Published online Nov 27, 2021. doi: 10.4240/wjgs.v13.i11.1361
Peer-review started: June 8, 2021
First decision: June 30, 2021
Revised: July 1, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: November 27, 2021
Processing time: 171 Days and 4.2 Hours
Chylous ascites following right colectomy has a high incidence which is a critical challenge. At present, there are few studies on the factors affecting chylous ascites after right colectomy and especially after D3 Lymphadenectomy. A predictive model for chylous ascites has not yet been established. Therefore, we created the first nomogram to predict the incidence of chylous ascites after right hemi
To analyze the risk factors for chylous ascites after right colectomy and establish a nomogram to predict the incidence of chylous ascites.
We retrospectively collected patients who underwent right hemicolectomy between January 2012 and May 2021 and were pathologically diagnosed with cancer. Multivariate logistic regression was used to analyze the influencing factors of chylous ascites and a nomogram was established. The predictive ability was assessed by the area under the receiver operating characteristic (ROC) curve.
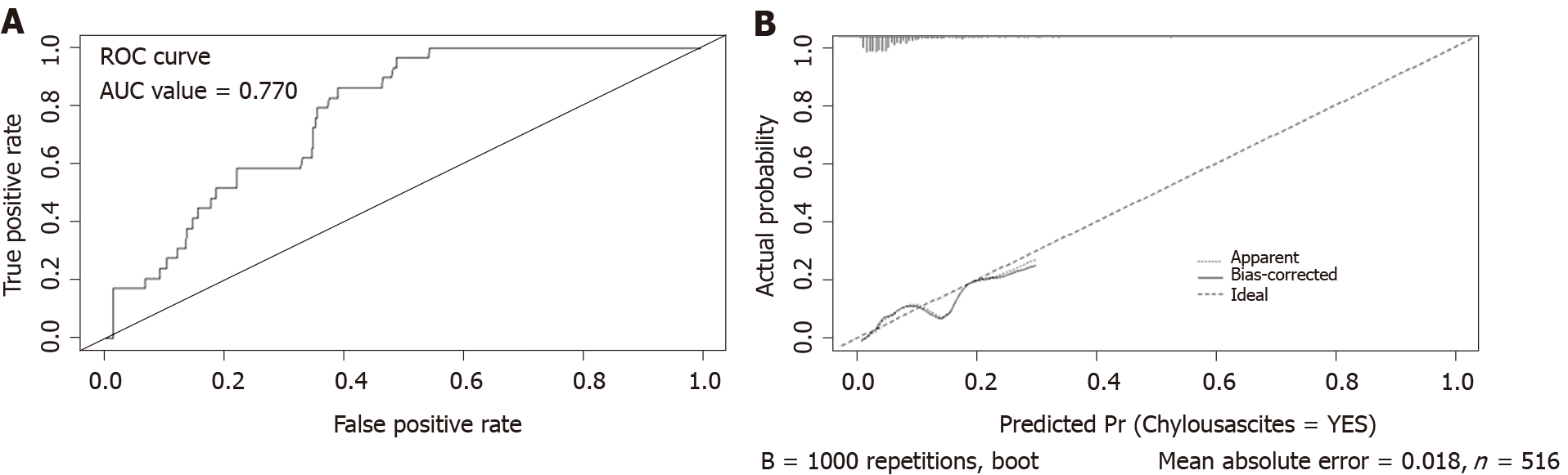
Operative time, the type of operation (standard or extended), the number of lymph nodes retrieved, and somatostatin administration were considered important risk factors. Multivariate logistic regression and nomograms can be used to accurately predict whether chylous ascites occurs. The area under the ROC curve of the model is 0.770. The C-statistic of this model is 0.770 which indicates that it has a relatively moderate ability to predict the risk of chylous ascites.
We found a novel set of risk factors, created a nomogram, and validated it. The nomogram had a relatively accurate forecasting ability for chylous ascites after right hemicolectomy and can be used as a reference for risk assessment of chylous ascites and whether to prevent it after surgery.
Core Tip: The article retrospectively analyzed the incidence of chylous ascites after right colectomy, and through multivariate analysis, the operative time, the type of operation (standard or extended), and the number of lymph nodes retrieved were identified as risk factors, while the administration of somatostatin or synthetic analogs after surgery was a protective factor. Based on these factors, we created a nomogram with moderate ability to predict the risk of chylous ascites.
- Citation: Zheng HD, Liu YR, Chen ZZ, Sun YF, Xu CH, Xu JH. Nomogram for predicting chylous ascites after right colectomy. World J Gastrointest Surg 2021; 13(11): 1361-1371
- URL: https://www.wjgnet.com/1948-9366/full/v13/i11/1361.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i11.1361
Colorectal cancer is the second most common cause of cancer-related death worldwide[1]. At present, although there are many treatment methods, surgical treatment is still the most important approach. Recent studies have shown that the incidence of colon cancer or rectal cancer on the left has remained stable or decreased and the survival rate of right-sided colon cancer is significantly lower than that of left colon cancer[2]. Moreover, there are differences in the incidence of complications, especially the incidence of chylous ascites.
Chylous ascites (CA) is defined as milky or milky peritoneal fluid that is rich in triglycerides[3]. It is generally believed that the main reason for the occurrence of CA is direct damage to the chylous duct, chylous branches or lymph nodes caused by surgery[4]. Complete mesocolic excision with D3 Lymph node dissection is regarded as a priority choice for right colectomy as more lymph nodes can be removed[5]. In clinical work and literature review, we were surprised to discover that the incidence of CA after right colectomy was significantly higher than that after other colorectal surgeries. Baek Se-Jin conducted a retrospective analysis of 779 patients after colorectal surgery and found that the incidence of CA after right colectomy was as high as 10.5%, which was significantly higher than the incidences of 5.75% after left colectomy and 4.6% of chyle leakage after rectal surgery[6]. Professor Chipan analyzed CA after colorectal surgery and the results showed that the incidence of CA following right hemicolectomy could be as high as 13.3%[7]. Therefore, according to the existing research, CA has become a complication that cannot be ignored after right colectomy. Because chyle drainage fluid contains many nutrients, CA may cause malnutrition, dehydration, electrolyte disorders, and delayed healing of incisions. In addition, CA contains lymph fluid that is enriched in lymphocytes and immunoglobulins; therefore, severe and long-term CA may lead to weakened immunity, which can cause severe infection and even death due to sepsis[8].
Thus, it is very important to establish a predictive model of CA after right hemicolectomy and to determine the risk reduction factors in a controllable strategy. At present, there are few studies evaluating the risk factors for CA after colorectal cancer surgery. The few existing studies suggested that the possible risk factors for CA were age, tumor location, preoperative albumin level, number of lymph nodes retrieved, operative time, intraoperative blood loss, D3 Lymphadenectomy, and surgeon[6,7,9,10]. However, no one has established a predictive model for CA that made sense in terms of preventive decision-making and risk assessment. This study aims to construct a nomogram to predict the incidence of CA after right hemicolectomy. Clinicians can eventually personalize the management of patients and take effective preventive measures through the nomogram and improve the prognosis and quality of life while reducing the length of hospitalization and costs.
This retrospective study was approved by the Ethics Committee. We collected 516 consecutive patients who underwent right hemicolectomy in the Second Affiliated Hospital of Fujian Medical University from January 2012 to May 2021. The inclusion criteria were pathologically confirmed right colon adenocarcinoma and right colectomy. The exclusion criterion was that the operation was an emergency operation. Ultimately, we collected a total of 516 patients. We divided patients into two groups: CA and without CA.
All operations were performed by clinical colorectal surgeons with extensive experience. D3 Lymphadenectomy is defined as the removal of the main lymph nodes at the roots of the blood vessels (ileocolonic vessels and the middle colon artery or the right branch of the middle colon artery) and then ligation of the blood vessels at the origin site. Tumor staging was reidentified according to the eighth edition of the American Joint Committee on Cancer staging system (AJCC).
All patients completed the necessary preoperative examinations, including colonoscopy with biopsy, to confirm the diagnosis. The perioperative treatments were essentially the same. Some patients were administered somatostatin or its synthetic analogs for 3 d after surgery in the collected data. In the following, the term somatostatin includes its synthetic analogs, such as octreotide.
CA is defined as milky or milky white ascites without infectious exudation in the drainage tube, with a volume of ≥ 200 mL/d and a triglyceride (TG) level of ≥ 110 mg/dL. When CA occurs, there should be no signs of fever, peritonitis, or other signs of infection to rule out the possibility of anastomotic leakage or other abdominal infections[6,7,9,10]. All patients with CA were cured after conservative treatment, avoiding a second operation.
Variables analyzed as risk factors for CA included age, sex, body mass index, history of abdominal surgery, neoadjuvant therapy, ASA score, combined organ resection, type of surgery, surgical approach, blood loss, operative time, number of positive lymph nodes (LNs), number of lymph nodes retrieved, tumor diameter (cm), preoperative albumin, preoperative CEA, pathological T stage, pathological N stage, metastasis, differentiation, vascular invasion, perineural invasion, and somatostatin administration.
Statistical analysis was performed using IBM SPSS Statistics 22.0. Continuous variables are presented as the mean ± SD and were compared using the Mann-Whitney U test (2-tailed). Categorical variables were represented by numbers (percentage), which were compared by using the χ2 test or Fisher’s exact test. Potential univariate and multivariate factors were analyzed by logistic regressions. Variables with a P value < 0.1 were included in the multivariate model.
We used R (version 4.0.5) to build a nomogram, and bootstraps with 1000 resamples were used to validate the internal nomogram. The coordination statistic (C-statistic) was used to measure the performance of the nomogram. A calibration curve was used to express the relationship between the observed frequency and the predicted probability, which was assessed by the area under the receiver operating characteristic (ROC) curve (AUC). The differences were considered statistically significant when the P value < 0.05.
After reviewing all included data, 29 patients were diagnosed with CA. Table 1 summarizes the characteristics of patients. A long operative time (P = 0.032), number of LNs retrieved (P = 0.005), standard or extended surgery (P = 0.012), and somatostatin administration (P = 0.039) were related to CA. However, intraoperative blood loss and open or laparoscopic surgery seemed not to be significantly related to the occurrence of CA.
| Variables | No CA (n = 487) | CA (n = 29) | P value |
| Age (yr) | 64.3 ± 11.5 | 66.0 ± 10.3 | 0.436 |
| Sex | 0.384 | ||
| Male | 245 (93.5%) | 17 (6.5%) | |
| Female | 242 (95.3%) | 12 (4.7%) | |
| BMI (kg/m2) | 0.122 | ||
| ≤ 25 | 337 (93.4%) | 24 (6.6%) | |
| > 25 | 150 (96.8%) | 5 (3.2%) | |
| History of abdominal surgery | 0.406 | ||
| Yes | 70 (97.2%) | 2 (2.8%) | |
| No | 417 (93.9%) | 27 (6.1%) | |
| Neoadjuvant therapy | 0.142 | ||
| Yes | 10 (83.3%) | 2 (16.7%) | |
| No | 477 (94.6%) | 27 (5.4%) | |
| ASA score | 0.565 | ||
| 1 or 2 | 363 (94.0%) | 23 (6.0%) | |
| ≥ 3 | 124 (95.4%) | 6 (4.6%) | |
| Combined organ resection | 0.495 | ||
| Yes | 38 (92.7%) | 3 (7.3%) | |
| No | 449 (94.5%) | 26 (5.5%) | |
| Type of surgery | 0.012 | ||
| Standard | 357 (96.0%) | 15 (4.0%) | |
| Extended | 130 (90.3%) | 14 (9.7%) | |
| Surgical approach | 0.094 | ||
| Open | 94 (97.9%) | 2 (2.1%) | |
| Laparoscopy | 391 (93.5%) | 27 (6.5%) | |
| Blood loss (mL) | 83.8 ± 81.5 | 94.5 ± 64.2 | 0.252 |
| Operative time (min) | 155.0 ± 21.5 | 164.1 ± 19.3 | 0.032 |
| Number of positive LNs | 2.79 ± 5.3 | 2.3 ± 7.0 | 0.668 |
| Number of LNs retrieved | 25.69 ± 8.7 | 29.79 ± 6.6 | 0.005 |
| Tumor diameter (cm) | 5.4 ± 2.4 | 5.6 ± 1.9 | 0.377 |
| Preoperative albumin | 38.5 ± 5.5 | 39.7 ± 4.1 | 0.163 |
| Preoperative CEA | 22.3 ± 81.7 | 5.0 ± 4.0 | 0.110 |
| Pathological T stage | 0.220 | ||
| T0-T2 | 50 (90.9%) | 5 (9.1%) | |
| T3-T4 | 437 (94.8%) | 24 (5.2%) | |
| Pathological N stage | 0.101 | ||
| N0 | 226 (92.6%) | 18 (7.4%) | |
| N1-N2 | 261 (96.0%) | 11 (4%) | |
| Metastasis | 0.749 | ||
| Yes | 46 (93.9%) | 3 (6.1%) | |
| No | 441 (94.4%) | 26 (5.6%) | |
| Differentiation | 0.299 | ||
| w/d, m/d | 302 (93.5%) | 21 (6.5%) | |
| p/d | 30 (100.0%) | 0 (0.0%) | |
| Lymphovascular Invasion | 0.098 | ||
| Negative | 259 (92.8%) | 20 (7.2%) | |
| Positive | 228 (96.2%) | 9 (3.8%) | |
| Perineural invasion | 0.665 | ||
| Negative | 369 (94.1%) | 23 (5.9%) | |
| Positive | 118 (95.2%) | 6 (4.8%) | |
| Somatostatin administration | 0.039 | ||
| Yes | 156 (97.5%) | 4 (2.5%) | |
| No | 331 (93.0%) | 25 (7.0%) |
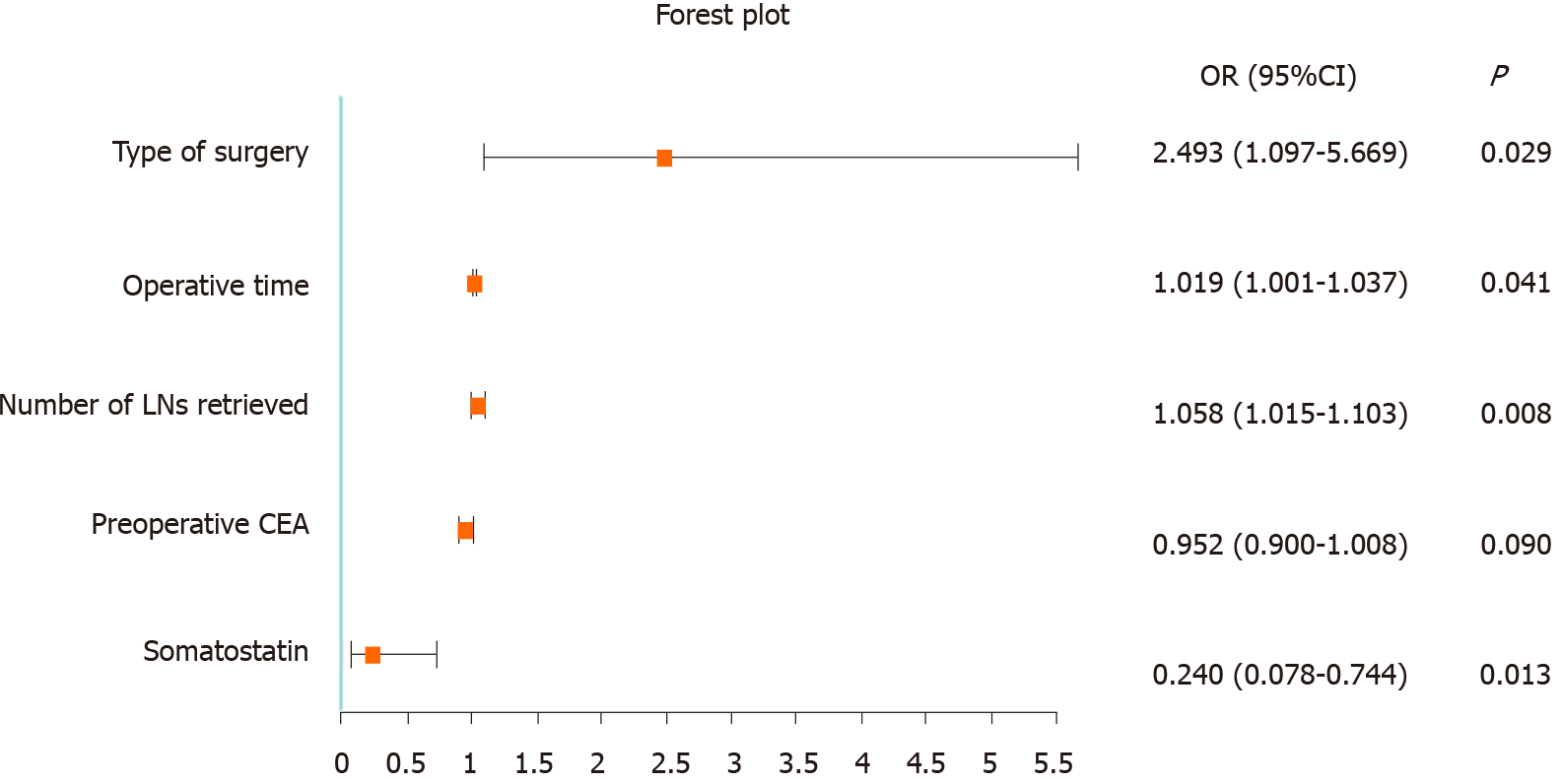
As shown in Table 2, after univariate logistic regression analysis, a long operative time, number of LNs retrieved, type of surgery (standard or extended surgery), somatostatin administration and preoperative CEA were associated with CA. Multivariate analysis showed that a long operative time (OR = 1.019, 95%CI: 1.001-1.037; P = 0.041), number of LNs retrieved (OR = 1.058, 95%CI: 1.015-1.103; P = 0.008), type of surgery (OR = 2.493, 95%CI: 1.097-5.669; P = 0.029), and somatostatin administration (OR = 0.240, 95%CI: 0.078-0.744; P = 0.013) were independent influencing factors of CA. According to these results, a forest plot was established (Figure 1).
| Univariate | Multivariate | |||
| P value | OR (95% CI) | P value | ||
| Age (yr) | 0.449 | |||
| Sex | Male or female | 0.386 | ||
| BMI (kg/m2) | > 25 vs ≤ 25 | 0.130 | ||
| History of abdominal surgery | Absent vs present | 0.272 | ||
| Neoadjuvant therapy | Absent vs present | 0.114 | ||
| ASA score | 1 or 2 vs ≥ 3 | 0.566 | ||
| Combined organ resection | Absent vs present | 0.624 | ||
| Type of surgery | Standard vs extended | 0.015 | 2.493 (1.097-5.669) | 0.029 |
| Surgical approach | Open vs laparoscopy | 0.112 | ||
| Blood loss (mL) | 0.492 | |||
| Operative time (min) | 0.028 | 1.019 (1.001-1.037) | 0.041 | |
| Number of positive LNs | 0.668 | |||
| Number of LNs retrieved | 0.014 | 1.058 (1.015-1.103) | 0.008 | |
| Tumor diameter (cm) | 0.199 | |||
| Preoperative albumin | 0.228 | |||
| Preoperative CEA | 0.087 | 0.952 (0.900-1.008) | 0.090 | |
| Pathological T stage | T0-T2 vs T3-T4 | 0.243 | ||
| Pathological N stage | N0 vs N1-N2 | 0.106 | ||
| Metastasis | Absent vs present | 0.873 | ||
| Differentiation | w/d, m/d vs p/d | 0.784 | ||
| Lymphovascular Invasion | Absent vs present | 0.103 | ||
| Perineural invasion | Absent vs present | 0.665 | ||
| Administration of somatostatin | Absent vs present | 0.048 | 0.240 (0.078-0.744) | 0.013 |
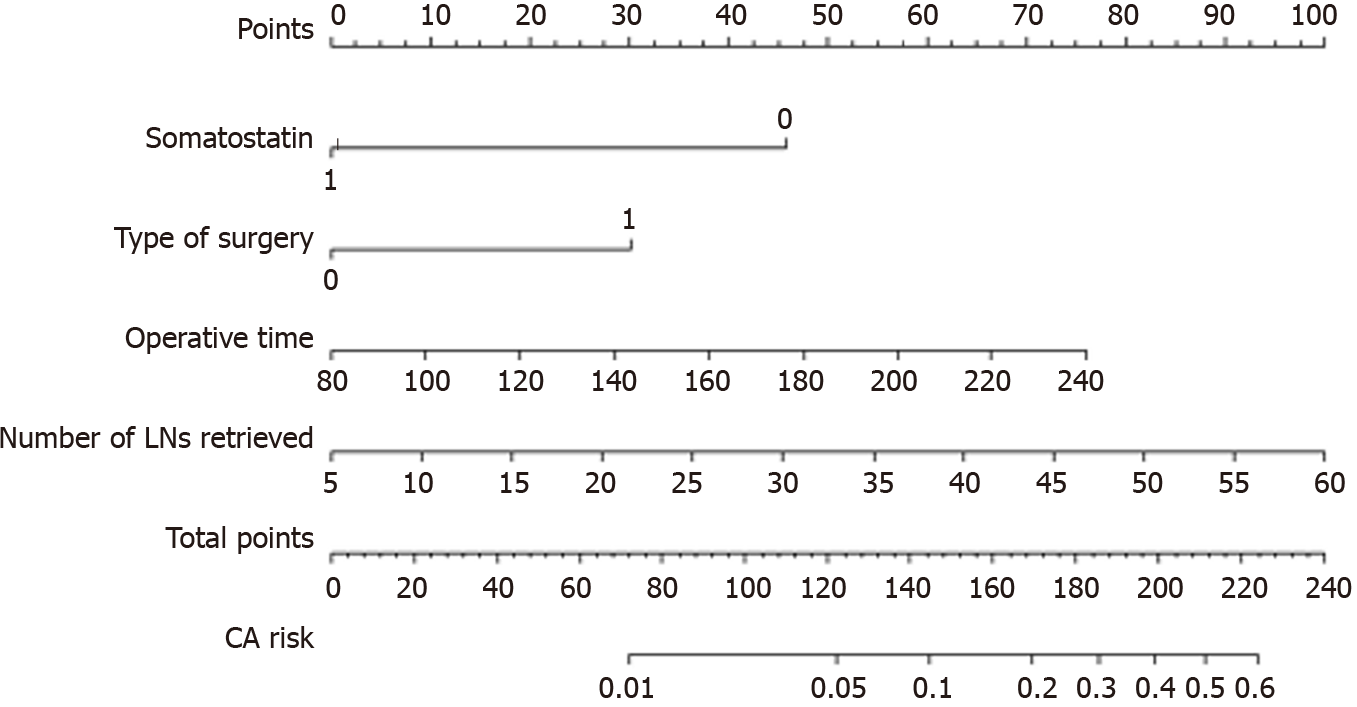
According to the multivariate logistic regression analysis, we established a nomogram to predict the risk of CA after right hemicolectomy and showed that operative time, number of LNs retrieved, type of surgery, and somatostatin had greater impacts on CA (Figure 2). The higher the total points assigned based on each factor in the nomogram, the higher the risk of CA. For example, a patient with a long operative time (160 min), a D3 type of operation, extended lymphadenectomy, with 30 LNs retrieved and without somatostatin administration postoperatively would have a total of 157.5 points (37.5 points for operative time, 30 points for type of operation, 45 points for number of LNs retrieved, and 45 points for without somatostatin administration postoperatively), for a predicted risk of CA of 15%. According to ROC analysis, the AUC of the model was 0.770. Because it was a binary variable model, the C-statistic of the model was 0.770, indicating that the model had considerable predictive potential (Figure 3A). The calibration curve showed that there was optimal agreement between the results predicted by the nomogram and actual observations, indicating good calibration (Figure 3B).
To the best of our knowledge, few articles have specifically studied the occurrence of CA after right colectomy and no one has established a nomogram to predict the occurrence of CA. At present, no auxiliary examination that can accurately predict CA has been proposed. This study analyzed the possible risk factors for CA after right colectomy in 516 cases and created a nomogram to predict the incidence of CA. We observed that the probability of CA after right colectomy was 5.6%. A long operative time, number of lymph nodes retrieved, and type of surgery (standard or extended surgery) were independent risk factors for CA after right colectomy, and somatostatin administration was a protective factor. All patients with CA were cured by conservative treatment and the short- and long-term survival results of patients were not affected by CA[9].
Looking back at previous research reports, the incidence of CA after major abdominal surgery ranges from 0.2% to 11.0%[11]. In our data, the incidence of CA fell in that range. However, because of the protective effect of somatostatin after surgery, the incidence was low compared to other studies. After we removed patients who were administered somatostatin after surgery, the incidence of CA was 7.0%. Why the incidence of CA after right colectomy with D3 Lymphadenectomy was so high, even as high as 13.3%, is closely associated with the anatomy of the region[6]. It is generally believed that the chyle cistern is located on the right side of the aorta, the anterior side of the first or second lumbar vertebrae has abundant lymphatic branches nearby, and it is closely related to the surgical area of D3 dissection[12]. In the process of D3 Lymph node dissection, the retroperitoneal lymph vessels and fat were removed, which resulted in the cutting and interruption of lymphatic drainage[13,14]. As the area for lymph node dissection expands, the number of lymph nodes retrieved increases, and the incidence of CA increases, which is consistent with previous reports on the risk factors for CA[6,7,9-11].
A previous study stated that CA occurrence was affected by various factors. For patients who have undergone right colectomy, there are few large-scale studies to provide risk factors for CA. We found that a long operative time, number of LNs retrieved, type of surgery (standard or extended surgery), and somatostatin administration were independent influencing factors. The operative time was related to many factors including the surgeon, the operation method, and whether the abdominal cavity had adhesions. The differences in the skills of the surgeon would cause differences in the operative time. If surgeons had not mastered a comprehensive understanding of vascular anatomy or advanced laparoscopic techniques[15] or if the abdominal cavity was severely adhered[16], the surgeon may enter the wrong anatomy level, leading to the destruction of a larger area of the lymphatic network and lymphatic vessels, prolonging the operative time, and increasing the incidence of CA, which was inconsistent with previous reports that considered short operative times; additionally, the occurrence of CA may be caused by incomplete sealing of lymphatic vessels and energy devices due to insufficient sealing time during laparoscopic surgery[6,9]. However, laparoscopy was not a risk factor in our study (P > 0.05). The increase in the incidence of postoperative CA may be directly related to more extensive and more detailed lymph node dissection; thus, the number of LNs retrieved can be explained as an independent risk factor. As the principle of D3 Lymphadenectomy is to remove more LNs in the mesenteric root, the number of LNs retrieved was quite large, but it was still less than in the study by Liang et al[17] (25.9 ± 8.7 vs 34.4 ± 8.4, P < 0.05). Due to the larger area of lymph node removal in D3 extended mesenterectomy, CA was more likely to occur, which was confirmed in Agustsdottir EES’s study that the incidence of CA after D3 extended mesenterectomy was 41.0%[18], which was higher than our data (41.0% vs 9.7%). Somatostatin, or its synthetic analog octreotide, which can reduce the absorption of triglycerides and inhibits visceral circulation and gastrointestinal motility, thereby reducing the concentration of triglycerides in the thoracic duct and reducing the lymphatic flow of the main lymphatic vessels[19]. It can provide valuable time for the healing of damaged lymphatic vessels and can thus play a preventive role.
There are few articles in the literature addressing the prevention of CA. At present, the main methods of prevention are that the injured lymphatics must be controlled and ligated intraoperatively during the operation[20], and the diet must be controlled. The surgical habit of our center was to use a slow range and double cauterization of the ultrasonic scalpel when performing laparoscopic lymphadenectomy, especially in the root of the main artery, and follow the integrity principle of lymphadenectomy to avoid partial lymphadenectomy. In open surgery, the use of an electrosurgical scalpel should be minimized for thicker lymphatic ducts or lymph nodes near the lymphatic trunk, and silk suture or ligation should be used. This may be part of the reason why our incidence of CA was lower than that in other studies. The study by Agustsdottir et al[18] considered that a routine fat-reduced diet (FRD) had a prophylactic effect and prevented the lymphatic vessels from collapsing, thereby reducing the occurrence of CA. According to our study, somatostatin played a protective role, indicating that somatostatin administration can reduce the occurrence of CA, but there is currently no relevant research to confirm this hypothesis. In our study, the average time for CA to occur was 3.9 d, and it often appeared after eating. However, Lizaola et al[21] believed that CA may occur in the early period (approximately 1 wk) after abdominal surgery because of the rupture of lymphatic vessels. In our study, the reason for the earlier appearance of CA was the accelerated rehabilitation surgery (ERAS) we implemented, which led to the patient eating earlier. Therefore, early intervention in the occurrence of CA is significant.
There are two modalities for the conservative treatment of CA: nutritional support and the use of somatostatin. Pan et al[22] revealed that in treating CA, enteral nutrition (EN) + medium-chain triglyceride (MCT) instead of total parenteral nutrition (TPN) was the best nutritional support and somatostatin should be used immediately. Aalami et al[23] published a large review of 156 cases and concluded that the resolution rate of conservative treatment was 67% while the remaining 33% of patients required surgical intervention such as lymphangiography and embolization[24]. The patients with CA that we included in this study were all cured by conservative treatment, such as diet control and the administration of somatostatin or its synthetic derivatives but the hospital stay was significantly prolonged with an average length of stay of 15.7 d, leading to a significant increase in hospital costs and was not conducive to the turnover rate of hospital beds.
We focused on patients after right colectomy who were reported to have a high risk of CA. According to the data of multiple logistic regression analysis, we established a nomogram that included operative time, type of surgery, number of LNs retrieved, and somatostatin administration. The nomogram can provide clinicians with an accurate approximation of CA risk after right colectomy because it is composed of data throughout the perioperative period which will make it convenient and feasible for doctors to better control the occurrence of CA and surgeons will be able to judge whether a patient is at high risk through our model so that the patient can take preventive measures. Considering that the cost of an extra day of hospitalization far exceeds the cost of somatostatin or octreotide administration, and there are potential economic effects of low risk, high benefit, and shortening the length of hospitalization[11]. For patients who are assessed as high-risk for CA by nomogram, it is recommended to start administering somatostatin or octreotide for 3 d immediately after the operation and to implement a 3-d fat-reduced diet at the same time[18].
The advantage of our study was that it focused on the high incidence of CA after right hemicolectomy, which has rarely been reported. We analyzed multiple factors that may be related to CA and created a nomogram that can provide a valuable prediction of the risk of CA after surgery. However, the study has several limitations. First, this is a retrospective, single-center study with a small sample size. Second, the mechanisms of most of the risk factors for CA we discussed were hypothetical and have not been confirmed on a scientific basis. For example, whether somatostatin can truly prevent CA has not been reported in the previous literature; carefully designed prospective clinical trials will be needed to confirm our results. Finally, it is currently uncertain whether the nomogram we created can be used by all surgeons because its effectiveness has not been evaluated in another study cohort. We expect to conduct forward-looking, large-sample, multicenter research to improve the reliability and value of the prediction model in future research.
In our study, the risk factors for CA after right hemicolectomy were screened by multivariate analysis and a nomogram was constructed to predict the possibility of CA. The nomogram had a good predictive ability for CA which can provide a reference for whether preventive measures need to be taken after the operation.
Chylous ascites is a relatively rare postoperative complication but its incidence in patients after right hemicolectomy is relatively high.
If it is possible to assess which postoperative patients are at high risk for chylous ascites, appropriate preventive measures can be taken which will greatly speed up the recovery of patients and reduce hospitalization costs.
To identify the risk factors for chylous ascites and to establish a novel nomogram for predicting chylous ascites after right colectomy.
A hospital-based retrospective study was conducted. Multivariate logistic regression was used to analyze the risk factors for chylous ascites and a novel nomogram was created. We used the receiver operating characteristic curve to assess the predictive ability of the model.
Operative time, the type of operation (standard or extended), and the number of lymph nodes retrieved were risk factors and somatostatin administration was considered a protective factor. Multivariate logistic regression and nomogram had relatively moderate abilities to predict the risk of chylous ascites.
The nomogram had a relatively accurate predictive ability for chylous ascites. Thus, we can use this model to assess the risk of patients for developing chylous ascites.
A multicenter prospective study should be performed to improve the practicality of the model.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luo X S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wu RR
| 1. | Geijsen AJMR, van Roekel EH, van Duijnhoven FJB, Achaintre D, Bachleitner-Hofmann T, Baierl A, Bergmann MM, Boehm J, Bours MJL, Brenner H, Breukink SO, Brezina S, Chang-Claude J, Herpel E, de Wilt JHW, Gicquiau A, Gigic B, Gumpenberger T, Hansson BME, Hoffmeister M, Holowatyj AN, Karner-Hanusch J, Keski-Rahkonen P, Keulen ETP, Koole JL, Leeb G, Ose J, Schirmacher P, Schneider MA, Schrotz-King P, Stift A, Ulvik A, Vogelaar FJ, Wesselink E, van Zutphen M, Gsur A, Habermann N, Kampman E, Scalbert A, Ueland PM, Ulrich AB, Ulrich CM, Weijenberg MP, Kok DE. Plasma metabolites associated with colorectal cancer stage: Findings from an international consortium. Int J Cancer. 2020;146:3256-3266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 2. | Nakagawa-Senda H, Hori M, Matsuda T, Ito H. Prognostic impact of tumor location in colon cancer: the Monitoring of Cancer Incidence in Japan (MCIJ) project. BMC Cancer. 2019;19:431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Lu J, Wei ZQ, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M. Small-volume chylous ascites after laparoscopic radical gastrectomy for gastric cancer: results from a large population-based sample. World J Gastroenterol. 2015;21:2425-2432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Han D, Wu X, Li J, Ke G. Postoperative chylous ascites in patients with gynecologic malignancies. Int J Gynecol Cancer. 2012;22:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Hwang DY, Lee GR, Kim JH, Lee YS. Laparoscopic complete mesocolic excision with D3 lymph node dissection for right colon cancer in elderly patients. Sci Rep. 2020;10:12633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Baek SJ, Kim SH, Kwak JM, Kim J. Incidence and risk factors of chylous ascites after colorectal cancer surgery. Am J Surg. 2013;206:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 7. | Sun YW, Chi P, Lin HM, Lu XR, Huang Y, Xu ZB, Huang SH. [Risk factors of postoperative chyle leak following complete mesocolic excision for colon cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:328-331. [PubMed] |
| 8. | Sriram K, Meguid RA, Meguid MM. Nutritional support in adults with chyle leaks. Nutrition. 2016;32:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Lee SY, Kim CH, Kim YJ, Kim HR. Chylous ascites after colorectal cancer surgery: risk factors and impact on short-term and long-term outcomes. Langenbecks Arch Surg. 2016;401:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Nishigori H, Ito M, Nishizawa Y, Koyama A, Koda T, Nakajima K, Minagawa N, Kobayashi A, Sugito M, Saito N. Postoperative chylous ascites after colorectal cancer surgery. Surg Today. 2012;42:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Weniger M, D'Haese JG, Angele MK, Kleespies A, Werner J, Hartwig W. Treatment options for chylous ascites after major abdominal surgery: a systematic review. Am J Surg. 2016;211:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | van der Gaag NA, Verhaar AC, Haverkort EB, Busch OR, van Gulik TM, Gouma DJ. Chylous ascites after pancreaticoduodenectomy: introduction of a grading system. J Am Coll Surg. 2008;207:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Garcia-Granero A, Pellino G, Giner F, Frasson M, Grifo Albalat I, Sánchez-Guillén L, Valverde-Navarro AA, Garcia-Granero E. A Proposal for Novel Standards of Histopathology Reporting for D3 Lymphadenectomy in Right Colon Cancer: The Mesocolic Sail and Superior Right Colic Vein Landmarks. Dis Colon Rectum. 2020;63:450-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Solmaz U, Turan V, Mat E, Dereli ML, Ekin A, Peker N, Tosun G, Dogan A, Gokcu M, Sanci M. Chylous ascites following retroperitoneal lymphadenectomy in gynecologic malignancies: incidence, risk factors and management. Int J Surg. 2015;16:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Gaupset R, Nesgaard JM, Kazaryan AM, Stimec BV, Edwin B, Ignjatovic D. Introducing Anatomically Correct CT-Guided Laparoscopic Right Colectomy with D3 Anterior Posterior Extended Mesenterectomy: Initial Experience and Technical Pitfalls. J Laparoendosc Adv Surg Tech A. 2018;28:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Strik C, Stommel MW, Schipper LJ, van Goor H, Ten Broek RP. Risk factors for future repeat abdominal surgery. Langenbecks Arch Surg. 2016;401:829-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Liang JT, Lai HS, Huang J, Sun CT. Long-term oncologic results of laparoscopic D3 lymphadenectomy with complete mesocolic excision for right-sided colon cancer with clinically positive lymph nodes. Surg Endosc. 2015;29:2394-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Agustsdottir EES, Stimec BV, Stroemmen TT, Sheikh AE, Elaiyarajah I, Lindstroem JC, Ignjatovic D. Preventing chylous ascites after right hemicolectomy with D3 extended mesenterectomy. Langenbecks Arch Surg. 2020;405:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Swanson MS, Hudson RL, Bhandari N, Sinha UK, Maceri DR, Kokot N. Use of Octreotide for the Management of Chyle Fistula Following Neck Dissection. JAMA Otolaryngol Head Neck Surg. 2015;141:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Yol S, Bostanci EB, Ozogul Y, Ulas M, Akoglu M. A rare complication of D3 dissection for gastric carcinoma: chyloperitoneum. Gastric Cancer. 2005;8:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Lizaola B, Bonder A, Trivedi HD, Tapper EB, Cardenas A. Review article: the diagnostic approach and current management of chylous ascites. Aliment Pharmacol Ther. 2017;46:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Pan W, Cai SY, Luo HL, Ouyang SR, Zhang WD, Wei ZR, Wang DL. The application of nutrition support in conservative treatment of chylous ascites after abdominal surgery. Ther Clin Risk Manag. 2016;12:607-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Aalami OO, Allen DB, Organ CH Jr. Chylous ascites: a collective review. Surgery. 2000;128:761-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 277] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Tai E, Min A, Rajan DK. A Single-Center Experience With Percutaneous Interventional Management of Refractory Chylous Ascites. Can Assoc Radiol J. 2020;846537120929429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |