucinous colorectal carcinoma: clues from etiology. Ann Surg Oncol. 2014;21:2963-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Lin J, Qiu M, Xu R, Dobs AS. Comparison of survival and clinicopathologic features in colorectal cancer among African American, Caucasian, and Chinese patients treated in the United States: Results from the surveillance epidemiology and end results (SEER) database. Oncotarget. 2015;6:33935-33943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Du W, Mah JT, Lee J, Sankila R, Sankaranarayanan R, Chia KS. Incidence and survival of mucinous adenocarcinoma of the colorectum: a population-based study from an Asian country. Dis Colon Rectum. 2004;47:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Nitsche U, Zimmermann A, Späth C, Müller T, Maak M, Schuster T, Slotta-Huspenina J, Käser SA, Michalski CW, Janssen KP, Friess H, Rosenberg R, Bader FG. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg. 2013;258:775-82; discussion 782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 25. | Perez RO, Bresciani BH, Bresciani C, Proscurshim I, Kiss D, Gama-Rodrigues J, Pereira DD, Rawet V, Cecconnello I, Habr-Gama A. Mucinous colorectal adenocarcinoma: influence of mucin expression (Muc1, 2 and 5) on clinico-pathological features and prognosis. Int J Colorectal Dis. 2008;23:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Melis M, Hernandez J, Siegel EM, McLoughlin JM, Ly QP, Nair RM, Lewis JM, Jensen EH, Alvarado MD, Coppola D, Eschrich S, Bloom GC, Yeatman TJ, Shibata D. Gene expression profiling of colorectal mucinous adenocarcinomas. Dis Colon Rectum. 2010;53:936-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Vernmark K, Sun XF, Holmqvist A. Mucinous and Non-Mucinous Rectal Adenocarcinoma-Differences in Treatment Response to Preoperative Radiotherapy. J Pers Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 29. | Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 383] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 30. | Jonckheere N, Skrypek N, Van Seuningen I. Mucins and tumor resistance to chemotherapeutic drugs. Biochim Biophys Acta. 2014;1846:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Amini A, Masoumi-Moghaddam S, Ehteda A, Morris DL. Secreted mucins in pseudomyxoma peritonei: pathophysiological significance and potential therapeutic prospects. Orphanet J Rare Dis. 2014;9:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Reynolds IS, Fichtner M, McNamara DA, Kay EW, Prehn JHM, Burke JP. Mucin glycoproteins block apoptosis; promote invasion, proliferation, and migration; and cause chemoresistance through diverse pathways in epithelial cancers. Cancer Metastasis Rev. 2019;38:237-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, Kim KC. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol. 2008;38:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1026] [Cited by in RCA: 1113] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 35. | Ahmad R, Raina D, Joshi MD, Kawano T, Ren J, Kharbanda S, Kufe D. MUC1-C oncoprotein functions as a direct activator of the nuclear factor-kappaB p65 transcription factor. Cancer Res. 2009;69:7013-7021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 36. | Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2227] [Cited by in RCA: 2406] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 37. | Ahmad R, Alam M, Hasegawa M, Uchida Y, Al-Obaid O, Kharbanda S, Kufe D. Targeting MUC1-C inhibits the AKT-S6K1-elF4A pathway regulating TIGAR translation in colorectal cancer. Mol Cancer. 2017;16:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Mukherjee P, Pathangey LB, Bradley JB, Tinder TL, Basu GD, Akporiaye ET, Gendler SJ. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine. 2007;25:1607-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci USA. 2007;104:16209-16214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 40. | Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 235] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 41. | Krishn SR, Kaur S, Smith LM, Johansson SL, Jain M, Patel A, Gautam SK, Hollingsworth MA, Mandel U, Clausen H, Lo WC, Fan WT, Manne U, Batra SK. Mucins and associated glycan signatures in colon adenoma-carcinoma sequence: Prospective pathological implication(s) for early diagnosis of colon cancer. Cancer Lett. 2016;374:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Li C, Zuo D, Yin L, Lin Y, Li C, Liu T, Wang L. Prognostic Value of MUC2 Expression in Colorectal Cancer: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2018;2018:6986870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Gratchev A, Siedow A, Bumke-Vogt C, Hummel M, Foss HD, Hanski ML, Kobalz U, Mann B, Lammert H, Mansmann U, Stein H, Riecken EO, Hanski C. Regulation of the intestinal mucin MUC2 gene expression in vivo: evidence for the role of promoter methylation. Cancer Lett. 2001;168:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Bu XD, Li N, Tian XQ, Li L, Wang JS, Yu XJ, Huang PL. Altered expression of MUC2 and MUC5AC in progression of colorectal carcinoma. World J Gastroenterol. 2010;16:4089-4094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Jonckheere N, Van Der Sluis M, Velghe A, Buisine MP, Sutmuller M, Ducourouble MP, Pigny P, Büller HA, Aubert JP, Einerhand AW, Van Seuningen I. Transcriptional activation of the murine Muc5ac mucin gene in epithelial cancer cells by TGF-beta/Smad4 signalling pathway is potentiated by Sp1. Biochem J. 2004;377:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Jonckheere N, Vincent A, Franquet-Ansart H, Witte-Bouma J, Korteland-van Male A, Leteurtre E, Renes IB, Van Seuningen I. GATA-4/-6 and HNF-1/-4 families of transcription factors control the transcriptional regulation of the murine Muc5ac mucin during stomach development and in epithelial cancer cells. Biochim Biophys Acta. 2012;1819:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Park ET, Gum JR, Kakar S, Kwon SW, Deng G, Kim YS. Aberrant expression of SOX2 upregulates MUC5AC gastric foveolar mucin in mucinous cancers of the colorectum and related lesions. Int J Cancer. 2008;122:1253-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Raja SB, Murali MR, Devaraj H, Devaraj SN. Differential expression of gastric MUC5AC in colonic epithelial cells: TFF3-wired IL1 β/Akt crosstalk-induced mucosal immune response against Shigella dysenteriae infection. J Cell Sci. 2012;125:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Kocer B, Soran A, Erdogan S, Karabeyoglu M, Yildirim O, Eroglu A, Bozkurt B, Cengiz O. Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol Int. 2002;52:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Pothuraju R, Rachagani S, Krishn SR, Chaudhary S, Nimmakayala RK, Siddiqui JA, Ganguly K, Lakshmanan I, Cox JL, Mallya K, Kaur S, Batra SK. Molecular implications of MUC5AC-CD44 axis in colorectal cancer progression and chemoresistance. Mol Cancer. 2020;19:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 51. | De Palma FDE, D'Argenio V, Pol J, Kroemer G, Maiuri MC, Salvatore F. The Molecular Hallmarks of the Serrated Pathway in Colorectal Cancer. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 52. | Crockett SD, Nagtegaal ID. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology. 2019;157:949-966.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 53. | Cancer Genome Atlas Network. . Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6643] [Article Influence: 511.0] [Reference Citation Analysis (0)] |
| 54. | Khaidakov M, Lai KK, Roudachevski D, Sargsyan J, Goyne HE, Pai RK, Lamps LW, Hagedorn CH. Gastric Proteins MUC5AC and TFF1 as Potential Diagnostic Markers of Colonic Sessile Serrated Adenomas/Polyps. Am J Clin Pathol. 2016;146:530-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1115] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 56. | Reynolds IS, Furney SJ, Kay EW, McNamara DA, Prehn JHM, Burke JP. Meta-analysis of the molecular associations of mucinous colorectal cancer. Br J Surg. 2019;106:682-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 57. | Leopoldo S, Lorena B, Cinzia A, Gabriella DC, Angela Luciana B, Renato C, Antonio M, Carlo S, Cristina P, Stefano C, Maurizio T, Luigi R, Cesare B. Two subtypes of mucinous adenocarcinoma of the colorectum: clinicopathological and genetic features. Ann Surg Oncol. 2008;15:1429-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1345] [Article Influence: 67.3] [Reference Citation Analysis (1)] |
| 59. | Yurgelun MB, Kulke MH, Fuchs CS, Allen BA, Uno H, Hornick JL, Ukaegbu CI, Brais LK, McNamara PG, Mayer RJ, Schrag D, Meyerhardt JA, Ng K, Kidd J, Singh N, Hartman AR, Wenstrup RJ, Syngal S. Cancer Susceptibility Gene Mutations in Individuals With Colorectal Cancer. J Clin Oncol. 2017;35:1086-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 369] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 60. | Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073-2087.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1677] [Cited by in RCA: 1534] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 61. | Boland PM, Yurgelun MB, Boland CR. Recent progress in Lynch syndrome and other familial colorectal cancer syndromes. CA Cancer J Clin. 2018;68:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 62. | Ogino S, Cantor M, Kawasaki T, Brahmandam M, Kirkner GJ, Weisenberger DJ, Campan M, Laird PW, Loda M, Fuchs CS. CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut. 2006;55:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 290] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 63. | Wu C, Bekaii-Saab T. CpG Island Methylation, Microsatellite Instability, and BRAF Mutations and Their Clinical Application in the Treatment of Colon Cancer. Chemother Res Pract. 2012;2012:359041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Li ZH, You DY, Gao DP, Yang GJ, Dong XX, Zhang DF, Ding YY. Role of CT scan in differentiating the type of colorectal cancer. Onco Targets Ther. 2017;10:2297-2303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Chand M, Yu S, Swift RI, Brown G. Mucinous carcinoma of the rectum: a distinct clinicopathological entity. Tech Coloproctol. 2014;18:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Kim MJ, Park JS, Park SI, Kim NK, Kim JH, Moon HJ, Park YN, Kim WH. Accuracy in differentiation of mucinous and nonmucinous rectal carcinoma on MR imaging. J Comput Assist Tomogr. 2003;27:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Yu SK, Chand M, Tait DM, Brown G. Magnetic resonance imaging defined mucinous rectal carcinoma is an independent imaging biomarker for poor prognosis and poor response to preoperative chemoradiotherapy. Eur J Cancer. 2014;50:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 68. | Cao W, Wu L, Zhao Y, Zhou J, Li W, Wang X, Xu J, Zhou Z, Liang C. A New MRI-Defined Biomarker for Rectal Mucinous Adenocarcinoma: Mucin Pool Patterns in Determining the Efficacy of Neoadjuvant Therapy. Front Oncol. 2020;10:1425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Horvat N, Hope TA, Pickhardt PJ, Petkovska I. Mucinous rectal cancer: concepts and imaging challenges. Abdom Radiol (NY). 2019;44:3569-3580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 70. | Nagtegaal I, Gaspar C, Marijnen C, Van De Velde C, Fodde R, Van Krieken H. Morphological changes in tumour type after radiotherapy are accompanied by changes in gene expression profile but not in clinical behaviour. J Pathol. 2004;204:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Patel UB, Blomqvist LK, Taylor F, George C, Guthrie A, Bees N, Brown G. MRI after treatment of locally advanced rectal cancer: how to report tumor response--the MERCURY experience. AJR Am J Roentgenol. 2012;199:W486-W495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 72. | Wnorowski AM, Menias CO, Pickhardt PJ, Kim DH, Hara AK, Lubner MG. Mucin-Containing Rectal Carcinomas: Overview of Unique Clinical and Imaging Features. AJR Am J Roentgenol. 2019;213:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | National Health Commission Of The People's Republic Of China. National guidelines for diagnosis and treatment of colorectal cancer 2020 in China (English version). Chin J Cancer Res. 2020;32:415-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 74. | Dos Anjos DA, Habr-Gama A, Vailati BB, Rossi CB, Coturel AE, Perez RO, São Julião GP, de Sousa JB, Buchpiguel CA. (18)F-FDG uptake by rectal cancer is similar in mucinous and nonmucinous histological subtypes. Ann Nucl Med. 2016;30:513-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Barbaro B, Leccisotti L, Vecchio FM, Di Matteo M, Serra T, Salsano M, Poscia A, Coco C, Persiani R, Alfieri S, Gambacorta MA, Valentini V, Giordano A, Bonomo L. The potential predictive value of MRI and PET-CT in mucinous and nonmucinous rectal cancer to identify patients at high risk of metastatic disease. Br J Radiol. 2017;90:20150836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 76. | Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol. 2000;174:1005-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 250] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 77. | Li X, Sun K, Liao X, Gao H, Zhu H, Xu R. Colorectal carcinomas with mucinous differentiation are associated with high frequent mutation of KRAS or BRAF mutations, irrespective of quantity of mucinous component. BMC Cancer. 2020;20:400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Song IH, Hong SM, Yu E, Yoon YS, Park IJ, Lim SB, Kim JC, Yu CS, Kim J. Signet ring cell component predicts aggressive behaviour in colorectal mucinous adenocarcinoma. Pathology. 2019;51:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Fields AC, Lu P, Goldberg J, Irani J, Bleday R, Melnitchouk N. The role of adjuvant chemotherapy in stage II and III mucinous colon cancer. J Surg Oncol. 2019;120:1190-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Huang Y, Ge K, Fu G, Chu J, Wei W. Mucinous Histology Might Be an Indicator for Enhanced Survival Benefit of Chemotherapy in Stage II Colon Cancer. Front Med (Lausanne). 2020;7:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Yu F, Huang L, Shen F, Wu S, Chen J. Prognostic implications of mucinous histology in stage III colon cancer with the receipt of adjuvant chemotherapy. J Gastrointest Oncol. 2020;11:858-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Kim SH, Shin SJ, Lee KY, Kim H, Kim TI, Kang DR, Hur H, Min BS, Kim NK, Chung HC, Roh JK, Ahn JB. Prognostic value of mucinous histology depends on microsatellite instability status in patients with stage III colon cancer treated with adjuvant FOLFOX chemotherapy: a retrospective cohort study. Ann Surg Oncol. 2013;20:3407-3413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 83. | Catalano V, Loupakis F, Graziano F, Torresi U, Bisonni R, Mari D, Fornaro L, Baldelli AM, Giordani P, Rossi D, Alessandroni P, Giustini L, Silva RR, Falcone A, D'Emidio S, Fedeli SL. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer. 2009;100:881-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 84. | Maisano R, Azzarello D, Maisano M, Mafodda A, Bottari M, Egitto G, Nardi M. Mucinous histology of colon cancer predicts poor outcomes with FOLFOX regimen in metastatic colon cancer. J Chemother. 2012;24:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Mekenkamp LJ, Heesterbeek KJ, Koopman M, Tol J, Teerenstra S, Venderbosch S, Punt CJ, Nagtegaal ID. Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur J Cancer. 2012;48:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 86. | McCawley N, Clancy C, O'Neill BD, Deasy J, McNamara DA, Burke JP. Mucinous Rectal Adenocarcinoma Is Associated with a Poor Response to Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2016;59:1200-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 87. | Tan Y, Fu D, Li D, Kong X, Jiang K, Chen L, Yuan Y, Ding K. Predictors and Risk Factors of Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Population-Based Analysis. Front Oncol. 2019;9:497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 88. | Smith JJ, Paty PB, Garcia-Aguilar J. Watch and Wait in Rectal Cancer or More Wait and See? JAMA Surg. 2020;155:657-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Hugen N, van de Velde CJ, Bosch SL, Fütterer JJ, Elferink MA, Marijnen CA, Rutten HJ, de Wilt JH, Nagtegaal ID. Modern Treatment of Rectal Cancer Closes the Gap Between Common Adenocarcinoma and Mucinous Carcinoma. Ann Surg Oncol. 2015;22:2669-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Chand M, Rasheed S, Bhangu A, Stamp GW, Swift RI, Tekkis PP, Brown G. Adjuvant chemotherapy improves overall survival after TME surgery in mucinous carcinoma of the rectum. Eur J Surg Oncol. 2014;40:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Sugarbaker PH. Patient selection and treatment of peritoneal carcinomatosis from colorectal and appendiceal cancer. World J Surg. 1995;19:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426-2432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 765] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 93. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1509] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 94. | Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, Marchal F, Loi V, Meeus P, Juzyna B, de Forges H, Paineau J, Glehen O; UNICANCER-GI Group and BIG Renape Group. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:256-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 513] [Article Influence: 128.3] [Reference Citation Analysis (2)] |
| 95. | Klempner SJ, Ryan DP. HIPEC for colorectal peritoneal metastases. Lancet Oncol. 2021;22:162-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 96. | Malhotra V, Perry MC. Classical chemotherapy: mechanisms, toxicities and the therapeutic window. Cancer Biol Ther. 2003;2:S2-S4. [PubMed] |
| 97. | De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P, Penault-Llorca F, Rougier P, Vincenzi B, Santini D, Tonini G, Cappuzzo F, Frattini M, Molinari F, Saletti P, De Dosso S, Martini M, Bardelli A, Siena S, Sartore-Bianchi A, Tabernero J, Macarulla T, Di Fiore F, Gangloff AO, Ciardiello F, Pfeiffer P, Qvortrup C, Hansen TP, Van Cutsem E, Piessevaux H, Lambrechts D, Delorenzi M, Tejpar S. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1491] [Cited by in RCA: 1654] [Article Influence: 110.3] [Reference Citation Analysis (1)] |
| 98. | De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M, Piessevaux H, Van Cutsem E, O'Callaghan CJ, Khambata-Ford S, Zalcberg JR, Simes J, Karapetis CS, Bardelli A, Tejpar S. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 584] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 99. | Peeters M, Douillard JY, Van Cutsem E, Siena S, Zhang K, Williams R, Wiezorek J. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol. 2013;31:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 100. | Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1728] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 101. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 102. | Rao CV, Janakiram NB, Madka V, Kumar G, Scott EJ, Pathuri G, Bryant T, Kutche H, Zhang Y, Biddick L, Gali H, Zhao YD, Lightfoot S, Mohammed A. Small-Molecule Inhibition of GCNT3 Disrupts Mucin Biosynthesis and Malignant Cellular Behaviors in Pancreatic Cancer. Cancer Res. 2016;76:1965-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Rao CV, Janakiram NB, Mohammed A. Molecular Pathways: Mucins and Drug Delivery in Cancer. Clin Cancer Res. 2017;23:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 104. | Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res. 2016;22:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 671] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 105. | Masugi Y, Nishihara R, Yang J, Mima K, da Silva A, Shi Y, Inamura K, Cao Y, Song M, Nowak JA, Liao X, Nosho K, Chan AT, Giannakis M, Bass AJ, Hodi FS, Freeman GJ, Rodig S, Fuchs CS, Qian ZR, Ogino S. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017;66:1463-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 106. | Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4375] [Cited by in RCA: 4591] [Article Influence: 459.1] [Reference Citation Analysis (0)] |
| 107. | Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6945] [Cited by in RCA: 7516] [Article Influence: 751.6] [Reference Citation Analysis (0)] |
| 108. | Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11762] [Article Influence: 784.1] [Reference Citation Analysis (0)] |
| 109. | Kim JH, Park HE, Cho NY, Lee HS, Kang GH. Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br J Cancer. 2016;115:490-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 110. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7215] [Article Influence: 721.5] [Reference Citation Analysis (0)] |
| 111. | Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, Pardoll DM, Brahmer JR, Topalian SL. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 431] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 112. | Rozek LS, Schmit SL, Greenson JK, Tomsho LP, Rennert HS, Rennert G, Gruber SB. Tumor-Infiltrating Lymphocytes, Crohn's-Like Lymphoid Reaction, and Survival From Colorectal Cancer. J Natl Cancer Inst. 2016;108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 113. | Schwitalle Y, Linnebacher M, Ripberger E, Gebert J, von Knebel Doeberitz M. Immunogenic peptides generated by frameshift mutations in DNA mismatch repair-deficient cancer cells. Cancer Immun. 2004;4:14. [PubMed] |
| 114. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4924] [Article Influence: 615.5] [Reference Citation Analysis (0)] |
| 115. | Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wu CS, Gregory KM, Freedman-Cass D. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 566] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 116. | Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1460] [Article Influence: 208.6] [Reference Citation Analysis (0)] |
| 117. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1786] [Article Influence: 357.2] [Reference Citation Analysis (0)] |
| 118. | Smith SM, Wachter K, Burris HA 3rd, Schilsky RL, George DJ, Peterson DE, Johnson ML, Markham MJ, Mileham KF, Beg MS, Bendell JC, Dreicer R, Keedy VL, Kimple RJ, Knoll MA, LoConte N, MacKay H, Meisel JL, Moynihan TJ, Mulrooney DA, Mulvey TM, Odenike O, Pennell NA, Reeder-Hayes K, Smith C, Sullivan RJ, Uzzo R. Clinical Cancer Advances 2021: ASCO's Report on Progress Against Cancer. J Clin Oncol. 2021;39:1165-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 119. | Catalano V, Loupakis F, Graziano F, Bisonni R, Torresi U, Vincenzi B, Mari D, Giordani P, Alessandroni P, Salvatore L, Fornaro L, Santini D, Baldelli AM, Rossi D, Giustini L, Silva RR, Falcone A, D'Emidio S, Rocchi M, Luzi Fedeli S. Prognosis of mucinous histology for patients with radically resected stage II and III colon cancer. Ann Oncol. 2012;23:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 120. | Ma Y, Luo Y, Lin N, Lv Y, Zhou Y, Li B, Han K, Jiang S, Gao J. Prognostic impact of the number of lymph nodes examined in different stages of colorectal mucinous adenocarcinoma. Onco Targets Ther. 2018;11:3659-3670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 121. | Lochhead P, Kuchiba A, Imamura Y, Liao X, Yamauchi M, Nishihara R, Qian ZR, Morikawa T, Shen J, Meyerhardt JA, Fuchs CS, Ogino S. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 122. | Nguyen M, Tipping Smith S, Lam M, Liow E, Davies A, Prenen H, Segelov E. An update on the use of immunotherapy in patients with colorectal cancer. Expert Rev Gastroenterol Hepatol. 2021;15:291-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |