Published online Oct 27, 2021. doi: 10.4240/wjgs.v13.i10.1245
Peer-review started: April 19, 2021
First decision: August 9, 2021
Revised: August 21, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 27, 2021
Processing time: 189 Days and 16.7 Hours
The prognosis of advanced hepatocellular carcinoma (HCC) that is not indicated for curative hepatectomy remains poor, despite advances in the treatment of HCC, including the development of tyrosine kinase inhibitors (TKIs). The outcomes of reduction hepatectomy and multidisciplinary postoperative treatment for advanced HCC that is not indicated for curative hepatectomy, including those of recently treated cases, should be investigated.
To examine the outcomes of combination treatment with reduction hepatectomy and multidisciplinary postoperative treatment for advanced HCC that is not indicated for curative hepatectomy.
Thirty cases of advanced HCC that were not indicated for curative hepatectomy, in which reduction hepatectomy was performed between 2000 and 2018 at the Department of Gastroenterological Surgery I, Hokkaido University Graduate School of Medicine, were divided into postoperative complete remission (POCR) (+) and POCR (-) groups, depending on whether POCR of all evaluable lesions was achieved through postoperative treatment. The cases in the POCR (-) group were subdivided into POCR (-) TKI (+) and POCR (-) TKI (-) groups, depending on whether TKIs were administered postoperatively.
The 5-year overall survival rate and mean survival time (MST) after reduction hepatectomy were 15.7% and 28.40 mo, respectively, for all cases; 37.5% and 56.55 mo, respectively, in the POCR (+) group; and 6.3% and 14.84 mo, respectively, in the POCR (-) group (P = 0.0041). Tumor size, major vascular invasion, and the number of tumors in the remnant liver after the reduction hepatectomy were also found to be related to survival outcomes. The number of tumors in the remnant liver was the only factor that differed significantly between the POCR (+) and POCR (-) groups, and POCR was achieved significantly more frequently when ≤ 3 tumors remained in the remnant liver (P = 0.0025). The MST was 33.52 mo in the POCR (-) TKI (+) group, which was superior to the MST of 10.74 mo seen in the POCR (-) TKI (-) group (P = 0.0473).
Reduction hepatectomy combined with multidisciplinary postoperative treatment for unresectable advanced HCC that was not indicated for curative hepatectomy was effective when POCR was achieved via multidisciplinary postoperative therapy. To achieve POCR, reduction hepatectomy should aim to ensure that ≤ 3 tumors remain in the remnant liver. Even in cases in which POCR is not achieved, combined treatment with reduction hepatectomy and multidisciplinary therapy can improve survival outcomes when TKIs are administered.
Core Tip: This was a retrospective study examining the outcomes of combination treatment with reduction hepatectomy and multidisciplinary postoperative treatment for advanced hepatocellular carcinoma (HCC). When reduction hepatectomy is performed for unresectable advanced HCC that is not indicated for curative hepatectomy, achieving postoperative complete remission (POCR) via postoperative multidisciplinary therapy is the key to success, with the 5-year overall survival rate and mean survival time for the POCR (+) group being 37.5% and 56.55 mo, respectively. To achieve POCR, reduction hepatectomy should be performed with the aim of reducing the number of tumors in the remnant liver to ≤ 3. Even in cases in which POCR is not achieved, tyrosine kinase inhibitor treatment might improve the prognosis of advanced HCC after reduction hepatectomy.
- Citation: Asahi Y, Kamiyama T, Kakisaka T, Orimo T, Shimada S, Nagatsu A, Aiyama T, Sakamoto Y, Kamachi H, Taketomi A. Outcomes of reduction hepatectomy combined with postoperative multidisciplinary therapy for advanced hepatocellular carcinoma. World J Gastrointest Surg 2021; 13(10): 1245-1257
- URL: https://www.wjgnet.com/1948-9366/full/v13/i10/1245.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i10.1245
Hepatocellular carcinoma (HCC) is the most common primary malignant hepatic tumor, accounting for 85%-90% of primary malignant hepatic tumors[1]. Advanced HCC is defined as progressive malignant HCC, which is hard to treat in a single hepatectomy procedure[2,3]. This is one reason for the poor prognosis of advanced HCC because hepatectomy is an important curative option. In fact, it is more effective at achieving local control of advanced HCC than any other treatment[4]. Local ablation therapy (LAT), including radiofrequency ablation (RFA) and microwave coagulation therapy (MCT), can also result in long survival periods; however, LAT is designed to treat less advanced HCC than hepatectomy[5]. Other treatment options include transarterial infusion (TAI) therapies, such as transarterial chemoembolization (TACE) and intraarterial chemotherapy (IAC), and the systemic administration of tyrosine kinase inhibitors (TKIs); however, the outcomes of these treatments are unsatisfactory. For instance, the survival period after TKI treatment ranges from 10 to 11 mo for advanced HCC[6,7].
Postoperative multidisciplinary therapy for HCC can include additional surgery, LAT (RFA or MCT), TAI (TACE or IAC), and TKI treatment. A retrospective study reported mean survival times (MSTs) of 31.8 and 18.6 mo for Barcelona Clinic Liver Cancer (BCLC) stage B and C HCC after reduction hepatectomy followed by postoperative local therapy targeting the liver, such as additional hepatectomy, LAT, and TAI[8]. In another retrospective study, the prognosis of patients who exhibited remnant extrahepatic lesions after reduction hepatectomy was reported to be poor (3-year overall survival [OS] rate: 0%)[9]. However, the latter study only included 6 cases of HCC with extrahepatic lesions, and postoperative TKI treatment was not mentioned. Although there are various treatment options for HCC, there are few reports about reduction hepatectomy followed by multidisciplinary postoperative therapy for cases of advanced HCC that are not indicated for curative hepatectomy, and the utility of this treatment strategy should be evaluated.
In the present study, we evaluated the efficacy of combination treatment involving reduction hepatectomy followed by multidisciplinary therapy, including TKI treatment, for unresectable advanced HCC that was not indicated for curative hepatectomy.
Of 828 hepatectomies performed for HCC between 2000 and 2018 at our department, the clinical data for 30 patients who underwent reduction hepatectomy for BCLC stage B or C advanced HCC that was not indicated for curative hepatectomy were retrospectively analyzed. The preoperative investigations and hepatectomy were carried out according to the method described in our previous report[10]. Major vascular invasion, major portal vein invasion, and major hepatic vein invasion were found in 17, 15, and 2 cases, respectively. All 30 patients were preoperatively evaluated using 3-phase dynamic contrast-enhanced computed tomography (CT). The preoperative whole-liver volume and tumor volume, the estimated volume of the remnant liver, and the effective resection ratio of the liver were calculated preoperatively using a 3D workstation. Liver function was evaluated through blood tests, the indocyanine green retention rate at 15 min (ICGR15), and technetium-99m diethylenetriamine pentaacetic acid galactosyl human serum albumin (Tc-GSA) scintigraphy. HCC was considered to not be indicated for curative hepatectomy if resection of all of the evaluable lesions was not possible, or the predicted remnant liver volume after reduction hepatectomy was considered to be insufficient, according to the Hokkaido University algorithm for hepatic resection[10]. This algorithm indicates: (1) If the ICGR15 is < 15%, the effective resection ratio of the liver has to be < 60% for hemihepatectomy or extended hemihepatectomy to be performed; (2) If the ICGR15 ranges from 15% to 20%, sectionectomy can be performed; (3) If the ICGR15 ranges from 20% to 25%, segmentectomy can be performed; (4) If the ICGR15 ranges from 25% to 40%, a limited resection can be performed; and (5) If the ICGR15 is > 40%, hepatectomy is contraindicated. Reduction hepatectomy was performed for patients with unresectable advanced HCC that 1) were not indicated for curative hepatectomy, 2) were in a good general condition, and 3) were considered to be eligible for postoperative treatment, providing that it was considered that reduction hepatectomy of the main tumor would eliminate the most important poor prognostic factor (even if residual tumors remained in the liver), according to the Hokkaido University algorithm for hepatic resection. In all 30 cases, residual tumor (s) were present in the remnant liver after hepatectomy, and 3 patients had extrahepatic metastases (in the lungs in 2 cases and in the bone in 1 case). The pre- and postoperative treatments employed after the reduction hepatectomy, OS, prognostic factors for OS, and whether the postoperative treatments resulted in postoperative complete remission (POCR) of all evaluable lesions were also examined. POCR was considered to have been achieved when no evaluable lesions were detected during the imaging study performed to evaluate the effects of treatment.
This research was approved by the institutional review board of Hokkaido University Hospital (approval number: 019-0115), and all analyses of the clinical data were carried out according to the ethical guidelines of Hokkaido University.
During the first 1 to 2 mo after treatment, imaging studies were performed with contrast-enhanced CT or gadolinium-ethoxybenzyl-diethylenetriaminepentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI) to evaluate the effects of LAT or TAI treatment. All cases were divided into two groups, according to whether POCR was achieved at least once in the postoperative period. The cases in which POCR was achieved were included in the POCR (+) group, and those in which POCR was not achieved were included in the POCR (-) group.
Some clinical data were converted to categorical variables. Pearson’s chi-square test was used for the statistical analyses, except for variables with expected counts of ≤ 5, for which Fisher’s exact test was used instead. OS was calculated using the Kaplan-Meier method and compared between the groups using the Wilcoxon test in the univariate analyses. Two-sided p-values of < 0.05 were considered significant. All analyses were performed with the software JMP (JMP Pro, version 14; SAS Institute Inc., Cary, NC).
Table 1 summarizes the clinical data for the 30 cases. The in-hospital and 90-day mortality data were excluded from Table 1 because no deaths occurred in hospital or within 90 days. The subjects’ mean age was 62.8 ± 11.8 years old (44-89 years old). The mean serum levels of alpha-fetoprotein (AFP), protein induced by vitamin K absence/antagonist-II (PIVKA-II), total bilirubin (T-Bil), and albumin (Alb) were 10228.33 ± 7287.26 ng/mL (35-217390), 52534.8 ± 22566.31 mAU/mL (17-664680), 0.88 ± 0.08 mg/dL (0.4-1.9), and 3.71 ± 0.08 g/dL (2.9-4.6), respectively; the mean prothrombin time (PT) was 90.19% ± 2.75% (69.8-115.8); and the mean ICGR15 was 17.34% ± 1.96% (2.6-43.8). The mean size of the largest tumor was 10.13 ± 1.02 cm (2.0-24.0). Anatomical hepatectomy was conducted in 27 cases, and non-anatomical hepatectomy (partial resection of the liver) was carried out in the remaining 3 cases. The median surgical time was 340 min (188-911), and the median amount of intraoperative blood loss was 690 mL (0-35820). The median follow-up time was 17.41 mo (1.02-111.04).
| Clinical data | Surgical data | ||||
| Age (yr) | 62.8 ± 11.8 | Anatomical hepatectomy | - | 3 | |
| + | 27 | ||||
| Sex | Male | 28 | Operation time (min) | 340 | |
| Female | 2 | ||||
| HBV/HCV | - | 6 | Blood loss (mL) | 690 | |
| + | 24 | ||||
| Alb (g/dL) | 3.71 ± 0.08 | Number of tumors in the remnant liver | 1-3 | 12 | |
| ≥ 4 | 18 | ||||
| T-Bil (mg/dL) | 0.88 ± 0.08 | POCR | + | 8 | |
| - | 22 | ||||
| PT (%) | 90.19 ± 2.75 | ||||
| ICGR15 (%) | 17.34 ± 1.96 | ||||
| AFP (ng/mL) | 102288 ± 7287 | ||||
| PIVKA-II (AU/mL) | 52534 ± 22566 | ||||
| Child-pugh class | A | 27 | |||
| B | 3 | ||||
| Number of tumors | St | 0 | |||
| Mt | 30 | ||||
| Tumor size (cm) | 10.13 ± 1.02 | ||||
| Differentiation | Wel | 1 | |||
| Mod | 16 | ||||
| Por | 13 | ||||
| pN | - | 28 | |||
| + | 2 | ||||
| Macrovascular invasion | - | 5 | |||
| + | 25 | ||||
| BCLC stage | B | 12 | |||
| C | 18 | ||||
| Distant metastasis | - | 27 | |||
| + | 3 | ||||
Table 2 summarizes the peri-surgical treatments, including both the preoperative and postoperative treatments, employed in the 30 cases. Preoperative treatment was performed in 6 cases. Postoperative treatment was employed in 28 cases. In one case, postoperative treatment was not employed, as the patient’s general condition deteriorated due to a postoperative cerebral infarction. In another case, clinical information was lacking after a follow-up period of 1.01 mo because the postoperative treatment was not performed at our institution. POCR was and was not achieved during the postoperative period in 8 cases [26.7%; POCR (+) group] and 22 cases [73.3%; POCR (-) group], respectively. POCR was achieved in the following cases: 1 of 1 cases that were treated with a second hepatectomy, partial lung resection, TAI therapy, and chemotherapy after the reduction hepatectomy; 1 of 1 cases that were treated with a second hepatectomy, TAI therapy, chemotherapy, and external beam radiotherapy (ERT) for palliative purposes after the reduction hepatectomy; 1 of 1 cases that were treated with partial lung resection, LAT, TAI therapy, chemotherapy, and ERT after the reduction hepatectomy; 2 of 2 cases that were treated with LAT and TAI therapy with/or without ERT after the reduction hepatectomy; 1 of 6 cases that were treated with TAI therapy and chemotherapy with/or without ERT after the reduction hepatectomy; and 2 of 9 cases that were treated with TAI therapy with/or without ERT after the reduction hepatectomy. All 6 patients that received postoperative TKI treatment were included in the POCR (-) group.
| Preoperative treatment | ||
| No treatment | 24 | |
| TAI | 4 | |
| TAI + ERT | 1 | |
| TAI + TKI1 + ERT | 1 | |
| Postoperative treatment | Total | POCR (+) |
| TAI + hepatectomy + lung resection + chemo | 1 | 1 |
| Hepatectomy + chemo + TAI + ERT | 1 | 1 |
| Lung resection + LAT + TAI + chemo + ERT | 1 | 1 |
| Lung resection + LAT | 1 | 0 |
| Brain tumor resection + TAI + TKI2 + chemo + ERT | 1 | 0 |
| LAT + TAI + ERT | 2 | 2 |
| TAI + TKI3 + chemo ( + ERT) | 3 | 0 |
| TAI + TKI4 | 2 | 0 |
| TAI + chemo ( + ERT) | 6 | 1 |
| TAI ( + ERT) | 9 | 2 |
| ERT | 1 | 0 |
| No treatment | 1 | 0 |
| Unknown | 1 | 0 |
| Total | 30 | 8 |
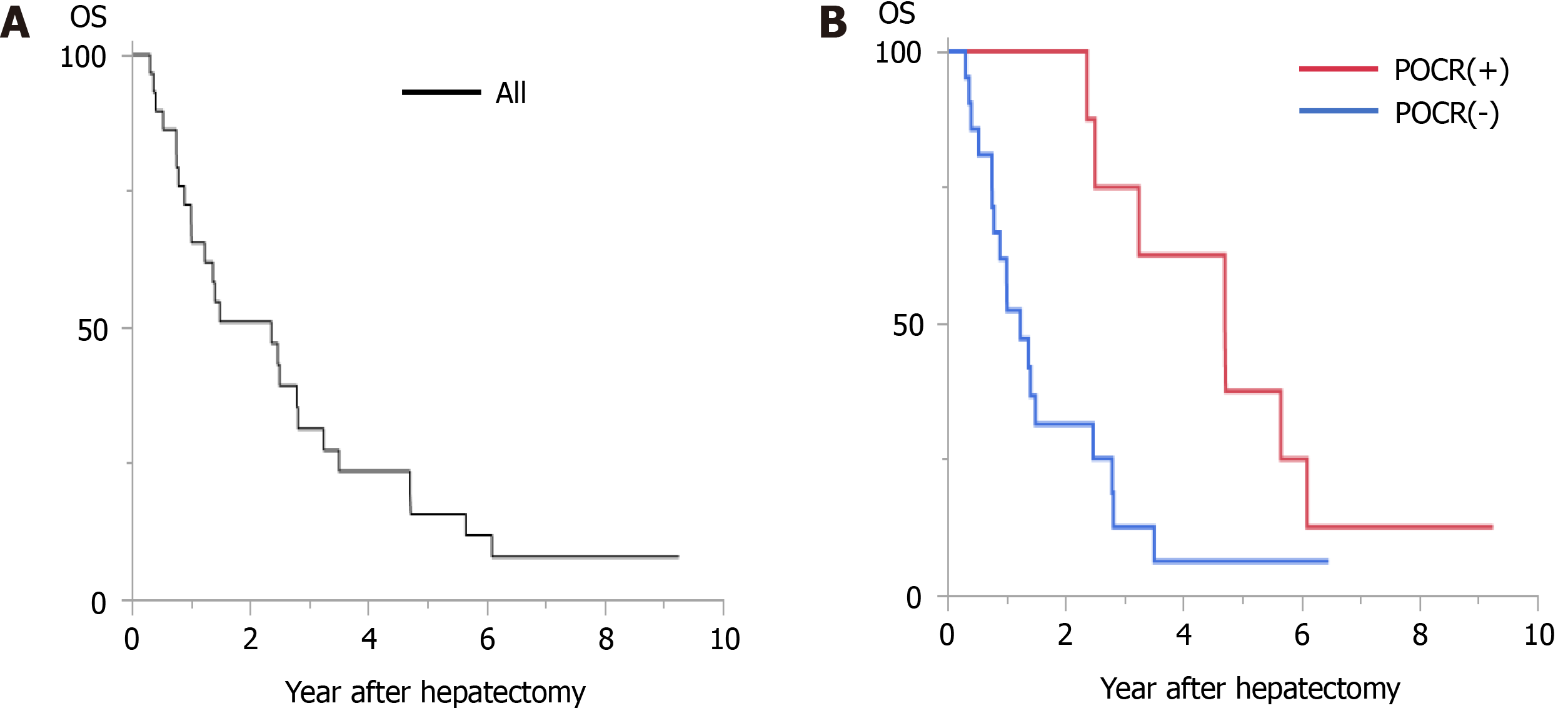
Among all 30 cases, the 1-year, 3-year, and 5-year OS rates after reduction hepatectomy were 72.4%, 31.3%, and 15.7%, respectively, and the MST after reduction hepatectomy was 28.40 mo. In the POCR (+) group, the 1-year, 3-year, and 5-year OS rates were 100%, 75.0%, and 37.5%, respectively, and the MST was 56.55 mo, whereas in the POCR (-) group the 1-year, 3-year, and 5-year OS rates were 61.9%, 12.6%, and 6.3%, respectively, and the MST was 14.84 mo (P = 0.0041, Figure 1). Univariate analyses revealed significant intergroup differences in the Child-Pugh class (P < 0.0001), tumor size (P = 0.0485), the frequency of major vascular invasion (P = 0.0053), the number of tumors in the remnant liver (P = 0.0283), and the frequency of POCR (P = 0.0041) (Table 3).
| Clinicopathological data | Surgical data | ||||||||
| Category | n | MST (m) | P value | Category | n | MST (m) | P value | ||
| Age (yr) | ≥ 60 | 18 | 17.9 | 0.9267 | Anatomical hepatectomy | - | 3 | 39.0 | 0.2162 |
| < 60 | 12 | 29.7 | + | 27 | 16.9 | ||||
| Sex | Male | 28 | 17.9 | 0.1584 | Operation time (min) | < 340 | 15 | 28.4 | 0.4177 |
| Female | 2 | - | ≥ 340 | 15 | 14.8 | ||||
| HBV/HCV | - | 6 | 15.0 | 0.2674 | Blood loss (mL) | < 690 | 15 | 29.7 | 0.6355 |
| + | 24 | 29.7 | ≥ 690 | 15 | 16.4 | ||||
| Alb (g/dL) | ≥ 3.7 | 15 | 33.5 | 0.3444 | Number of tumors in the remnant liver | 1-3 | 12 | 56.5 | 0.0283a |
| < 3.7 | 15 | 17.4 | ≥ 4 | 18 | 14.8 | ||||
| T-Bil (mg/dL) | ≤ 0.8 | 19 | 28.4 | 0.5131 | POCR | + | 8 | 56.6 | 0.0041a |
| > 0.8 | 11 | 16.5 | - | 22 | 14.8 | ||||
| PT (%) | ≥ 90 | 17 | 17.9 | 0.7839 | |||||
| < 90 | 13 | 28.4 | |||||||
| ICGR15 (%) | ≥ 15 | 12 | 29.7 | 0.6790 | |||||
| < 15 | 18 | 16.5 | |||||||
| AFP (ng/mL) | < 200 | 16 | 29.7 | 0.5569 | |||||
| ≥ 200 | 14 | 17.9 | |||||||
| PIVKA-II (AU/mL) | < 100 | 3 | 73.2 | 0.0584 | |||||
| ≥ 100 | 27 | 16.9 | |||||||
| Child-pugh class | A | 27 | 29.7 | < 0.0001 | |||||
| B | 3 | 6.4 | |||||||
| Tumor size (cm) | ≥ 10 cm | 15 | 12.2 | 0.0485 | |||||
| < 10 cm | 15 | 29.7 | |||||||
| Differentiation | wel/mod | 17 | 17.9 | 0.5449 | |||||
| por | 13 | 28.4 | |||||||
| pN | - | 28 | 17.9 | 0.2335 | |||||
| + | 2 | 56.5 | |||||||
| Macrovascular invasion | - | 5 | 9.5 | 0.0053 | |||||
| + | 25 | 30.0 | |||||||
| BCLC stage | B | 12 | 33.8 | 0.7652 | |||||
| C | 18 | 16.9 | |||||||
| Distant metastasis | - | 27 | 28.4 | 0.6013 | |||||
| + | 3 | 12.1 | |||||||
The clinical data for the POCR (+) and POCR (-) groups are shown in Table 4. Only the number of tumors in the remnant liver exhibited significant intergroup differences. The proportion of cases in which ≤ 3 tumors were seen in the remnant liver was higher in the POCR (+) group than in the POCR (-) group (P = 0.0025).
| Category | POCR (+) | POCR (-) | P value | |
| Age | < 60/≥ 60 | 1/7 | 11/11 | 0.0994 |
| Sex | Male/female | 7/1 | 21/1 | 0.4690 |
| HBV and/or HCV | -/ + | 0/8 | 6/16 | 0.1550 |
| Alb (g/dL) | < 3.7/≥ 3.7 | 5/3 | 10/12 | 0.6817 |
| T-Bil (mg/dL) | ≤ 0.8/> 0.8 | 6/2 | 13/9 | 0.6722 |
| PT (%) | < 90/≥ 90 | 4/4 | 9/13 | 0.6976 |
| ICGR15 (%) | < 15/≥ 15 | 4/4 | 14/8 | 0.6779 |
| AFP (ng/mL) | < 200/> 200 | 4/4 | 12/10 | 1.0000 |
| PIVKA-II | < 100/≥ 100 | 2/6 | 1/21 | 0.1655 |
| Child-pugh class | A/B | 8/0 | 19/3 | 0.5448 |
| Tumor size (cm) | < 10/≥ 10 | 5/3 | 10/12 | 0.6817 |
| Differentiation | wel or mod/por | 5/3 | 12/10 | 1.0000 |
| pN | -/ + | 7/1 | 21/1 | 0.4690 |
| Macrovascular invasion | -/ + | 0/8 | 5/17 | 0.2868 |
| Distant metastasis | -/ + | 8/0 | 19/3 | 0.5448 |
| BCLC stage | B/C | 3/5 | 9/13 | 1.0000 |
| Anatomical hepatectomy | -/ + | 2/6 | 1/21 | 0.1665 |
| Number of tumors in the remnant liver | 1-3/≥ 4 | 7/1 | 5/17 | 0.0025a |
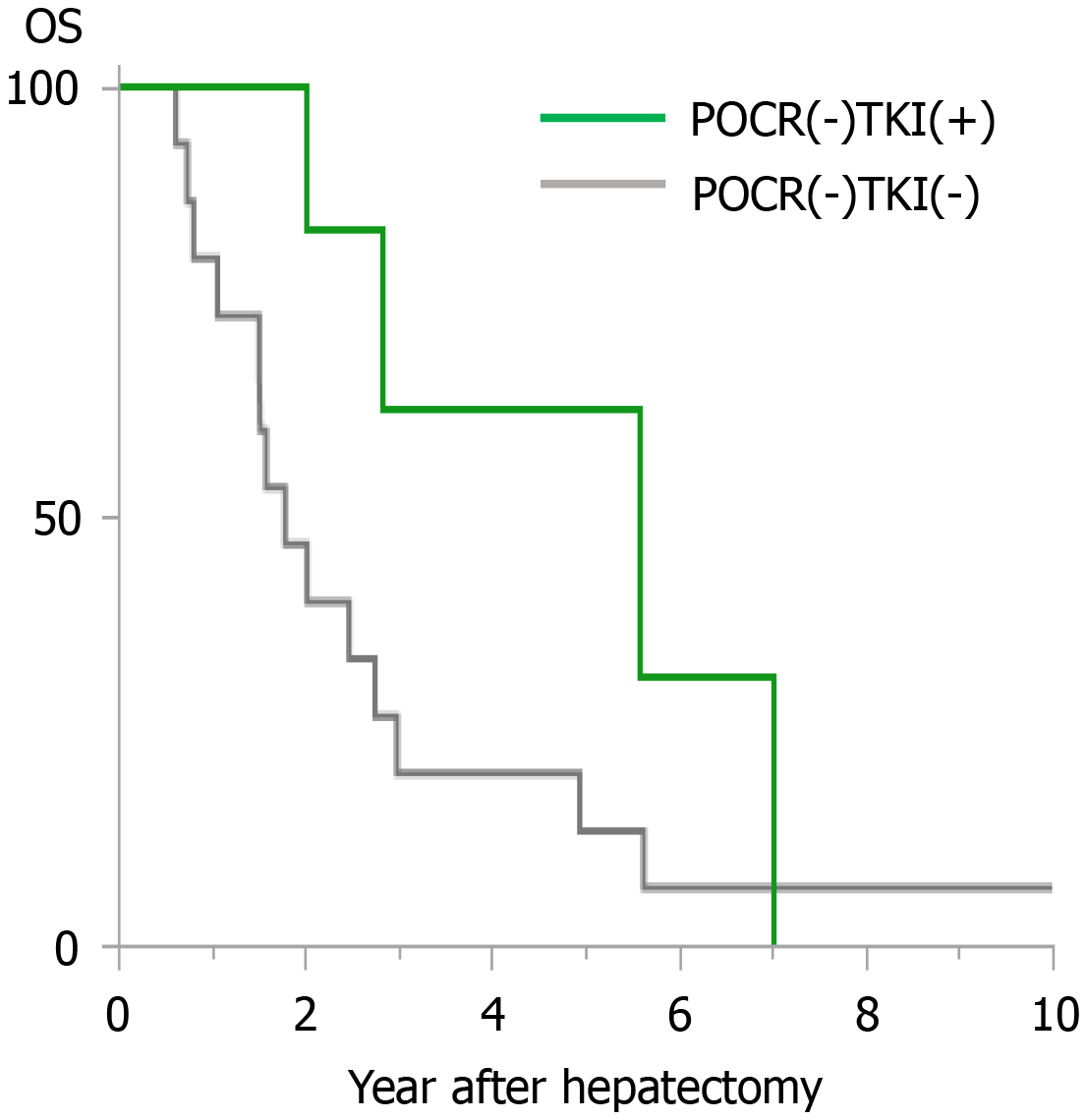
The cases in the POCR (-) group were subdivided into two groups according to whether postoperative TKI treatment was administered. Cases involving TKI treatment were included in the POCR (-) TKI (+) group, and those that did not involve TKI treatment were included in the POCR (-) TKI (-) group. In the POCR (-) TKI (+) group, the 1-year, 3-year, and 5-year OS rates after reduction hepatectomy were 100%, 31.3%, and 0%, respectively, and the MST was 33.52 mo, whereas in the POCR (-) TKI (-) group the 1-year, 3-year, and 5-year OS rates after reduction hepatectomy were 46.7%, 6.7%, and 6.7%, respectively, and the MST was 10.74 mo (P = 0.0473, Figure 2). There were no significant differences between the clinicopathological data of the POCR (-) TKI (+) and POCR (-) TKI (-) groups (Table 5).
| Category | POCR (-)/TKI (+) | POCR (-)/TKI (-) | P value | |
| Age | < 60/≥ 60 | 4/2 | 7/9 | 0.6351 |
| Sex | Male/female | 6/0 | 15/1 | 1.0000 |
| HBV and/or HCV | -/ + | 2/4 | 4/12 | 1.0000 |
| Alb (g/dL) | < 3.7/≥ 3.7 | 1/5 | 9/7 | 0.1619 |
| T-Bil (mg/dL) | ≤ 0.8/> 0.8 | 4/2 | 9/7 | 1.0000 |
| PT (%) | < 90/≥ 90 | 2/4 | 7/9 | 1.0000 |
| ICGR15 (%) | < 15/≥ 15 | 4/2 | 10/6 | 1.0000 |
| AFP (ng/mL) | < 200/> 200 | 4/2 | 8/8 | 0.6462 |
| PIVKA-II | < 100/≥ 100 | 0/6 | 1/15 | 1.0000 |
| Child-pugh class | A/B | 6/0 | 13/3 | 0.5325 |
| Tumor size (cm) | < 10/≥ 10 | 4/2 | 6/10 | 0.3476 |
| Differentiation | Wel or mod/por | 4/2 | 8/8 | 0.6462 |
| pN | -/ + | 5/1 | 16/0 | 0.2727 |
| Macrovascular invasion | -/ + | 0/6 | 5/11 | 0.2663 |
| Distant metastasis | -/ + | 5/1 | 14/2 | 1.0000 |
| BCLC stage | B/C | 2/4 | 7/9 | 1.0000 |
| Anatomical hepatectomy | -/ + | 0/6 | 1/15 | 1.0000 |
| Number of tumors in the remnant liver | 1-3/≥ 4 | 0/6 | 5/11 | 0.2663 |
In the present study, the survival rate of the POCR (+) group was better than that of the POCR (-) group (P = 0.0041), suggesting that achieving POCR after reduction hepatectomy could have an important impact on survival in patients with advanced HCC that is not indicated for curative hepatectomy. Moreover, even in the cases in which POCR was not achieved the administration of TKIs resulted in an improvement in survival outcomes; i.e., the survival rate of the POCR (-) TKI (+) group was better than that of the POCR (-) TKI (-) group (P = 0.0473). Thus, reduction hepatectomy could be effective against advanced HCC that is not indicated for curative hepatectomy, especially when POCR is achieved via postoperative multidisciplinary therapy. Even in cases in which POCR is not achieved, the administration of TKIs should be considered in the postoperative period.
In ovarian carcinoma, the maximal resection of any primary or metastatic carcinoma followed by postoperative chemotherapy has become the standard treatment strategy[11]. However, there are only a limited number of reports about reduction hepate
The potential prognostic factors identified in the univariate analyses in the current study were the Child-Pugh class, tumor size, major vascular invasion, the number of tumors in the remnant liver, and whether POCR was achieved. The Child-Pugh class was the only independent prognostic factor that exhibited significance in the multivariate analysis (data not shown). However, we decided to focus on POCR, as it can be set as an aim of multidisciplinary therapy after reduction hepatectomy.
Achieving POCR using postoperative multidisciplinary treatment had an important impact on survival in the current cases. When the cases were limited to those in which POCR was achieved, the 1-year, 3-year, and 5-year OS rates after reduction hepatectomy were 100%, 75.0%, and 37.5%, respectively, and the MST was 56.55 mo. This suggests that reduction hepatectomy followed by postoperative treatment that aims to achieve POCR could be an effective treatment strategy for advanced HCC that is not indicated for curative hepatectomy.
The postoperative treatments employed after reduction surgery for HCC are different from those used to treat other malignancies. Firstly, the recovery of the remnant liver after hepatectomy enables further treatment for tumors in the remnant liver, which is considered to affect prognosis in most cases of HCC[15]. In fact, tumors were detected in the remnant liver after reduction hepatectomy in all of the present cases, but extrahepatic metastases were only detected in 3 cases. Secondly, there are established additional non-surgical treatments for HCC localized in the liver, such as LAT and TAI therapy. RFA is indicated for cases of HCC involving ≤ 3 tumors and a maximum tumor size of ≤ 3 cm and is sometimes employed as an alternative to hepatectomy[5]. TACE is indicated for cases of unresectable HCC involving large or multifocal tumors without major vascular invasion or extrahepatic metastases[5]. R0 resection is the first-choice treatment for some advanced malignancies, even in cases involving distant metastasis. For distant metastases from HCC, there are not enough data supporting the validity of this approach, and the efficacy of surgical resection for lung metastases[16,17], adrenal gland metastases[18], and brain metastases[19] is disputed.
In the present study, the number of tumors in the remnant liver after reduction hepatectomy was the only factor that differed significantly between the POCR (+) and POCR (-) groups. This indicates that it is important that reduction hepatectomy is performed with the aim of reducing the number of tumors in the remnant liver to ≤ 3, which agrees with the conclusion of the study by Hai et al[9]. According to the present study, POCR might not need to be achieved via surgery alone, and even patients in whom POCR is achieved using LAT or TAI therapy can be good candidates for reduction surgery. Furthermore, the findings of the current study suggest that some patients would benefit from reduction hepatectomy even if POCR is not achieved. Patients that are likely to benefit from TKI treatment can also be good candidates for reduction hepatectomy.
In the current study, there were only 6 cases in which TKIs were orally administered. Two reasons are considered as possible explanations for the low frequency of TKI treatment. The first is the small number of cases included in the present study; i.e., only 30. Second, the treatment options for HCC changed during the study period. The most important change was the introduction of TKIs as a treatment option. Sorafenib, a TKI, was reported to improve the prognosis of HCC in 2008[20], and it started to be used in the clinical setting in Japan in 2009. Moreover, lenvatinib, another TKI, was reported to be non-inferior to sorafenib in the REFLECT trial in 2018[6]. In the present study, TKIs were only administered in the cases in which POCR was not achieved. This might have been due to the fact that TKIs were mainly administered when surgery, LAT, and TAI therapy were not indicated; i.e., TKIs were used when the abovementioned treatments were not expected to be effective. The MST of the POCR (-) TKI (+) group was 33.52 mo, which is superior to the outcomes described in other studies in which unresectable HCC was treated with TKIs alone[6,20].
Some cases that were successfully converted to downstaging hepatectomy after the preoperative administration of the TKIs sorafenib and lenvatinib have been reported[21-23]. Although these cases were successfully treated with conversion hepatectomy, the actual conversion rate due to the downstaging effects of TKIs remains unknown, and the response rate of HCC to TKI therapy (a complete response rate of 2% and a partial response rate of 38% can be achieved with lenvatinib[6]) is still insufficient to enable TKIs to be used for downstaging purposes as part of the standard treatment strategy for advanced HCC.
This study had several limitations. The first is the inevitable selection bias caused by the study’s retrospective and single-center design, although one of the most important processes in reduction hepatectomy for advanced HCC that is not indicated for curative hepatectomy is the selection of cases that would benefit from such treatment. Two of the inclusion criteria for reduction hepatectomy for advanced HCC employed in the present study were similar to criteria reported by Komatsu et al[8]. The first was that it must be considered that reduction hepatectomy of the main tumor would eliminate the most important poor prognostic factor, and the second was that the patient’s condition must be good enough to make them eligible for postoperative treatment. In the study by Komatsu et al[8], postoperative local treatment produced an obvious survival benefit. Surgical safety also has an important impact on whether postoperative multidisciplinary treatment can be performed. In the present study, postoperative treatment could not be performed in one case due to the deterioration of the patient’s general condition because of a postsurgical complication. At the same time, the safety of surgery should be considered to be the most important factor from an ethical viewpoint. There were no surgery-related deaths in the present study. Other limitations of this study include the small number of cases and the short follow-up periods in some of the cases. Another limitation of the present study was the small number of cases it included; therefore, a study involving more cases from multiple institutions should be performed in the future.
When reduction hepatectomy is performed for unresectable advanced HCC that is not indicated for curative hepatectomy, achieving POCR via postoperative multidisciplinary therapy is the key to success, with the 5-year OS rate and MST for the POCR (+) group being 37.5% and 56.55 mo, respectively. To achieve POCR, reduction hepatectomy should be performed with the aim of reducing the number of tumors in the remnant liver to ≤ 3. Even in cases in which POCR is not achieved, TKI treatment might improve the prognosis of advanced HCC after reduction hepatectomy.
Reduction hepatectomy combined with multidisciplinary postoperative treatment should be considered as a treatment option for unresectable advanced hepatocellular carcinoma (HCC) that is not indicated for curative hepatectomy. A well designed and/or larger cohort study is required to further evaluate this treatment strategy.
Reduction hepatectomy combined with multidisciplinary postoperative treatment for unresectable advanced HCC that was not indicated for curative hepatectomy was effective when postoperative complete remission (POCR) was achieved through multidisciplinary postoperative therapy. To achieve POCR, reduction hepatectomy should aim to ensure that ≤ 3 tumors remain in the remnant liver. In cases in which POCR is not achieved, tyrosine kinase inhibitors (TKIs). can improve survival outcomes when administered as part of postoperative multidisciplinary therapy after reduction hepatectomy.
The 5-year overall survival rate and mean survival time (MST) for all cases after reduction hepatectomy were 15.7% and 28.40 mo, respectively. POCR, tumor size, major vascular invasion, and the number of tumors in the remnant liver after the reduction hepatectomy were found to be related to survival outcomes. In the POCR (+) and POCR (-) groups, the MST was 56.55 mo and 14.84 mo, respectively (P = 0.0041). POCR was achieved significantly more frequently when ≤ 3 tumors remained in the remnant liver (P = 0.0025). The MST was 33.52 mo in the POCR (-) TKI (+) group, which was superior to the MST of 10.74 mo seen in the POCR (-) TKI (-) group (P = 0.0473).
Thirty cases of advanced HCC, in which reduction hepatectomy was performed between 2000 and 2018 at the Department of Gastroenterological Surgery I, Hokkaido University Graduate School of Medicine, were retrospectively investigated. These 30 cases were divided into two groups, the POCR (+) and POCR (-) groups, according to whether postoperative complete remission (POCR) of the evaluable lesions was achieved through postoperative treatment. Further analyses were performed after dividing the POCR (-) cases into two groups, the POCR (-) TKI (+) and POCR (-) TKI (-) groups, depending on whether TKIs were administered postoperatively.
To investigate the outcomes of combination treatment with reduction hepatectomy and multidisciplinary postoperative treatment for advanced HCC that is not indicated for curative hepatectomy.
To date, few studies have evaluated combination treatment with reduction hepatectomy and multidisciplinary postoperative treatment for advanced HCC that is not indicated for curative hepatectomy.
The prognosis of advanced HCC that is not indicated for curative hepatectomy remains poor, despite advances in the treatment of HCC including the development of tyrosine kinase inhibitors.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng HW, Wang YF S-Editor: Wang LL L-Editor: A P-Editor: Li JH
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4265] [Article Influence: 236.9] [Reference Citation Analysis (2)] |
| 2. | Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 396] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 3. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 561] [Reference Citation Analysis (1)] |
| 4. | Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 503] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 5. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1644] [Article Influence: 205.5] [Reference Citation Analysis (0)] |
| 6. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 561] [Reference Citation Analysis (1)] |
| 7. | von Felden J. New systemic agents for hepatocellular carcinoma: an update 2020. Curr Opin Gastroenterol. 2020;36:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Komatsu S, Kido M, Tanaka M, Kuramitsu K, Tsugawa D, Awazu M, Gon H, Toyama H, Ueno K, Fukumoto T. Clinical Relevance of Reductive Hepatectomy for Barcelona Clinic Liver Cancer Stages B and C Advanced Hepatocellular Carcinoma: A Single-Center Experience of 102 Patients. World J Surg. 2019;43:2571-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Hai S, Hatano E, Okada T, Uyama N, Suzumura K, Fujimoto J. Is Noncurative Hepatic Resection Justified for Advanced Hepatocellular Carcinoma? Am Surg. 2018;84:1938-1944. [PubMed] |
| 10. | Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, Taniguchi M, Shimamura T, Matsushita M, Todo S. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Makar AP, Tropé CG, Tummers P, Denys H, Vandecasteele K. Advanced Ovarian Cancer: Primary or Interval Debulking? Oncologist. 2016;21:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Yamamoto M, Iizuka H, Matsuda M, Nagahori K, Miura K, Itakura J. The indications for tumor mass reduction surgery and subsequent multidisciplinary treatments in stage IV hepatocellular carcinoma. Surg Today. 1993;23:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Wakabayashi H, Ushiyama T, Ishimura K, Izuishi K, Karasawa Y, Masaki T, Watanabe S, Kuriyama S, Maeta H. Significance of reduction surgery in multidisciplinary treatment of advanced hepatocellular carcinoma with multiple intrahepatic lesions. J Surg Oncol. 2003;82:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Inoue K, Nakamura T, Kinoshita T, Konishi M, Nakagohri T, Oda T, Takahashi S, Gotohda N, Hayashi T, Nawano S. Volume reduction surgery for advanced hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:362-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, Goto T, Omata M, Yoshida H, Koike K. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475-4483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 327] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 16. | Nakagawa T, Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Matsushita M, Todo S. Pulmonary resection for metastases from hepatocellular carcinoma: factors influencing prognosis. J Thorac Cardiovasc Surg. 2006;131:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Wang C, Yang L, Liang Z, Liu Y, Liu S. Long-Term Survival and Prognostic Factors of Pulmonary Metastasectomy in Liver Cancer: A Systematic Review and Meta-Analysis. World J Surg. 2018;42:2153-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Ha TY, Hwang S, Ahn CS, Kim KH, Lee YJ, Moon DB, Song GW, Jung DH, Park GC, Lee SG. Resection of metachronous adrenal metastasis after liver resection and transplantation for hepatocellular carcinoma. Dig Surg. 2014;31:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Park ES, Kwon DH, Park JB, Lee DH, Cho YH, Kim JH, Kim CJ. Gamma Knife surgery for treating brain metastases arising from hepatocellular carcinomas. J Neurosurg. 2014;121 Suppl:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10268] [Article Influence: 604.0] [Reference Citation Analysis (2)] |
| 21. | Kim TS, Kim JH, Kim BH, Lee YS, Yoo YJ, Kang SH, Suh SJ, Jung YK, Seo YS, Yim HJ, Yeon JE, Byun KS. Complete response of advanced hepatocellular carcinoma to sorafenib: another case and a comprehensive review. Clin Mol Hepatol. 2017;23:340-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Yokoo H, Takahashi H, Hagiwara M, Iwata H, Imai K, Saito Y, Matsuno N, Furukawa H. Successful hepatic resection for recurrent hepatocellular carcinoma after lenvatinib treatment: A case report. World J Hepatol. 2020;12:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Ohya Y, Hayashida S, Tsuji A, Kuramoto K, Shibata H, Setoyama H, Hayashi H, Kuriwaki K, Sasaki M, Iizaka M, Nakahara O, Inomata Y. Conversion hepatectomy for advanced hepatocellular carcinoma after right portal vein transection and lenvatinib therapy. Surg Case Rep. 2020;6:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |