PATHOGENESIS AND PATHOGENIC FACTORS OF PC
To date, the specific pathogenesis of PC has not been clear. At present, it is certain that the occurrence and development of PC are the result of a combined effect of lifestyle and the internal and external environments. A large number of clinical and epidemiological studies have confirmed that the risk factors for PC include smoking, chronic diabetes and obesity[8-10]. High alcohol consumption, diet and a family history are also associated with PC[11]. Japan’s 2016 version of pancreatic ductal adenocarcinoma (PDAC) clinical guidelines[12] pointed out that risk factors for PDAC include a family history of PC, hereditary PC syndrome, pancreatic complications of diabetes/chronic pancreatitis/intraductal papillary mucinous neoplasm/pancreatic cysts/obesity, an unhealthy lifestyle (smoking, excessive alcohol intake) and occupational factors.
TREATMENT AND PROGNOSIS OF PHC
Surgical resection is still the most important and the only curative treatment for PHC. The prognosis of patients with surgically resected PHC is significantly better than that of unresectable PHC[13]. The National Comprehensive Cancer Network (NCCN) guidelines[14] recommend neoadjuvant therapy only for borderline resectable PC. Currently, pancreaticoduodenectomy (PD) is the standard surgical procedure for radical treatment of PHC, and the long-term recurrence rate and long-term survival rate of patients with PD are mainly dependent on radical R0 resection[15,16]. Even for patients unable to achieve an R0 resection, R1 resection can still improve the prognosis and achieve an improved quality of life. In the past 10 years, the perioperative mortality of pancreatic cancer has been reduced to 3%. Moreover, the expanded surgical indications for locally advanced tumors have also increased the 5-year overall survival (OS) rate close to 30%.
With the development of laparoscopic technology, the safety and feasibility of laparoscopic resection for pancreatic body and tail tumors has been confirmed, and laparoscopic resection has become the first choice for patients with resectable benign or low-grade malignant tumors in the pancreatic body and tail[17]. For pancreatic head cancer, due to the technical difficulty of the operation, the application of laparoscopic PD (LPD) is still hard to promote widely[18-20]. For LPD, R0 resection is the only way for patients with PHC to obtain long-term survival. Whether R0 resection can be achieved is the key factor for the prognosis of PHC[21]. Unfortunately, it has been reported that 20%–86% of patients with PHC undergoing surgical resection do not achieve R0 resection[22,23]; most of which are related to the insufficient removal of retroperitoneal tissue mainly concentrated in the retropancreatic tissue space, a well-known anatomical space recognized as the mesopancreas[16]. Recently, R1 resection in many PHCs has been believed to be caused by incomplete resection of the mesopancreas. Accordingly, the concept of total mesopancreas excision (TMpE) has been proposed, which is suggested to increase the R0 resection rate of pancreaticoduodenal surgery[24-26].
CONCEPT OF MESOPANCREAS AND SURGICAL PROGRESS OF TMpE
The pancreas has always been considered an extraperitoneal organ. In 2007, Gockel et al[27] proposed the concept of the mesopancreas and TMpE by analogizing total mesorectal excision based on the autopsy results of five corpses. According to this concept, TMpE requires resection of the entire pancreatic tumor and the peripancreatic lymph nodes and adipose tissue layer en bloc to achieve a negative retroperitoneal margin and to improve the R0 resection rate and the prognosis of the patients. Gockel et al[27] considered the mesopancreas to be the fibrous connective tissue between the pancreas neck and the mesenteric blood vessels. Although the exact anatomical boundary was not described, the mesopancreas was confirmed to be an anatomical region containing nerves, blood vessels, and lymphoid tissues, the most likely metastatic region of pancreatic cancer. In contrast, Agrawal et al[28] pointed out that although loose areolar tissue, adipose tissue, peripheral nerves, a nerve plexus, lymphatic ducts and capillaries could be found extending from the head, neck and uncinate process of the pancreas to the aortocaval groove in the retropancreatic tissue in 20 patients with autopsy, no fibrous sheath or fascia was detected around these structures. Therefore, the concept of the mesopancreas could not be confirmed anatomically. However, Popescu and Dumitrascu[29] insisted that even without a complete fascial structure, the mesopancreas could still be considered an anatomical structure. To date, there has been no consensus on the existence of a mesopancreas and whether it could be called a mesostructure. Adham and Singhirunnusorn[30] specifically described the superior, inferior, anterior and posterior resection margins of TMpE and characterized them as an inverted triangle, the mesopancreas triangle, whose anatomical boundaries are represented by a base lying on the posterior surface of the superior mesenteric vein (SMV) and portal vein (PV), a summit lying on the anterior surface of the aorta between the celiac trunk and superior mesenteric artery (SMA) origin, and limited on each side by the right semicircumferences of the celiac trunk and SMA plexus (Figure 1). Adham and Singhirunnusorn[30] emphasized thorough removal of all tissues in this area to achieve R0 resection (Figure 2). Kawabata et al[31] proposed the concept of the mesopancreatoduodenum, which consisted of a cluster of soft connective tissues along the inferior pancreaticoduodenal artery (IPDA) and the first jejuna artery (FJA). The mesoduodenum (fed by the IPDA) and the jejunal mesentery (dominated by the FJA) were demonstrated to form a common mesentery named the mesopancreatoduodenum in the resected specimens. Correspondingly, the concept of total mesopancreatoduodenum excision with an expanded range relative to the aforementioned mesopancreas was put forward to emphasize the circumferential resection of SMA. In view of the unclear mesopancreas boundary and various modes of local pancreatic invasion, Peparini et al[32] proposed that extending the resection as much as possible (including dissection of the para-aortic lymph nodes such as 16a2 and 16bl) should be implemented. In terms of pancreaticoduodenal resection, Wu et al[33] divided the mesopancreas at the head of the pancreas into the anterior and posterior parts, which mainly refers to the lymphatic connective tissues that attach to the anterior side of the abdominal aorta and surround the SMA and SMV and the celiac trunk. According to the Japan Pancreas Society General Rules for the Study of Pancreatic Cancer (7th edition)[34], the mesopancreas is defined as the pancreatic head plexus, which is more in compliance with anatomy. The existence and range of the mesopancreas is still controversial.
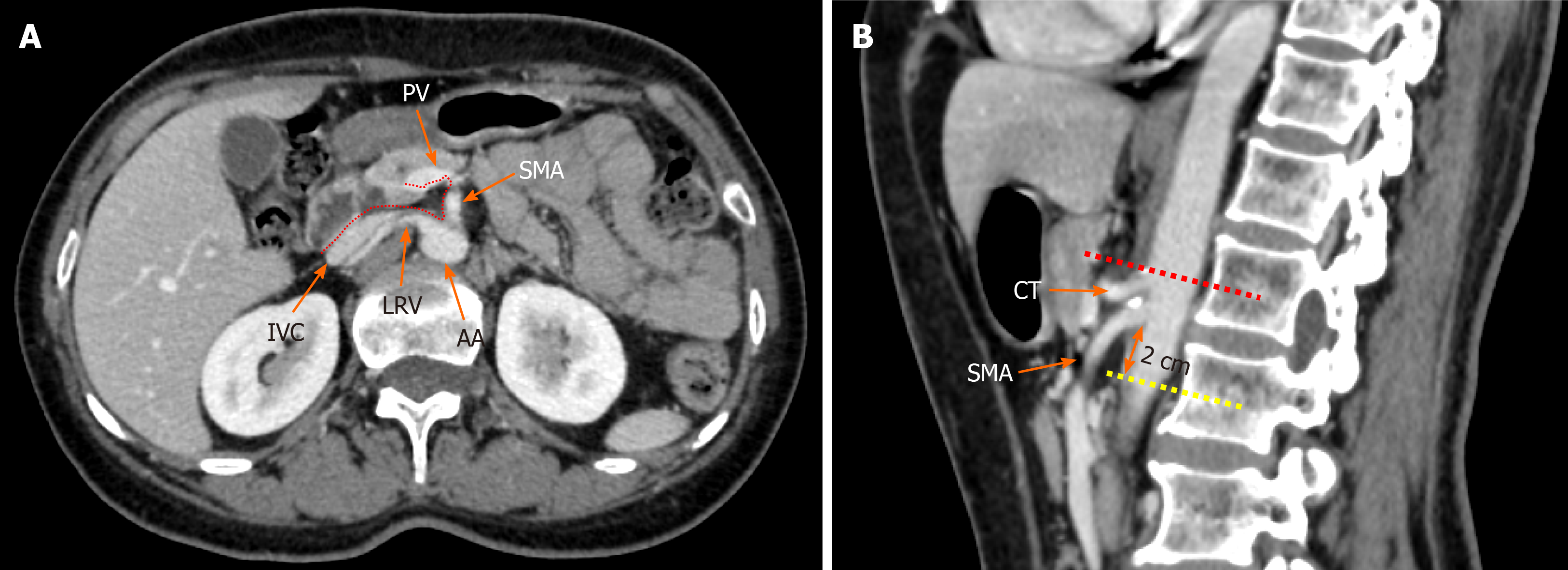
Figure 1 Radiological depiction of the mesopancreas in computed tomography.
A: The dotted line outlines the boundary of the mesopancreas, a region identified as the retro pancreatic retro portal tissue; B: The inferior boundary of the mesopancreas is 2 cm below the origin of superior mesenteric artery. PV: Portal vein; SMA: Superior mesenteric artery; LRV: Left renal vein; IVC: Inferior vena cava; AA: Aorta artery; CT: Celiac trunk.
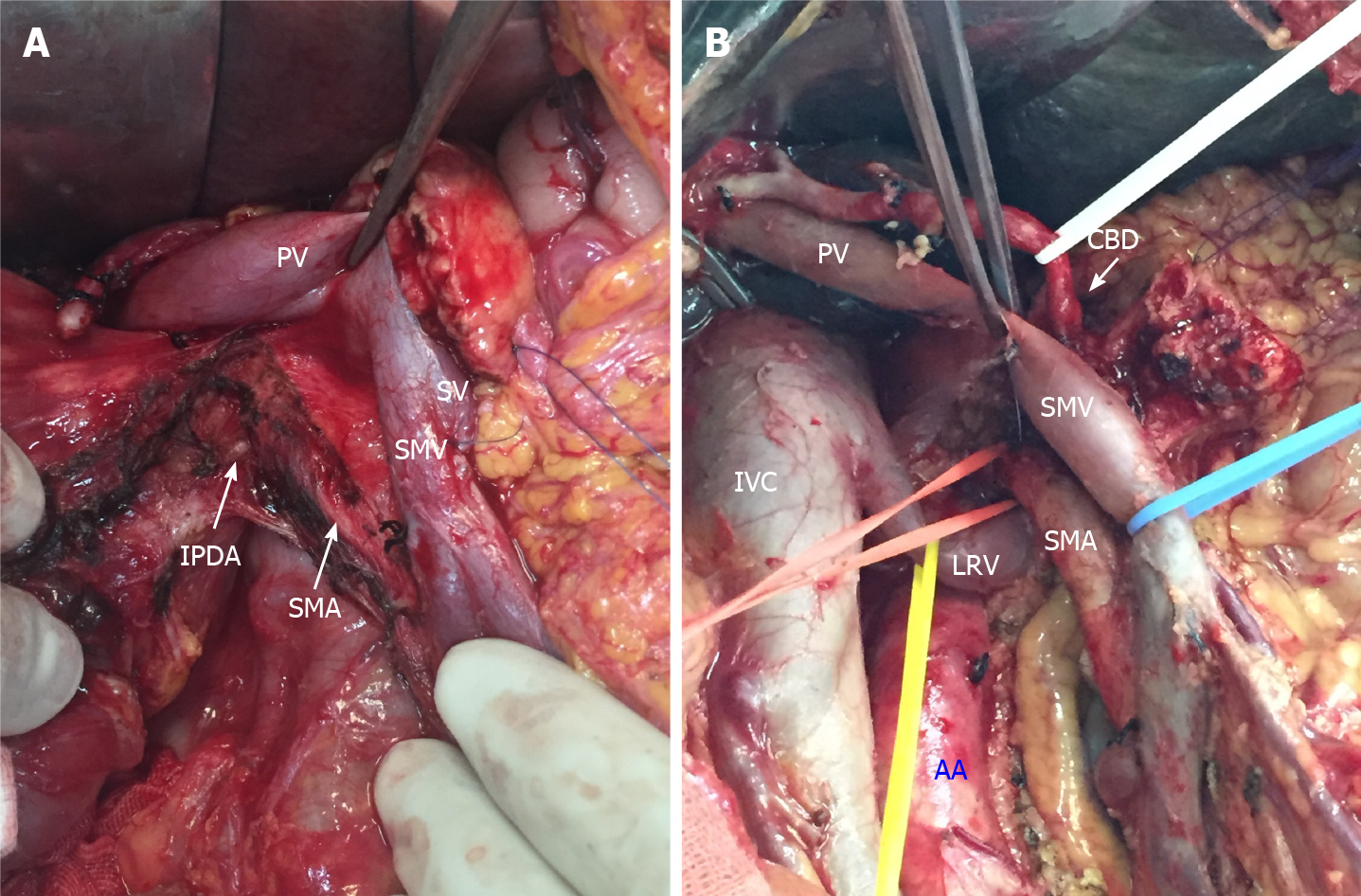
Figure 2 Key steps of total mesopancreas excision.
A: Dissection of the right semi-circumference of the superior mesenteric artery and celiac trunk; B: Clearance of the retropancreatic retroportal space (mesopancreas triangular). PV: Portal vein; SV: Splenic vein; SMV: Superior mesenteric vein; SMA: Superior mesenteric artery; IPDA: Inferior pancreaticoduodenal arteries; IVC: Inferior vena cava; LRV: Left renal vein; AA: Aorta artery; CBD: Common bile duct.
To date, the safety and effectiveness of TMpE have not been thoroughly discussed. Adham and Singhirunnusorn[30] found that TMpE showed no significance in terms of operating time, intraoperative blood loss, incidence of postoperative complications, length of hospitalization or perioperative mortality compared with other surgical methods for PHC. Meanwhile, postoperative pathology confirmed a positive mesopancreas invasion by PHC rate of 23%, a median lymph node dissection of 24%, and an overall R0 resection rate of 80.7% for PHC after TMpE. Therefore, TMpE is believed to be an effective surgical method to reduce the positive rate of the posterior peritoneal resection margin and increase the R0 resection rate of PHC. Kawabata et al[31] compared the surgical methods of TMpE in 14 PHC patients with standard PD performed in 25 patients during the same period and achieved a higher number of lymph node dissections (26 vs 18, P = 0.027) and a higher R0 resection rate (93% vs 60%, P = 0.019) in the TMpE group. Some studies have also demonstrated that TMpE could significantly increase the R0 resection rate of pancreaticoduodenal surgery for PHC[29,30,35-38].
Known as the most common sites for invasion and metastasis of PHC, the region posterior to the head of the pancreas, the SMV, posterior PV, SMA, the right semicircumference of the celiac trunk, and the anterior and right sides of the abdominal aorta are the most likely sites of residual tumor and local recurrence after surgical resection, making it the key site of current radical resection of PHC. In fact, because the mesopancreas is included in the resection range of lymph node dissection for PHC, TMpE does not contradict traditional lymph node dissection and just differs in the emphasis of the importance of resection of the region near the posterior head of the pancreas. Currently, the absence of a definite anatomical boundary for the soft tissue posterior to the head of the pancreas brings challenges to precise resection of the mesopancreas in TMpE. This uncertainty may also increase the heterogeneity among studies and affect the evaluation of TMpE.
To our knowledge, until now, only a few studies have compared TMpE with traditional PD, and these studies mainly focused on OS and disease-free survival (DFS)[39-41]. Kurosaki et al[41] reported similar short-term survival but prolonged 3-year survival rates in patients with successful TMpE. Quero et al[43] also reported benefits in terms of DFS for TMpE. However, some studies found that TMpE did not influence short-term outcomes[35,39,42]. Quero et al[43] also reported a similar estimated 5-year OS between these two techniques, even though a slight advantage was observed in the TMpE group.
IMAGING STUDIES ON PHC AND THE MESOPANCREAS
As a tumor with rapid progression, the early diagnosis of PHC has many difficulties. Approximately 80%–85% of patients are diagnosed with locally advanced tumors or distant metastasis, and only 15%–20% of patients are suitable for surgical resection[44]. Enhanced computed tomography (CT) and magnetic resonance imaging (MRI) are currently the most commonly used examination methods for the diagnosis of PHC.
Typical imaging manifestations of PHC
Consistent with the microscopic pathological characteristics of PC, mixed tumor cells and a large amount of interstitial fibrosis with an irregular proportion, the direct signs of typical PHC include an isodense or slightly low-density mass in the pancreatic head area on noncontrast CT images and an avascular tumor with a lower density than normal pancreatic parenchyma in contrast imaging (Figure 3). On MRI, most tumors show a low signal on T1-weighted imaging (WI), an equal/low/slightly higher signal on T2WI, and a slightly higher signal on diffusion-weighted imaging (DWI). The signal intensity of most PHCs is lower than that of the pancreatic parenchyma in the early stage of enhancement and becomes iso-signal intensity in the later stage (Figure 4). Indirect signs of PHC include changes in the contour of the pancreas, parenchymal atrophy of the pancreatic body and tail, pancreatic and bile duct truncation sign, double duct sign[45] (Figure 4D), and secondary retention cyst.
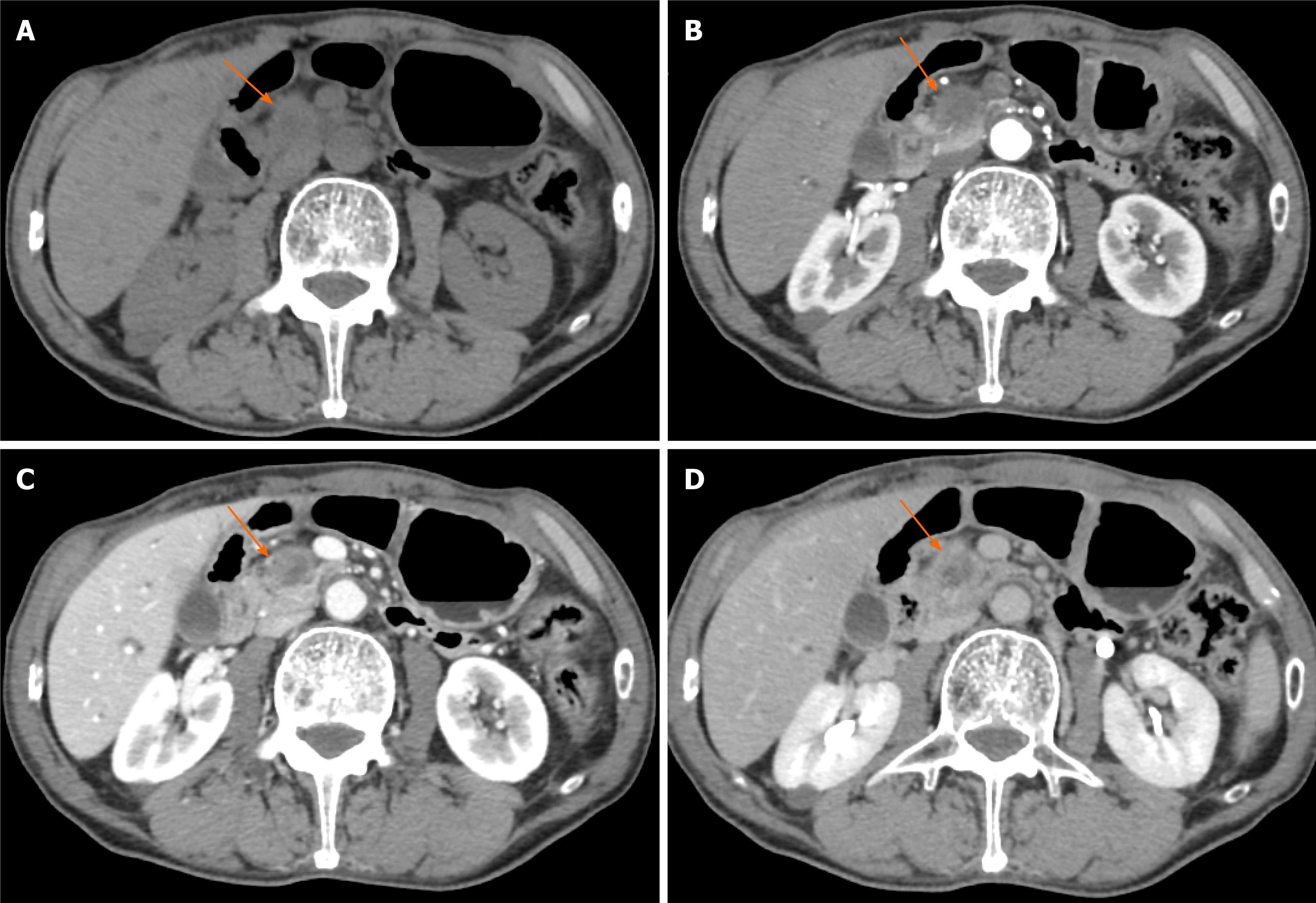
Figure 3 Typical CT features of pancreatic head carcinoma in a 73-year-old male patient.
A: On noncontrast CT imaging a slightly low-density mass (arrow) in the pancreatic head area was identified; B–D: On contrast CT images, the tumor shows an avascular tumor with a lower density than normal pancreatic parenchyma on arterial phase (B), venous phase (C), and delay phase (D). CT: Computed tomography.
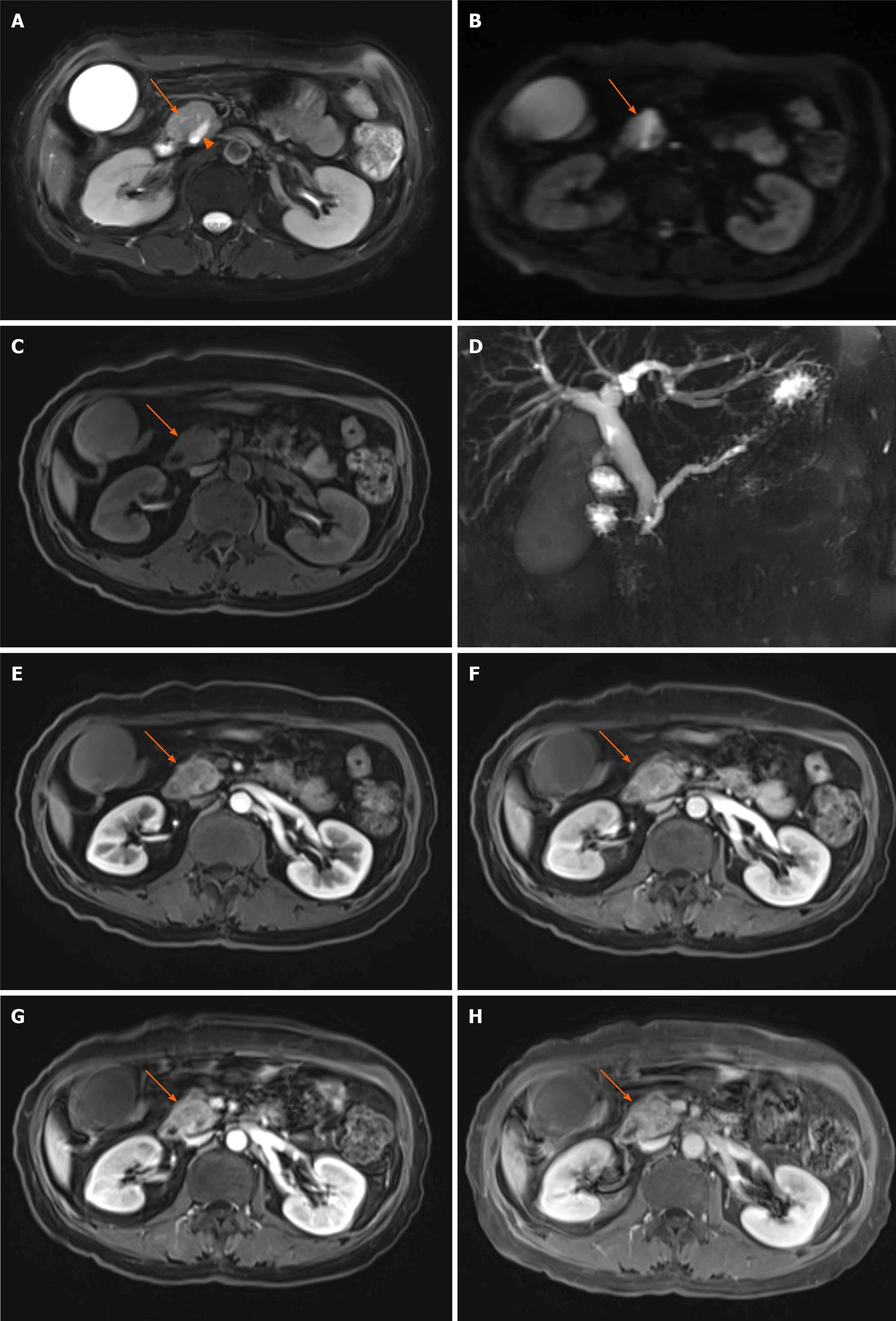
Figure 4 Typical magnetic resonance features of pancreatic head carcinoma in a 57-year-old female patient.
A–C: Swollen pancreatic head (arrow) with slightly higher signal on T2-weighted imaging (WI) (A) and diffusion-weighted imaging (B), and low signal on T1WI (C) was detected; D: Notes that the dilation of pancreatic duct (arow head) and double duct sign on magnetic resonance cholangiopancreatography; E–H: After administration of contrast agent, the tumor shows a progressive enhancement pattern similar to computed tomography on early arterial phase (E), late arterial phase (F), venous phase (G), and delay phase (H).
It should be noted that 10%–14% of tumors on multidetector CT (MDCT) are isodense on both noncontrast and contrast scans[46], which is more common in histopathologically highly differentiated tumors. Early PHC is usually well differentiated and presents as an isodense tumor. Approximately 88% of isodense PCs with a diameter < 2 cm are accompanied by indirect signs, with an incidence of pancreatic duct truncation sign, double duct sign, and pancreatic atrophy of 59%, 63% and 21%[47]. Therefore, indirect signs are important for the early diagnosis of PHC, especially in cases without a definite tumor, and further examination should be performed to avoid a missed diagnosis. MRI using multiple sequences can significantly improve the sensitivity and specificity of the early diagnosis of pancreatic cancer[48,49].
Imaging evaluation of resectability of PHC
At present, surgical resection is the only possible radical curative treatment for PHC. However, only 15%–20% of patients with PHC are suitable for surgery due to local involvement or distant metastases. Therefore, accurate judgment of the resectability of PHC is of importance when selecting treatment options to avoid unnecessary surgery. Currently, the standard evaluation for the resectability of pancreatic cancer is the NCCN Clinical Practice Guidelines for Pancreatic Cancer (2017, 1st edition), including the evaluation of the peripancreatic artery, vein, pancreatic head, body and tail cancers and the contact surface between the tumor and blood vessels. Moreover, with a combination of tumor location, the type of involved blood vessels and the contact angle, tumors can be divided into resectable pancreatic cancer (RPC), borderline RPC (BRPC), locally advanced pancreatic cancer (LAPC) and metastatic PC[50]. Meanwhile, the importance of enhanced CT and MRI in the evaluation of PC has also been highlighted. High-quality images are especially significant for accurately assessing the relationship between pancreatic cancer and adjacent blood vessels, which is of guiding significance for the assessment of the resectability of PC and the selection of treatment options.
Unfortunately, there are still some problems with the NCCN guidelines. First, Hong et al[51] evaluated 371 PC patients with preoperative CT according to the NCCN guidelines and found that tumors with a diameter > 4 cm and tumor contact with PV were high risk factors for R1/2 resection margins. For those 104 patients diagnosed with BRPC by preoperative CT, 47 patients were pathologically confirmed as R1/2 margins after surgery. Therefore, the NCCN guidelines based on anatomical standards have significant limitations in the evaluation of resectability and cannot promise benefit from surgical treatment in some patients. Second, according to the 8th edition of the American Joint Cancer Committee in 2018, PC is distinguished as T1, T2 and T3 stages according to the largest tumor diameter and T4 stage (with involvement of celiac trunk, SMA, and/or the common hepatic artery, regardless of tumor size). This new T staging system undoubtedly promises more objectivity and reproducibility for MDCT, but it also brings challenges to radiologists. The sensitivity of MDCT in T staging differs in tumors of different sizes, with a sensitivity of 90%–98% for tumors with a diameter > 2 cm and 60%–77% for smaller tumors[52]. Therefore, difficulties still exist in the detection and further staging of T1 tumors for MDCT. MRI provides multisequence imaging, which is better than MDCT in the diagnosis of small PHCs. Third, the N staging system recommended in the NCCN guidelines is based on the number of metastatic lymph nodes: N0 (no regional lymph node metastasis); N1 (1–3 regional lymph node metastases); and N2 (> 4 lymph node metastases). Nevertheless, there are still some limitations in the imaging diagnosis of metastatic lymph nodes. As metastatic lymph nodes have no obvious correlation with tumor size or the tumor T stage, it is difficult to identify metastatic lymph nodes based only on morphological changes and distinguish them from inflammatory hyperplasia. As a functional imaging technology that can provide not only anatomical but also functional/ metabolic features, MR-DWI may help detect metastatic lymph nodes combined with the apparent diffusion coefficient[53].
Positron emission tomography–CT has also been reported to be able to enable a qualitative diagnosis based on the uptake of 18F-fluorodeoxyglucose in the lymph nodes with better sensitivity and specificity than MDCT[54]. In addition, the use of MR-targeted lymphatic imaging technology and radiomics may also help improve the accuracy of the diagnosis of metastatic lymph nodes in the future. Despite these developments in imaging modalities, the absence of pathological sampling standards for peripancreatic lymph nodes and the lack of a unified definition of regional lymph nodes make it difficult to establish a point-to-point correspondence between pathologically positive lymph nodes and positive lymph nodes on images, resulting in a disconnect among preoperative imaging evaluations, surgical lymph node dissections, and pathological assessments. In conclusion, although MDCT or MRI can show the tumor morphology, adjacent vascular invasion, lymph node and distant metastasis well, there are still some limitations in the current imaging assessment based on the TNM staging system, which may affect the treatment options and prognosis of patients to some extent.
Application status of imaging in TMpE
With the novel conception of the mesopancreas and advances in TMpE, all of the structures in the mesopancreas should be accurately preoperatively evaluated. However, the assessment of the resectability of PHC in previous studies has mainly focused on the size of the tumor, invasion of the peripheral blood vessels, and the presence of lymph node metastasis and distant metastasis. Current studies on TMpE are mostly focused on surgical methods, treatment effects, and patient prognosis.
In addition, with the proposal of a mesopancreas and developments in TMpE, peripancreatic vessel invasion is no longer an absolute contraindication for radical resection of PHC; therefore, the evaluation principles of preoperative imaging need to be changed correspondingly. Consistent with the anatomical concept of the mesopancreas, preoperative imaging evaluation of PHC should include all of the nerves, lymphatic vessels, and fatty tissues in the mesopancreas instead of only evaluating masses, vascular invasion, lymph nodes and distant metastasis. Wu[55] found an important anatomical level for TMpE, the posterior pancreatic space, containing a large number of nerves, lymphatic vessels, adipose tissue, and dense fibrous connective tissue surrounding the CA and the SMA within it. As a particularly important anatomical region for the occurrence of lymphatic and nerve metastasis, preoperative imaging evaluation should pay more attention to the posterior pancreatic space and the structures around the CA and SMA to achieve R0 resection. Liang et al[56] proposed 3D visualization technology in TMpE, which could help make more accurate preoperative assessments and select reasonable surgery approaches, thus, increasing the R0 resection rate of PHC.
However, there are many controversies regarding the scope and boundary of the mesopancreas because of the absence of a definite anatomical fascial structure. A definitely complete posterior capsule posterior to the head of the pancreas and the duodenum was found in a recent study[57], which has brought new challenges to clinical imaging. Similar to the mesorectum, which could be identified as low signal intensity on MRI, the mesopancreas should also be detectable. Unfortunately, whether the mesopancreas could be identified by imaging is questionable and requires extensive research with a comprehensive comparison of gross anatomy, surgery, pathology, and imaging. Furthermore, perineuronal invasion is characteristic of pancreatic cancer, generally first invading the intrapancreatic nerves and then to the nerve plexus outside the pancreas by spreading along the nerve bundles. It has been reported that a longer survival period could only be achieved in patients with peripancreatic nerve resection[58,59]; therefore, the evaluation of preoperative peripheral nerve invasion and postoperative resection margin is of great importance for the efficacy of TMpE. However, until now, neither CT nor MRI has been able to clearly visualize the nerve plexus of the pancreas. Further improvements in imaging technologies should be developed to achieve precise assessment of the perineuronal invasion of PHC.