Published online Oct 27, 2021. doi: 10.4240/wjgs.v13.i10.1285
Peer-review started: June 2, 2021
First decision: July 1, 2021
Revised: July 11, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: October 27, 2021
Processing time: 145 Days and 20.5 Hours
Esophageal adenocarcinoma (EAC) derived from long-segment Barrett’s esophagus (LSBE) is extremely rare in Asia. LSBE-related EAC is often difficult to diagnose in the horizontal extent. If the tumor has spread throughout the LSBE, whole circumferential endoscopic submucosal dissection (ESD) should be performed, which is difficult to complete safely. Additionally, whole circumferential ESD can bring refractory postoperative stenosis. We hereby report a case of EAC involving the whole circumference of the LSBE, achieving complete endoscopic removal without complications.
An 85-year-old man with the chief complaint of dysphagia underwent esophagogastroduodenoscopy. We suspected a flat-type cancerous lesion that extended the whole circumference of the LSBE (C 3.5, M 4.0) using narrow-band imaging magnification endoscopy (NBI-M). We achieved circumferential en bloc resection of the lesion safely with special ESD techniques. Histology of the ESD specimens demonstrated that the superficial EAC extended the whole circumference of the LSBE, and papillary or well-differentiated tubular adenocarcinoma was confined in the lamina propria mucosa showing a vertical negative margin. To prevent post-ESD stenosis, we performed endoscopic local injection of steroids, followed by oral administration of steroids. There was no evidence of esophageal refractory stenosis or tumor recurrence 30 mo after ESD. In summary, we experienced a rare case of LSBE-related EAC. The horizontal tumor extent was accurately diagnosed by NBI-M. Additionally, we achieve whole circumferential ESD safely without postoperative refractory stenosis.
NBI-M, ESD, and steroid therapy enabled the curative resection of superficial full circumferential LSBE-related EAC without refractory postoperative stenosis.
Core Tip: Esophageal adenocarcinoma (EAC) arising from long-segment Barrett’s esophagus is rare and tends to be diffuse. Preoperative diagnosis of the horizontal tumor extent and postoperative stenosis after endoscopic submucosal dissection (ESD) could be problematic in this case. We accurately diagnosed the horizontal extent of the EAC lesion by narrow-band imaging magnification endoscopy and achieved complete en bloc R0 resection via whole circumferential ESD. We also succeeded in preventing refractory stenosis after whole circumferential ESD by prophylactic steroid therapy combing local injection and oral administration.
- Citation: Abe K, Goda K, Kanamori A, Suzuki T, Yamamiya A, Takimoto Y, Arisaka T, Hoshi K, Sugaya T, Majima Y, Tominaga K, Iijima M, Hirooka S, Yamagishi H, Irisawa A. Whole circumferential endoscopic submucosal dissection of superficial adenocarcinoma in long-segment Barrett's esophagus: A case report. World J Gastrointest Surg 2021; 13(10): 1285-1292
- URL: https://www.wjgnet.com/1948-9366/full/v13/i10/1285.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i10.1285
The incidence of Barrett's esophagus-related adenocarcinoma has increased rapidly in western countries[1-3], and has also been gradually increasing in Asia[4]. In the west, the carcinoma has been reported to develop from long-segment Barrett’s esophagus (LSBE) in more than half of cases[5], while in Asia, LSBE is extremely rare[6]. LSBE-related adenocarcinomas tend to be diffuse[7] and flat[8], and diagnosing the horizontal extent of the tumor can be more difficult than in short-segment Barrett’s esophagus-related adenocarcinoma, which tends to have solitary or localized carcinogenesis[7]. Postoperative refractory stenosis can occur even if endoscopic submucosal dissection (ESD) can be performed for superficial esophageal adenocarcinoma (EAC) involving the whole circumference of the LSBE[9,10].
We hereby report a rare case of superficial EAC with suspected involvement of the whole circumference of the LSBE via narrow-band imaging magnification endoscopy (NBI-M). We achieved en bloc R0 resection with special ESD techniques. Additionally, we could prevent refractory postoperative stenosis by prophylactic steroid combination therapy.
An 85-year-old man complained of hoarseness and dysphagia. He was referred to our hospital for further medical work-up and treatment.
The patient underwent esophagogastroduodenoscopy; A nodular aggregated protruded lesion was found in the lower esophagus. Histological analysis of the biopsy samples obtained from the protruded lesion showed adenocarcinoma.
The patient had a history of left glottic cancer and prostatic cancer, which were treated with radiation therapy and hormonal therapy, respectively.
He had a smoking history of 40 cigarettes per day for 40 years. There was no remarkable family medical history.
The patient presented in a normal nutritional state and the physical examination was unremarkable.
Laboratory studies, including total blood count, analysis of markers of kidney and liver failure, and analysis of tumor makers did not reveal any abnormalities.
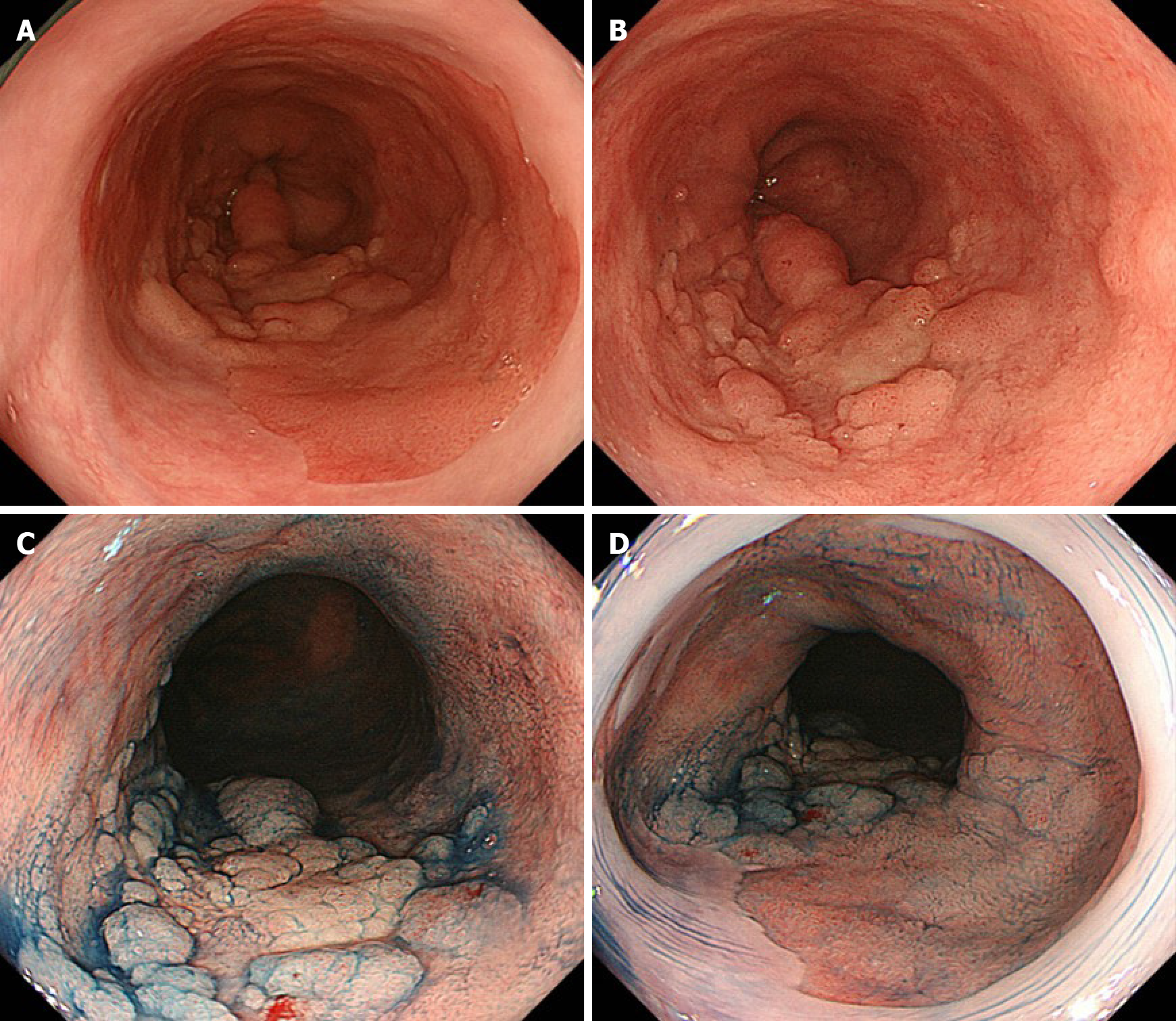
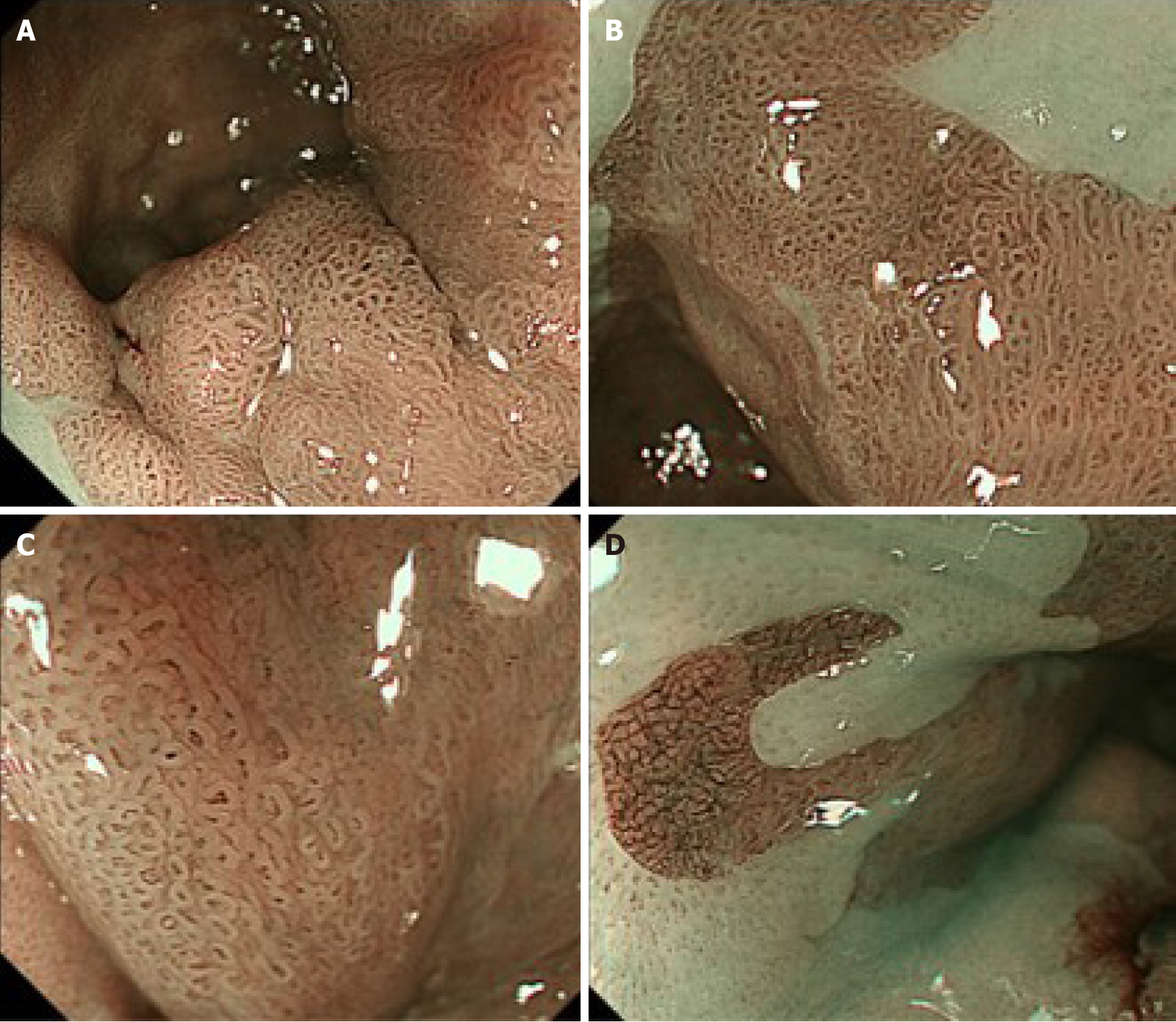
Conventional white-light endoscopy (CWE) showed a protruded lesion in the LSBE (C 3.5 M 4; Figure 1A and B). CWE and indigo carmine chromoendoscopy could not visualize a definitive lesion other than the protruded lesion (Figure 1), whereas NBI-M visualized extensive irregular mucosal/vascular patterns in the flat areas surrounding the nodular aggregated protruded lesion (Figure 2). The NBI-M findings were suggestive of a flat-type neoplastic lesion extending the whole circumference of the LSBE. The flat-type neoplastic lesion was suspected to longitudinally extend up to the esophagogastric junction. Adenocarcinoma and neoplastic glands were observed in the biopsy specimens obtained from the protruded and flat lesions, respectively. We predicted a diagnosis of superficial tumors spreading extensively along the whole circumference of the LSBE. The protruded lesion did not show poor distensibility or an expanding appearance but was semi-pedunculated. These findings suggested that the protruded tumor was confined to the mucosal layer. Contrast-enhanced computed tomography showed no metastatic lesions in the thorax and abdomen.
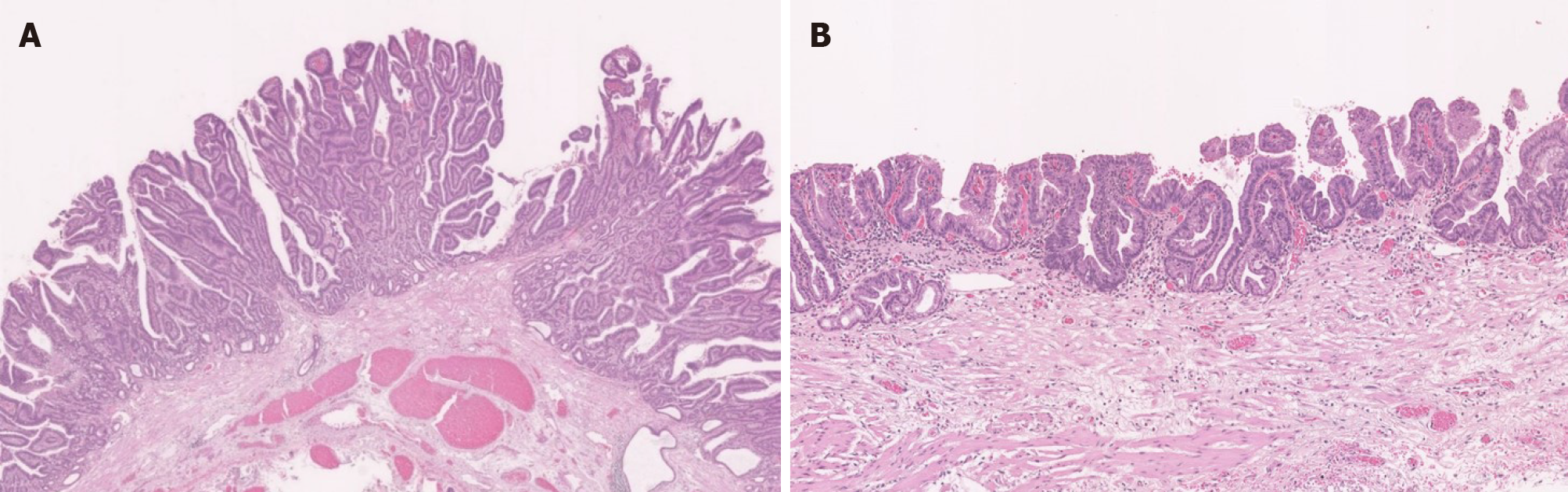
Histological analysis of the ESD specimen demonstrated that the superficial EAC extended the whole circumference of the LSBE (Figure 3). The protruding and flat extending tumors showed papillary and well-differentiated tubular adenocarcinoma, respectively (Figure 3). We achieved en bloc R0 resection with horizontal and vertical margins that were negative for cancer cells. Tumor invasion was confined to the superficial muscularis mucosa without lymphatic or vascular involvement.
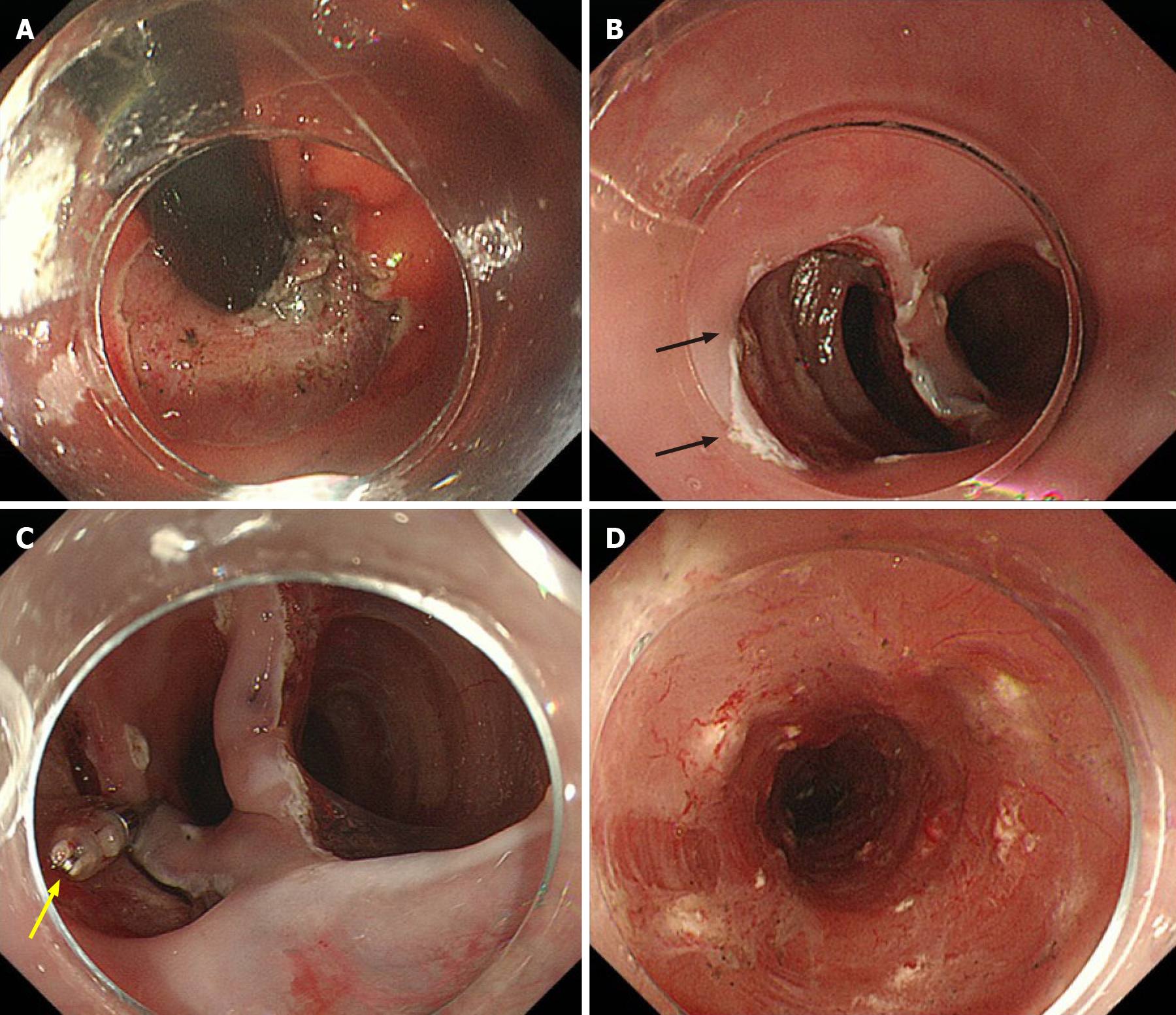
We achieved whole circumferential ESD with en bloc removal of the whole LSBE safely. We injected 0.4% sodium hyaluronate (MucoUp; Boston Scientific, Marlborough, MA, United States) into the submucosal layer during the ESD. We used special ESD techniques, including the submucosal tunneling method and a thread-traction method[11,12]. We created three submucosal tunnels from the oral side and dissected the submucosal tissue between the tunnels (Figure 4). The ESD specimen was 98 mm × 54 mm in diameter (Figure 5).
We injected a steroid solution containing 80 mg triamcinolone into the remaining submucosal layer immediately after ESD as prophylactic therapy for postoperative stenosis. Additionally, oral prednisolone was administered at an initial dose of 20 mg/d [0.5 mg/body weight (kg)] beginning on the second day post-ESD, which was gradually tapered every 2 wk, and completed 12 wk later.
Although the patient had mild narrowing of the esophageal lumen and did not complain of severe dysphagia, esophagogastroduodenoscopy showed mild stenosis in the lower esophagus. We performed prophylactic endoscopic balloon dilatation with a 12-15 mm balloon diameter (CRE balloon; Boston Scientific, Boston, United States) at 3 mo, 5 mo, and 6 mo post-ESD. The patient has a regular diet and no tumor recurrence 30 mo after ESD.
LSBE is a rare disease in Asia, including in Japan, and carcinogenesis from LSBE is even rarer. In EAC derived from LSBE, the histological distribution of dysplasia and EAC tends to be multiple and diffuse[7]. This often makes diagnosing the horizontal extent of the dysplasia and superficial EAC difficult. Unlike in western countries, ESD is commonly used in Japan to treat superficial EAC in the Barrett's esophagus. When the tumor has spread the whole circumference of the LSBE, as in the present case, the ESD procedure can be extremely challenging because of the high likelihood of perforation, severe hemorrhage, and refractory stenosis after whole circumferential ESD.
Using NBI-M, we were able to accurately diagnose the horizontal extent of this superficial EAC involving the whole circumference of LSBE and achieved complete en bloc R0 resection. In this case, an extensive flat-type tumor lesion surrounded the protruded tumor lesion. Diagnosing the horizontal extent of the flat-type tumor can sometimes be difficult by CWE or indigo carmine chromoendoscopy. Previous studies have shown that NBI-M is useful in the diagnosis of the flat-type superficial EAC lesions[13-15]. However, little is known about utility of NBI-M for LSBE-related superficial adenocarcinoma that tends to be diffuse and flat. The horizontal tumor extent of the LSBE-related superficial EAC was accurately diagnosed using NBI-M in the present case. Additionally, we utilized the following two ESD techniques and successfully completed a highly difficult procedure of whole circumferential ESD. The first ESD technique is a tunneling method[16,17], and the other is a thread-traction method[11]. The tunneling method creates tunnels in the submucosal layer of a lesion, which allow for the submucosal layer to be dissected easily and safely. In this case, after creating three tunnels, the mucosa at the entrance of a tunnel was pulled towards the oral side with a thread and clip. Combining the thread-traction method with the tunneling method enable the safe completion of ESD with good traction whilst maintaining a clear the view of the operative field. Refractory postoperative stenosis commonly occurs in patients who undergo extensive endoscopic resection of ≥ 75% of the circumference, and these patients often require repeated endoscopic balloon dilation[18].
Recently, studies have shown that steroid injection therapy and oral steroid administration prevented post-operative stenosis after extensive esophageal ESD (≥ 75% circumference)[19,20]. As alternative techniques for preventing post-ESD stenosis, other than steroid injection, polyglycolic acid (PGA) sheets and oral epithelial cell sheets may have the potential to prevent esophageal stricture after ESD[21-23]. However, these methods have not been widely used as a prophylactic measure for preventing stenosis because the PGA has a prolonged time for endoscopic delivery and fixation, and providing oral mucosal epithelial cell sheets in every hospital would be technically and financially difficult. We considered that this case had a considerably high risk for refractory postoperative stenosis because whole circumferential ESD was performed. Consequently, we conducted combination therapy with local steroid injection and oral steroid administration, which enabled us to prevent refractory stenosis.
This case suggested that minimally invasive and radical treatment could be achieved for superficial EAC involving the whole circumference of the LSBE using ESD. NBI-M and steroid combination therapy enabled us to diagnose horizontal tumor extension accurately and prevent refractory postoperative stenosis, respectively.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lorenzo-Zúñiga V, Samanta J, Suresh Kumar VC S-Editor: Wu YXJ L-Editor: A P-Editor: Liu JH
| 1. | Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 2. | Caygill CP, Royston C, Charlett A, Wall CM, Gatenby PA, Ramus JR, Watson A, Winslet M, Bardhan KD. Mortality in Barrett's esophagus: three decades of experience at a single center. Endoscopy. 2012;44:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 4. | Committee for Scientific Affairs; The Japanese Association for Thoracic Surgery. Masuda M, Endo S, Natsugoe S, Shimizu H, Doki Y, Hirata Y, Kobayashi J, Motomura N, Nakano K, Nishida H, Okada M, Saiki Y, Saito A, Sato Y, Tanemoto K, Toh Y, Tsukihara H, Wakui S, Yokomise H, Yokoi K, Okita Y. Thoracic and cardiovascular surgery in Japan during 2015 : Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2018;66:581-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | American Gastroenterological Association. Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 383] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 6. | Ogiya K, Kawano T, Ito E, Nakajima Y, Kawada K, Nishikage T, Nagai K. Lower esophageal palisade vessels and the definition of Barrett's esophagus. Dis Esophagus. 2008;21:645-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Cameron AJ, Carpenter HA. Barrett's esophagus, high-grade dysplasia, and early adenocarcinoma: a pathological study. Am J Gastroenterol. 1997;92:586-591. [PubMed] |
| 8. | Yamasaki A, Shimizu T, Kawachi H, Yamamoto N, Yoshimizu S, Horiuchi Y, Ishiyama A, Yoshio T, Hirasawa T, Tsuchida T, Sasaki Y, Fujisaki J. Endoscopic features of esophageal adenocarcinoma derived from short-segment vs long-segment Barrett's esophagus. J Gastroenterol Hepatol. 2020;35:211-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Kaneko M, Mitoro A, Yoshida M, Sawai M, Okura Y, Furukawa M, Namisaki T, Moriya K, Akahane T, Kawaratani H, Kitade M, Kaji K, Takaya H, Sawada Y, Seki K, Sato S, Fujii T, Yamao J, Obayashi C, Yoshiji H. Treatment of long-segment Barrett's adenocarcinoma by complete circular endoscopic submucosal dissection: a case report. BMC Gastroenterol. 2018;18:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Iizuka T, Kikuchi D, Hoteya S, Kaise M. Effectiveness of modified oral steroid administration for preventing esophageal stricture after entire circumferential endoscopic submucosal dissection. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Linghu E, Feng X, Wang X, Meng J, Du H, Wang H. Endoscopic submucosal tunnel dissection for large esophageal neoplastic lesions. Endoscopy. 2013;45:60-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Koike Y, Hirasawa D, Fujita N, Maeda Y, Ohira T, Harada Y, Suzuki K, Yamagata T, Tanaka M. Usefulness of the thread-traction method in esophageal endoscopic submucosal dissection: randomized controlled trial. Dig Endosc. 2015;27:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Mannath J, Subramanian V, Hawkey CJ, Ragunath K. Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett's esophagus: a meta-analysis. Endoscopy. 2010;42:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Sharma P, Bergman JJ, Goda K, Kato M, Messmann H, Alsop BR, Gupta N, Vennalaganti P, Hall M, Konda V, Koons A, Penner O, Goldblum JR, Waxman I. Development and Validation of a Classification System to Identify High-Grade Dysplasia and Esophageal Adenocarcinoma in Barrett's Esophagus Using Narrow-Band Imaging. Gastroenterology. 2016;150:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 15. | Goda K, Fujisaki J, Ishihara R, Takeuchi M, Takahashi A, Takaki Y, Hirasawa D, Momma K, Amano Y, Yagi K, Furuhashi H, Shimizu T, Kanesaka T, Hashimoto S, Ono Y, Yamagata T, Fujiwara J, Azumi T, Nishikawa M, Watanabe G, Ohkura Y, Oyama T. Newly developed magnifying endoscopic classification of the Japan Esophageal Society to identify superficial Barrett's esophagus-related neoplasms. Esophagus. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Arantes V, Albuquerque W, Freitas Dias CA, Demas Alvares Cabral MM, Yamamoto H. Standardized endoscopic submucosal tunnel dissection for management of early esophageal tumors (with video). Gastrointest Endosc. 2013;78:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Gan T, Yang JL, Zhu LL, Wang YP, Yang L, Wu JC. Endoscopic submucosal multi-tunnel dissection for circumferential superficial esophageal neoplastic lesions (with videos). Gastrointest Endosc. 2016;84:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 19. | Yamaguchi N, Isomoto H, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S, Nakao K. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 2011;73:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Hashimoto S, Kobayashi M, Takeuchi M, Sato Y, Narisawa R, Aoyagi Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1389-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 21. | Iizuka T, Kikuchi D, Yamada A, Hoteya S, Kajiyama Y, Kaise M. Polyglycolic acid sheet application to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Endoscopy. 2015;47:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Sakaguchi Y, Tsuji Y, Ono S, Saito I, Kataoka Y, Takahashi Y, Nakayama C, Shichijo S, Matsuda R, Minatsuki C, Asada-Hirayama I, Niimi K, Kodashima S, Yamamichi N, Fujishiro M, Koike K. Polyglycolic acid sheets with fibrin glue can prevent esophageal stricture after endoscopic submucosal dissection. Endoscopy. 2015;47:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Yamaguchi N, Isomoto H, Kobayashi S, Kanai N, Kanetaka K, Sakai Y, Kasai Y, Takagi R, Ohki T, Fukuda H, Kanda T, Nagai K, Asahina I, Nakao K, Yamato M, Okano T, Eguchi S. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep. 2017;7:17460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |