Published online Nov 27, 2021. doi: 10.4240/wjgs.v13.i11.1390
Peer-review started: April 7, 2021
First decision: June 14, 2021
Revised: July 6, 2021
Accepted: October 24, 2021
Article in press: October 24, 2021
Published online: November 27, 2021
Processing time: 233 Days and 9.9 Hours
Controversy over the issue that No. 12a lymph node involvement is distant or regional metastasis remains, and the possible inclusion of 12a lymph nodes in D2 lymphadenectomy is unclear. As reported, gastric cancer (GC) located in the lower third is highly related to the metastasis of station 12a lymph nodes.
To investigate whether the clinicopathological factors and metastasis status of other perigastric nodes can predict station 12a lymph node metastasis and evaluate the prognostic significance of station 12a lymph node dissection in patients with lower-third GC.
A total of 147 patients with lower-third GC who underwent D2 or D2+ lymphadenectomy, including station 12a lymph node dissection, were included in this retrospective study from June 2003 to March 2011. Survival prognoses were compared between patients with or without station 12a lymph node metastasis. Logistic regression analyses were used to clarify the association between station 12a lymph node metastasis and clinicopathological factors or metastasis status of other perigastric nodes. The metastasis status of each regional lymph node was evaluated to identify the possible predictors of station 12a lymph node metastasis.
Metastasis to station 12a lymph nodes was observed in 18 patients with lower-third GC, but not in 129 patients. The incidence of station 12a lymph node involvement was reported as 12.2% in patients with lower-third GC. The overall survival of patients without station 12a lymph node metastasis was significantly better than that of patients with station 12a metastasis (P < 0.001), which could also be seen in patients with or without extranodal soft tissue invasion. Station 12a lymph node metastasis and extranodal soft tissue invasion were identified as independent predictors of poor prognosis in patients with lower-third GC. Advanced pN stage was defined as independent risk factor significantly correlated with station 12a lymph node positivity. Station 3 lymph node staus was also proven to be significantly correlated with station 12a lymph node invo
Metastasis of station 12a lymph nodes could be considered an independent prognosis factor for patients with lower-third GC. The dissection of station 12a lymph nodes may not be ignored in D2 or D2+ lymphadenectomy due to difficulties in predicting station 12a lymph node metastasis.
Core Tip: The possible inclusion of 12a lymph nodes in D2 lymphadenectomy remains unclear. As reported, gastric cancer (GC) located in the lower third was highly related to the metastasis of station 12a lymph nodes. The clinicopathological factors related to station 12a lymph node metastasis in patients with lower-third GC were investigated. The results showed that station 3 lymph node status was highly related to station 12a lymph node metastasis. The poor prognosis of patients with station 12a lymph node metastasis compared with those without 12a indicated that station 12a lymph node dissection must be considered.
- Citation: Dong YP, Cai FL, Wu ZZ, Wang PL, Yang Y, Guo SW, Zhao ZZ, Zhao FC, Liang H, Deng JY. Risk of station 12a lymph node metastasis in patients with lower-third gastric cancer. World J Gastrointest Surg 2021; 13(11): 1390-1404
- URL: https://www.wjgnet.com/1948-9366/full/v13/i11/1390.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i11.1390
Gastric cancer (GC) prevails as the fourth most common malignancy worldwide, and its mortality ranks second among all cancer-related deaths in China[1]. Surgery, including radical gastrectomy and lymphadenectomy, still plays a dominant role in patients with operable GC[2]. However, controversy has persisted for decades over the issue of performing D2 lymphadenectomy because of the high perioperative morbidity and mortality rate of D2 dissection[3-5]. Nevertheless, considerably extensive lymph node excision (D2 or D2+) helped reduce the cancer burden and identify the N status of patients[6]. Thus, D2 Lymphadenectomy combined with radical gastrectomy has become the standard treatment for advanced GC in Japan[7-9].
Station 12a lymph nodes are defined as the hepatoduodenal ligament lymph nodes along the proper hepatic artery. However, whether station 12a lymph node metastasis should be regarded as regional or distant and whether resection of this station should be included in D2 lymphadenectomy remain controversial. Station 12a lymph node metastasis, which was categorized as distant metastasis by the 7th American Joint Committee on Cancer (AJCC) classification[10,11], was reclassified as regional lymph node metastasis by the 8th AJCC classification[12]. However, the concept of station 12a lymph node involvement as regional metastasis once occurred in the 6th AJCC[13] and the 3rd Japanese classifications of gastric carcinoma[14]. Station 12a lymph nodes, as well as stations 1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, and 11p lymph nodes, are all indispensable for D2 lymphadenectomy during distal or total gastrectomy despite the aforementioned classifications. Additionally, D2 lymphadenectomy plus dissection of any of stations 8p, 10, 11d, 12b, 12p, 13, 14v, 16a2, and 16b1, so-called D2+ lymphadenectomy, as a more extended dissection, was an option for selected patients. D2 lymphadenectomy is essential to lower-third GC according to the 5th Japanese treatment guidelines[15]. Nevertheless, other studies indicated that station 12a lymph node dissection during D2 lymphadenectomy is unnecessary due to the absence of survival benefits with the additional 12a lymph node dissection[16,17].
As reported, with an incidence ranging from 1.7% to 18.2%[18-23], station 12a lymph node metastasis was highly related to the lower third tumor[23]. Therefore, in the present study some patients with lower-third GC were retrospectively reviewed to investigate the risk factors for station 12a lymph node metastasis and evaluate the survival outcomes of station 12a lymph node dissection.
A total of 705 patients with lower-third GC at the Department of Gastroenterology of the Tianjin Medical University Cancer Hospital (TJMUCH) were recruited in this retrospective study between June 2003 and March 2011. All eligible patients delivered written informed consent, and the study was approved by the institutional review board of the TJMUCH. The eligibility criteria were as follows: (1) Histological confirmation of primary gastric adenocarcinoma located in the lower third; (2) D2 or D2+ lymphadenectomy with station 12a lymph node dissection; (3) Radical gastrectomy with pathologically negative margin (R0 resection); and (4) Negative peritoneal lavage cytology without peritoneal metastasis or other distant metastasis. The exclusion criteria were as follows: (1) History of gastric surgery; (2) Prior chemotherapy, radiotherapy, or endocrine therapy for any malignancy; (3) Psychologically diagnosed disorders or other life-threatening diseases; or (4) Part of the stomach tumor pathologically diagnosed as stromal tumor or lymphoma.
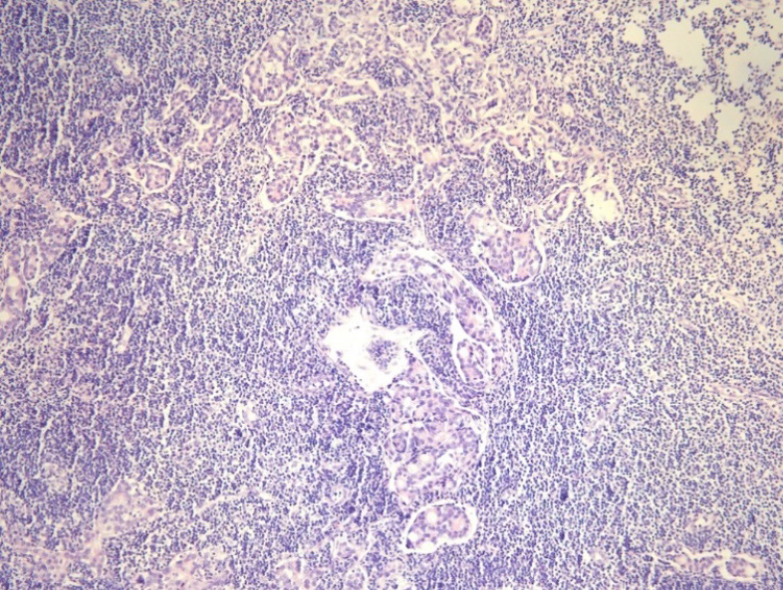
The following 16 clinicopathologic factors were reviewed from the medical and pathological record: Sex, age at surgery, Lauren classification, Borrmann type, maximum diameter, examined lymph node count, station 12a lymph node metastasis, pT stage, pN stage, extranodal soft tissue invasion [defined as the presence of tumor cells in an isolated tumor nodule between extranodal adipose tissues that was discontinuous with either the primary lesion and beyond the capsule of the lymph node (Figure 1)], perineuronal invasion, vessel invasion, adjuvant chemotherapy, histopathological subtype, surgical procedure, and blood transfusion. The postoperative pathological stages of all included cases were determined following the 8th AJCC gastric cancer guidelines.
Curative gastrectomy and lymphadenectomy were delivered to all included patients by experienced surgeons according to the guidelines of the Japanese Gastric Cancer Association. Almost all the patients underwent open surgery. Primary tumors were resected en bloc by gastrectomy plus D2 or D2+ lymphadenectomy with the dissection of station 12a lymph nodes because the surgical procedures were mainly based on the Japanese Gastric Cancer Treatment Guidelines[15].
After surgery, the patients were followed at 3-mo to 6-mo intervals up to the first 2 years, every 6 mo for the next 3 years, and annually thereafter until the end of the study (November 2015) or death. The median follow-up duration for the entire cohort was 42 (range, 2-145) mo. The main endpoint of the study was overall survival (OS), which was recorded from the date of surgery to the death of subjects or the latest follow-up. A total of 109 patients (74.1%) died during the follow-up period.
SPSS software (version 19.0, SPSS Inc, Chicago, IL, United States) was employed for all statistical analyses. Kaplan-Meier methods were performed to generate the survival curves, and log-rank tests were applied to compare the OS by corresponding clinicopathological factors. These factors, which might be associated with station 12a lymph node metastasis, were evaluated by univariate and multivariate logistic regression analyses. Factors with significance (P value < 0.05) in the univariate analysis were included in the subsequent multivariate analysis. Cox proportional hazards model was used for multivariate survival analysis to identify independent risk factors for prognosis in patients with lower-third GC. Moreover, χ2 test, McNemar paired-sample test, or Fisher’s test was applied to compare the sensitivity, specificity, and false-negative and false-positive rates between other regional lymph node metastasis and station 12a lymph node metastasis to identify the possible predictors of station 12a lymph node involvement. P < 0.05 was considered statistically significant.
Overall, 147 patients with lower-third GC underwent R0 gastrectomy with D2 or D2+ lymphadenectomy, including station 12a lymph node dissection, were eligible for this study inclusion. Moreover, of all eligible patients, 129 were histopathologically diagnosed without station 12a lymph node involvement and 18 had station 12a lymph node metastasis. The mean age for all patients was 52.9 (range, 26-79) years. Among these included patients, 18 patients (12.2%) had station 12a lymph node metastasis. The mean number of station 12a lymph node metastases was 1.33 ± 0.59 (range: 1-3). The characteristics of the patients and clinicopathological variables are shown in Table 1.
| Characteristic | n | Station 12a lymph nodes | χ2 | P value | |
| Negative (n = 129) | Positive (n = 18) | ||||
| Gender | 0.061 | 0.806 | |||
| Male | 91 | 82 | 9 | ||
| Female | 56 | 47 | 9 | ||
| Age at surgery, yr | 0.242 | 0.622 | |||
| < 60 | 84 | 74 | 10 | ||
| ≥ 60 | 63 | 55 | 8 | ||
| Lauren classification | 5.911 | 0.052 | |||
| Intestinal | 78 | 69 | 9 | ||
| Diffuse | 68 | 60 | 8 | ||
| Mixed | 1 | 0 | 1 | ||
| Borrmann type | 1.165 | 0.761 | |||
| I | 7 | 6 | 1 | ||
| II | 52 | 47 | 5 | ||
| III | 81 | 71 | 10 | ||
| IV | 7 | 5 | 2 | ||
| Maximum diameter | 4.491 | 0.034 | |||
| 4 cm or less | 77 | 73 | 4 | ||
| More than 4 cm | 70 | 56 | 14 | ||
| Examined lymph node count | 1.241 | 0.538 | |||
| < 16 | 47 | 42 | 5 | ||
| 16-30 | 69 | 61 | 8 | ||
| > 30 | 31 | 26 | 5 | ||
| pT stage | 10.112 | 0.039 | |||
| T1 | 2 | 2 | 0 | ||
| T2 | 28 | 28 | 0 | ||
| T3 | 10 | 9 | 1 | ||
| T4a | 102 | 86 | 16 | ||
| T4b | 5 | 4 | 1 | ||
| pN stage | 61.092 | < 0.001 | |||
| N0 | 56 | 56 | 0 | ||
| N1 | 22 | 22 | 0 | ||
| N2 | 36 | 32 | 4 | ||
| N3a | 19 | 10 | 9 | ||
| N3b | 14 | 9 | 5 | ||
| Soft tissue invasion | 19.249 | < 0.001 | |||
| Yes | 31 | 23 | 8 | ||
| No | 115 | 105 | 10 | ||
| Perineuronal invasion | 0.719 | 0.397 | |||
| Yes | 1 | 1 | 0 | ||
| No | 146 | 128 | 18 | ||
| Vessel invasion | 0.279 | 0.597 | |||
| Yes | 2 | 2 | 0 | ||
| No | 145 | 127 | 18 | ||
| Adjuvant chemotherapy | 5.997 | 0.014 | |||
| Yes | 97 | 82 | 15 | ||
| No | 50 | 47 | 3 | ||
| Blood transfusion | 1.394 | 0.238 | |||
| Yes | 19 | 17 | 2 | ||
| No | 128 | 112 | 16 | ||
Univariate analysis showed that station 12a lymph node metastasis, extranodal soft tissue invasion, large tumor diameter (maximum diameter > 4 cm), advanced pT and pN category, and no adjuvant chemotherapy were significantly associated with a poor prognosis in patients with lower-third GC (Table 1). Multivariate analysis revealed that pN stage and extranodal soft tissue were regarded as independent risk factors for the OS of GC patients. However, station 12a lymph node metastasis was defined as a part of pN stage, and pN stage exhibited a significant correlation with station 12a lymph node metastasis (Table 2). Therefore, pN stage were excluded in multivariate analysis to avoid multicollinearity. The results excluding the pN stage revealed that station 12a lymph node metastasis and extranodal soft tissue invasion were both independent prognostic factors for the OS of patients with lower-third GC (Table 3).
| Variables | Univariate analysis | Multivariate analysis1 | Multivariate analysis2 | ||||||
| OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | |
| Gender | 1.745 | 0.648-4.700 | 0.271 | ||||||
| Age at surgery | 1.076 | 0.399-2.906 | 0.885 | ||||||
| Lauren classification | 1.427 | 0.549-3.709 | 0.465 | ||||||
| Borrmann type | 1.404 | 0.643-3.066 | 0.396 | ||||||
| Maximum diameter | 4.562 | 1.424-14.619 | 0.011 | 2.838 | 0.743-10.840 | 0.127 | 4.012 | 1.231-13.078 | 0.021 |
| Examined lymph node count | 1.268 | 0.641-2.510 | 0.495 | ||||||
| pT stage | 2.935 | 1.807-7.924 | 0.034 | 1.456 | 0.389-5.449 | 0.577 | 2.055 | 0.749-5.642 | 0.162 |
| pN stage | 3.336 | 1.971-5.648 | < 0.001 | 3.322 | 1.962-5.625 | < 0.001 | |||
| Soft tissue invasion | 3.500 | 1.249-9.804 | 0.017 | 1.201 | 0.350-4.121 | 0.771 | 2.912 | 1.007-8.420 | 0.048 |
| Perineuronal invasion | 0.000 | 0.000 | 1.000 | ||||||
| Vessel invasion | 0.000 | 0.000 | 0.999 | ||||||
| Surgical options | 0.568 | 0.240-1.343 | 0.197 | ||||||
| BMI | 1.400 | 0.519-3.779 | 0.507 | ||||||
| Adjuvant chemotherapy | 2.866 | 0.789-10.415 | 0.11 | ||||||
| Blood transfusion | 0.824 | 0.174-3.903 | 0.807 | ||||||
| Variable | Cox regression analysis1 | Cox regression analysis2 | ||
| 95%CI | P value | 95%CI | P value | |
| Station 12a lymph node metastasis | 0.775-2.576 | 0.260 | 1.659-5.043 | < 0.001 |
| Maximum diameter | 0.690-1.559 | 0.859 | 0.722-1.644 | 0.682 |
| pT stage | 0.852-1.439 | 0.446 | 0.926-1.524 | 0.175 |
| pN stage | 1.291-1.809 | < 0.001 | ||
| Soft tissue invasion | 1.189-3.063 | 0.007 | 1.334-3.386 | 0.002 |
| Adjuvant chemotherapy | 0.841-2.028 | 0.234 | 0.823-1.969 | 0.279 |
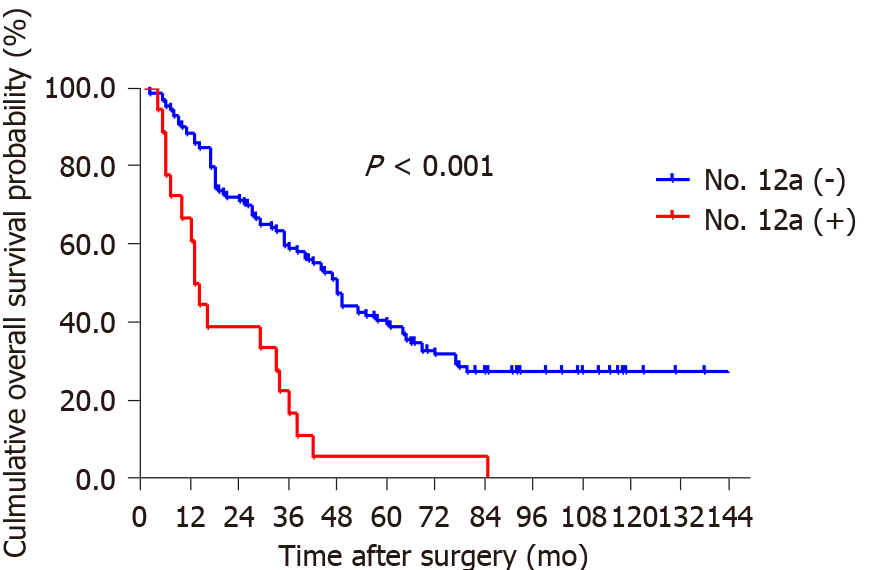
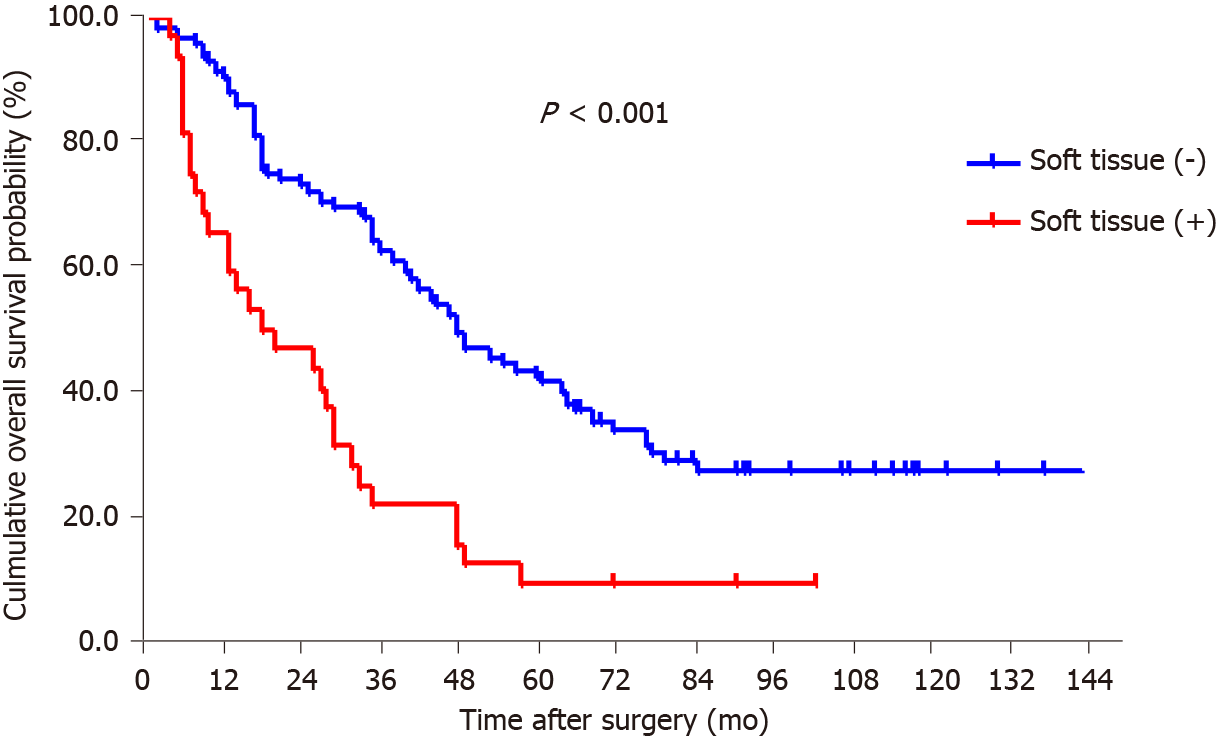
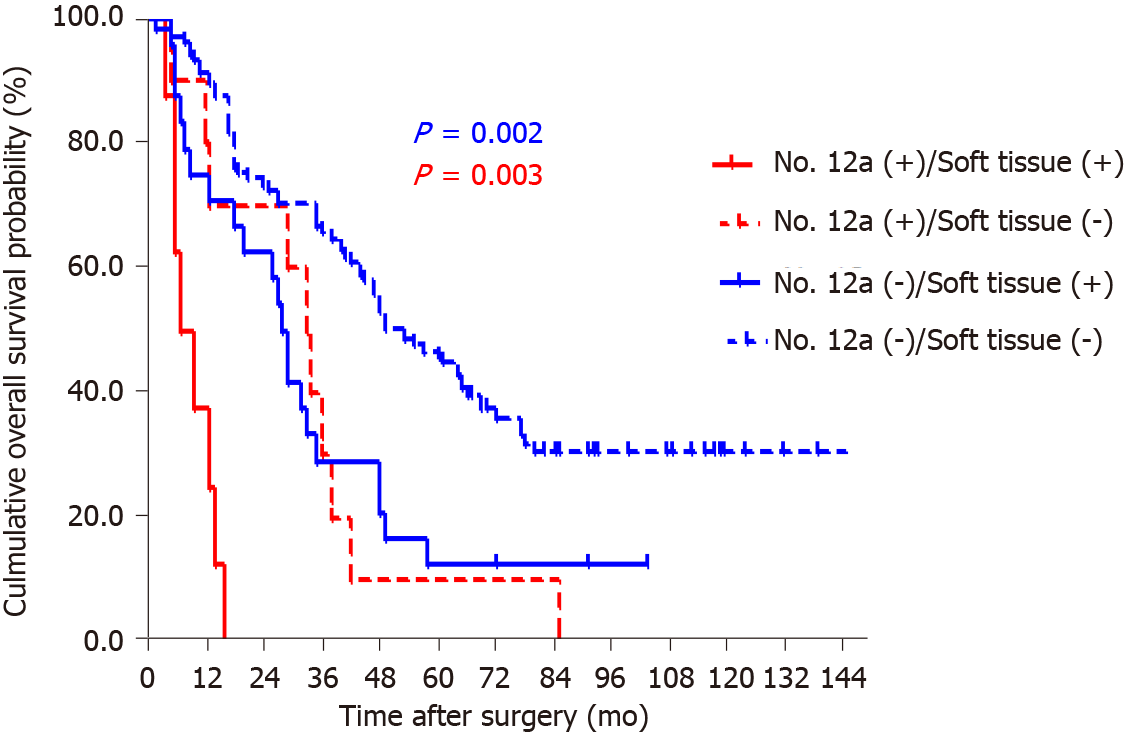
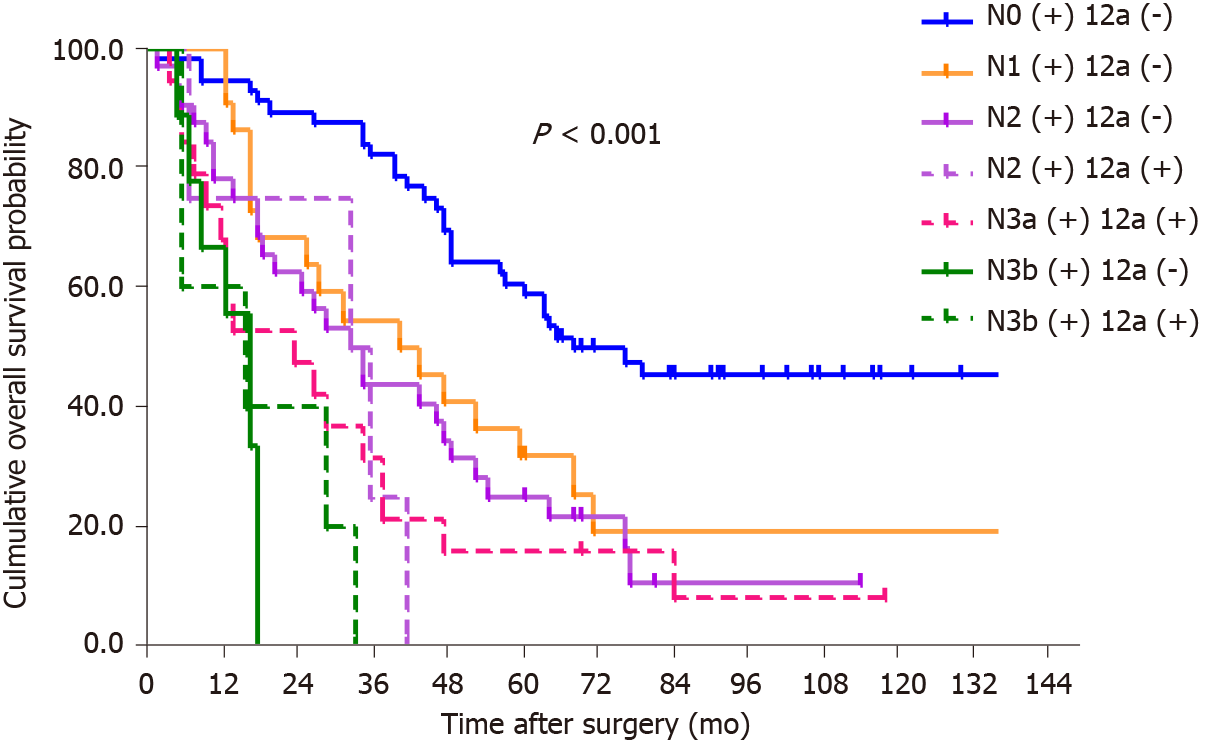
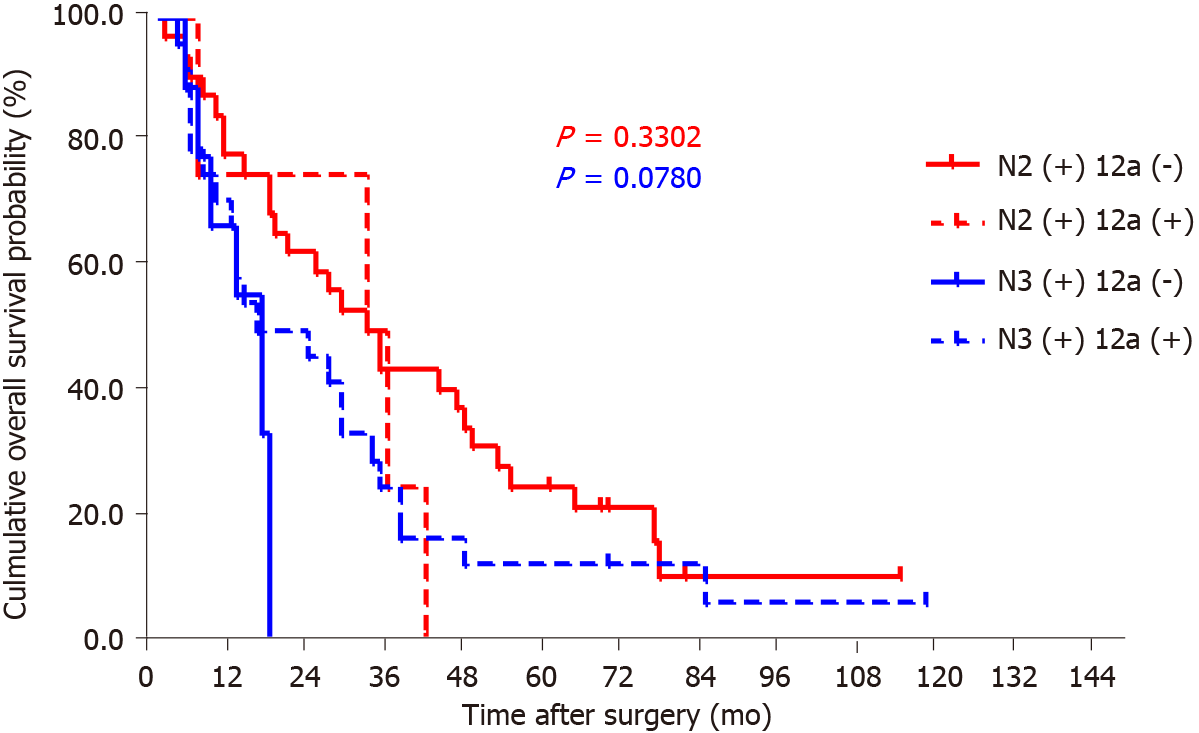
By the end of follow-up, all 18 patients with station 12a lymph node metastasis died, and the mean OS for patients with and without station 12a lymph node metastasis was 22.4 and 74.8 mo, respectively. The 5-year survival rate (5-YSR) for patients with or without station 12a lymph node metastasis was 5.6% and 39.5%, respectively. The patients with station 12a lymph node involvement showed a poorer prognosis compared with those without (P < 0.001; Figure 2). Moreover, all factors related to station 12a lymph node metastasis were included in the survival analysis for patients with positive or negative 12a lymph nodes (Table 4). OS rates were all significantly associated with soft tissue invasion despite the presence or absence of station 12a lymph node metastasis, which were both defined as independent predictors of OS (Figures 3 and 4). However, station 12a lymph node metastasis was unavailable for patients with pN0 and pN1 stages (Figure 5). By contrast, no statistically significant difference was found between patients with and without station 12a lymph node metastasis for patients with pN2 stage. Moreover, similar results could be obtained for patients with pN3 stage (Figure 6).
| Variable | No. 12a (+) | No. 12a (-) | ||
| 5-YSR (%) | P value | 5-YSR (%) | P value | |
| Maximum diameter | 0.408 | 0.142 | ||
| 4 cm or less | 0 | 45.2 | ||
| More than 4 cm | 7.1 | 32.1 | ||
| pT stage | 0.152 | 0.158 | ||
| T1 | - | 100.0 | ||
| T2 | - | 60.7 | ||
| T3 | 0 | 55.6 | ||
| T4a | 6.3 | 31.4 | ||
| T4b | 0 | 0 | ||
| pN stage | 0.619 | < 0.001 | ||
| N0 | - | 60.7 | ||
| N1 | - | 31.8 | ||
| N2 | 0 | 31.3 | ||
| N3a | 11.1 | 20.0 | ||
| N3b | 0 | 0 | ||
| Soft tissue invasion | 0.003 | 0.002 | ||
| + | 0 | 12.5 | ||
| - | 10 | 45.7 | ||
| No. 1 LNs | 0.873 | 0.292 | ||
| + | 0 | 28.6 | ||
| - | 7.7 | 40.2 | ||
| No. 2 LNs | 0.171 | 0.407 | ||
| + | 33.3 | 20.0 | ||
| - | 0 | 40.3 | ||
| No. 3 LNs | 0.950 | < 0.001 | ||
| + | 7.7 | 12.5 | ||
| - | 0 | 48.5 | ||
| No. 4sb LNs | 0.408 | 0.042 | ||
| + | 14.3 | 0 | ||
| - | 0 | 42.1 | ||
| No. 6 LNs | 0.290 | < 0.001 | ||
| + | 0 | 10.8 | ||
| - | 14.3 | 51.1 | ||
| No. 7 LNs | 0.143 | 0.028 | ||
| + | 0 | 23.1 | ||
| - | 10 | 43.7 | ||
| No. 8a LNs | 0.173 | < 0.001 | ||
| + | 0 | 6.3 | ||
| - | 8.3 | 44.2 | ||
Station 12a lymph node metastasis was significantly related to the maximum diameter of tumor (more than 4 cm), pT stage, advanced pN stage, and extranodal soft tissue invasion by univariate analyses. However, the results of multiple logistic regression analysis, including the four above-mentioned factors, only indicated that pN stage was significantly correlated with station 12a lymph node metastasis (P < 0.001; Table 3). The maximum diameter of tumor (more than 4 cm) and extranodal soft tissue invasion were both significantly correlated with 12a lymph node metastasis (P = 0.021 and P = 0.048, respectively) during multivariate analysis with the exclusion of pN to avoid multicollinearity (Table 2).
The univariate analyses indicated that the status of stations 1, 2, 3, 4sb, 6, 7, and 8a lymph node was significantly associated with station 12a lymph node metastasis (P < 0.05; Table 5). Station 3 lymph node status was found to be significantly related to station 12a lymph node metastasis by multivariate analysis. However, the correlation between the status of each regional lymph node and station 12a lymph node metastasis displayed high false-negative ratios ranging from 1%-10%. Therefore, significant predictors with relatively high kappa values were absent based on the consistency analysis. Such finding may be due to the small sample size of patients with other regional lymph node metastases (Table 6).
| Variables | No. 12a metastasis | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | ||
| No. 1 LNs | |||||||
| + | 5 | 6.703 | 1.860-24.163 | 0.004 | |||
| - | 13 | ||||||
| No. 2 LNs | |||||||
| + | 3 | 4.960 | 1.076-22.868 | 0.040 | |||
| - | 15 | ||||||
| No. 3 LNs | |||||||
| + | 13 | 7.881 | 2.608-23.821 | < 0.001 | 7.881 | 2.608-23.821 | < 0.001 |
| - | 5 | ||||||
| No. 4sa LNs | |||||||
| + | 3 | 3.025 | 0.723-12.657 | 0.130 | |||
| - | 15 | ||||||
| No. 4sb LNs | |||||||
| + | 7 | 4.836 | 1.626-14.383 | 0.005 | |||
| - | 11 | ||||||
| No. 4d LNs | |||||||
| + | 0 | 0.000 | 0.000 | 0.999 | |||
| - | 18 | ||||||
| No .5 LNs | |||||||
| + | 4 | 3.065 | 0.860-10.929 | 0.084 | |||
| - | 14 | ||||||
| No. 6 LNs | |||||||
| + | 11 | 3.907 | 1.407-10.853 | 0.009 | |||
| - | 7 | ||||||
| No. 7 LNs | |||||||
| + | 8 | 3.169 | 1.138-8.828 | 0.027 | |||
| - | 10 | ||||||
| No. 8a LNs | |||||||
| + | 6 | 3.531 | 1.163-10.726 | 0.026 | |||
| - | 12 | ||||||
| No. 8p LNs | |||||||
| + | 0 | 0.000 | 0.000 | 0.999 | |||
| - | 18 | ||||||
| No. 9 LNs | |||||||
| + | 2 | 1.115 | 0.230-5.403 | 0.892 | |||
| - | 16 | ||||||
| No. 10 LNs | |||||||
| + | 0 | 0.000 | 0.000 | 0.999 | |||
| - | 18 | ||||||
| No. 11p LNs | |||||||
| + | 1 | 3.735 | 0.321-43.427 | 0.292 | |||
| - | 17 | ||||||
| No. 11d LNs | |||||||
| + | 0 | 0.000 | 0.000 | 1.000 | |||
| - | 18 | ||||||
| Possible predictor | Sensitivity | Specificity | False negative | False positive | P value | Kappa value |
| No. 1 | 27.8 | 94.6 | 72.2 | 5.4 | 0.263 | 0.261 |
| No. 2 | 16.7 | 96.1 | 83.3 | 3.9 | 0.041 | 0.168 |
| No. 3 | 72.2 | 75.2 | 27.8 | 24.8 | 0.000 | 0.288 |
| No. 4d | 0.0 | 97.7 | 100.0 | 2.3 | 1.000 | -0.036 |
| No. 4sa | 16.7 | 93.8 | 83.3 | 6.2 | 0.2101 | 0.126 |
| No. 4sb | 38.9 | 88.4 | 61.1 | 11.6 | 0.557 | 0.249 |
| No. 5 | 22.2 | 91.5 | 77.8 | 8.5 | 0.148 | 0.690 |
| No. 6 | 61.1 | 72.1 | 38.9 | 27.9 | 0.000 | 0.196 |
| No. 7 | 44.4 | 79.8 | 55.6 | 20.2 | 0.011 | 0.176 |
| No. 8a | 33.3 | 87.6 | 66.7 | 12.4 | 0.191 | 0.572 |
| No. 8p | 0.0 | 97.7 | 100.0 | 2.3 | 1.0001 | -0.036 |
| No. 9 | 11.1 | 89.9 | 88.9 | 10.1 | 0.011 | 0.711 |
| No. 10 | 0.0 | 98.4 | 100.0 | 1.6 | 1.0001 | -0.025 |
| No. 11d | 0.0 | 99.2 | 100.0 | 0.8 | 1.0001 | -0.013 |
| No. 11p | 5.6 | 98.4 | 94.4 | 1.6 | 0.001 | 0.062 |
Lymph node metastasis was considered a significant prognostic factor for GC patients. The incidence of station 12a lymph node metastasis varied from 1.7% to 18.2% among studies[18-23]. However, many studies found that tumor located in the lower third was significantly associated with metastasis to station 12a lymph nodes. Moreover, patients with lower-third GC even showed a high incidence of station 12a lymph node metastasis[23,24]. The present study reported that the incidence of station 12a lymph node involvement was as high as 12.2% in patients with lower-third GC. Station 12a lymph node metastasis and extranodal soft tissue were defined in this study as independent prognosis factors for patients with lower-third GC. By contrast, station 12a lymphadenectomy was suggested as an independent prognostic factor for stage III patients by other investigations[24].
In addition to the tumor located in the lower third, many other clinicopathological factors were also significantly associated with station 12a lymph node metastasis. For example, lesser curvature or circumferential involvement and tumor diameter of more than 81.5 mm were identified as independent risk factors for station 12a lymph node metastasis[23]. Moreover, N and M stages were reported to be significantly correlated with the metastasis of station 12a lymph nodes[24], while T and N stages were proven to have significant associations with station 12a lymph node metastasis. However, while excluding the pN stage to avoid multicollinearity, the maximum diameter of tumor (more than 4 cm) and extranodal soft tissue invasion were verified in this study to have a significant correlation with the metastasis of station 12a lymph nodes. Nonetheless, only pN stage was significantly associated with 12a lymph node metastasis while including pN stage in the multivariate analysis. These differences may come from the included cohorts of the current study, which focused on patients with lower-third GC. This study showed that patients with early-stage GC did not present with station 12a lymph node metastasis, including pT1-2, pN0-1, and Ia-IIb stages, which may cause a significant relation to station 12a lymph node metastasis.
Controversy over performing D2 lymphadenectomy with or without station 12a lymph node dissection has persisted for decades possibly due to the uncertainty that station 12a lymph node positivity should be regarded as distant or regional metastasis. As mentioned above, station 12a lymph node positivity was considered to be a distant metastasis by the 7th AJCC classification[10,11]. Meanwhile, such positivity was not assigned to D2 lymphadenectomy according to the 7th AJCC staging and guidelines of the National Cancer Comprehensive Network of GC (Version 3, 2015)[25]. Nevertheless, station 12a lymph node positivity was regarded as a regional metastasis by the 8th AJCC staging[12], and the dissection of station 12a lymph nodes should be included in D2 lymphadenectomy with distal or total gastrectomy according to the 5th Japanese treatment guidelines[15], which were also supported by the studies of Shirong et al[22] and Lee et al[26] studies. Several studies argued about this issue for long periods. Moreover, some studies suggested that excluding the dissection of station 12a lymph nodes would not affect survival compared with standard D2 lymphadenectomy[16,17]. All patients underwent station 12a lymph node dissection in the current study, but the 5-YSR of patients with station 12a lymph node metastasis was substantially lower than that of patient without station 12a lymph node metastasis. This finding may be due to the advanced pN stage for patients with station 12a lymph node metastasis. However, the poor prognosis of station 12a lymph node metastasis and the survival benefits of station 12a lymph node dissection for patients with station 12a lymph node metastasis from previous studies[23] revealed the possible consideration of the dissection of station 12a lymph nodes in D2 lymphadenectomy for GC patients. As well, considering that none of enrolled patients with station 12a lymph node metastasis underwent any neoadjuvant therapy, preoperative enhanced computed tomography (CT) and endoscopic ultrasonography must be performed to evaluate preoperative CT stage, and preoperative chemotherapy should be given to patients with station No. 12a lymph node metastases to improve their survival rate.
Station 12a lymph nodes are located around the common hepatic artery, and the portal vein must be exposed during the dissection, thus posing a risk for major vessel damage during the operation. Therefore, confirming whether the metastasis-free status of other regional lymph nodes could be identified as a predictor to avoid station 12a dissection is necessary. Kumagai et al[23] reported that station 11p lymph node status demonstrated a significant correlation to 12a metastasis. By contrast, station 5 lymph node status was significantly associated with the metastasis of 12a lymph nodes in the study of Yang et al[24]. Shirong et al[22] also demonstrated a significant relation of stations 3, 5, and 6 lymph node involvement to 12a metastasis. However, in our study, station 3 lymph node involvement was certified as an independent predictor of station 12a lymph node metastasis in patients with GC in the lower third. However, these differences may come from the small sample size and different inclusion criteria. Now, the lymphatic drainage to station 12a lymph nodes remains unclear. Therefore, large-scale studies should be conducted to further investigate relevant regional lymph nodes as predictors of station 12a lymph node metastasis.
Nevertheless, this study has some limitations. The small sample size constrained the number of patients with other positive regional lymph nodes. Moreover, the number of patients with station 12a lymph node metastasis was remarkably small. Thus, obtaining additional significant outcomes, including survival benefit and safety of station 12a lymph node dissection for GC patients, is difficult.
Overall, the obtained results reveal that station 12a lymph node metastasis is an independent risk factor for patients with lower third GC. Extranodal soft tissue invasion and the maximum diameter of tumor (more than 4 cm) are independent risk factors significantly correlated with the metastasis of station 12a lymph nodes. Station 3 lymph node status is significantly correlated with station 12a lymph node involvement. However, no regional lymph node was defined as an effective predictor of station 12a lymph node metastasis, indicating the necessity of large multicenter prospective randomized controlled studies in the future. However, station 12a lymph nodes should be resected in D2 gastrectomy due to increased difficulties in predicting station 12a lymph node metastasis.
Controversy over the issue that station 12a lymph node involvement is distant or regional metastasis remains, and whether station 12a lymph nodes should be included in D2 lymphadenectomy or not is unclear.
To investigate the risk factors for station 12a lymph node metastasis and evaluate the survival outcomes of station 12a lymph node dissection in patients with lower-third gastric cancer (GC).
To investigate whether the clinicopathological factors and metastasis status of other perigastric lymph nodes can predict station 12a lymph node metastasis and evaluate the prognostic significance of station 12a lymph node dissection in patients with lower-third GC.
Survival prognoses were compared between patients with or without station 12a lymph node metastasis. Logistic regression analyses were used to clarify the association between station 12a lymph node metastasis and clinicopathological factors or metastasis status of other perigastric lymph nodes.
The incidence of station 12a lymph node involvement was reported as 12.2% in patients with lower-third GC. The overall survival of patients without station 12a lymph node metastasis was significantly better than that of patients with station 12a lymph node metastasis (P < 0.001), which could also be seen in patients with or without extranodal soft tissue invasion. Advanced pN stage was defined as an independent risk factor significantly correlated with station 12a lymph node positivity. Station 3 lymph node status was also proven to be significantly correlated with station 12a lymph node involvement.
The dissection of station 12a lymph nodes may not be ignored in D2 or D2+ lymphadenectomy due to difficulties in predicting station 12a lymph node metastasis.
Controversy over the issue that station 12a lymph node involvement is distant or regional metastasis remains, and the possible inclusion of station 12a lymph nodes in the D2 lymphadenectomy is unclear. As reported, GC located in the lower third was highly related to the metastasis of station 12a lymph nodes. The clinicopathological factors related to station 12a lymph node metastasis in patients with lower-third GC were investigated in this study. The results showed that station 3 lymph node status was highly related to station 12a lymph node metastasis. The poor prognosis of patients with station 12a lymph node metastasis compared with those without indicated that station 12a lymph node dissection must be considered. This study further validated the significance of the study of station 12a lymph node metastasis in patients with lower third GC.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pruthi DS, Sato T S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Gao CC
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13187] [Article Influence: 1465.2] [Reference Citation Analysis (3)] |
| 2. | Van Cutsem E, Dicato M, Geva R, Arber N, Bang Y, Benson A, Cervantes A, Diaz-Rubio E, Ducreux M, Glynne-Jones R, Grothey A, Haller D, Haustermans K, Kerr D, Nordlinger B, Marshall J, Minsky BD, Kang YK, Labianca R, Lordick F, Ohtsu A, Pavlidis N, Roth A, Rougier P, Schmoll HJ, Sobrero A, Tabernero J, Van de Velde C, Zalcberg J. The diagnosis and management of gastric cancer: expert discussion and recommendations from the 12th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2010. Ann Oncol. 2011;22 Suppl 5:v1-v9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 793] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 4. | Sierra A, Regueira FM, Hernández-Lizoáin JL, Pardo F, Martínez-Gonzalez MA, A-Cienfuegos J. Role of the extended lymphadenectomy in gastric cancer surgery: experience in a single institution. Ann Surg Oncol. 2003;10:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 471] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 6. | Dudeja V, Habermann EB, Abraham A, Zhong W, Parsons HM, Tseng JF, Al-Refaie WB. Is there a role for surgery with adequate nodal evaluation alone in gastric adenocarcinoma? J Gastrointest Surg. 2012;16:238-46; discussion 246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1894] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 8. | Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg. 1987;11:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 442] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Kodera Y, Schwarz RE, Nakao A. Extended lymph node dissection in gastric carcinoma: where do we stand after the Dutch and British randomized trials? J Am Coll Surg. 2002;195:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 11. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual (7th ed). New York: Springer, 2010. |
| 12. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer, LR. AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2016. |
| 13. | Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC cancer staging manual. 6th edition. New York: Springer, 2002. |
| 14. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2857] [Article Influence: 204.1] [Reference Citation Analysis (0)] |
| 15. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1317] [Article Influence: 329.3] [Reference Citation Analysis (2)] |
| 16. | Galizia G, Lieto E, De Vita F, Castellano P, Ferraraccio F, Zamboli A, Mabilia A, Auricchio A, De Sena G, De Stefano L, Cardella F, Barbarisi A, Orditura M. Modified vs standard D2 Lymphadenectomy in total gastrectomy for nonjunctional gastric carcinoma with lymph node metastasis. Surgery. 2015;157:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Ichikura T, Chochi K, Sugasawa H, Mochizuki H. Modified radical lymphadenectomy (D1.5) for T2-3 gastric cancer. Langenbecks Arch Surg. 2005;390:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Maruyama K, Gunvén P, Okabayashi K, Sasako M, Kinoshita T. Lymph node metastases of gastric cancer. General pattern in 1931 patients. Ann Surg. 1989;210:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 311] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Kong SH, Yoo MW, Kim JW, Lee HJ, Kim WH, Lee KU, Yang HK. Validation of limited lymphadenectomy for lower-third gastric cancer based on depth of tumour invasion. Br J Surg. 2011;98:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Keller E, Stützer H, Heitmann K, Bauer P, Gebbensleben B, Rohde H. Lymph node staging in 872 patients with carcinoma of the stomach and the presumed benefit of lymphadenectomy. German Stomach Cancer TNM Study Group. J Am Coll Surg. 1994;178:38-46. [PubMed] |
| 21. | Wei ZW, Xia GK, Wu Y, Schwarz RE, Smith DD, He YL, Zhang CH. Evaluation of skeletonization of the hepatoduodenal ligament for the lower third gastric cancer by propensity score analysis. Hepatogastroenterology. 2013;60:1789-1796. [PubMed] |
| 22. | Shirong C, Jianhui C, Chuangqi C, Kaiming W, Xinhua Z, Wu S, Yulong H. Survival of proper hepatic artery lymph node metastasis in patients with gastric cancer: implications for D2 Lymphadenectomy. PLoS One. 2015;10:e0118953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Kumagai K, Hiki N, Nunobe S, Irino T, Ida S, Ohashi M, Yamaguchi T, Sano T. Metastasis to the lymph nodes along the proper hepatic artery from adenocarcinoma of the stomach. Langenbecks Arch Surg. 2016;401:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Yang K, Chen HN, Liu K, Zhang WH, Chen XZ, Chen XL, Zhou ZG, Hu JK. The survival benefit and safety of No. 12a lymphadenectomy for gastric cancer patients with distal or total gastrectomy. Oncotarget. 2016;7:18750-18762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA, Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:1286-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 677] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 26. | Lee SL, Lee HH, Ko YH, Song KY, Park CH, Jeon HM, Kim SS. Relevance of hepatoduodenal ligament lymph nodes in resectional surgery for gastric cancer. Br J Surg. 2014;101:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |