Published online Oct 27, 2021. doi: 10.4240/wjgs.v13.i10.1267
Peer-review started: February 14, 2021
First decision: March 16, 2021
Revised: March 25, 2021
Accepted: August 4, 2021
Article in press: August 4, 2021
Published online: October 27, 2021
Processing time: 253 Days and 19.6 Hours
There is limited evidence on the safety of immunotherapy use after liver transplantation and its efficacy in treating post-liver transplant hepatocellular carcinoma (HCC) recurrence.
To assess the safety of immunotherapy after liver transplant and its efficacy in treating post-liver transplant HCC recurrence.
A literature review was performed to identify patients with prior liver transplantation and subsequent immunotherapy. We reviewed the rejection rate and risk factors of rejection. In patients treated for HCC, the oncological outcomes were evaluated including objective response rate, progression-free survival (PFS), and overall survival (OS).
We identified 25 patients from 16 publications and 3 patients from our institutional database (total n = 28). The rejection rate was 32% (n = 9). Early mortality occurred in 21% (n = 6) and was mostly related to acute rejection (18%, n = 5). Patients who developed acute rejection were given immunotherapy earlier after transplantation (median 2.9 years vs 5.3 years, P = 0.02) and their graft biopsies might be more frequently programmed death ligand-1-positive (100% vs 33%, P = 0.053). Their PFS (1.0 ± 0.1 mo vs 3.5 ± 1.1 mo, P = 0.02) and OS (1.0 ± 0.1 mo vs 19.2 ± 5.5 mo, P = 0.001) compared inferiorly to patients without rejection. Among the 19 patients treated for HCC, the rejection rate was 32% (n = 6) and the overall objective response rate was 11%. The median PFS and OS were 2.5 ± 1.0 mo and 7.3 ± 2.7 mo after immunotherapy.
Rejection risk is the major obstacle to immunotherapy use in liver transplant recipients. Further studies on the potential risk factors of rejection are warranted.
Core Tip: A literature review was performed to identify patients with prior liver transplantation and subsequent immunotherapy. Among the 28 included patients, the rejection rate was 32% (n = 9). Patients who developed acute rejection were given immunotherapy earlier after transplantation (median 2.9 years vs 5.3 years, P = 0.02) and their graft biopsies might be more frequently programmed death ligand-1 positive (100% vs 33%, P = 0.053). Among the 19 patients treated for hepatocellular carcinoma (HCC), the overall objective response rate was 11%. Rejection risk is the major obstacle to immunotherapy for post-liver transplant HCC recurrence.
- Citation: Au KP, Chok KSH. Immunotherapy after liver transplantation: Where are we now? World J Gastrointest Surg 2021; 13(10): 1267-1278
- URL: https://www.wjgnet.com/1948-9366/full/v13/i10/1267.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i10.1267
Post-liver transplant hepatocellular carcinoma (HCC) recurrence represents a therapeutic challenge. Prognosis is generally poor while tumor progression is unrestrained with suppressed host immunity. Thanks to recent advances in oncological treatment and improved immunosuppression, the outlook of these patients has improved[1,2], and long-term survival is no longer impossible. Nevertheless, reduced immune surveillance remains the Achilles heel for tumor control.
Over the last decade, immunotherapy has revolutionized cancer treatment. By disengaging immune checkpoints pathways, host immune response is augmented and directed towards the tumor. Immunotherapy is also characterized by a favorable side-effect profile compared to targeted therapy, which has been extensively investigated for post-transplant HCC recurrence. Modest efficacy was observed, but significant adverse effect has often led to dose reduction or discontinuation[3-6]. While immunotherapy has demonstrated satisfactory outcomes in patients with advanced primary HCC[7,8], its role in post-transplant HCC recurrence has not been investigated. There are two major obstacles to immunotherapy use in this setting. First, the possibility of enhancing alloimmunity and inducing rejection has raised safety concern. Second, efficacy is also questionable because concomitant immunosuppression potentially interferes with the immunomodulatory pathways involved. Given these concerns, liver transplant patients have been excluded from cancer immunotherapy trials, and limited data exist on the role of immune checkpoint inhibitors for post-liver transplant HCC recurrence.
In this study, we reviewed the literature for the record of patients who had undergone prior liver transplantation and received immunotherapy. In addition, we reviewed the liver transplant recipients who had been treated with immunotherapy in our institution. The objective was to summarize the existing experience and provide further insights on safety and efficacy of immunotherapy for post-transplant HCC recurrence.
A literature search was performed on PubMed (United States National Library of Medicine, National Institutes of Health, United States) for relevant English articles with a combination of keywords: “liver transplantation” with “immunotherapy” or “checkpoint inhibitors” or “programmed cell death 1” or “PD-1” or “cytotoxic T lymphocyte associated 4” or “CTLA-4.” The full text of potentially relevant articles was reviewed. Original case reports, case series, observation studies, and review articles were included if they described immune checkpoint inhibitor therapy in a patient with prior liver transplantation. Laboratory studies without clinical subjects were excluded. References in the included studies were reviewed for additional relevant articles. Patient data was extracted including demographics, timing and indication of immunotherapy, concomitant immunosuppression, programmed death ligand-1 (PD-L1) status, adverse events, treatment response, and survival. Subjects were cross-checked to ensure no individual patient was included twice. In addition, we reviewed the records of liver transplant recipients who underwent immunotherapy in Queen Mary Hospital, the University of Hong Kong during the period from January 2016 to December 2020. Patient data were retrieved from a prospectively maintained institutional database.
We assessed the safety of immunotherapy by reviewing the rejection rate and mortality in all identified patients treated for various indications. We also looked into patients treated for recurrent HCC after liver transplantation to investigate the efficacy of immunotherapy in this setting. We reviewed the best treatment response, rate of early mortality, progression-free survival (PFS), and overall survival (OS) after immunotherapy. Early mortality was defined as mortality within 30 d from immunotherapy. Treatment response was defined according to the Response Evaluation Criteria in Solid Tumors 1.1[9]. Data was summarized with descriptive statistics. Continuous variables were expressed with medians and interquartile ranges (IQRs). Parametric and non-parametric variables were compared with the Student’s t-test and Mann-Whitney U test where appropriate. Categorical variables were expressed in frequencies and percentages and were compared with the chi-square test. Survival data was analyzed with the Kaplan-Meier method and compared using the log-rank test. Data were analyzed using Statistical Package for the Social Sciences 16.0 (SPSS) for Windows (SPSS Inc., Chicago, IL, United States). Statistical significance was defined by P < 0.05.
Using PubMed, we identified 16 publications describing 25 patients who had a prior liver transplantation and subsequently received immunotherapy[10-25]. From the institutional database, there were 3 patients fulfilling the same inclusion criteria. These 28 patients formed the basis of this study (Table 1).
| Ref. | Drug | No. of cycles | Sex | Age | Indication | Year from transplant | Line of therapy | Rejection | Early mortality | PD-L1 status | Immunosuppression | Best response | PFS (mo) | OS (mo) | |
| Graft | Tumor | ||||||||||||||
| De Toni and Gerbes[10] | Nivolumab | 15 | M | 41 | HCC | NA | 1 | No | No | NA | 0% | Tacrolimus | PD | 3.5 | 7 |
| Friend et al[11] | Nivolumab | 2 | M | 20 | HCC | 4 | 2 | Yes | Yes | Pos | Pos | Sirolimus | NA | 1 | 1 |
| Friend et al[11] | Nivolumab | 1 | M | 14 | HCC | 3 | 3 | Yes | Yes | Pos | Pos | Tacrolimus | NA | 1 | 1 |
| Varkaris et al[12] | Pembrolizumab | NA | M | 70 | HCC | 8 | NA | No | No | NA | NA | Tacrolimus | PD | NA | NA |
| Munker and De Toni[13] | Nivolumab | NA | M | 57 | HCC | 2.7 | 3 | No | No | NA | 10% | Tacrolimus | PD | 2.2 | 1.2 (surviving) |
| Munker and De Toni[13] | Nivolumab | NA | M | 56 | HCC | 7.8 | 4 | No | No | 5% | NA | Sirolimus/MMF | PD | 0.7 | 1.1 (surviving) |
| Munker and De Toni[13] | Nivolumab | NA | F | 35 | HCC | 3.7 | 5 | No | No | 0% | 0% | Tacrolimus | PD | 1.3 | 1.3 (surviving) |
| Munker and De Toni[13] | Nivolumab | NA | M | 64 | HCC | 1.2 | 2 | No | Yes | NA | 0% | Tacrolimus | NA | 0.3 | 0.3 |
| Munker and De Toni[13] | Nivolumab | NA | M | 68 | HCC | 1.1 | 2 | Yes | Yes | 30% | 0% | Sirolimus | NA | 0.9 | 0.9 |
| Al Jarroudi et al[14] | Nivolumab | 4 | M | 70 | HCC | 2.75 | 3 | Yes | No | NA | NA | Tacrolimus | NA | 4 | 4 |
| Al Jarroudi et al[14] | Nivolumab | 5 | F | 62 | HCC | 1 | 4 | No | No | NA | NA | Tacrolimus | PD | 2.5 | NA |
| Al Jarroudi et al[14] | Nivolumab | 6 | M | 66 | HCC | 5 | 4 | No | No | NA | NA | Tacrolimus | SD | 3 | NA |
| Rammohan et al[15] | Pembrolizumab | 14 | M | 57 | HCC | 4.3 | 2 | No | No | NA | NA | Tacrolimus/mTOR inhibitor | CR | 10 (no progression) | 10 (surviving) |
| Gassmann et al[16] | Nivolumab | 1 | F | 53 | HCC | 3 | 2 | Yes | Yes | NA | NA | Everolimus | NA | 0.8 | 0.8 |
| Nasr et al[17] | Pembrolizumab | 35 | M | 63 | HCC | 4.6 | 2 | No | No | NA | NA | Tacrolimus/MMF | CR | 25 (no progression) | 25 (surviving) |
| Wang et al[18] | Pembrolizumab | 1 | M | 48 | HCC | 1 | 1 | Yes | No | NA | NA | Tacrolimus/Everolimus | NA | NA | 8 (surviving) |
| Au (current research) | Nivolumab | 4 | M | 62 | HCC | 2.2 | 3 | No | No | NA | NA | Tacrolimus/Everolimus | PD | 4.0 | 7.3 |
| Au (current research) | Nivolumab | 6 | M | 53 | HCC | 6.0 | 2 | No | No | NA | NA | Sirolimus | PD | 2.8 | 10.6 |
| Au (current research) | Pembrolizumab | 16 | M | 77 | HCC | 32 | 1 | No | No | NA | NA | Tacrolimus/Everolimus | SD | 12.4 | 19.2 |
| Ranganath and Panella[19] | Ipilimumab | 4 | F | 59 | Melanoma | 8 | NA | No | No | NA | NA | Sirolimus | PR | 5 | 9 (surviving) |
| Morales et al[20] | Ipilimumab | 4 | M | 67 | Melanoma | 8 | 2 | No | No | NA | NA | Sirolimus/MMF | PR | 4 (no progression) | 14 (surviving) |
| Munker and De Toni[13] | Pembrolizumab | NA | M | 55 | Melanoma | 5.5 | 2 | No | No | 0% | 5% | Everolimus/MMF | CR | 21.1 (no progression) | 21.1 (surviving) |
| Munker and De Toni[13] | Pembrolizumab | NA | M | 64 | Melanoma | 3.1 | 2 | Yes | No | 25% | NA | MMF/Prednisolone | NA | NA | 0.7 (surviving) |
| Kuo et al[21] | Ipilimumab/Pembrolizumab | 4/25 | M | 62 | Melanoma | 6 | NA | No | No | NA | NA | Sirolimus | PR | 24 (no progression) | 24 (surviving) |
| Dueland et al[22] | Ipilimumab | 1 | F | 67 | Melanoma | 1.5 | 1 | Yes | No | NA | NA | Prednisolone | PD | 3 (no progression) | 4 |
| Schvartsman et al[23] | Pembrolizumab | 2 | M | 35 | Melanoma | 20 | 1 | No | No | NA | NA | Tacrolimus | CR | 6 | 6 (surviving) |
| Tio et al[24] | Pembrolizumab | 1 | F | 63 | Melanoma | NA | NA | Yes | Yes | NA | NA | Ciclosporin | NA | NA | NA |
| Biondani et al[25] | Nivolumab | 3 | M | 54 | SCC lung | 13 | 1 | No | No | NA | NA | Tacrolimus/Everolimus | PD | 2.25 | 15 |
The descriptive characteristics are shown in Table 2. There was a male predominance (79%), and the median age was 61 (IQR 53-66). Nineteen patients (68%) were treated for recurrent HCC, 8 (29%) for de novo melanoma, and 1 (4%) for squamous cell carcinoma of the lung. Most received immunotherapy after failure of prior systemic therapy (median line of systemic treatment 2, IQR 1-3). Twenty-five patients (89%) received a programmed cell death protein-1 (PD-1) inhibitor (nivolumab 54%; pembrolizumab 36%). Four patients (14%) received cytotoxic T lymphocyte antigen-4 (CTLA-4) inhibitor (ipilimumab) and they were all indicated for melanoma. One patient received ipilimumab followed by pembrolizumab.
| All | Rejection | No rejection | P value | |
| Total (%) | 28 | 9 (32) | 19(68) | |
| Gender (M/F; %M) | 22/6 (79) | 6/3 (67) | 16/3 (84) | 0.29 |
| Age | 61 (53-66) | 63 (34-67.5) | 59 (54-64) | 1.00 |
| Year after transplant | 3.9 (2.5-6.5) | 2.9 (1.2-3.1) | 5.3 (2.7-8.0) | 0.02 |
| Indication (%) | 0.93 | |||
| HCC | 19 (68) | 6 (67) | 13 (68) | |
| Melanoma | 8 (29) | 3 (33) | 5 (26) | |
| SCC of lung | 1 (4) | 0 (0) | 1 (5) | |
| Line of systemic therapy | 2 (1-3) | 2 (1-3) | 2 (1-4) | 0.52 |
| Immunotherapy by drug (%) | 0.92 | |||
| Nivolumab | 15 (54) | 5 (56) | 10 (53) | |
| Pembrolizumab | 10 (36) | 3 (33) | 7 (37) | |
| Ipilimumab | 4 (14) | 1 (11) | 3 (16) | |
| Immunotherapy by class (%) | 1.00 | |||
| PD1/PD-L1 | 24 (86) | 8 (89) | 16 (84) | |
| CTLA-4 | 3 (11) | 1 (11) | 2 (11) | |
| Both | 1 (4) | 0 (0) | 1 (5) | |
| PD-L1 positivity (%) | ||||
| Graft | 5/7 (71) | 4/4 (100) | 1/3 (33) | 0.053 |
| Tumor | 4/8 (50) | 2/3 (67) | 2/5 (40) | 0.47 |
| Immunosuppression (%) | ||||
| Single agent tacrolimus | 10 (36) | 2 (22) | 8 (42) | 0.31 |
| Single agent mTOR-inhibitor | 6 (21) | 3 (33) | 3 (16) | 0.29 |
| Tacrolimus with mTOR-inhibitor | 5 (18) | 1 (11) | 4 (21) | 0.52 |
| Others | 7 (25) | 3 (33) | 4 (21) | 0.48 |
| Acute rejection (%) | 9 (32) | |||
| Mortality in 30 d (%) | 6 (21) | 5 (56) | 1 (5) | 0.002 |
| Progression-free survival | 3 ± 0.6 | 1.0 ± 0.1 | 3.5 ± 1.1 | 0.02 |
| Overall survival | 10.6 ± 5.3 | 1.0 ± 0.1 | 19.2 ± 5.5 | 0.001 |
Seven graft liver and eight tumor tissues were tested for PD-L1 status. Among the tested samples, the rates of positive PD-L1 staining were 71% for graft liver and 50% for tumor. Ten patients (36%) received tacrolimus monotherapy as immunosuppression. Six patients (21%) received a mammalian target of rapamycin (mTOR) inhibitor as single agent while 5 patients (18%) received combination therapy with tacrolimus and an mTOR inhibitor.
The rate of acute rejection following immunotherapy was 32% (n = 9). Early mortality occurred in 21% (n = 6), and most were related to acute rejection (18%, n = 5). Patients who developed acute rejection were given immunotherapy earlier after transplantation (median 2.9 years vs 5.3 years, P = 0.02). Among the patients with acute rejection, graft PD-L1 positivity was possibly more frequent but not statistically evident (100% vs 33%, P = 0.053). Otherwise, patients with and without rejection were comparable in terms of age (63 vs 59, P = 1.00), indication of immunotherapy (P = 0.93), proportion of PD-1 vs CTLA-4 blockade (P = 1.00), and immunosuppressive therapy received (P = 0.29-0.48). Excluding one patient who received both PD-1 and CTLA-4 blockade, the rejection rate was similar between patients receiving PD-1 (8/24) and CTLA-4 blockade (1/3) (both 33%, P = 1.00).
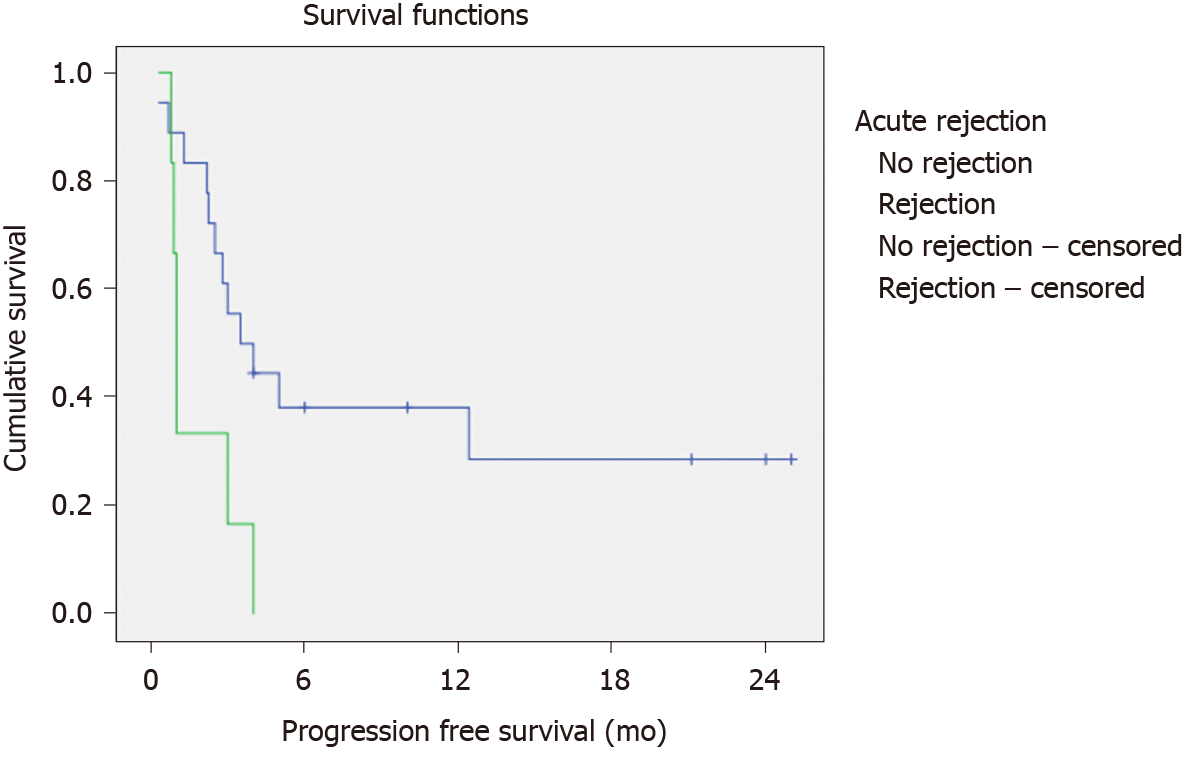
Patients with acute rejection suffered from more early mortalities (56% vs 5%, P = 0.002). Their PFS (1.0 ± 0.1 mo vs 3.5 ± 1.1 mo, P = 0.02) and OS (1.0 ± 0.1 vs 19.2 ± 5.5 mo, P = 0.001) compared inferiorly to patients without rejection (Figures 1 and 2).
Patients who received immunotherapy for HCC recurrence were treated with immunotherapy earlier after transplant than those treated for de novo malignancies (median time from transplant 3.3 years vs 7 years, P = 0.03). They received immunotherapy as a median of second-line systemic therapy (IQR 1-3) (Table 3). Six patients (32%) suffered rejection and one patient (5%) suffered early mortality unrelated to rejection. Treatment response was not evaluated for these patients. The proportion of patients with complete response, partial response, stable disease, and progressive disease were 11% (n = 2), 0% (n = 0), 11% (n = 2), and 42% (n = 8) respectively. The overall objective response rate was 11%. The median PFS and OS were 2.5 ± 1.0 and 7.3 ± 2.7 mo after immunotherapy.
| All | Nivolumab | Pembrolizumab | P value | |
| Total (%) | 19 | 14 (74) | 5 (26) | |
| Rejection (%) | 6 (32) | 5 (36) | 1 (20) | 0.52 |
| Early mortality (%) | 5 (26) | 5 (36) | 0 (0) | 0.12 |
| Line of systemic therapy | 2 (1-3) | 3 (2-4) | 2 (1-2) | 0.03 |
| Tumour PD-L1 positivity (%) | 3/7 (43) | 3/7 (43) | 0/0 (-) | |
| Best treatment response (%) | ||||
| Complete response | 2 (11) | 0 (0) | 2 (40) | 0.03 |
| Partial response | 0 (0) | 0 (0) | 0 (0) | 0.64 |
| Stable disease | 2 (11) | 1 (7) | 1 (20) | 0.58 |
| Progressive disease | 8 (42) | 7 (50) | 1 (20) | 0.03 |
| Progression-free survival | 2.5 ± 1.0 | 1.3 ± 1.1 | 12.4 | 0.004 |
| Overall survival | 7.3 ± 2.7 | 4.0 ± 3.4 | 19.2 | 0.006 |
We compared the relative efficacy of nivolumab and pembrolizumab for recurrent HCC after liver transplantation. Pembrolizumab was used as an earlier line of therapy (median third line vs second line, P = 0.03). Pembrolizumab was associated with a higher complete response (0% vs 40%, P = 0.03), less progressive disease (50% vs 20%, P = 0.03), and better PFS (1.3 ± 1.1 vs 12.4 mo, P = 0.004) and OS (4.0 ± 3.4 vs 19.2 mo, P = 0.006). Pembrolizumab was potentially associated with fewer early mortalities but this was not statistically evident (36% vs 0%, P = 0.12).
We found that immunotherapy could be associated with fatal graft rejection. The rejection rate was relatively high (32%), and more importantly, was associated with a high rate of organ failure and early mortality (56% in patients with rejection). A more malignant clinical course was observed opposed to spontaneous acute rejection, which was usually treatment responsive and seldom resulted in irreversible consequences[26-28]. To optimize patient selection, we investigated the potential clinical factors associated with acute rejection in the identified patient sample. These factors included the timing of immunotherapy, the role of PD-1 vs CTLA-4 blockade, the effect of PD-L1 positivity on the liver graft biopsy, and the strength of the immunosuppressive regimen during immunotherapy.
We observed that patients with long-term liver transplantation were less liable to rejection when treated with immunotherapy. From our cohort, patients with rejection received immunotherapy earlier after transplantation (median time from transplant 2.9 years vs 5.3 years, P = 0.02). After transplant, immune tolerance towards the liver graft increases with time[29,30]. The underlying mechanism is the dissemination and persistence of donor leukocytes from the liver graft to the recipient, leading to systemic chimerism[31]. This explains why most spontaneous acute rejection occurs early after liver transplant[32], allowing immunosuppression to be tapered with time. The protective effect of time was consistently observed in the setting of immunotherapy, however to a lesser extent. While the risk of spontaneous rejection is largely reduced beyond the first year after transplant[32], the risk of post-immunotherapy rejection persists further. Patients who developed post-immunotherapy rejection were given immunotherapy at a median time of 2.9 years after transplant. Existing data are too limited to conclude the safe time interval before immunotherapy that can safely be used. However, it appears that the risk of rejection cannot be neglected in the first few years after transplantation.
Most HCC recurrence occurs early after liver transplantation[33]. From the current series, patients who received immunotherapy for HCC recurrence were treated with immunotherapy earlier after transplant than those treated for de novo malignancies (median time from transplant 3.3 years vs 7 years, P = 0.03). From our experience, patients with early HCC recurrence also have a poorer prognosis[1]. While the use of immunotherapy for post-transplant HCC recurrence is investigational, it is reasonable to reserve immunotherapy to patients with late recurrence. With reduced rejection risk and better tumor biology, better outcomes can be expected.
Researchers have proposed that PD-1 inhibition is potentially associated with a higher risk of rejection and graft loss compared to CTLA-4 blockade[34]. In a cohort of 12 transplant recipients, rejection occurred in 4 of the 8 patients receiving anti-PD-1 therapy but in none of the 4 patients receiving anti-CTLA-4 treatment[35]. It is hypothesized that the PD-1 pathway plays a more integral role in allograft immune tolerance[35,36]; however, our data did not support this hypothesis. In the current cohort, patients who received anti-PD-1 agents had a rejection rate that was very similar to those receiving CTLA-4 blockade (33% vs 33%, P = 1.00). In comparison, our study was characterized by inclusion of liver transplant recipients only, and a better sample size (n = 28). Though insufficient to indicate the relative safety profile of both classes of immune checkpoint inhibitor, our observation showed that CTLA-4 blockade is not without risk of liver graft rejection. Given its established efficacy in primary HCC, anti-PD-1 agents should remain the agent of choice when immunotherapy is contemplated for treatment of post-transplant HCC recurrence[7,8].
Allograft PD-L1 staining was evaluated in 7 patients treated with immunotherapy. Patients with rejection were more frequently observed to have positive graft PD-L1 staining, though statistical significance was not reached. Our data are suggestive of a potential role of graft PD-L1 positivity predicting rejection. However, many of these allograft biopsies were taken during rejection. To allow risk stratification before commencement of therapy, a baseline allograft biopsy may be more valuable. In our institution, protocolled graft biopsy is taken during transplant after implantation. To better study the significance of graft PD-L1 status, these implant biopsies could be reviewed for PD-L1 status when immunotherapy is contemplated.
Immunosuppression is usually tapered upon diagnosis of cancer to preserve anti-tumor immunity[33]. Upon recurrence, some patients had calcineurin inhibitors weaned off and were maintained on an mTOR-inhibitor. In these patients, we did not observe a higher rejection rate following immunotherapy. However, the current study was underpowered to compare heterogenous immunosuppressive regimens. Dosage and drug level information was also incomplete for evaluation. The ideal immunosuppression for patients undergoing immunotherapy requires extensive investigation into the interaction between anti-tumor immunity and alloimmunity, which warrants future laboratory and clinical studies.
In non-organ transplant recipients, mild immune-related adverse events can often be observed or treated with steroids while continuing immunotherapy[37]. Although antagonizing mechanisms between immune checkpoint inhibitor and steroid have been described in cellular models[38], clinical studies have not consistently concluded a nefarious interaction between them[39]. In contrast, liver transplant recipients often suffer irreversible liver failure after immunotherapy induces graft rejection, despite high doses of steroid and prompt withdrawal of immunotherapy. Given the serious consequences of graft rejection, continuation of immunotherapy could not be recommended based on the current experience.
The overall response rate for immunotherapy for post-transplant HCC recurrence was low (11%). A significant proportion of patients developed rejection (32%), leading to mortality or premature discontinuation of treatment. These results suggest that safety of immunotherapy must be addressed before its potential efficacy can be fully assessed. Of note, the 5 patients who received pembrolizumab had a better overall response rate and survival. The comparably lower rate of rejection (36% vs 20%, P = 0.52) could have partly contributed. However, pembrolizumab was commenced earlier in the course of disease, while nivolumab was usually given after failure of multiple lines of systemic therapy. The disease status of these patients was not available for comparison. Their potential confounding effects should be considered when interpreting the outcomes. In the current series, patient numbers were too limited to assess the relationship between tumor PD-L1 status and treatment response. In future studies, explant tumor PD-L1 status can be reviewed when patients are contemplated for immunotherapy.
The current study was limited by its methodology. Subjects were sampled from individual case reports and series with low homogeneity, and data analysis is vulnerable to publication bias. Patients with extreme outcomes were preferentially reported and the rejection rate could have been overestimated. The included patients had heterogenous immunosuppressive regimen, which potentially affect rejection and tumor response. The small sample size largely limited the analytical power.
From the limited experience in the literature, we conclude that rejection remains the major obstacle to immunotherapy use in the setting of post-liver transplant HCC recurrence. It is associated with considerable risk of organ failure and mortality. Before immunotherapy can be recommended for post-transplant HCC recurrence, it is essential to determine which patients are at risk of developing rejection. We have identified a short duration from transplant and graft PD-L1 positivity as potential risk factors. We suggest establishing an international registry to allow information regarding immunotherapy for post-liver transplant HCC recurrence to be systemically collected. With better understanding and insights, we could better select the suitable patients and achieve more desirable outcomes.
Evidence on the safety of immunotherapy in liver transplant recipient is limited. Its efficacy on treating post-liver transplant hepatocellular carcinoma (HCC) recurrence is unknown.
To study the potential role of immunotherapy in the setting of post-liver transplant HCC recurrence.
To assess the safety of immunotherapy after liver transplantation and to assess its efficacy on treating post-liver transplant HCC recurrence.
A review of current literature describing immune checkpoint inhibitor therapy in a patient with prior liver transplantation. Patients from our institution were included for review.
There were 28 patients identified. The rejection rate was 32% (n = 9). Early mortality occurred in 21% (n = 6) and were mostly related to acute rejection (18%, n = 5). Patients with acute rejection were given immunotherapy earlier after transplantation (median 2.9 years vs 5.3 years, P = 0.02). Their progression-free survival (1.0 ± 0.1 vs 3.5 ± 1.1 mo, P = 0.02) and overall survival (1.0 ± 0.1 vs 19.2 ± 5.5 mo, P = 0.001) compared inferiorly to patients without rejection. Among the 19 patients treated for HCC, the rejection rate was 32% (n = 6) and the overall objective response rate was 11%.
Rejection risk is the major obstacle to immunotherapy use in liver transplant recipients.
Further studies on the potential risk factors of rejection are warranted.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boninsegna E, Yamaguchi K S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Au KP, Chok KSH. Mammalian target of rapamycin inhibitors after post-transplant hepatocellular carcinoma recurrence: Is it too late? World J Gastrointest Surg. 2020;12:149-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Au KP, Chiang CL, Chan ACY, Cheung TT, Lo CM, Chok KSH. Initial experience with stereotactic body radiotherapy for intrahepatic hepatocellular carcinoma recurrence after liver transplantation. World J Clin Cases. 2020;8:2758-2768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Mancuso A, Mazzola A, Cabibbo G, Perricone G, Enea M, Galvano A, Zavaglia C, Belli L, Cammà C. Survival of patients treated with sorafenib for hepatocellular carcinoma recurrence after liver transplantation: a systematic review and meta-analysis. Dig Liver Dis. 2015;47:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib vs best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | de'Angelis N, Landi F, Nencioni M, Palen A, Lahat E, Salloum C, Compagnon P, Lim C, Costentin C, Calderaro J, Luciani A, Feray C, Azoulay D. Role of Sorafenib in Patients With Recurrent Hepatocellular Carcinoma After Liver Transplantation. Prog Transplant. 2016;26:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Piñero F, Marciano S, Anders M, Ganem FO, Zerega A, Menéndez J, Mendizábal M, Baña MT, Gil O, Gerona S, de Santibañes E, Mastai R, Gadano A, Silva M. Sorafenib for Recurrent Hepatocellular Carcinoma after Liver Transplantation: A South American Experience. Acta Gastroenterol Latin. 2016;46:300-309. |
| 7. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3310] [Article Influence: 413.8] [Reference Citation Analysis (1)] |
| 8. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M Han KH, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Begic D, Chen G, Neely J, Anderson J, Sangro B. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30:v874-v875. [RCA] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 433] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 9. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21618] [Article Influence: 1351.1] [Reference Citation Analysis (1)] |
| 10. | De Toni EN, Gerbes AL. Tapering of Immunosuppression and Sustained Treatment With Nivolumab in a Liver Transplant Recipient. Gastroenterology. 2017;152:1631-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Friend BD, Venick RS, McDiarmid SV, Zhou X, Naini B, Wang H, Farmer DG, Busuttil RW, Federman N. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr Blood Cancer. 2017;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Varkaris A, Lewis DW, Nugent FW. Preserved Liver Transplant After PD-1 Pathway Inhibitor for Hepatocellular Carcinoma. Am J Gastroenterol. 2017;112:1895-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 13. | Munker S, De Toni EN. Use of checkpoint inhibitors in liver transplant recipients. United European Gastroenterol J. 2018;6:970-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Al Jarroudi O, Ulusakarya A, Almohamad W, Afqir S, Morere JF. Anti-Programmed Cell Death Protein 1 (PD-1) Immunotherapy for Metastatic Hepatocellular Carcinoma After Liver Transplantation: A Report of Three Cases. Cureus. 2020;12:e11150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Rammohan A, Reddy MS, Farouk M, Vargese J, Rela M. Pembrolizumab for metastatic hepatocellular carcinoma following live donor liver transplantation: The silver bullet? Hepatology. 2018;67:1166-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Gassmann D, Weiler S, Mertens JC, Reiner CS, Vrugt B, Nägeli M, Mangana J, Müllhaupt B, Jenni F, Misselwitz B. Liver Allograft Failure After Nivolumab Treatment-A Case Report With Systematic Literature Research. Transplant Direct. 2018;4:e376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 17. | Nasr F, AlGhoche A, Diab S, Janah M, Layal M, Ali K, El Karim GA. Pembrolizumab Monother-Apy in Relapsed Hepatocellular Carcinoma Post Living Donor Liver Transplantation and Sorafenib. Int J Oncol Res. 2018;1:009. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Wang G, Tang H, Yingcai Z. Programmed death receptor (PD)-11 monoclonal antibody-induced acute immune hepatitis in the treatment of recurrent hepatocellular carcinoma after liver transplantation: a case report. Organ Transplant. 2016;7:45-47. |
| 19. | Ranganath HA, Panella TJ. Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J Immunother. 2015;38:211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Morales RE, Shoushtari AN, Walsh MM, Grewal P, Lipson EJ, Carvajal RD. Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J Immunother Cancer. 2015;3:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Kuo JC, Lilly LB, Hogg D. Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: a case report and literature review. Melanoma Res. 2018;28:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Dueland S, Guren TK, Boberg KM, Reims HM, Grzyb K, Aamdal S, Julsrud L, Line PD. Acute liver graft rejection after ipilimumab therapy. Ann Oncol. 2017;28:2619-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Schvartsman G, Perez K, Sood G, Katkhuda R, Tawbi H. Immune Checkpoint Inhibitor Therapy in a Liver Transplant Recipient With Melanoma. Ann Intern Med. 2017;167:361-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Tio M, Rai R, Ezeoke OM, McQuade JL, Zimmer L, Khoo C, Park JJ, Spain L, Turajlic S, Ardolino L, Yip D, Goldinger SM, Cohen JV, Millward M, Atkinson V, Kane AY, Ascierto PA, Garbe C, Gutzmer R, Johnson DB, Rizvi HA, Joshua AM, Hellmann MD, Long GV, Menzies AM. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur J Cancer. 2018;104:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 25. | Biondani P, De Martin E, Samuel D. Safety of an anti-PD-1 immune checkpoint inhibitor in a liver transplant recipient. Ann Oncol. 2018;29:286-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Rodríguez-Perálvarez M, Rico-Juri JM, Tsochatzis E, Burra P, De la Mata M, Lerut J. Biopsy-proven acute cellular rejection as an efficacy endpoint of randomized trials in liver transplantation: a systematic review and critical appraisal. Transpl Int. 2016;29:961-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Thurairajah PH, Carbone M, Bridgestock H, Thomas P, Hebbar S, Gunson BK, Shah T, Neuberger J. Late acute liver allograft rejection; a study of its natural history and graft survival in the current era. Transplantation. 2013;95:955-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Jain A, Reyes J, Kashyap R, Dodson SF, Demetris AJ, Ruppert K, Abu-Elmagd K, Marsh W, Madariaga J, Mazariegos G, Geller D, Bonham CA, Gayowski T, Cacciarelli T, Fontes P, Starzl TE, Fung JJ. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 410] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 29. | Bohne F, Martínez-Llordella M, Lozano JJ, Miquel R, Benítez C, Londoño MC, Manzia TM, Angelico R, Swinkels DW, Tjalsma H, López M, Abraldes JG, Bonaccorsi-Riani E, Jaeckel E, Taubert R, Pirenne J, Rimola A, Tisone G, Sánchez-Fueyo A. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest. 2012;122:368-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 30. | Martínez-Llordella M, Lozano JJ, Puig-Pey I, Orlando G, Tisone G, Lerut J, Benítez C, Pons JA, Parrilla P, Ramírez P, Bruguera M, Rimola A, Sánchez-Fueyo A. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118:2845-2857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, Ramos H, Todo S, Tzakis A, Fung JJ. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 504] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 32. | Anand AC, Hubscher SG, Gunson BK, McMaster P, Neuberger JM. Timing, significance, and prognosis of late acute liver allograft rejection. Transplantation. 1995;60:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Au KP, Chok KSH. Multidisciplinary approach for post-liver transplant recurrence of hepatocellular carcinoma: A proposed management algorithm. World J Gastroenterol. 2018;24:5081-5094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Wanchoo R, Riella LV, Uppal NN, Lopez CA, Nair V, Devoe C, Jhaveri KD. Immune Checkpoint Inhibitors in the Cancer Patient with An Organ Transplant. J Onco-Nephrology. 2017;1:42-48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Kittai AS, Oldham H, Cetnar J, Taylor M. Immune Checkpoint Inhibitors in Organ Transplant Patients. J Immunother. 2017;40:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 36. | Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, Grimm M, Waaga AM, Ueno T, Padera RF, Yagita H, Azuma M, Shin T, Blazar BR, Rothstein DM, Sayegh MH, Najafian N. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179:5204-5210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 37. | Thompson JA. New NCCN Guidelines: Recognition and Management of Immunotherapy-Related Toxicity. J Natl Compr Canc Netw. 2018;16:594-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 38. | Xing K, Gu B, Zhang P, Wu X. Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: an insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunol. 2015;16:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 39. | Garant A, Guilbault C, Ekmekjian T, Greenwald Z, Murgoi P, Vuong T. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: A systematic review. Crit Rev Oncol Hematol. 2017;120:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |