Published online Oct 27, 2021. doi: 10.4240/wjgs.v13.i10.1235
Peer-review started: March 22, 2021
First decision: August 15, 2021
Revised: August 23, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: October 27, 2021
Processing time: 218 Days and 7.5 Hours
Neoadjuvant chemotherapy (NACT) and oesophagectomy is the standard of care for resectable oesophageal adenocarcinomas. Survival outcomes following resection have been improving over time while NACT remain largely unchanged. Indeed, a recent meta-analysis of randomized control trials did not demonstrate a survival benefit in adding NACT, raising the possibility that improved surgical techniques may be reducing the perceived effectiveness of NACT.
To compare the effect of addition of NACT to a standardized surgery and lymphadenectomy on overall and disease-free survival in patients undergoing curative oesophagectomy for oesophageal adenocarcinoma.
Patient data were analysed from a prospectively maintained surgical survival database. Demographic, surgical, and survival outcomes were compared between groups according to treatment and nodal count.
The data of 243 consecutive patients were identified. 79 patients were given NACT and 162 had surgery only. The NACT group were younger, and there was less frequent stage I adenocarcinoma. Overall survival was similar between NACT and surgery only groups (5YS: 48.7% vs 42.5%; P = 0.113), as was disease-free survival (5YS: 40.6% vs 39.9%; P = 0.635). There were ≥ 30 nodes removed in 46 patients, and < 30 in 197 patients, but were otherwise similar. There was improved survival in patients with ≥ 30 nodes removed than those with < 30 nodes (5YS: 64.4% vs 40.7%; P = 0.015), and a better disease-free survival that neared significance (5YS: 54.9% vs 36.6%; P = 0.078).
NACT did not appear to affect overall or disease-free survival. However, an overall survival benefit was observed in patients with ≥ 30 lymph nodes removed, and a benefit in disease-free survival which was not significant.
Core Tip: This study aimed to compare the effect of neoadjuvant chemotherapy to a standardized surgery and lymphadenectomy on survival outcomes in curative oesophagectomy for cancer. Overall and disease-free survival were similar between neoadjuvant chemotherapy (NACT) and surgery only groups. There was improved survival in patients with ≥ 30 nodes harvested compared to those with < 30 nodes. The possibility that improved lymphadenectomy techniques, as opposed to NACT, improves survival outcomes in curative resection of oesophageal adenocarcinoma warrants further investigation.
- Citation: Park JS, Van der Wall H, Kennedy C, Falk GL. Oesophageal adenocarcinoma: In the era of extended lymphadenectomy, is the value of neoadjuvant therapy being attenuated? World J Gastrointest Surg 2021; 13(10): 1235-1244
- URL: https://www.wjgnet.com/1948-9366/full/v13/i10/1235.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i10.1235
Oesophagectomy and lymphadenectomy remain a mainstay in the curative treatment of oesophageal adenocarcinoma. Neoadjuvant chemotherapy (NACT) or combined neoadjuvant chemoradiotherapy (NACRT) preceding surgical resection is now the standard in multimodal therapy aimed at curing disease.
Multiple randomized control trials have found neoadjuvant regimens to increase long-term survival compared to surgery alone[1,2]. However, a meta-analysis of eleven randomized controlled trials did not demonstrate a survival benefit when comparing NACT plus surgery vs surgery alone[3]. There is uncertainty as to whether it is only neoadjuvant therapy that provides an improvement to overall survival, or other factors such as pre-operative staging, patient selection, or extent of resection and lymphadenectomy.
The value of extended lymphadenectomy after neoadjuvant therapy is uncertain. It has been reported that extended lymphadenectomy affects survival in various studies[4-6], but the extent to which it improves overall and disease-free survival after neoadjuvant therapy remains unclear. It has been argued that if there is no survival benefit in removing more lymph nodes, a less extensive lymphadenectomy may be more acceptable[7].
The present study aimed to assess the effect of NACT preceding surgery vs surgery alone, with standardized extensive mediastinal dissection, as well as the extent of lymph node removal, on overall- and disease free-survival in participants that underwent oesophageal resection with curative intent for adenocarcinoma in a consecutive cohort of patients.
Data for adenocarcinoma were extracted from a prospective oesophageal cancer database maintained by the senior author (GLF). Patients who underwent curative oesophagectomy for oesophageal cancer between January 1990 to October 2019 were identified and included in this study. Patients were excluded if they underwent procedures in addition to oesophagectomy at the time of operation. Extracted data included baseline demographics, tumour location, histopathology, stage, perioperative outcomes and survival.
Curative surgery was offered if the patient treatment risk was considered reasonable and primary and nodal disease encompassed within the field of resection with expected clear (R0) margins. Neoadjuvant treatment was administered increasingly as evidence supporting its usage evolved, in the form of MAGIC protocol[8] chemotherapy for oesophageal adenocarcinoma in the form of epirubicin, cisplatin, and either fluorouracil or capecitabine. Patient demographics, clinicopathological data, and survival outcomes were compared amongst the study population. Patients were grouped and compared according to receipt of NACT, as well as whether they had ≥ 30 nodes resected in the pathologic specimen.
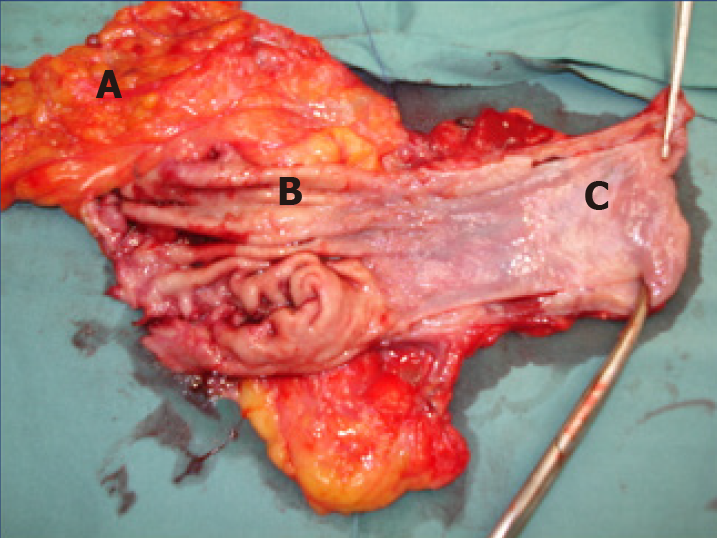
Surgery was performed 3-5 wk after completion of NACT. The standardized surgical management for mid-to-lower oesophageal and gastro-oesophageal junctional (GOJ) tumours was oesophageal resection performed with laparotomy with right thoracotomy in Ivor-Lewis fashion. Transthoracic, two-field lymphadenectomy was performed en bloc with the oesophageal resection. Figure 1 demonstrates an operative photograph of a representative oesophagectomy resection specimen, with en bloc lymphadenectomy of lesser sac lymph nodes. Oesophagectomy with the addition of a left cervical incision in McKeown fashion was infrequently utilized for adenocarcinomas in the middle third of the oesophagus.
Follow up was standardized, and was done through the senior surgeon (GF), or by proxy. Clinical history and examination was performed at three months for two years, then six months for the next three years, and then on an annual basis henceforth. Correspondence with the primary care doctor was performed when the patient was inaccessible or remote. Cross-sectional imaging was performed eighteen months post-operatively, or to assess the possibility of recurrences when clinically indicated. Pathologic staging was performed by specialist pathologists in accordance with the American Joint Committee on Cancer (AJCC, 8th edition)[9].
SPSS V24 (IBM Corp, NY) was utilized for statistical analysis. Data were expressed as medians and ranges. A post-hoc analysis of power was performed to ensure that the study number was sufficiently powered to provide statistical significance. Nominal and ordinal data were analyzed with the chi squared test. Non parametric continuous data were analyzed with the Mann-Whitney U test for dual variables, or Kruskal-Wallis test for multiple variables. Analyses of survival outcomes were assessed with the Kaplan-Meier method, and curves representing survival outcomes were assessed with the Breslow (Wilcoxon) test. Multivariate analyses were calculated with logistic regression modelling. A multivariable model tested various potential confounding variables: Age, sex, Barrett’s oesophagus, tumour location, AJCC stage, and histological tumour grade. A statistical analysis with P < 0.05 was considered significant.
Of 702 patients with oesophageal malignancy were managed by the senior author (GF) between June 1990 and October 2019. Curative oesophageal resection was performed in 395 of these patients. 39 patients had data unavailable due to loss to follow-up. 5 patients underwent operations in addition to oesophageal resection (for example, lung resection or colectomy), and were excluded from analysis. 243 patients had adenocarcinoma confirmed on histopathology of resected specimen, and formed the study cohort.
The cohort was analysed by whether they underwent oesophagectomy earlier in the series (1990-2004) or later in the series (2005-2019). 122 patients had surgery earlier in the series and 121 patients had surgery later in the series. These two groups were similar in terms of demographic features, sex, and age, and operative factors such as tumour location and number of lymph nodes harvested.
Surgery only was performed in 162 patients, and 79 patients had NACT preceding surgery. Two patients had incomplete data on NACT regimen. The NACT group was younger, with a median age of 62 years (range: 42-75) compared to a median age in the surgery only group of 69 years (range: 37-87; P < 0.001). Between the NACT and surgery only groups, there were similar distributions of males (P = 0.770) and Barrett’s oesophagus (P = 0.279) (Table 1).
| NACT (n = 79) | Surgery only (n = 162) | P value | |
| Age (median, range) | 62 (42-75) | 69 (37-87) | < 0.0011 |
| Sex | |||
| Male | 67 | 135 | 0.770 |
| Female | 12 | 27 | |
| Barrett’s oesophagus | 30 (38%) | 73 (45.1%) | 0.297 |
| Tumour location | |||
| Upper | 1 (1.3%) | 4 (2.5%) | 0.562 |
| Mid | 0 (0%) | 3 (1.9%) | 0.233 |
| Lower | 29 (38.7%) | 65 (40.6%) | 0.775 |
| GOJ | 45 (60%) | 88 (55%) | 0.471 |
| Tumour differentiation | |||
| Poor | 35 (46.1%) | 76 (48.7%) | 0.703 |
| Mod | 38 (50%) | 67 (42.9%) | 0.311 |
| Well | 3 (3.9%) | 13 (8.3%) | 0.216 |
| Stage1 | |||
| I | 12 (15.6%) | 43 (27.7%) | 0.0401 |
| II | 20 (26%) | 27 (17.4%) | 0.127 |
| III | 43 (55.8%) | 80 (51.6%) | 0.543 |
| IV | 1 (1.3%) | 5 (3.2%) | 0.384 |
| Nodes positive | 1 (0-20) | 1 (0-22) | 0.344 |
The NACT group had less patients that were stage I adenocarcinoma on pathologic assessment of resected surgical specimen compared with the surgery only group (15.6% vs 27.7%; P = 0.040). Tumour location was similar between NACT and surgery groups amongst upper, middle, and lower parts of the oesophagus as well as the GOJ. Tumour differentiation was also similarly distributed between the two groups. More lymph nodes were counted in the NACT group, a median of 24 (range: 3-61), compared to the surgery only group with a median of 18 (range: 0-45; P < 0.001). The proportion of patients who had ≥ 30 lymph nodes removed was also greater in the NACT compared to the surgery only group (32.9% vs 12.3%; P < 0.001). There was no difference in the number of nodes that were positive between the two groups (P = 0.344).
Median overall survival of the study cohort was 19.3 mo (range: 0.1-220.3). Overall survival at 1, 2, and 5 years was 75.7%, 58.2%, and 45%, respectively. 30-d mortality in the study cohort was 3.7%.
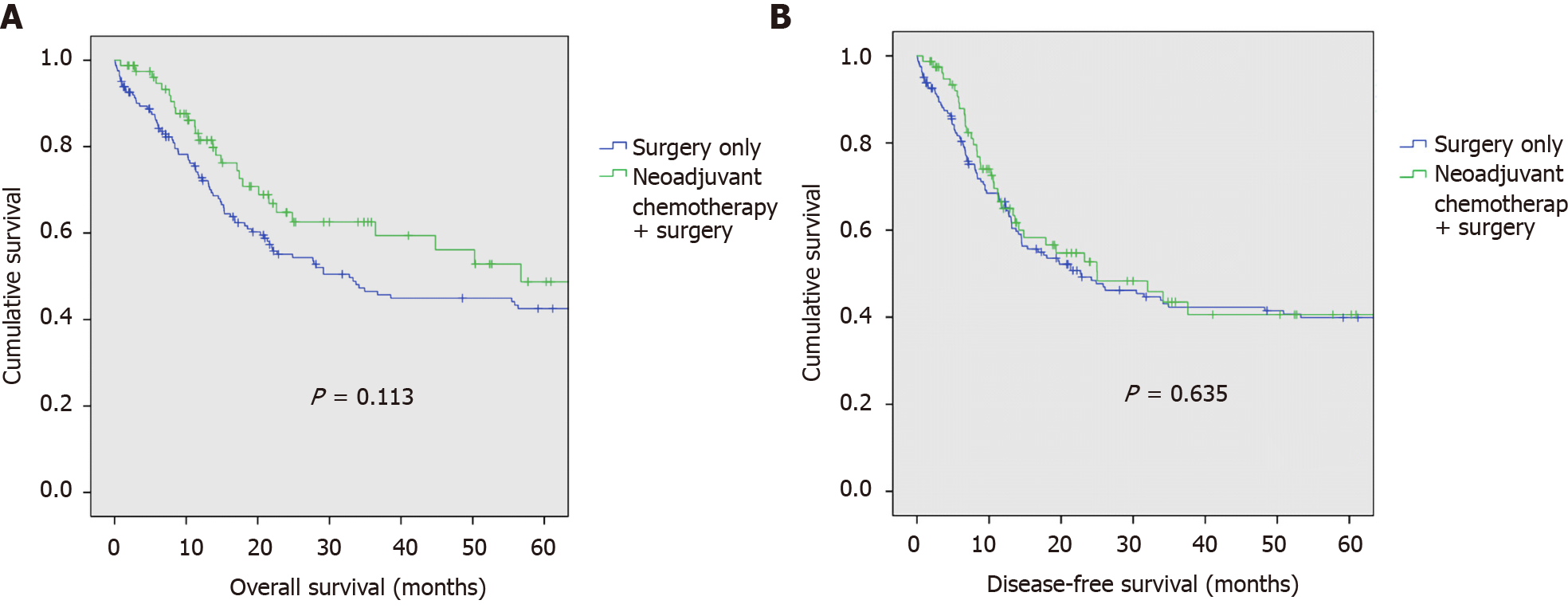
Median overall survival in the NACT group was 17.9 mo (range: 0.8-161.2), and in the surgery only group, 20.9 mo (range: 0.1-220.3). Kaplan-Meier analysis of overall-survival between NACT vs surgery-only groups is demonstrated in Figure 2A. There was no difference in overall survival between NACT and surgery-only populations. Overall survival at 1-, 2-, and 5-years in the NACT population was 81.5%, 64.8%, and 48.7%, respectively. Overall survival at 1-, 2-, and 5-years in the surgery-only population was 72.8%, 55.1%, and 42.5%, respectively (P = 0.113).
Median disease-free survival of the study cohort was 14.5 mo (range: 0.1-220.3). Disease-free survival at 1, 2, and 5 years was 66.3%, 50.1%, and 40%, respectively.
Median disease-free survival in the NACT group was 13.3 mo (range: 0.8-161.2), and in the surgery only group, 16.7 mo (range: 0.1-220.3). Kaplan-Meier analysis of disease-free-survival between NACT vs surgery-only groups is demonstrated in Figure 2B. There was no difference in disease-free survival between NACT and surgery-only populations. Disease-free survival at 1-, 2-, and 5-years in the NACT population was 64.9%, 52.7%, and 40.6%, respectively. Disease-free survival at 1-, 2-, and 5-years in the surgery-only population was 66.5%, 49.2%, and 39.9%, respectively (P = 0.635).
The study cohort was then separated into two groups by the number of nodes removed. There were 46 patients with ≥ 30 nodes removed, and 197 patients had < 30 nodes removed. Their demographic and clinicopathologic data are summarized in Table 2. The two groups were otherwise similar in terms of age, sex, presence of Barrett’s, NACT, tumour location, tumour grade, and pathologic AJCC 8th Edition staging.
| ≥ 30 nodes (n = 46) | < 30 nodes (n = 197) | P value | |
| Age (median, range) | 66.5 (45-87) | 66 (37-84) | 0.970 |
| Sex | |||
| Male | 40 (87%) | 163 (82.7%) | 0.488 |
| Female | 5 (13%) | 34 (17.3%) | |
| Barrett’s oesophagus | 20 (43.5%) | 85 (43.1%) | 0.967 |
| Tumour location | |||
| Upper | 1 (2.2%) | 4 (2.1%) | 0.953 |
| Mid | 0 (0%) | 3 (1.6%) | 0.399 |
| Lower | 14 (31.1%) | 80 (41.7%) | 0.193 |
| GOJ | 30 (66.7%) | 105 (54.7%) | 0.144 |
| Tumour differentiation | |||
| Poor | 19 (41.3%) | 93 (49.7%) | 0.305 |
| Mod | 23 (50%) | 82 (43.9%) | 0.453 |
| Well | 4 (8.7%) | 12 (6.4%) | 0.584 |
| Stage1 | |||
| I | 11 (23.9%) | 44 (23.5%) | 0.956 |
| II | 11 (23.9%) | 36 (19.3%) | 0.480 |
| III | 23 (50%) | 101 (54%) | 0.625 |
| IV | 1 (2.2%) | 5 (2.7%) | 0.848 |
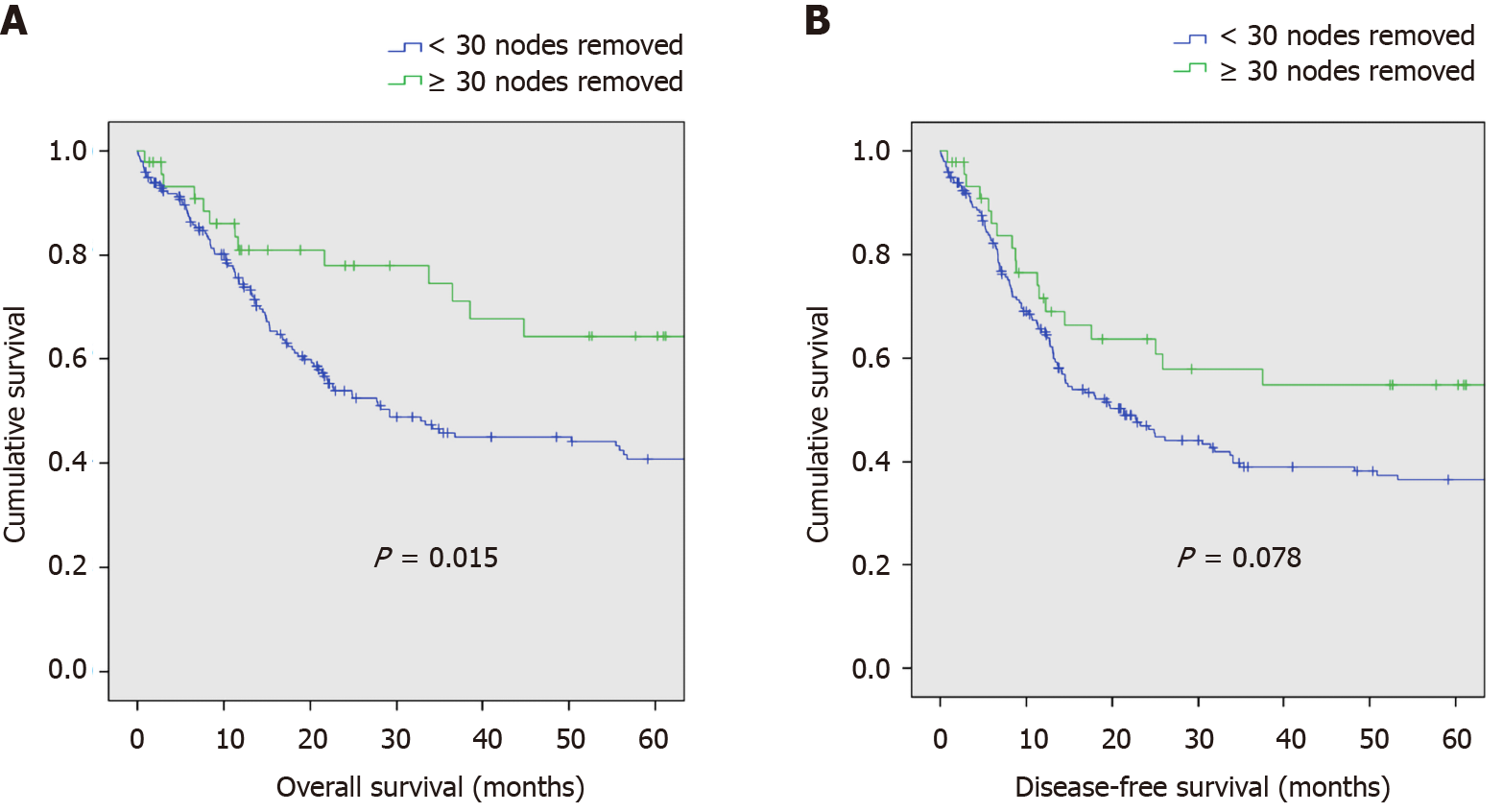
Median overall survival in patients who had ≥ 30 nodes removed was 31.4 mo (range: 0.8-176.5), and in patients who had < 30 nodes removed, 18 mo (0.1-220.3). The Kaplan-Meier survival curve for overall survival between patients with ≥ 30 nodes and < 30 nodes removed is shown in Figure 3A. Patients with ≥ 30 nodes had improved overall survival compared to those with < 30 nodes removed. Overall survival at 1, 2, and 5 years in the group of patients who had ≥ 30 nodes removed was 81%, 78%, and 64.4%, respectively. Overall survival at 1-, 2-, and 5-years in the population who had < 30 nodes removed was 74.5%, 53.9%, and 40.7%, respectively (P = 0.015).
Median disease-free survival in patients who had ≥ 30 nodes removed was 21.4 mo (range: 0.8-176.5), and in patients who had < 30 nodes removed, 13.7 mo (range: 0.1-220.3). The Kaplan-Meier survival curve for disease-free survival between patients with ≥ 30 nodes and < 30 nodes removed is shown in Figure 3B. There was no difference in disease-free survival between patients who had ≥ 30 nodes removed vs those that had less than 30 nodes removed. Disease-free survival at 1-, 2-, and 5-years in those who had ≥ 30 nodes removed was 71.6%, 63.7%, and 54.9%, respectively. In those with less than 30 nodes removed, disease-free survival was 65.1%, 47%, and 36.6%, respectively (P = 0.078).
By multivariate analysis, independent predictors for greater overall survival were AJCC stage (P < 0.001), histologic grade (P < 0.001), and more than 30 nodes removed (P = 0.016). Male sex (P = 0.642), age older than 75 years (P = 0.369), tumour location (P = 0.057), and Barrett’s oesophagus (P = 0.421) did not predict overall survival on multivariate analysis.
In this cohort of 243 patients with oesophageal adenocarcinoma treated with curative oesophagectomy and two-field lymphadenectomy, NACT plus surgery did not appear to affect overall (P = 0.113) or disease-free survival (P = 0.206). Instead, an overall survival benefit was observed in patients who had ≥ 30 lymph nodes removed (P = 0.015), and a benefit in disease-free survival neared significance (P = 0.078).
The apparent failure of NACT to improve overall and disease-free survival was surprising, but not without precedent. The Medical Research Council OEO2 trial, which reported 5-year survival of 23% in the NACT and surgery group compared to 17.1% in their surgery only group (P = 0.03), was pivotal in gaining acceptance of NACT. However, 9.4% of overall patients had unresectable disease at surgery and 15.2% had macroscopically involved (R2) margins. The proportion of patients with involved margins or unresectable tumours was considerably higher in the surgery only group, and may have biased results (26.4% vs 14.3%)[2] Resectability may also have been affected by NACT, so this may have been an instrumental difference in the OEO2 study. OEO2 contained a high number of squamous carcinoma which is likely to behave differently from adenocarcinoma, making direct comparison uncertain.
There is a trend in most published retrospective data showing that surgery has improved over time. Fontana et al[10] reported an improvement in survival outcomes following radical resection of oesophageal and gastric cancers over a decade, identifying a larger number of resected lymph nodes as a possible factor affecting survival[10]. Similarly, analysis of the SEER database has identified that survival of local oesophageal cancer has improved dramatically over the past 3 decades[11]. This has followed advances in the management of oesophageal adenocarcinoma such as improved staging with positron emission tomography, endoscopic ultrasound and later-generational thoracoabdominal computer tomography. Consequently, the survival data in the OEO2 trial is significantly worse than most current series.
Data from the Swedish population registry of oesophago-gastric resections published by Klevebro et al[12] did not demonstrate a survival benefit in patients with adenocarcinoma who underwent NACT and surgery compared with surgery alone[12]. Subgroup analysis of only fit patients without co-morbidities showed a strong trend towards improving survival with NACT and surgery, ultimately concluding that the benefit of NACT was reproducible only for fit and healthy patients. The North American intergroup study by Kelsen et al[13] randomized 440 patients to pre
Mariette et al[14] randomised 195 patients to NACRT plus surgery or surgery alone in treating locally advanced oesophageal cancer. When comparing NACRT plus surgery with surgery alone, they did not report a difference in overall survival (5 year survival 41.1% and 33.8%, respectively) or disease-free survival (5 year survival 35.6% and 27.7%, respectively) between the two groups. They also reported a significantly higher in-hospital post-operative mortality in the neoadjuvant arm (11.1% vs 3.4%; P = 0.049). The possibility is raised that higher quality surgery (complete microscopic (R0) resection rates, a high number of lymph nodes retrieved, and a low 30-d postoperative mortality rate) in the surgery-only group may have contributed to the apparent diminished effectiveness of neoadjuvant therapy[14]. The study however contained a large cohort of squamous cell carcinoma (SCC).
The present study demonstrates a survival benefit in patients who had ≥ 30 nodes removed, with a 5 year survival rate of 64.4% vs 40.7% in those with less nodes removed. Whether extended lymphadenectomy affects long-term survival following oesophagectomy remains controversial.
Several studies report that extended lymphadenectomy improves long-term survival. Kang et al[5] examined 233 patients who underwent oesophagectomy for oesophageal SCC without neoadjuvant therapy. In comparing three groups with varying degree of lymphadenectomy, they reported no difference in overall or disease-free survival[5]. Similarly, Koen Talsma et al[15] compared the effect of resected nodes on survival in patients with and without NACRT. They reported that the total number of resected nodes was significantly associated with survival for patients in the surgery-only arm when compared with NACRT only[15]. Kelty et al[16] showed improved survival according to the ratio of positive to negative nodes removed confirming the effectiveness of increasing nodal retrieval[16]. Multiple studies have examined the extent of lymphadenectomy needed for a survival benefit. The estimate for a minimum lymph node harvest to confer a survival benefit have ranges from 18 to 30[4,6].
Conversely, Lagergren et al[7] reported that the extent of lymphadenectomy did not influence survival following surgery for oesophageal cancer. They did not demonstrate a dose-response association between varying degrees of lymphadenectomy and 5-year overall survival. However, there were three surgeons conducting operations, with no consensus about the preferred extent of lymphadenectomy[7]. The issue of heterogenous operative technique is addressed by Phillips et al[17], who reported that the absolute number of lymph nodes removed did not improve survival in a cohort of patients who underwent transthoracic oesophagectomy and a standardized two-field lymphadenectomy[17].
Similarly, both NACT and NACRT have been shown to have variable efficacy when an extensive lymphadenectomy is performed[14]. Data from the CROSS trial confirms that the extent of lymphadenectomy has not been shown to make a difference after NACRT. It was noted by investigators that patients that did not receive NACRT had a significant survival benefit for every 10 lymph nodes harvested[15]. The authors speculated that micrometastases in patients not treated with NACRT may be controlled with lymphadenectomy. This would suggest a complimentary effect of lymphadenectomy and neoadjuvant therapy on involved lymph nodes. A meta-analysis of NACRT and surgery compared against surgery alone showed a smaller benefit than previously demonstrated in the CROSS study (8.7%)[18]. This may reflect improved surgical techniques, including lymphadenectomy, contributing to improved survival outcomes, reducing the effect of neoadjuvant therapies.
The main disadvantage of the present study is that it was simply a cohort study with prospective data storage and not randomized, and a long duration of data collection. An advantage is that adenocarcinoma only was examined and results pertain to this tumour type only. Additionally there was no difference between the first half of the series and the second half of the series in staging or for lymph node count, indicating a standardized operative technique throughout the cohort, and no variation in harvested lymph node count over time, meaning that the effect of NACT as an independent variable was more precisely observed.
NACT did not appear to affect overall or disease-free survival in our cohort. Instead, an overall survival benefit was observed in patients who had ≥ 30 lymph nodes removed, and a benefit in disease-free survival which neared significance. Such mixed data in multiple studies suggests the need for further randomised controlled trials of neoadjuvant therapy and surgery with lymphadenectomy compared with surgery with lymphadenectomy alone, in adenocarcinoma of the oesophagus and GOJ. Ideally, surgeons should aim to harvest more than 30 lymph nodes in the contemporary era.
A meta-analysis of eleven randomized controlled trials did not demonstrate a survival benefit when comparing neoadjuvant chemotherapy (NACT) plus surgery vs surgery alone. There is uncertainty as to whether it is only neoadjuvant therapy that provides an improvement to overall survival, or other factors such as pre-operative staging, patient selection, or extent of resection and lymphadenectomy.
Techniques in oesophagectomy are improving, but the regimen for neoadjuvant therapies has largely remained static.
The authors aimed to assess the effect of addition of NACT to a standardized surgery and lymphadenectomy on overall and disease-free survival in patients undergoing curative oesophagectomy for oesophageal adenocarcinoma.
Survival data in a prospectively maintained surgical database were interrogated to review demographic, surgical, and survival outcomes. These were compared between groups according to treatment and nodal count.
The authors found that overall and disease-free survival were similar between patients that had undergone NACT preceding surgery and surgery only groups. There was improved survival in patients with ≥ 30 nodes removed than those with < 30 nodes and a better disease-free survival that neared significance.
NACT did not appear to affect overall or disease-free survival in our cohort. Instead, an overall survival benefit was observed in patients who had ≥ 30 lymph nodes removed, and a benefit in disease-free survival which neared significance. Ideally, surgeons should aim to harvest more than 30 lymph nodes in the contemporary era.
Conflicting results and mixed data in multiple studies suggests the need for further randomised controlled trials of neoadjuvant therapy and surgery with lymphadenectomy compared with surgery with lymphadenectomy alone.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shiryajev YN S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1079] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 2. | Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062-5067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 751] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 3. | Urschel JD, Vasan H, Blewett CJ. A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2002;183:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Groth SS, Virnig BA, Whitson BA, DeFor TE, Li ZZ, Tuttle TM, Maddaus MA. Determination of the minimum number of lymph nodes to examine to maximize survival in patients with esophageal carcinoma: data from the Surveillance Epidemiology and End Results database. J Thorac Cardiovasc Surg. 2010;139:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Kang CH, Kim YT, Jeon SH, Sung SW, Kim JH. Lymphadenectomy extent is closely related to long-term survival in esophageal cancer. Eur J Cardiothorac Surg. 2007;31:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer. 2008;112:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Lagergren J, Mattsson F, Zylstra J, Chang F, Gossage J, Mason R, Lagergren P, Davies A. Extent of Lymphadenectomy and Prognosis After Esophageal Cancer Surgery. JAMA Surg. 2016;151:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 8. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy vs surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4564] [Article Influence: 240.2] [Reference Citation Analysis (0)] |
| 9. | Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 523] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 10. | Fontana E, Smyth EC, Cunningham D, Rao S, Watkins D, Allum WH, Thompson J, Waddell T, Peckitt C, Chau I, Starling N. Improved survival in resected oesophageal and gastric adenocarcinomas over a decade: the Royal Marsden experience 2001-2010. Gastric Cancer. 2016;19:1114-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Dubecz A, Gall I, Solymosi N, Schweigert M, Peters JH, Feith M, Stein HJ. Temporal trends in long-term survival and cure rates in esophageal cancer: a SEER database analysis. J Thorac Oncol. 2012;7:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Klevebro F, Lindblad M, Johansson J, Lundell L, Nilsson M. Outcome of neoadjuvant therapies for cancer of the oesophagus or gastro-oesophageal junction based on a national data registry. Br J Surg. 2016;103:1864-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, Estes N, Haller DG, Ajani J, Kocha W, Minsky BD, Roth JA. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 949] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 14. | Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, Boige V, Pezet D, Robb WB, Le Brun-Ly V, Bosset JF, Mabrut JY, Triboulet JP, Bedenne L, Seitz JF. Surgery alone vs chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32:2416-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 451] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 15. | Koen Talsma A, Shapiro J, Looman CW, van Hagen P, Steyerberg EW, van der Gaast A, van Berge Henegouwen MI, Wijnhoven BP, van Lanschot JJ; CROSS Study Group, Hulshof MC, van Laarhoven HW, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW. Lymph node retrieval during esophagectomy with and without neoadjuvant chemoradiotherapy: prognostic and therapeutic impact on survival. Ann Surg. 2014;260:786-92; discussion 792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Kelty CJ, Kennedy CW, Falk GL. Ratio of metastatic lymph nodes to total number of nodes resected is prognostic for survival in esophageal carcinoma. J Thorac Oncol. 2010;5:1467-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Phillips AW, Lagarde SM, Navidi M, Disep B, Griffin SM. Impact of Extent of Lymphadenectomy on Survival, Post Neoadjuvant Chemotherapy and Transthoracic Esophagectomy. Ann Surg. 2017;265:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V; Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1259] [Article Influence: 89.9] [Reference Citation Analysis (0)] |