Published online Oct 27, 2021. doi: 10.4240/wjgs.v13.i10.1166
Peer-review started: May 9, 2021
First decision: June 23, 2021
Revised: June 30, 2021
Accepted: August 9, 2021
Article in press: August 9, 2021
Published online: October 27, 2021
Processing time: 170 Days and 5.2 Hours
Duodenal gastrointestinal stromal tumors (D-GISTs) are uncommon mesen
Core Tip: Duodenal gastrointestinal stromal tumors are an uncommon subset of small intestinal tumors and may pose surgical challenges in curative-intent resection. I herein discuss the outcomes of current surgical resection techniques of duodenal gastro
- Citation: Lim KT. Current surgical management of duodenal gastrointestinal stromal tumors. World J Gastrointest Surg 2021; 13(10): 1166-1179
- URL: https://www.wjgnet.com/1948-9366/full/v13/i10/1166.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i10.1166
The World Health Organization histological classification of the small intestine tumors are categorized into epithelial, non-epithelial, malignant lymphomas, secondary tumors and polyps[1]. Under non-epithelial small intestine tumors, sarcomas account for about 14%, and a vast majority of duodenal mesenchymal tumors are gastro
GISTs are the commonest mesenchymal tumors of the gastrointestinal tract with the reported incidence at 10-15 per million per year[3,9-11]. The median age of diagnosis is in the mid-60s with 60% of cases age > 60 years[12]. GISTs have equal gender distribution in most studies. The anatomical location of GISTs is frequently in the stomach (60%-70%) and small bowel (25%-35% of which 4.5% is in duodenum) and are less commonly found in the colon and rectum (5%), esophagus (< 2%) and other/various locations (5.5%)[11,13-16].
The standard curative treatment for resectable primary GIST is complete surgical excision of the lesion with an adequate margin and no dissection of clinically negative lymph nodes[17]. An adequate margin can be defined as tumor-free margin or R0 resection. The invasion spread of these mesenchymal tumors behave differently to epithelial tumors, particularly the risk of lymphatic spread is rare. Hence lymphadenectomy is usually not warranted unless there is gross evidence of lymphadenopathy. Local recurrence of the tumor can occur in any residual positive microscopic R1 resection, and in almost 100% cases of tumor rupture and spillage[18]. Adjuvant therapy with tyrosine kinase inhibitors such as imatinib mesylate (IM) for 3 years is the standard treatment of patients with significant risk of recurrence according to the National Institutes of Health’s consensus criteria (Fletcher’s criteria based on size and mitotic count) and the Armed Forces Institute of Pathology criteria (Miettinen’s criteria based on size, mitotic count and tumor site) of risk prediction. For advanced or metastatic GISTs, the standard treatment is IM, whilst the decision for surgical resection should be individualized or considered for patients with limited disease progression while on IM[19-21].
Most patients diagnosed with duodenal GISTs (D-GISTs) present with gastrointestinal bleeding in the form of anemia or melena (42.7%) and abdominal pain (18.7%), whilst a minority present with abdominal mass (3.7%), abdominal discomfort (3.7%) and anorexia (2%). Incidental finding of D-GISTs reported on imaging studies in asymptomatic patients ranges from 5%-40%[11,12,22-24].
Due to their unique biological and molecular profile of GISTs, the possibilities of performing oncological adequate but limited resection in a variety of ways either by open, laparoscopic or endoscopic-assisted surgery were recognized[25]. Surgical resection of these D-GISTs may be difficult and challenging due to the complex anatomical proximity of surrounding organ structures such as the pancreas, hepatobiliary tree and mesenteric blood vessels. This surgical challenge is coupled by the low incidence of D-GISTs, the lack of surgical volume and the operative experience in most centers.
Accurate preoperative diagnosis and staging of D-GIST is therefore critical to guide the most appropriate treatment option and so to establish the prognosis. The tumor size and mitotic count of GISTs are good predictors of prognosis, whilst the surgical outcomes are related to the adequacy of surgical resection of D-GISTs.
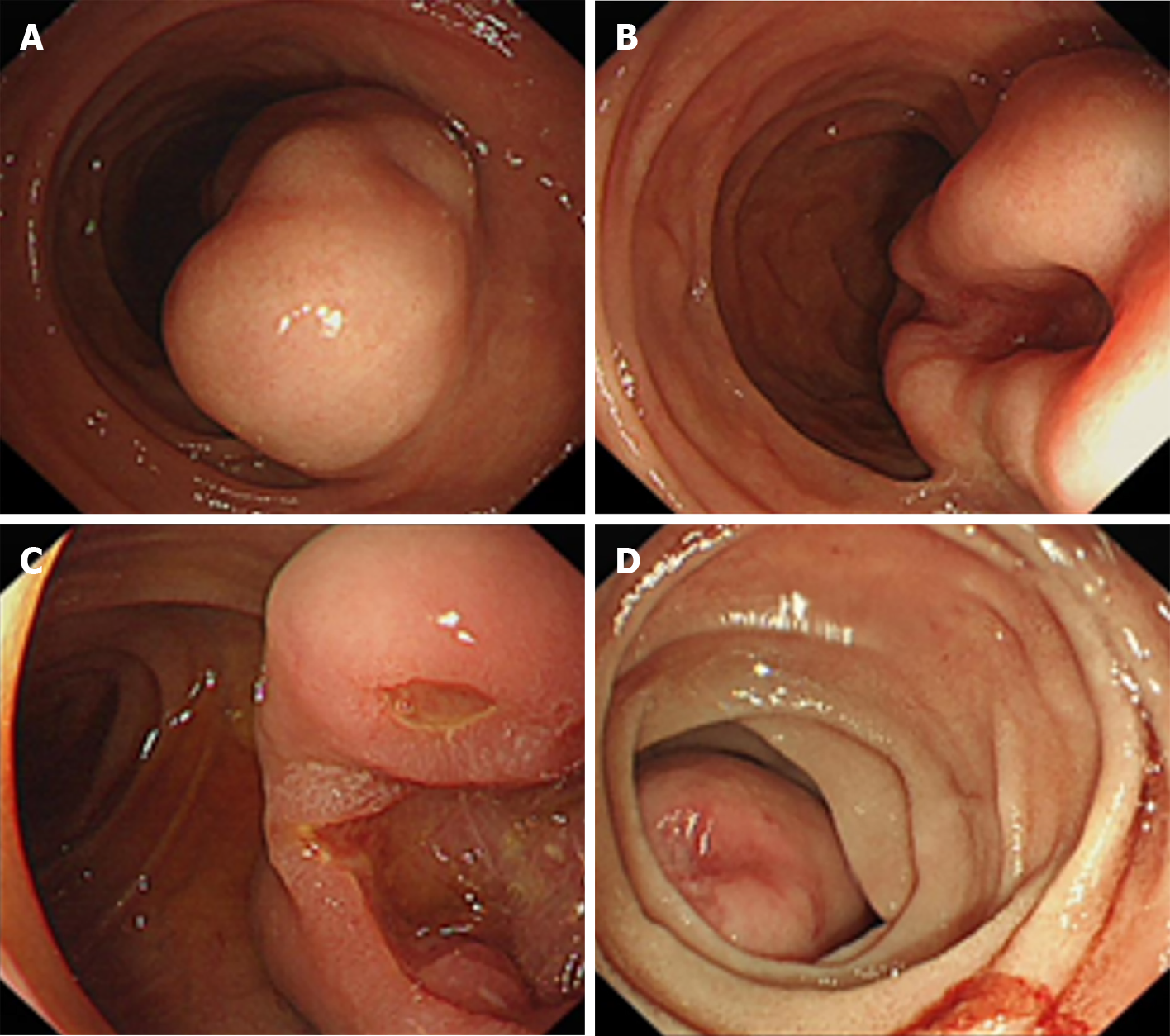
Esophagogastroduodenoscopy is routinely used to image-capture the features of submucosal tumors and to annotate any mucosal ulceration, intramural mass or bleeding (Figure 1). Standard endoscopic forceps biopsy has not provided reliable histological diagnosis due to submucosal location of GISTs and may even add additional risk of bleeding and perforation.
Endoscopic ultrasound scan (EUS) can add further endoscopic imaging evaluation of the hypoechoic mass, size, location, shape, layers of origin and vascularity of the D-GISTs. EUS-guided fine needle aspiration (FNA) or biopsy is useful for histological diagnosis prior to surgery planning, for neoadjuvant therapy or palliative-intent therapy for GISTs in general. However, the diagnostic cytology yield and sensitivity of EUS-guided FNA in a study of 37 patients to confirm D-GISTs was noted to be poor compared to gastric GISTs (0% vs 84.4%). This limitation was influenced by size, location, shape, and layer of origin[26].
Interestingly, a recent study of 142 patients diagnosed with D-GISTs showed that EUS has higher sensitivity and positive predictive value than computed tomography (CT) and magnetic resonance imaging (MRI) scans (P = 0.047 and P = 0.005, respectively). EUS-FNA also provided higher histological diagnosis of D-GISTs than conventional endoscopic biopsy (73% vs 33.3%, P = 0.006)[27]. These findings may be explained by the overall improvement of diagnostic equipment and operator experience over the years.
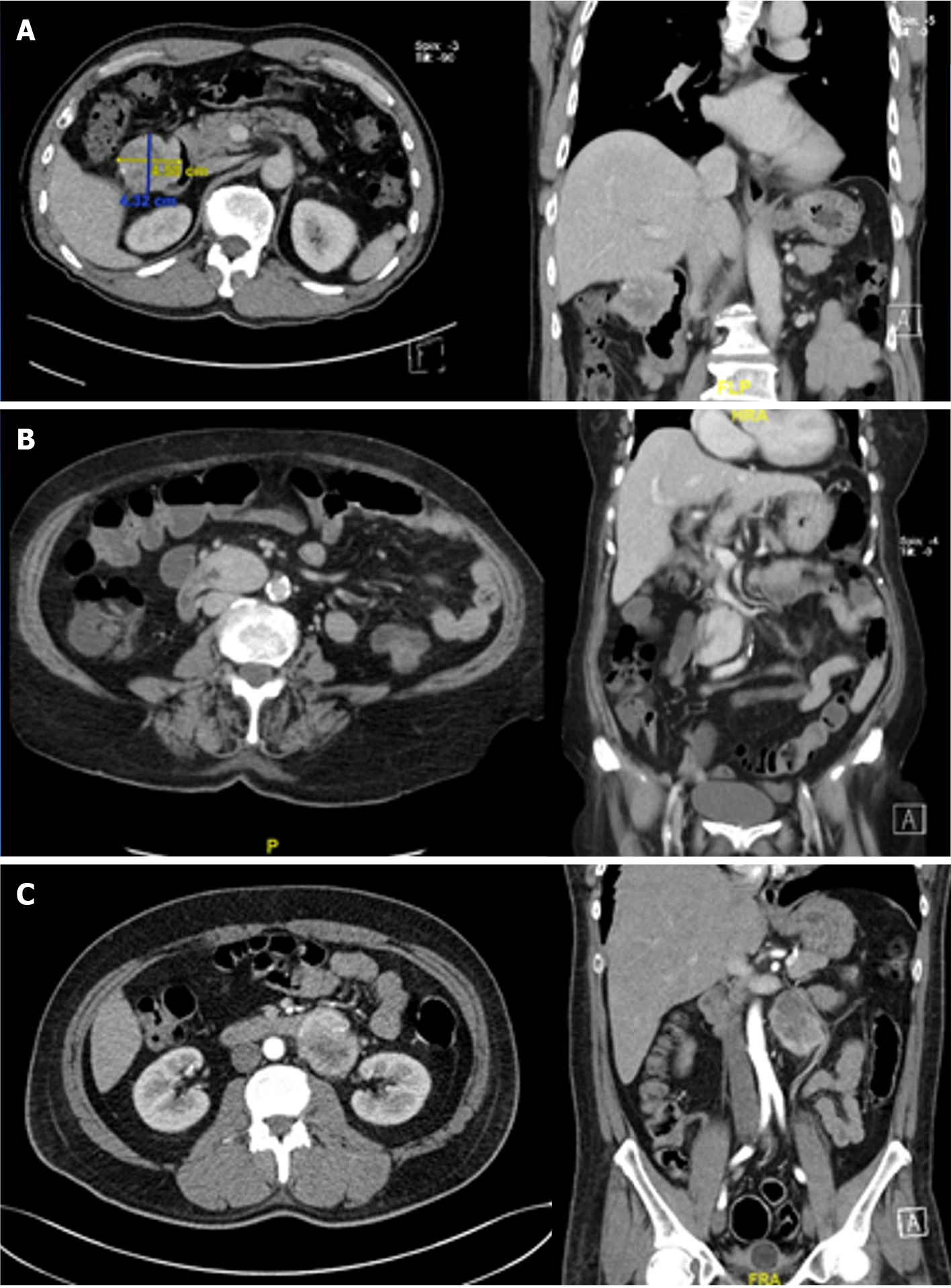
Staging CT and MRI scans are standard imaging modalities commonly used to evaluate the location and size of primary GISTs and to determine any invasion to local structures or distant metastatic disease (Figure 2). Multidetector CT has excellent discriminators of periampullary tumors in arterial phase for distinguishing duodenal adenocarcinoma and pancreatic ductal adenocarcinoma from D-GISTs[28]. Unlike EUS-FNA, CT- or US-guided transabdominal biopsy for resectable GISTs is not recommended due to the risk of pseudo-capsule rupture and tumor spillage in the peritoneal space[25].
Positron emission tomography scans may add further value by differentiating active tumor from inactive scar tissue and the likelihood of malignant tumor from benign tissue. It is a useful imaging modality to assess recurrent or metastatic GISTs before consideration for further surgical resection or second-line tyrosine kinase inhibitors therapy.
Another vital role of imaging studies either by EUS, CT or MRI scan is for interval surveillance of D-GIST < 2 cm in size. Patients with small tumor < 2 cm with benign EUS features are offered regular EUS surveillance or surgery if they wish. Any subsequent increase in size of D-GISTs would warrant consideration for surgical resection.
A study on EUS surveillance involving 93 patients with submucosal tumor for a mean period of 17.3 mo (range 6-42 mo) showed 3 patients (13.0%) had interval increase in tumor size, and surgery was performed[29]. It remains debatable whether EUS surveillance for small tumors originating from the muscularis propria in the upper gastrointestinal tract is useful. Nevertheless, EUS has better sensitivity than CT or MRI scan. It must be recognized that some patients with D-GIST < 2 cm do not wish to undergo invasive surveillance EUS but opted for non-invasive surveillance CT or MRI scan instead. Although the optimal follow up schedules are not known, the suggested frequency and imaging modality used for patients who underwent surgical resection of D-GISTs can follow the previously published algorithm for the management of GISTs[30].
According to the European Society for Medical Oncology and European Reference Network for Rare Adult Solid Cancers clinical practice guidelines for diagnosis, treatment and follow up on GISTs, duodenal nodules < 2 cm should have EUS assessment and then follow-up, whilst tumor > 2 cm should have biopsy or surgical excision[31]. For a resectable primary tumor, it is important to determine the location and the size of D-GISTs to guide the ideal surgical approach at the pancreaticoduodenal complex. The cohort studies of resected D-GISTs in terms of location and size are summarized in Table 1.
| Ref. | Duodenal location D1-D4 (%) | Median size (cm) in all patients | Surgical approach | Operative complications or morbidity & mortality | Pathological risk classification using NIH or AFIP criteria | Survival in all patients |
| Liu et al[12] (n = 300) | D1 (15.8); D2 (51.5); D3 (24.4); D4 (8.3) | 4 (0.1-28.0) | LR n = 199 (66.3%); PD n = 78 (26.0%); Not available n = 13 (4.3%); No surgery n = 10 (3.3%) | Not available | Very low n = 23 (12.8%); Low n = 87 (48.6%); Intermediate n = 2 (1.1%); High n = 67 (37.4%) | 1-, 3-, 5-, 10-yr DFS: 94.4%, 75.2%, 64.4%, 46.5%; 1-, 3-, 5-, 10-yr DSS: 99.5%, 93.4%, 80.9%, 54.5% |
| Liang et al[23] (n = 28) | D1 (14.3); D2 (60.7); D3 (17.9); D4 (7.1) | 5.8 (1.6-20.0) (95%CI: 5.3-8.6) | WR n = 5 (17.9%); SR n = 13 (46.3%); PD n = 10 (35.7%) | Morbidity 35.7%; Mortality 3.6% | Low n = 11 (39.3%); High n = 17 (60.7%) | 2- and 5-yr RFS: 83.3% and 50.0%; Median OS: 64.5 mo |
| Colombo et al[32] (n = 84) | D1 (11); D2 (39); D3 (30); D4 (20) | 5 (1-19) | LR n = 56 (66.6%); PD n = 28 (33.3%) | LR 9%; PD 36% | Low n = 35 (45%); Intermediate n = 4 (5%); High n = 39 (50%) | 3, 5 yr OS: 98%, 89%; 3-, 5-yr DFS: 67%, 64% |
| Daffaud et al[33] (n = 117) | D1 (7); D2 (33); D3 (24); D4 (13) | 5.0 (0.4-31.0) | Operated n = 109; LR n = 82 (74%); PD n = 23 (21%) | LR 18%; PD 26% | Very low n = 43 (39.0%); Low n = 52 (54.7%); High n = 19 (16.0%) | 2-, 5-yr EFS: 82.0%, 54.5%; 3-, 5-yr OS: 94.9%, 86.5% |
| Shen et al[34] (n = 74) | D1 (22.97); D2 (47.30); D3 (16.22); D4 (13.51) | 5.08 ± 2.90 | WR n = 18 (24.3%); SR n = 39 (52.7%); PD n = 17 (23.0%) | WR 5.6%; SR 2.6%; PD 23.5% | Low n = 32 (43.24%); Intermediate n = 8 (10.81%); High n = 34 (45.96%) | 1-, 3-, 5-yr RFS: 93.9%, 73.7%, 69.0%; 1-, 3-, 5-yr OS: 100%, 92.5%, 86.0% |
| Lee et al[35] (n = 60) | D1 (12); D2 (63); D3 (22); D4 (3) | 5.2 (3.5-8.8) | LR n = 37 (62%); PD n = 23 (38%) | LR 24%; PD 70% | Very low/Low n = 24 (40%); Intermediate n = 12 (20%); High n = 24 (40%) | 5-yr RpFS, RFS, OS: LR 56%, 53%, 72%, PD 81%, 64%, 76% |
| Zhang et al[36] (n = 52) | D1 (9.6); D2 (65.4); D3/4 (25.0) | 5.0 (0.5-13.5) | LR n = 45 (26.9%); PD n = 37 (71.2%) | LR 10.8%; PD 21.4% | Low n = 16 (45.7%); Intermediate n = 7 (20.0%); High n = 12 (34.3%) | 1-, 3-, 5-yr RFS: 93.5%, 77.8%, 72.9%; 1-, 3-, 5-yr OS: 100%, 94.6%, 89.1% |
| Lee et al[37] (n = 118) | D 1 (8.5); D2 (51.7); D3 (31.4); D4 (8.5) | 3.9 (3.0-5.4) | LR n = 73 (61.8%); PD n = 45 (38.1%) | LR 20.4%; PD 37.8% | Very low n = 13 (11.0%); Low n = 63 (53.4%); Intermediate n = 19 (16.1%); High n = 23 (43.2%) | 5-, 10-yr OS: 94.9%, 89.9% |
| Tien et al[38] (n = 25) | D1 (12); D2 (52); D3 (25); D4 (16) | 6.7 ± 5.2 | LR n = 16 (64%); PD n = 9 (26%) | LR 12.5%; PD 44.0% | Very low n = 3 (12%); Low n = 8 (32%); Intermediate n = 5 (20%); High n = 8 (32%) | 7 disease recurrence with median follow up 18-mo (9-92) |
| Kamath et al[39] (n = 41) | D1 (7.3); D2 (63.4); D3 (19.5); D4 (9.7) | 3.3-6.2 (0.5-17.0) | LR n = 19 (43.0%); SR n = 11 (26.8%); PD n = 11 (26.8%) | Morbidity 29.2%; Mortality 0% | Low n = 27 (65.8%); Intermediate n = 5 (12.1%); High n = 9 (21.9%) | 3-, 5-yr OS: 85%, 74%; 3-, 5-yr DFS: both 80% |
| Johnston et al[40] (n = 96) | D1 (8.4); D2 (49.0); D3/4 (42.7) | 4.0 (0.1-32.0) | LR n = 58 (60%); PD n = 38 (40%) | LR 29.3%; PD 57.9% | Very low n = 8 (8.3%); Low n = 46 (47.9%); Intermediate n = 25 (26.0%); High n = 16 (16.7%); Unknown n = 1 (1.0%) | 1-, 2-, 5-yr RFS: 94.2%, 82.3%, 67.3%; 1-, 2-, 5-yr OS: 98.3%, 87.4%, 82.0% |
| Zhou et al[54] (n = 48) | D1 (22.9); D1/2 (16.7); D2 (35.4); D2/3 (8.3); D3 (12.5); D4 (4.2) | 4.7 (2.0-15.0) | LR n = 34 (70.8%); PD n = 14 (29.2%) | LR 11.8%; PD 35.7%; Mortality in LR 5.9% (n = 2) | Low n = 28 (58.3%); Intermediate n = 11 (22.9%); High n = 9 (18.8%) | 1-, 3-yr DFS: 100%, 88% |
| Yang et al[56] (n = 22) | D1 (13.6); D2 (63.6); D3/4 (22.7) | 3.75 (1.40-14.00) | LR n = 10 (45.0%); SR n = 3 (13.6%); PD n = 6 (27.0%); PPPD n = 3 (13.6%) | LR 15.4%; PD 88.9% | Very low n = 3 (13.6%); Low n = 7 (31.8%); Intermediate n = 7 (31.8%); High n = 5 (22.7%) | 1-, 2-, 5-yr RFS: 95%, 89.5%, 86.7% |
| Shi et al[64] (n = 61) | D1 (14.8); D2 (54.1); D3 (21.3); D4 (9.8) | 4.0 (1.0-16.0) | LR n = 45 (73.8%); PD n = 16 (26.2%) | LR 33.3%; PD 56.3% | Very low n = 8 (13.1%); Low n = 29 (47.5%); Intermediate n = 14 (23.0%); High n = 10 (16.4%) | 3-, 5-yr RFS: 93.3%, 81.3% |
| Chen et al[66] (n = 64) | D1 (21.9); D2 (46.9); D3 (17.2); D4 (14.1) | 4.25 (1.00-15.00) | LR n = 41 (64%); PD n = 23 (36%) | LR 31.7%; PD 69.6% | Very low n = 4 (6.3%); Low n = 27 (42.2%); Intermediate n = 8 (12.5%); High n = 25 (39.1%) | 3-, 5-yr RFS: 62.9%, 44.3%; 3-, 5-yr OS: 85.7%, 59.5% |
| Sugase et al[69] (n = 25) | D1 (12); D2 (56); D3/4 (32) | 3.8 (1.5-16.0) | LR n = 16 (64%); PD n = 9 (36%) | LR 31%; PD 33% | Very low n = 4 (16%); Low n = 12 (48%); Intermediate n = 0 (0%); High n = 9 (36%) | 2-yr RFS, 2-yr OS, 5-yr OS: LR 85%, 100%, 89%; PD 34%, 80%, 45% |
The order of frequency of D-GISTs in most case series is highest at the second (D2) (33.00%-65.40%) followed by third (D3) (16.22%-31.40%), first (D1) (7.00%-22.97%) and fourth (D4) (3.00%-20.00%) part of duodenum[32-37]. The median size of resected D-GISTs ranges from 3.3 to 6.7 cm in some studies. The smallest size recorded was 1 mm, whilst the largest was 32 cm in diameter[12,38-40].
The indication for surgical resection of D-GIST is not only in asymptomatic patients with tumor size > 2 cm but also in those with symptoms at presentation such as gastrointestinal bleeding and abdominal pain regardless of the tumor size. The mainstay of resectable primary D-GIST is complete surgical resection with an adequate margin en bloc without breaching the pseudo-capsule. After considering the location, size and involvement of surrounding duodenal structures of D-GISTs, there are a few things to take note before embarking on surgical resection.
First, we need to consider the local expertise in utilizing the available instruments such as endoscopy, laparoscopy and robotic-assisted equipment. Second, we need to consider the route of access such as endo-luminal, open laparotomy, minimally invasive (laparoscopic or robotic-assisted) and hybrid endo-laparoscopic surgery. Third, we need to consider the future intact remnant and the size of the created duodenal defect. Fourth, we need to consider the type of reconstruction techniques to restore the gastrointestinal continuity and function restoration.
The use of laparoscopic or endo-laparoscopic surgery in managing GISTs have been increasingly adopted with the advancement of endoscopy, minimally invasive instruments and the development of safe technical skills in the last few decades[41,42]. However, open surgery remains an important surgical access for safety and oncologic reason especially in major complex resection and reconstruction[38,43,44].
Endoscopy has been widely used for diagnostic purposes for decades since the 1950s. It is now increasingly used for endoluminal therapeutic purposes such as endoscopic mucosal resection, endoscopic submucosal dissection, submucosal tunneling and endoscopic resection or per-oral endoscopic tumor resection and endoscopic full thickness resection in benign, pre-malignant or early malignant disease[45-48]. Although endoscopic mucosal resection and endoscopic submucosal dissection in the duodenum is possible, it is exceedingly difficult to safely performed, and hence D-GISTs are best managed by surgical resection[49]. The risks of incomplete resection and pseudo-capsule rupture precludes the use of pure endoscopic resection alone, and future research is needed in this area.
To overcome the limitations of pure endoscopic resection alone, the introduction of hybrid endo-laparoscopic surgery has been attractive. There are a few endo-laparoscopic techniques such as the laparoscopic-assisted endoscopic resection, laparoscopic endoscopic cooperative surgery, inverted laparoscopic endoscopic cooperative surgery, laparoscopic-assisted endoscopy full-thickness resection and endoscope-assisted laparoscopic wedge resection. These hybrid techniques have been described in the resection of gastric tumors and duodenal neuroendocrine tumors, adenoma and adenocarcinoma[42,50-53].
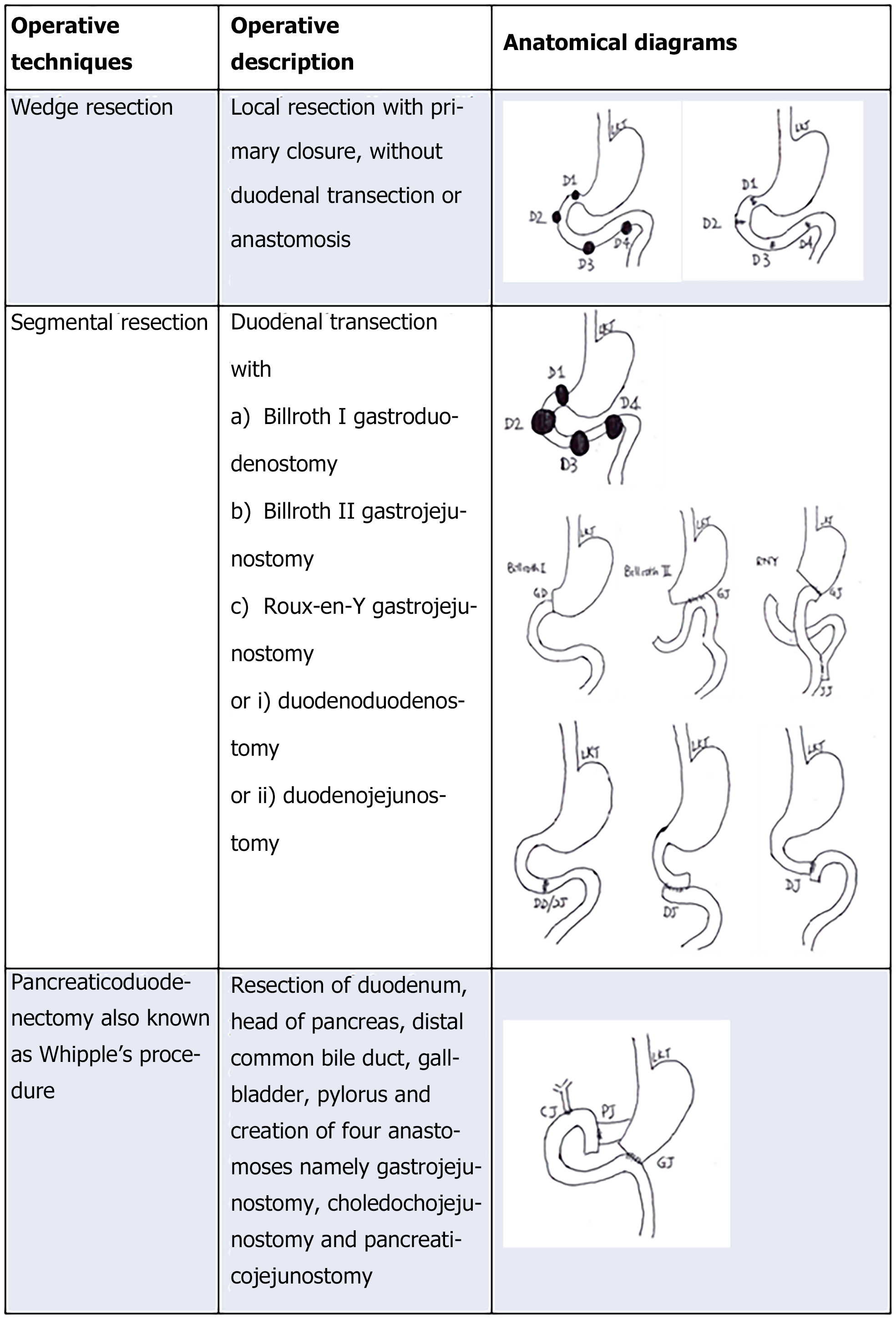
There are several operative techniques described in resecting D-GISTs in the literature with a spectrum of invasiveness and complexities shown in Figure 3. The operative description of limited resection (LR) of D-GISTs include local excision or wedge resection (WR) and segmental resection, whilst for more extended resection means requires pancreaticoduodenectomy (PD), also known as Whipple’s procedure or pylorus preserving pancreaticoduodenectomy[23,33,38,54,55].
WR is a local excision with primary closure without duodenal transection or anastomoses. Segmental resection involves duodenal transection with reconstruction. Reconstruction may be in the form of Billroth I gastroduodenostomy, Billroth II or Roux-en-Y gastrojejunostomy, end-to-end duodenoduodenostomy and end-to–end or end-to-side duodenojejunostomy (DJ) anastomosis[23,44]. PD is a complex procedure as it involves resection of duodenum, head of pancreas, common bile duct, gallbladder and sometimes pylorus and creation of three anastomoses namely gastrojejunostomy, choledochojejunostomy and pancreaticojejunostomy. Another equally effective procedure is pylorus preserving pancreaticoduodenectomy when the pyloric remnant is left intact[56].
For smaller-sized and mainly exophytic D-GIST, a longitudinal WR resulting in only limited defects of the duodenal wall can be primarily close in transverse direction. This surgical approach can be applied to any segment of the anti-mesenteric border of the duodenum including the D2 where ampulla of Vater can be retained. Traditionally, this technique was performed via open operation. Currently, there is evidence to suggest laparoscopic LR of D-GISTs is feasible and safe for both short- and long-term outcomes. In a case series of 6 consecutive patients with duodenal GISTs who underwent laparoscopic LR of D-GIST[42], there was minimal median blood loss of 10 mL, with median operative time of 2 h, no conversions to open surgery and no intraoperative or postoperative complications. All patients underwent curative resection with negative surgical margins, none had recurrence of their duodenal GISTs, and all patients were alive at the end of the follow-up period of 54 mo. In addition, in another study of 53 patients with D2/D3-GISTs, the laparoscopic LR group had less perioperative complications (16.7% vs 24.4%, P = 0.574) with shorter operative duration (155.0 min vs 218.8 min, P = 0.013) and postoperative length of stay (12.0 d vs 19.4 d, P = 0.036) than those in the open LR group[37].
An alternative LR for a larger-sized D-GIST located at the second or third part of the duodenum without the involvement of the ampulla of Vater is a partial duodenectomy and a side-to-side Roux-en-Y DJ at the site of the duodenal wall defect[43]. Similarly, for a larger-sized D-GIST located at the fourth part of the duodenum, a segmental duodenectomy and reconstruction with side-to-side Roux-en-Y DJ can be performed[22].
The indication for PD is reserved for D-GIST that invades the ampulla of Vater, pancreas or pancreatic duodenal wall. According to the retrospective analysis of combined series of 300 patients with D-GISTs, about two-thirds (66.3%) received LR. In the other one-third (33.7%) who received PD, the D-GISTs were found to be larger in size or arose from D2 (both P < 0.05)[12]. However, it is important to take note that PD has higher perioperative complications and longer postoperative length of stay compared to other LR options[40].
A recent study of 22 patients with D-GISTs located opposite the ampulla of Vater, both laparoscopic PD and laparoscopic pancreas-sparing duodenectomy have been shown to confer comparable safety and oncological benefits[57]. It is important to take note that the laparoscopic pancreas-sparing duodenectomy group had shorter operative duration (364.2 ± 58.7 vs 230.0 ± 12.3 min, P < 0.001), less blood loss (176.9 ± 85.7 vs 61.1 ± 18.2 mL, P < 0.001) and much shorter recovery time (10.9 ± 3.8 vs 20.6 ± 11.1 d, P = 0.021), resulting in lower total cost (76972.4 ± 11614.8 yuan vs 125628.7 ± 46356.8 yuan, P = 0.006). However, the authors concluded laparoscopic pancreas-sparing duodenectomy should only be performed in selected patients by experienced surgeons.
Recent case reports of robotic-assisted resection of large D-GISTs have demonstrated the feasibility with either primary closure or Roux-en-Y DJ re
Three systematic reviews and meta-analyses comparing patients who underwent LR vs PD for D-GISTs showed that LR was associated with lower surgical morbidity, less postoperative complications and better oncologic outcomes as shown in Table 2[61-63]. PD on the other hand was associated with longer operative duration, more intraoperative blood loss needing blood transfusion requirement, more surgical complications and longer length of hospital stay[62,63].
| Ref. | Outcome parameters | LR group | PD group |
| Systematic review and meta-analysis | |||
| Chok et al[61] (n = 162) | Surgical morbidity; Oncologic outcomes | Better (20.7%); Better DFS (HR 2.07, 95%CI: 1.07–4.01), lower rate of distant metastasis (8.9% vs 25.8%, OR 0.28, 95%CI: 0.13–0.59) | Worse (48.3%) (RR 2.34, 95%CI: 1.61–3.42). Worse: Related to large tumor (≥ 5 cm) (76.0% vs 36.6%, OR 5.49, 95%CI: 1.8–16.76), high mitotic count ≥ 5/50 HPF (33.7% vs 18.5%, OR 2.23, 95%CI: 1.22–4.08), high-risk classification (60.3% vs 32.0%, OR 3.23, 95%CI: 1.65–6.34) and which were located at D2 (80.5% vs 28.6%, OR 10.33, 95%CI: 5.22–20.47) |
| Shen et al[62] (n = 623) | Complications; Long term prognosis | Less; Better | More (OR 2.90; 95%CI: 1.90-4.42; P < 0.001); Worse (HR 1.93; 95%CI: 1.39-2.69; P < 0.001); Related to invasion of the D2, higher degree tumor mitosis (> 5/50 HPF) and high-risk classification (P < 0.001) |
| Zhou et al[63] (n = 1103) | Surgical outcomes | Better | Worse: Related to higher incidence of mitotic index > 5/50 HPF, high-risk classification, D2 tumor, tumor size, operative duration, intraoperative blood loss, blood transfusion requirement, morbidity, length of hospital stay and recurrence rate (P < 0.001) |
| Propensity score matching study | |||
| Wei et al[67] (n = 325) | Impact of surgical modalities on long term survival outcomes | Similar | Similar: OS (HR 1.160; 95%CI: 0.662-2.033); DSS (HR 1.208; 95%CI: 0.686-2.128) |
| Uppal et al[68] (n = 1084 of which 874 had resection) | Lymph node and stage; Survival; Adjuvant systemic therapy rate | Fewer and negative for disease; Better. 21.5% | Higher T3/4 stage, extra nodal involvement and performed more at academic center. Poorer, higher mortality, uninsured status. 31.3% |
It is reasonable to state that the factors contributing to the decision in the surgical approaches and the choice of LR vs PD are anatomical location, the size and the local invasion of D-GIST into the surrounding structures. Although minimally invasive techniques have shown to confer some benefits in surgical morbidity and postoperative length of stay, it is the complexity of surgical resection that determines the overall surgical morbidity and outcomes (Table 1).
When counselling the patients with D-GISTs for surgical resection, it is important to provide the information on the overall morbidity rate, which ranges from 8.2%-33.3% in LR group and 21.4%-88.9% in PD group, whilst the mortality rate is about 3.6%-6.0%[23,36,54,56,64].
It is debatable whether the location and size of the D-GISTs play a role in overall survival in comparison to gastric and other small bowel GISTs. A study comparing 202 patients with D-GISTs and 253 patients with gastric GISTs (G-GISTs) showed significantly different results with respect to tumor size, mitotic count and National Institutes of Health risk category (all P < 0.05). The 5-year disease-free survival (DFS) (64.4% vs 94.9%, P < 0.001) and disease-specific survival (80.9% vs 92.6%, P = 0.049) of D-GISTs were worse than that of G-GISTs (both P < 0.05)[12].
In contrast to another study analyzing the data extracted from the Surveillance, Epidemiology and End Results database from 1998 to 2011. The overall survival (OS) and cancer-specific survival (CSS) of patients with small bowel GIST were not statistically different from those with G-GIST when adjustment was made for confounding variables on a population-based level. Hence, the notion of small bowel GIST patients having a worse prognosis than that of G-GIST patients should be revisited, and the adjuvant treatment be reviewed[65].
Based on the anatomical location, the 5-year OS of all sizes of GISTs in stomach, duodenum, ileum/jejunum, colon, rectum and peritoneum were 86.3%, 88.2%, 85.0%, 68.4%, 89.0% and 68.8%, respectively. One must interpret the data carefully as the data comprised of 6% (n = 313) D-GIST, 25% (n = 1288) jejunal/ileal GISTs and 59% (n = 3011) G-GISTs, which could potentially lead to some bias. However, in multivariate analyses, the OS and CSS of patients with D-GISTs [OS, hazard ratio (HR) 0.95, 95% confidence interval (CI): 0.76-1.19; CSS, HR 0.99, 95%CI: 0.76-1.29] and the jejunal/ileal GISTs (OS, HR 0.97, 95%CI: 0.85-1.10; CSS, HR 0.95, 95%CI: 0.81-1.10) were similar to those of patients with G-GIST. The 5-year OS of non-metastatic D-GISTs of all sizes was 88.2%. More importantly, when the D-GISTs were categorized into ≤ 2 cm, > 2 to ≤ 5 cm, > 5 to ≤ 10 cm, > 10 cm, the 5-year OS was 100%, 97.4%, 83.5% and 78.5%, respectively[65].
Earlier studies have shown that tumor biology and tumor factors predict the prognosis and survival rather than the surgical approach[12,40]. Other studies have suggested the recurrence of D-GISTs was correlated to tumor biology rather than the type of operation performed such as organ invasion of D2, higher degree of tumor mitosis and higher malignant risk classification[32,54,61,62,66].
From these studies, it is not surprising to note that patients with D-GISTs in the PD group had a higher incidence of mitotic count > 5/50 high power fields, a higher incidence of high-risk classification, a higher incidence of tumors located at D2, a larger tumor size > 5 cm and an increased recurrence rate than those in the LR group.
A study of 114 cases of D-GISTs by the French Sarcoma group showed 5-year OS and event-free survival rates of 86.5% and 54.5%, respectively. More importantly, the event-free survival was similar in the LR and PD groups of patients (P > 0.05)[33]. In a Korean study of 118 patients with localized duodenal GISTs who underwent curative resection, the 5-year OS and DFS were 94.9% and 79.2%, respectively. The 5-year OS and DFS rates were not statistically significant between the LR and PD groups (OS: 91.9% vs 96.2%, P > 0.05, DFS: 84.0% vs 72.6%, P > 0.05)[37].
A more recent study using the Surveillance, Epidemiology and End Results database identified 325 patients who underwent surgery for D-GISTs between 1986 and 2016 showed 5-year OS and disease-specific survival in PD was significantly better than those in the LR group (71% vs 54.1%, P = 0.014; 66.6% vs 49.1%, P = 0.025). Propensity score matching performed after adjusting covariates and the type of surgery did not show any significant impact of the OS and disease-specific survival. These results may argue that surgical modalities do not have significant impact on long-term survival outcomes in patients with D-GISTs and should be dependent on tumor location and size[67].
However, in contrast to a study using the National Cancer Database examining the surgical resection of 874 cases of D-GISTs, it showed that local resection was associated with improved OS compared to radical resection after controlling for tumor factors and systemic treatment[68]. According to the National Cancer Database, most of the resected D-GIST patients did not receive adjuvant systemic therapy with only 31.3% in the PD group vs 21.5% in the LR group. This data may explain the reason neoadjuvant and adjuvant systemic therapy was not associated with improved OS.
As recommended by the National Comprehensive Cancer Network and European Society for Medical Oncology-European Reference Network for Rare Adult Solid Cancers guidelines of resected D-GIST, the intermediate risk group should receive adjuvant IM for at least 1 year, and the high-risk group should receive treatment for at least 3 years. However, not every population has access to IM readily and hence the risk of bias in the interpretation of survival after surgical resection of D-GISTs. For an example, only 3.0% to 5.2% of D-GISTs received neoadjuvant therapy and 13% to 17% received adjuvant therapy even when in the high-risk National Institutes of Health category, which accounted for 37.5% in the LR group vs 76.1% in the PD group[12].
The limitation in this review article on the overall survival outcomes of resected D-GIST is due to the heterogeneity of neoadjuvant and adjuvant IM therapy, which could influence the interpretation of these prognostication results. In summary, the 5-year OS of non-metastatic D-GISTs of all sizes ranges from 59.5% to 94.9%[37,66]. When taking the surgical approach into account, the 5-year OS is 72%-89% in the LR group vs 45-76% in the PD group[32,35,69].
The surgical management of D-GISTs can be performed safely with good oncological outcomes provided an adequate resection margin can be achieved. The surgical option of resectable primary D-GISTs varies with increasing complexity depending on the location, size and involvement of surrounding structures.
The surgical approach for D-GISTs located at D1 and D2 proximal to the ampulla is distal gastroduodenectomy with Billroth II or Roux-en-Y gastrojejunostomy anastomosis. WR resulting in only a limited defect of the duodenal wall can be closed primarily for smaller D-GISTs located at anti-mesenteric border of the duodenum where the ampulla can be retained. Segmental resection with DJ anastomosis is indicated for larger D-GISTs located at D3 and D4 distal to the ampulla. Any large D-GISTs located at D1 and D2 involving the ampulla, a PD is the treatment of choice. Laparoscopic approaches for LR and PD have been shown to be feasible and safe with good oncological outcomes in experienced hands. The minimally invasive techniques including robotic-assisted approach will likely increase in the future.
D-GISTs have a prognosis comparable to gastric and other small bowel GISTs. The use of LR when conditions allow is recommended due to lower surgical morbidity, less postoperative complications and better oncologic outcomes.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: An T, Sakamoto Y S-Editor: Zhang H L-Editor: Filipodia P-Editor: Li X
| 1. | Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system. Lyon, France: IARC, 2000. |
| 2. | Thomas RM, Sobin LH. Gastrointestinal cancer. Cancer. 1995;75:154-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 280] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 5. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1722] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 6. | Agaram NP, Wong GC, Guo T, Maki RG, Singer S, Dematteo RP, Besmer P, Antonescu CR. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47:853-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Gaal J, Stratakis CA, Carney JA, Ball ER, Korpershoek E, Lodish MB, Levy I, Xekouki P, van Nederveen FH, den Bakker MA, O'Sullivan M, Dinjens WN, de Krijger RR. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol. 2011;24:147-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Miettinen M, Lasota J. Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs) - a review. Int J Biochem Cell Biol. 2014;53:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 866] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 10. | Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 437] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 520] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 12. | Liu Z, Zheng G, Liu J, Liu S, Xu G, Wang Q, Guo M, Lian X, Zhang H, Feng F. Clinicopathological features, surgical strategy and prognosis of duodenal gastrointestinal stromal tumors: a series of 300 patients. BMC Cancer. 2018;18:563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Agaimy A, Wünsch PH. Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours. Langenbecks Arch Surg. 2006;391:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors presenting as omental masses--a clinicopathologic analysis of 95 cases. Am J Surg Pathol. 2009;33:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Miettinen M, Felisiak-Golabek A, Wang Z, Inaguma S, Lasota J. GIST Manifesting as a Retroperitoneal Tumor: Clinicopathologic Immunohistochemical, and Molecular Genetic Study of 112 Cases. Am J Surg Pathol. 2017;41:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Marano L, Boccardi V, Marrelli D, Roviello F. Duodenal gastrointestinal stromal tumor: From clinicopathological features to surgical outcomes. Eur J Surg Oncol. 2015;41:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Hohenberger P, Ronellenfitsch U, Oladeji O, Pink D, Ströbel P, Wardelmann E, Reichardt P. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. 2010;97:1854-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Fiore M, Palassini E, Fumagalli E, Pilotti S, Tamborini E, Stacchiotti S, Pennacchioli E, Casali PG, Gronchi A. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur J Surg Oncol. 2009;35:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Rutkowski P, Gronchi A, Hohenberger P, Bonvalot S, Schöffski P, Bauer S, Fumagalli E, Nyckowski P, Nguyen BP, Kerst JM, Fiore M, Bylina E, Hoiczyk M, Cats A, Casali PG, Le Cesne A, Treckmann J, Stoeckle E, de Wilt JH, Sleijfer S, Tielen R, van der Graaf W, Verhoef C, van Coevorden F. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol. 2013;20:2937-2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Wang J, Yin Y, Shen C, Yin X, Cai Z, Pu L, Fu W, Wang Y, Zhang B. Preoperative imatinib treatment in patients with locally advanced and metastatic/recurrent gastrointestinal stromal tumors: A single-center analysis. Medicine (Baltimore). 2020;99:e19275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Hoeppner J, Kulemann B, Marjanovic G, Bronsert P, Hopt UT. Limited resection for duodenal gastrointestinal stromal tumors: Surgical management and clinical outcome. World J Gastrointest Surg. 2013;5:16-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Liang X, Yu H, Zhu LH, Wang XF, Cai XJ. Gastrointestinal stromal tumors of the duodenum: surgical management and survival results. World J Gastroenterol. 2013;19:6000-6010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Popivanov G, Tabakov M, Mantese G, Cirocchi R, Piccinini I, D'Andrea V, Covarelli P, Boselli C, Barberini F, Tabola R, Pietro U, Cavaliere D. Surgical treatment of gastrointestinal stromal tumors of the duodenum: a literature review. Transl Gastroenterol Hepatol. 2018;3:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Gervaz P, Huber O, Morel P. Surgical management of gastrointestinal stromal tumours. Br J Surg. 2009;96:567-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Du H, Ning L, Li S, Lou X, Chen H, Hu F, Shan G, Zhang F, Xu G. Diagnosis and Treatment of Duodenal Gastrointestinal Stromal Tumors. Clin Transl Gastroenterol. 2020;11:e00156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 28. | Lu J, Hu D, Tang H, Hu X, Shen Y, Li Z, Peng Y, Kamel I. Assessment of tumor heterogeneity: Differentiation of periampullary neoplasms based on CT whole-lesion histogram analysis. Eur J Radiol. 2019;115:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Lok KH, Lai L, Yiu HL, Szeto ML, Leung SK. Endosonographic surveillance of small gastrointestinal tumors originating from muscularis propria. J Gastrointestin Liver Dis. 2009;18:177-180. [PubMed] |
| 30. | Lim KT, Tan KY. Current research and treatment for gastrointestinal stromal tumors. World J Gastroenterol. 2017;23:4856-4866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 31. | Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY; ESMO Guidelines Committee and EURACAN. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv68-iv78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 289] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 32. | Colombo C, Ronellenfitsch U, Yuxin Z, Rutkowski P, Miceli R, Bylina E, Hohenberger P, Raut CP, Gronchi A. Clinical, pathological and surgical characteristics of duodenal gastrointestinal stromal tumor and their influence on survival: a multi-center study. Ann Surg Oncol. 2012;19:3361-3367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Duffaud F, Meeus P, Bachet JB, Cassier P, Huynh TK, Boucher E, Bouché O, Moutardier V, le Cesne A, Landi B, Marchal F, Bay JO, Bertucci F, Spano JP, Stoeckle E, Collard O, Chaigneau L, Isambert N, Lebrun-Ly V, Mancini J, Blay JY, Bonvalot S. Conservative surgery vs. duodeneopancreatectomy in primary duodenal gastrointestinal stromal tumors (GIST): a retrospective review of 114 patients from the French sarcoma group (FSG). Eur J Surg Oncol. 2014;40:1369-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Shen C, Chen H, Yin Y, Chen J, Han L, Zhang B, Chen Z. Duodenal gastrointestinal stromal tumors: clinicopathological characteristics, surgery, and long-term outcome. BMC Surg. 2015;15:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Lee SY, Goh BK, Sadot E, Rajeev R, Balachandran VP, Gönen M, Kingham TP, Allen PJ, D'Angelica MI, Jarnagin WR, Coit D, Wong WK, Ong HS, Chung AY, DeMatteo RP. Surgical Strategy and Outcomes in Duodenal Gastrointestinal Stromal Tumor. Ann Surg Oncol. 2017;24:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Zhang S, Tian Y, Chen Y, Zhang J, Zheng C, Wang C. Clinicopathological Characteristics, Surgical Treatments, and Survival Outcomes of Patients with Duodenal Gastrointestinal Stromal Tumor. Dig Surg. 2019;36:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Lee SJ, Song KB, Lee YJ, Kim SC, Hwang DW, Lee JH, Shin SH, Kwon JW, Hwang SH, Ma CH, Park GS, Park YJ, Park KM. Clinicopathologic Characteristics and Optimal Surgical Treatment of Duodenal Gastrointestinal Stromal Tumor. J Gastrointest Surg. 2019;23:270-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Tien YW, Lee CY, Huang CC, Hu RH, Lee PH. Surgery for gastrointestinal stromal tumors of the duodenum. Ann Surg Oncol. 2010;17:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Kamath AS, Sarr MG, Nagorney DM, Que FG, Farnell MB, Kendrick ML, Reid Lombardo KM, Donohue JH. Gastrointestinal stromal tumour of the duodenum: single institution experience. HPB (Oxford). 2012;14:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Johnston FM, Kneuertz PJ, Cameron JL, Sanford D, Fisher S, Turley R, Groeschl R, Hyder O, Kooby DA, Blazer D 3rd, Choti MA, Wolfgang CL, Gamblin TC, Hawkins WG, Maithel SK, Pawlik TM. Presentation and management of gastrointestinal stromal tumors of the duodenum: a multi-institutional analysis. Ann Surg Oncol. 2012;19:3351-3360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Zioni T, Dizengof V, Kirshtein B. Laparoscopic resection of duodenal gastrointestinal stromal tumour. J Minim Access Surg. 2017;13:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Ojima T, Nakamura M, Hayata K, Kitadani J, Katsuda M, Takeuchi A, Tominaga S, Yamaue H. Laparoscopic Limited Resection for Duodenal Gastrointestinal Stromal Tumors. J Gastrointest Surg. 2020;24:2404-2408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Goh BK, Chow PK, Ong HS, Wong WK. Gastrointestinal stromal tumor involving the second and third portion of the duodenum: treatment by partial duodenectomy and Roux-en-Y duodenojejunostomy. J Surg Oncol. 2005;91:273-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Chung JC, Kim HC, Hur SM. Limited resections for duodenal gastrointestinal stromal tumors and their oncologic outcomes. Surg Today. 2016;46:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Joo MK, Park JJ, Kim H, Koh JS, Lee BJ, Chun HJ, Lee SW, Jang YJ, Mok YJ, Bak YT. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc. 2016;83:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 46. | Kappelle WFW, Backes Y, Valk GD, Moons LMG, Vleggaar FP. Endoscopic full-thickness resection of gastric and duodenal subepithelial lesions using a new, flat-based over-the-scope clip. Surg Endosc. 2018;32:2839-2846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Chiu PWY, Yip HC, Teoh AYB, Wong VWY, Chan SM, Wong SKH, Ng EKW. Per oral endoscopic tumor (POET) resection for treatment of upper gastrointestinal subepithelial tumors. Surg Endosc. 2019;33:1326-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Liu J, Wang Y, Liu Z, Lv L, Ren Z, Hu J, Qin W, Zhong Y, Zhou P, Li Q. Submucosal tunneling endoscopic resection treatment of multiple gastrointestinal submucosal tumors. J Gastroenterol Hepatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Gaspar JP, Stelow EB, Wang AY. Approach to the endoscopic resection of duodenal lesions. World J Gastroenterol. 2016;22:600-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 50. | Kato M, Nakajima K, Nishida T, Yamasaki M, Tsutsui S, Ogiyama H, Yamamoto S, Yamada T, Mori M, Doki Y, Hayashi N. Local resection by combined laparoendoscopic surgery for duodenal gastrointestinal stromal tumor. Diagn Ther Endosc. 2011;2011:645609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Ueda K, Hijioka M, Lee L, Igarashi H, Niina Y, Osoegawa T, Nakamura K, Takahashi S, Aishima S, Ohtsuka T, Takayanagi R, Ito T. A synchronous pancreatic neuroendocrine tumor and duodenal gastrointestinal stromal tumor. Intern Med. 2014;53:2483-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Ohata K, Murakami M, Yamazaki K, Nonaka K, Misumi N, Tashima T, Minato Y, Shozushima M, Mitsui T, Matsuhashi N, Fu K. Feasibility of endoscopy-assisted laparoscopic full-thickness resection for superficial duodenal neoplasms. ScientificWorldJournal. 2014;2014:239627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Ntourakis D, Mavrogenis G. Cooperative laparoscopic endoscopic and hybrid laparoscopic surgery for upper gastrointestinal tumors: Current status. World J Gastroenterol. 2015;21:12482-12497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 54. | Zhou B, Zhang M, Wu J, Yan S, Zhou J, Zheng S. Pancreaticoduodenectomy versus local resection in the treatment of gastrointestinal stromal tumors of the duodenum. World J Surg Oncol. 2013;11:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Cananzi FCM, Ruspi L, Samà L, Sicoli F, Gentile D, Minerva EM, Cozzaglio L, Quagliuolo V. Short-term outcomes after duodenal surgery for mesenchymal tumors: a retrospective analysis from a single tertiary referral center. Updates Surg. 2019;71:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Yang F, Jin C, Du Z, Subedi S, Jiang Y, Li J, Di Y, Zhou Z, Tang F, Fu D. Duodenal gastrointestinal stromal tumor: clinicopathological characteristics, surgical outcomes, long term survival and predictors for adverse outcomes. Am J Surg. 2013;206:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Lu C, Jin W, Mou Y, Shao H, Wu X, Li S, Xu B, Wang Y, Zhu Q, Xia T, Zhou Y. Optimal Laparoscopic Management and Oncological Outcomes of Gastrointestinal Stromal Tumors in Duodenum: Pancreaticoduodenectomy or Pancreas-Sparing Duodenectomy? Cancer Manag Res. 2020;12:4725-4734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Marano A, Allisiardi F, Perino E, Pellegrino L, Geretto P, Borghi F. Robotic Treatment for Large Duodenal Gastrointestinal Stromal Tumor. Ann Surg Oncol. 2020;27:1101-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | McGuirk M, Gachabayov M, Gogna S, Da Dong X. Robotic duodenal (D3) resection with Roux-en-Y duodenojejunostomy reconstruction for large GIST tumor: Step by step with video. Surg Oncol. 2021;36:130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Cho HJ, Jang JY, Jeong SY, Kang IC, Lee SH, Choi SH. Robotic limited local resection of duodenal juxta-ampullary neoplasms. Int J Med Robot. 2021;17:e2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Chok AY, Koh YX, Ow MY, Allen JC Jr, Goh BK. A systematic review and meta-analysis comparing pancreaticoduodenectomy versus limited resection for duodenal gastrointestinal stromal tumors. Ann Surg Oncol. 2014;21:3429-3438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 62. | Shen Z, Chen P, Du N, Khadaroo PA, Mao D, Gu L. Pancreaticoduodenectomy versus limited resection for duodenal gastrointestinal stromal tumors: a systematic review and meta-analysis. BMC Surg. 2019;19:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Zhou Y, Wang X, Si X, Wang S, Cai Z. Surgery for duodenal gastrointestinal stromal tumor: A systematic review and meta-analysis of pancreaticoduodenectomy versus local resection. Asian J Surg. 2020;43:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Shi HP, Huang ML, Wang ZQ, Zheng YN, Zhu ZL, Sah BK, Liu WT, Yan M, Zhu ZG, Li C. Clinicopathological and Prognostic Features of Surgical Management in Duodenal Gastrointestinal Stromal Tumors. Dig Surg. 2018;35:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Guller U, Tarantino I, Cerny T, Ulrich A, Schmied BM, Warschkow R. Revisiting a dogma: similar survival of patients with small bowel and gastric GIST. A population-based propensity score SEER analysis. Gastric Cancer. 2017;20:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Chen P, Song T, Wang X, Zhou H, Zhang T, Wu Q, Kong D, Cui Y, Li H, Li Q. Surgery for Duodenal Gastrointestinal Stromal Tumors: A Single-Center Experience. Dig Dis Sci. 2017;62:3167-3176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Wei YZ, Cai ZB, Zhu CL, Zhou YM, Zhang XF. Impact of Surgical Modalities on Long-term Survival Outcomes of Patients with Duodenal Gastrointestinal Stromal Tumor. Ann Surg Oncol. 2021;28:4668-4674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Uppal A, Wang M, Fischer T, Goldfarb M. Duodenal GI stromal tumors: Is radical resection necessary? J Surg Oncol. 2019;120:940-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Sugase T, Takahashi T, Nakajima K, Hirota S, Masuzawa T, Nishida T, Kimura Y, Miyazaki Y, Makino T, Kurokawa Y, Yamasaki M, Takiguchi S, Mori M, Doki Y. Clinicopathological Characteristics, Surgery and Survival Outcomes of Patients with Duodenal Gastrointestinal Stromal Tumors. Digestion. 2016;94:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |