INTRODUCTION
Surgeons across the globe embraced minimally invasive surgery (MIS) by the end of the 20th century, but pancreatic surgery was one of the last frontiers for its application. Many factors make laparoscopic pancreatic surgery difficult: The organ is deep in the retroperitoneum, attached to the duodenum, intimately related to large mesenteric vessels and draped by viscera. In addition, its soft glandular consistency and its ability to fibrose surrounding tissues in the setting of pathology make it a treacherous contender for laparoscopy.
An additional consideration is the fact that multiple studies demonstrated that patient safety and outcomes are better when pancreatic operations are performed in high volume centers by specialized surgical teams[1,2]. This limits the experience with laparoscopic pancreatic surgery to a small cadre of institutions, mostly in high-income, resource-rich countries. In this paper, we review the evolution of minimally invasive pancreatic surgery and discuss our experience incorporating this into low-volume, resource-poor countries such as those in the Caribbean.
LITERATURE REVIEW
The first report of a laparoscopic operation on the pancreas was published by Gagner et al[3] who completed a pancreaticoduodenectomy (PD) in 1994. Their operation was complicated by a jejunal ulcer, delayed gastric emptying and a prolonged 30-d post-operative hospitalization, forcing the authors to conclude that “although technically feasible, the laparoscopic Whipple procedure did not improve the postoperative outcome or shorten the postoperative recovery”[1]. It was interesting that Gagner and Pomp chose this operation for their initial attempt at laparoscopic pancreatic surgery, considering that a PD is longer and technically more complex than left-sided resections.
Most of the subsequent reports in the 1990’s focused on laparoscopic distal pancreatectomies (LDP), demonstrating that it was feasible for benign endocrine lesions and chronic pancreatitis[4-11]. By the end of the 20th century, it appeared that laparoscopy was being seriously entertained for benign pancreatic diseases. In 1998, Cuschieri et al[11] wrote “laparoscopic distal pancreatic resections have been entirely favorable, with benefit to the patient in terms of postoperative recovery, minimal morbidity and short hospital stay”. And in 1999, Park et al[10] stated that “patients appear to benefit from laparoscopic distal pancreatic resections.”
MINIMALLY INVASIVE DISTAL PANCREATECTOMY
In the first decade of the 21st century, more robust publications began to appear proving that LDP was feasible, technically reproducible and accompanied by encouraging short-term outcomes[12-18]. One decade later, sufficient data had accumulated to allow large metanalyses[19-21].
Venkat et al[19] published a metanalysis in 2012 that compared LDP and open distal pancreatectomy (ODP) in 1814 patients across 18 studies. They demonstrated that both techniques had similar operative times, margin positivity, postoperative pancreatic fistula and mortality, but LDP brought statistically significant reductions in blood loss, hospital stay, overall morbidity and surgical site infection. Venkat et al[19] wrote that the “improved complication profile of LDP, taken together with the lack of compromise of margin status, suggests that this technique is a reasonable approach in selected cancer patients.”
In 2013 Nakamura et al[20] published a meta-analysis of 2904 distal pancreatectomies across 24 studies. Compared with ODP, LDP showed statistically significant reductions in blood loss, transfusion requirements, wound infection rates, morbidity rates and hospitalization. Based on this, Nakamura et al[20] wrote “LDP showed significantly better perioperative outcomes and is a reasonable operative method for benign tumors and some ductal carcinomas in the pancreas”.
Riviere et al[21] then published a 2016 Cochrane Database Systematic Review that compared ODP and LDP in 1576 patients. They noted that hospital stay was 2.43 d shorter in the laparoscopic group, but lamented that existing data were from observational and case-control studies with confounders that did not allow definitive conclusions. Riviere et al[21] called for prospective randomized trials to evaluate this further. The Dutch Pancreatic Cancer Group responded and published results of the LEOPARD trial in 2019 that randomized patients to LDP or ODP, blinding patients with a large abdominal dressing[22]. In this trial, patients who had LDP had statistically significant reductions in the time to functional recovery (4 d vs 6 d), operative blood less (150 mL vs 400 mL) and incidence of delayed gastric emptying (6% vs 20%). They also had better quality of life after LDP. Although the time to complete LDP was significantly longer (217 min vs 179 min), it did not increase the overall cost of care.
The data in support of LDP continued to accrue, but as laparoscopic surgeons pushed the boundaries of pancreatic surgery another development occurred simultaneously. The approval of Intuitive’s DaVinci surgical robot by the United States Food and Drug Administration (FDA) in the year 2000[23] ushered in the robotic surgical revolution. In 2003, Melvin et al[24] published a report of the first robotic distal pancreatectomy (RDP) and this was followed by a publication from Guilianotti et al[25] in 2003 documenting 13 robotic pancreatic operations (among a series of 193 varied robotic operations) that included 5 RDPs and 8 robotic-assisted pancreaticoduodenectomy (RPDs). They reported good outcomes with RDP, with 270 min operating time, 20% overall morbidity and no mortality. Within a few years, the robotic approach became popular in resource-rich countries and small RDP series with good results were published[26-29].
Within a decade, sufficient data were accrued to allow meta-analyses to be performed[30-35]. The first was published by Gavriilidis et al[30] in 2016 and compared RDP vs LDP in 637 patients across 9 studies. They found no significant difference in operative time, conversions, grade B–C pancreatic fistula, morbidity, spleen preservation, perioperative mortality or R0 surgical margins. There was a reduction in hospitalization by one day when patients underwent RDP, but this was countered by significantly increased readmission rates. Therefore, Gavriilidis et al[30] concluded that both were reasonable techniques with similar feasibility, safety and oncological adequacy.
In 2017, Huang et al[31] compared LDP and RDP in more than 1100 patients across 9 studies and found no difference in operating time, conversions, pancreatic fistulae, spleen preservation, transfusion rates or post-operative hospitalization between the two approaches. Huang et al[31] also concluded that RDP was a “safe and effective alternative”.
In 2017, Guerrini et al[32] compared RDP and LDP in a metanalysis of 813 patients across 10 studies. They were able to show definite advantages for RDP, with significantly greater spleen preservation, less conversions and shorter hospitalization. Although there was greater higher cost associated with RDP, Guerrini et al[32] concluded that RDP was “safe and comparable to LDP” and suggested that the increase in cost was balanced by the improved peri-operative profile.
In 2019, Gavriilidis et al[33] published an updated metanalysis comparing the oncological adequacy and efficacy between RDP, LDP and ODP in 6796 patients across 36 studies. Both RDP and LDP brought significantly less blood loss, shorter length of stay and better R0 margins compared to open surgery. When they compared LDP and RDP directly, RDP had lower conversion rates, reduced blood loss and shorter hospital stay. In their conclusion, however, they acknowledged that the data were “underpowered and did not permit conclusions about oncological safety for pancreatic adenocarcinoma.”
Hu et al[34] published another metanalysis comparing RDP and LDP across 22 studies in 2020. In this study, robotic surgery significantly increased spleen preservation, reduced conversions and shortened hospitalization, at the expense of increased cost. There were no differences in blood loss, overall morbidity, node harvest, transfusions, grade B-C pancreatic fistula, margin positivity or mortality. Hu et al[34] concluded that “both RDP and LDP are safe and feasible alternatives” and suggested that the advantages of RDP balanced the increase in cost.
Finally, in 2020 Zhou et al[35] compared RDP and ODP in 2264 patients in a meta-analysis of 7 studies. They demonstrated that RDP significantly reduced blood loss, transfusion rates, postoperative mortality and length of hospital stay. There was no difference in operating time, node harvest, margin positivity, spleen preservation, severe morbidity or grade B-C pancreatic fistula between the groups. They concluded that RDP was a “safe and feasible alternative in centers with expertise in robotic surgery.”
It appears that within the first two decades of the 21st century, there was a rapid swing of the pendulum, moving from open to laparoscopic to robotic distal pancreatectomy. Indeed, the conclusions of early authors seem to have been dismissed and many now propone LDP as standard of care, and RDP as a safe and feasible alternative. While the qualities of a surgical robot (better 3-dimentional visualization, tremor filtration, motion scaling, improved ergonomics and better freedom of motion) appear attractive compared to conventional laparoscopy, the exorbitant cost is prohibitive even in the health care systems of high-income economies. Many developing countries are not be able to afford the high cost, and in the English-speaking Caribbean there are no surgical robots available for use.
MINIMALLY INVASIVE WHIPPLE’S PD
Although LDP gained footing in the late 1990s, there was reluctance to embrace laparoscopy for PD. In 1998 Cuschieri et al[11] wrote “the experience with laparoscopic PD has been unfavorable. With the current technology, the laparoscopic approach for this procedure is too prolonged and does not seem to offer any benefit to the patient.” Similarly, in 1999 Parks et al[10] wrote that “patients benefit from laparoscopic distal pancreatic resection but not from laparoscopic PD”. And in 2001, Gentileschi et al[12] wrote that laparoscopic PD “is not associated with patient benefit and may be accompanied by increased morbidity.” The general theme during the late 1990s was to dissuade the surgical community in its pursuit of minimally invasive PD.
The turn of the 21st century saw publication of small series demonstrating that LPD was technically feasible and associated with reasonable short-term outcomes[36,37]. And by the second decade of the 21st century there were increasing numbers of larger, more robust studies comparing LPD and open pancreaticoduodenectomy (OPD) being published[38-49]. Most publications reported significantly longer operating time[20,42,43] and increased cost[20,39,41-43]. But the data supported LPD by showing benefit with significantly reduced post-operative pain[42], quicker return of bowel function[42], reduced overall morbidity[43], shorter high dependency unit or intensive care unit (HDU/ICU) stay[38,40], shorter hospitalization[38,39,42,43] and lower blood loss[20,38,39,43]. It is clear that within 2 decades most authors adopted conclusions that were opposite to those in the late 1990s[42-49].
Although there now seemed to be a general embrace of the minimally invasive approach, the Dutch LEOPARD trials[50] mounted a challenge. The Dutch LEOPARD trial was a multi-centre randomized blinded trial that randomized 99 patients to OPD or LPD[50]. The surgeons in this trial were highly skilled pancreatic surgeons who had to complete at least 20 LPDs in an approved training programme before they participated in the study. Patients who underwent LPD had a trend toward greater 90-d mortality (10% vs 2%; P = 0.02; RR 4.9; 95%CI: 0.59-40.4), but no difference in median time to functional recovery (10 d vs 8 d; 95%CI: 7-9), no difference in major morbidity (50% vs 39%; RR 1.29; 95%CI: 0.82-2.02; P = 0.26) and no difference in grade B/C pancreatic fistula (28% vs 24%; RR 1.14; 95%CI: 0.59-2.22; P = 0.69). Although there was no statistically significant difference between the two groups, the study was concluded early based on the rationale that the findings were “unexpected and worrisome, especially in the setting of trained surgeons working in centres performing 20 or more pancreatoduodenectomies annually.”
But before consensus was achieved, the direction again shifted soon after the FDA approval of Intuitive’s DaVinci surgical robot[23]. Guilianotti et al[25] in 2003 published the first series of 193 robotic operations that included 8 RPDs. Within a few years, RPD gained traction in resource-rich countries and their outcomes data were published[30,47,48,51]. Four authors attempted to collectively evaluate the existing data in meta-analyses to compare the robotic approach with OPD[52-55].
Peng et al[52] reported on a meta-analysis of 435 patients undergoing OPD vs 245 undergoing RPD across 9 non-randomized studies. Patients in the RPD group had significantly lower overall morbidity, significantly better R0 margin clearance, lower surgical site infections and a shorter duration of post-operative hospital stay. This was achieved without any difference in operation time, node harvest, pancreatic fistulae or mortality. Peng et al[52] concluded that RPD was safe and efficient, but noted that multi-centre randomized, controlled trials were lacking.
Zhao et al[53] published a meta-analysis that compared RPD (assisted) and OPD across 11 non-randomized controlled trials. The robotic approach had longer operative times, but was accompanied by statistically significant reductions in blood loss, surgical site infections, R1 margin involvement, overall morbidity and time to return of post-operative activity. Compared to OPD, there was equivalent lymph node harvest, post-operative pancreatic fistula, hospitalization and mortality rates. Zhao et al[53] concluded that RPD is a “safe and feasible alternative to OPD with regard to perioperative outcomes. However, due to the lack of high-quality randomized controlled trials, the evidence is still limited”.
Shin et al[54] published a systematic review comparing RPD or LPD and OPD. Both techniques had similar oncologic outcomes, but the robotic approach had significantly longer operative times, less intraoperative blood loss and shorter hospital stay. Shin et al[54] concluded that RPD was “feasible and oncologically safe”, but lamented the paucity of robust data.
Podda et al[55] published the most recent metanalysis to date compared 1593 patients who underwent RPD to 12046 patients who underwent OPD across 18 non-randomized studies. They found that both techniques had similar outcomes in mortality, overall morbidity, post-operative pancreatic fistula rates, haemorrhage, bile leaks, nodal harvest and positive margin status. While RPD did require significantly longer operating time (461 min vs 384 min), it did have the advantage of significantly lower operative blood loss (174 mL vs 352 mL). Based on this, Podda et al[55] concluded that RPD was a “safe and feasible alternative” to open surgery.
Simultaneously, two meta-analyses evaluated the existing data to compare the robotic and laparoscopic approaches to PD[47,56]. In 2014 Boggi et al[47] published a systematic review of 746 LPDs done across 25 published articles. These included pure laparoscopic (386), robot assisted (243), laparoscopic assisted (121) and hand-assisted (5) cases. Interestingly, they were able to show that pure LPD was associated with a significant reduction in operative time, blood loss and pancreatic fistulae vs cases completed with laparoscopic assistance and robotic assistance.
Kamarajah et al[56] published a metanalysis comparing outcomes in 2437 patients undergoing LPD and 1025 patients undergoing RPD across 44 studies. They noted that RPD was associated with significantly less conversions, lower transfusion requirements and shorter post-operative hospitalization (11 d vs 12 d). But there was no difference in blood loss, (220 mL vs 287 mL), operating time (405 min vs 418 min), overall morbidity, pancreatic fistula or margin involvement. Kamarajah et al[56] noted that RPD did have advantages, but the data was limited and so had to be considered to “offerequivalent clinical outcomes.”
In summary, it appears that there was a similar swing of the pendulum for PD from open to laparoscopic and robotic approaches, although the movement took longer to gain momentum. Although some authors have documented good outcome data in select patients at experienced centres, most agree that minimally invasive PD, whether purely laparoscopic, hybrid (laparoscopic dissection with open or robotic reconstruction) or wholly robotic, remain in the hands of experienced pancreatic surgeons in high-volume centers.
CARIBBEAN EXPERIENCE
Although the first reports of laparoscopy in the Anglophone Caribbean date back to 1991 with a cholecystectomy in Trinidad & Tobago, MIS remained relatively dormant and did not gain traction in the Caribbean until 2005[57]. As it relates to service centralization, regional referral centers for pancreatic diseases in the Caribbean were only established in 2010 under the auspices of the Caribbean Chapter of the Americas Hepaticopancreaticobiliary Association (AHPBA)[58]. Therefore, a situation exists where both centralization for pancreatic surgery and the minimally invasive surgical revolution are in their infancy in the Caribbean.
The surgeons attached to the pancreatic referral centers all completed formal fellowship training at high-volume hospitals in Canada (4), the United Kingdom (1) and India (1). In these centers they gained sufficient experience to overcome learning curves for the full range of open pancreatic operations and select minimally invasive operations[58]. It is important to appreciate that these training centers all ran accredited fellowships in hepatopancreaticobiliary surgery, but they were high-volume hospitals that operated in different healthcare environments. Although the local surgeons generally surpassed their learning curves, they repatriated to the resource-poor settings with many challenges: scarce blood products, high competition for ICU/HDU beds, an undersupply of consumables and referral bias. Consequently, pancreatic operations are still performed at low volumes in the region. To illustrate this point, consider data from the largest Caribbean hepatopancreatobiliary referral center in Trinidad & Tobago, where only 12.8 pancreaticoduodenectomies were performed annually[58]. This falls short of the “high-volume” mark of 15-20 procedures generally quoted in the medical literature[1,2]. Pancreatic operations outside of these centers are performed in very small volumes by general surgeons with even less experience in pancreatic surgery and with varied MIS exposure.
Although the surgeons in regional referral centers received sufficient exposure during fellowship training to overcome the learning curve for LDP, LPD was not performed regularly during their training[58]. Consequently, LPD is not popular in the region. To date there have only been one published case report of totally LPD[59] and three (un-published) laparoscopic-assisted PDs performed in Trinidad & Tobago. Otherwise, the reports of minimally invasive pancreatic surgery in the Anglophone Caribbean have been limited to three small series of laparoscopic distal pancreatectomies[57,60,61] and two reports of laparoscopic cysto-gastrostomy[62,63]. Up to the year 2021, there were no surgical robots in any English-speaking Caribbean country.
Unpublished data from the registry maintained by the Caribbean Chapter of the AHPBA revealed that only 13 LDPs were performed over the four-year period between January 1, 2014 and December 30, 2017 for trauma (2), adenocarcinoma (2), neuroendocrine tumours (3) and Frantz tumours (3). Generally, the small numbers (3.25 distal pancreatectomies annually) are reflective of low case volumes in the Caribbean. It is hoped that the volumes will increase once the centralization concept is embraced and there is continued progress in MIS in the region. In the region, we experience obstacles that are similar to other developing countries that wish to commence minimally invasive pancreatic surgery: (1) scarce consumables for MIS surgery; (2) lack of universal health insurance for Caribbean populations; (3) paucity of operating list time; (4) limited ICU/HDU space; and (5) poor attitudes toward MIS[58].
Despite the existing challenges, we believe that LDP is an operation that can be done with minimal consumables and in similar time to the open approach. It is attractive to healthcare administrators because it can prevent lengthy hospitalizations, thereby saving limited resources. An intermediate MIS surgeon should have safe dissection skills (since there are no anastomoses) and sufficient familiarity with the instrumentation to make this is a feasible operation to integrate in to a resource-limited environment.
Therefore, for the remainder of this paper, we focus on LDP, cystogastrosomy and enucleations because we believe these are realistic operations to be learned and practiced in developing countries. The authors advocate these as the initial operations to be introduced in expanding MIS programs because it does not require specialized instruments, does not require a keen grasp of intracorporal suturing and treats that part of the pancreas that is most maneuverable and easiest to control if hemorrhage were to occur.
TECHNICAL ASPECTS
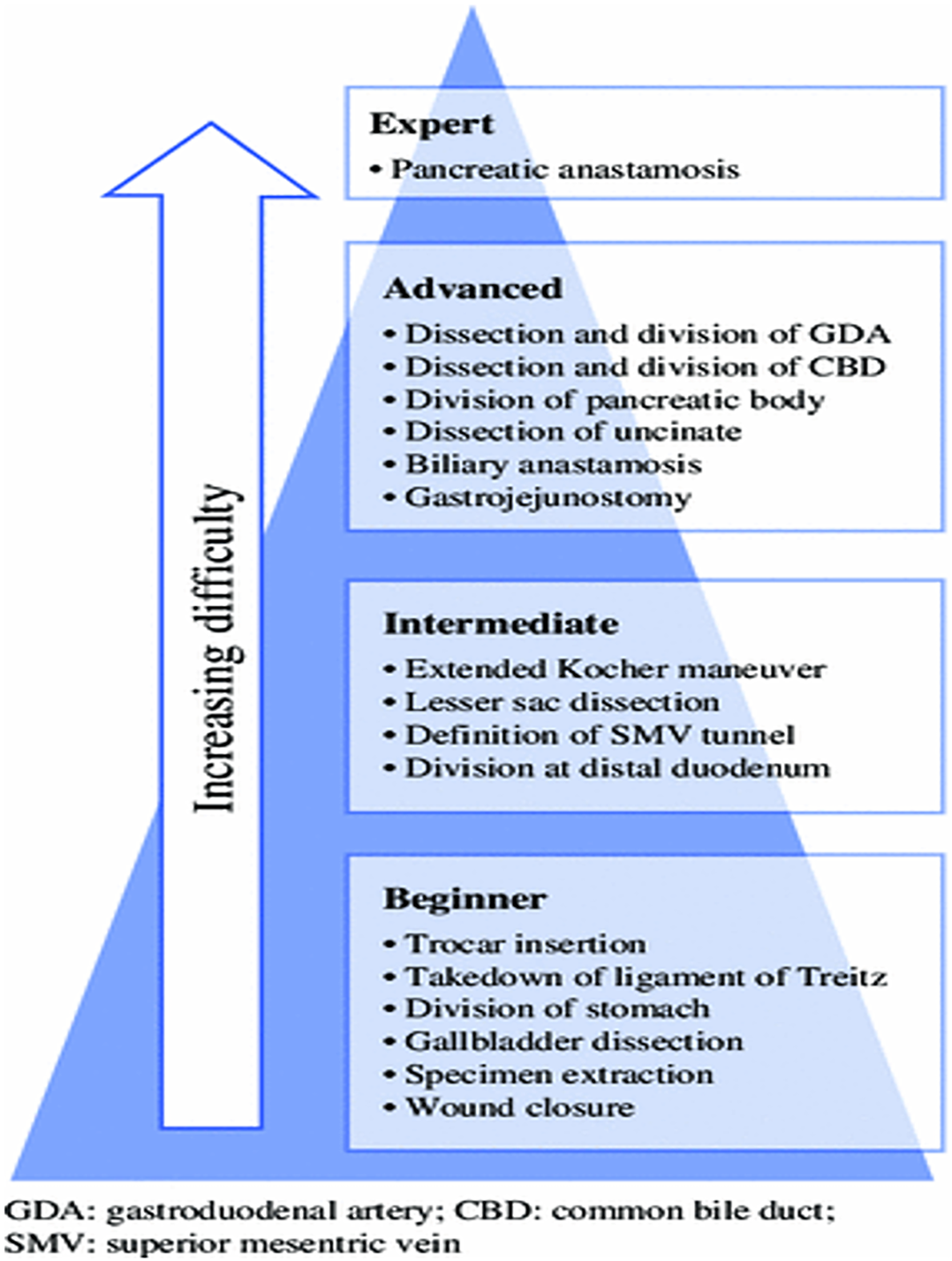
Surgeons seeking to incorporate minimally invasive pancreatic surgery into their practice should be intimately familiar with pancreatic anatomy as well as common anatomic variants. Facility with laparoscopic foregut surgery is an advantage when beginning, including appreciation of optimal patient positioning, port placement, intracorporeal suturing skills and proficiency with laparoscopic ultrasonography. We advocate for a team-based approach, where an advanced laparoscopic surgeon is paired with an experienced pancreatic surgeon. The authors also suggest mastery of the operations in a step-wise fashion, starting with completing the simpler dissections initially and gradually rising to the most difficult step (Figure 1). Initial un-proctored exposure should aim for the most straightforward anatomy possible, and as the learning curve levels off, more challenging cases can be attempted. Finally, there should be no shame or bruised ego when conversion to an open procedure is required as patient welfare and oncologic principles must come first.
Figure 1 Learning curve pyramid illustrating the suggested mastery of pancreatic operations in a step-wise fashion, starting with simpler dissections initially and gradually rising to the most difficult operations.
Citation: Speicher PJ, Nussbaum DP, White RR, Zani S, Mosca PJ, Blazer DG 3rd, Clary BM, Pappas TN, Tyler DS, Perez A. Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol 2014; 21(12): 4014-4019. Copyright © Speicher PJ et al 2014. Published by Springer Nature[74].
Patient selection
Because the pancreas is a retroperitoneal organ, patients with lower body mass index, less visceral fat and no previous abdominal operations are more straightforward laparoscopic candidates. At the same time, thin or short patients may present the surgeon with diminished working space. As skill and comfort develops, less ideal patients can be considered. Of course, a patient with compromised pulmonary mechanics or severe acid-base disorders may not be the best choice for a prolonged laparoscopic procedure, where CO2 retention can be considerable.
Favorable lesions include those located toward the pancreatic tail, requiring less retroperitoneal dissection and laying comfortably away from major vascular structures (celiac trunk, superior mesenteric artery, inferior mesenteric vein, superior mesenteric and portal veins). Benign diseases tend to result in easier dissection planes, as do small neuroendocrine lesions. In contrast, “benign” acute or chronic pancreatitis may present obscure tissue planes and rock-hard fibrosis making dissection exceedingly challenging. Malignant lesions also can range in the spectrum from small tumors with minimal desmoplastic reaction to large immobile tumors which obscure a laparoscopic camera view.
Distal pancreatectomies are the most straightforward cases to integrate, especially where instrumentation, personnel and operative time may be limited. When the surgeon is prepared to tackle more complex cases, patient selection is again paramount. Even many experienced laparoscopic or robotic surgeons will not plan a minimally invasive approach for lesions that abut or invade major vascular structures.
Patient positioning and trocar placement
Careful patient positioning is a critical step for successful minimally invasive pancreatectomy. The first consideration is where the operating surgeon will stand. To perform a distal (left-sided) pancreatectomy, the surgeon may either stand to the patient’s right side or between the legs in a modified lithotomy position. A surgeon performing midline (central) pancreatectomy or pancreaticoduodenectomy may be better served standing between split legs or on the patient’s left side. Steep reverse Trendelenberg position is useful to allow viscera to fall, facilitating exposure of the lesser sac. To ensure patient safety, footboards should be in place and safety straps should be used to prevent slipping. An electric operating table is useful (but not essential) to provide lateral tilt for gravity-assisted retraction of viscera.
Optimal trocar placement relies on the principle of triangulation, where the visual access, operator and assistant are arranged in a triangle centered on the target pathology; the pathology should be considered the apex of a diamond with the instruments as the remaining three points. Thus, in a left upper quadrant procedure, one or two working trocars should be midline/right abdomen, the viewing port left mid-abdomen, and a retraction port left lateral abdomen. However, a large pannus often distorts anatomy, with the umbilicus far more caudad, so the trocars may be better positioned more cephalad than external landmarks indicate. Handedness of the operating surgeon may also play a role in trocar position and size. The size of the trocars depends on the instruments used; often the authors will start with all 5 mm trocars and upsize when it is clear where the stapler, suturing device or extraction site will be best positioned. Most of the dissection can be done with 5 mm instruments and energy devices. All trocars are typically placed off midline for these cases; the umbilical trocar used for appendectomy or cholecystectomy is rarely needed.
Technical aspects of dissection including instrumentation
There are many types of laparoscopic instruments available, and surgeons should accumulate a representative selection for the task at hand. We typically use 30 angled laparoscopes and pneumoperitoneum pressures ranging from 12-15 mmHg, depending on body habitus and respiratory physiology.
Atraumatic graspers should be used to grasping bowel and stomach. Note that most pancreata are fragile and should not be grasped, but rather nudged or held by surrounding fatty tissue. Finer dissectors, either needle-nosed, round-nosed or curved, can be used for blunt dissection and one might consider a few bariatric length instruments for use on the proximal short gastric vascular bundles and around the spleen. Fine electrocautery tips or hooks are handy tools for rendering hemostasis on raw surfaces. Energy instruments are useful for hemostatic peripancreatic dissection and the surgeon should ultimately choose ones they are comfortable with, remaining aware of the capabilities and precautions of each.
As with the energy devices, the surgeon should choose the stapling device they wish to use based on: what is available in their setting, one that they are comfortable with, that is easy for them to handle and fire, and reasonable for the operating room staff to load. One prerequisite is that the stapler should be able to articulate and rotate, as the retroperitoneal space does not offer much flexibility for stapler positioning. Prolonged compression before slow firing may aid in hemostasis and has even been proposed as a maneuver to decrease the incidence of pancreatic leaks[64,65]. Buttress materials for staplers are also available, and there is data suggesting that they decrease the incidence of pancreatic fistula[66]. We advocate using different staple heights based on the types of tissues and texture of the pancreas, though we acknowledge that there is no data to guide this. For very thick pancreata, we prefer to transect with energy devices and suture the duct because a stapled closure may not be robust. In fact, in some cases the stapler may not even close over the organ or it may fracture the gland[67,68]. This technique may be feasible for low resource centres where staplers are not readily available.
Intracorporeal suturing is one of the more challenging minimally invasive skills to acquire. Therefore, laparoscopically assisted dissection and resection can be considered followed by a mini-laparotomy for the reconstruction. Alternatively, there are several enabling devices are available to facilitate suturing such as Endo360 (Endoevolution, LLC, Raynam, MA, United States) or EndoStitch (Covidien Ltd, Minneapolis, MN, United States) devices. As a note, it also takes practice to master these instruments so the surgeon should make an individualized decision on their use, weighing the instrument capabilities and cost with free hand suturing.
Exposure of the pancreas
We were being by using an energy device to open the gastrocolic ligament in an avascular plane, preserving the gastroepiploic vessels along the greater curvature of stomach. If there is an option to preserve the spleen, the short gastric vascular bundles should be preserved as long as possible to allow a Warshaw-type spleen preservation if the main splenic artery and vein need to be sacrificed[69]. If splenectomy is planned or visualization is poor, the short gastric vascular bundles can be divided early. There are often adhesions between the pancreas and the posterior stomach to the pancreas that can be safely divided with energy. The stomach will then need to be retracted to expose the pancreas and this can be achieved either with laparoscopic self-retaining retractors or using a marionette technique to suspending it with sutures placed along the posterior gastric body and exiting through the abdominal wall[70]. This allows the surgeon and assistant to use both hands for the operation.
Dissection of the body and tail of the gland from the retroperitoneum
As the pancreas is often a soft and fragile organ, grasping it should be kept to a minimum, if at all. One may consider placing a small gauze sponge inside the abdomen, for quick access in case unexpected bleeding is encountered or as a gentle retractor held by another instrument. We usually start the pancreatic dissection 2 cm proximal to the intended resection margin, or at the superior mesenteric vein for formal distal pancreatectomy, by lifting the inferior edge using gentle, blunt dissection aided by energy devices as needed. The objective is to make a tunnel behind the pancreas from caudad to cephalad direction. The surgeon must heed the splenic vein, especially during the initial exposure, taking care to avoid direct application of energy to this large vein. The splenic artery is usually easy to separate from the gland, but the splenic vein is not and it is laden with many tiny branches all along the pancreas. Proper identification of the origin of the splenic artery as well as the common hepatic and left gastric branches is critical for dissections near the neck of the gland. We find an articulating instrument such as an esophageal dissector to be useful when creating the retro-pancreatic tunnel and then we routinely pass an umbilical tape through the tunnel to allow retraction and facilitate further dissection.
Division of the pancreas
There is no reconstruction required in LDP so the surgeon aims to divide with an intention to seal. This can be achieved by linear stapling, with or without buttress material, or by dividing with an energy device followed by suturing the cut edge. In a recent multi-institutional retrospective study of fistula after distal pancreatectomy, none of the following operative techniques independently affected the occurrence of fistulae: method of pancreas transection, suture ligation of the pancreatic duct, staple size, the use of staple line reinforcement, tissue patches, biologic sealants, or prophylactic octreotide[71]. Although the study was primarily (70%) comprised of open cases, there is no reason to expect differences for a minimally invasive approach.
Suturing
This is generally more straightforward when suturing mobile organs on mesenteries (stomach, bowel), but becomes increasingly difficult when mobility is limited. And that is compounded when suturing small, fragile pancreatic and bile ducts. To add an extra layer of complexity, many of the previously mentioned suture-assist devices are not well-suited for these anastomoses. The small, straight needles used in these devices are inappropriate for securing a thick pancreas margin, or for passing through fragile pancreatic tissue for a pancreatic anastomosis. Similarly, barbed sutures are generally too traumatic for soft pancreata. Realistically, minimally invasive surgeons intending to perform a pancreatic anastomosis must be practiced in intracorporeal suturing and knot-tying with conventional curved needles. The choice of suture (monofilament vs braided, absorbable vs nonabsorbable) and technique (running vs interrupted) can mirror the surgeon’s choice in open surgery.
PROCEDURES
Pancreatic tumor enucleation
Well-chosen tumor enucleations may be excellent cases to start with during the initial minimally invasive experience. Generally, these are small (< 2 cm), tumors with well-defined borders, in the body and tail of the pancreas. In addition, a reasonable distance from the pancreatic duct (at least 2 mm) is suggested to avoid duct disruption, either directly during dissection or postoperatively due to duct ischemia. The pancreas may not need to be mobilized much, if at all for anterior lesions. Visible lesions are simpler to extract; those requiring ultrasound guidance require a slightly greater degree of sophistication and comfort with the technology. After the tumor is removed the magnification afforded by laparoscopy is excellent to survey the pancreatic bed for evidence of ductal disruption and for hemostasis. In the absence of intra-operative ultrasound, these procedures should be avoided because determination of distance from the main pancreatic duct cannot be determined increasing the risk of pancreatic leak and complications, as in general, enucleations have the highest risk of fistula.
Distal pancreatectomy
Much of the technique for distal pancreatectomy without reconstruction has been reviewed in the previous sections. Splenic preservation should always be considered for benign lesions by either technique: splenic artery and vein preservation (Kimura technique) or maintenance of the short gastric vessels and transection of the splenic artery and vein (Warshaw technique)[69,72,73].
Drainage of symptomatic pancreatic pseudocysts–cystogastrostomy, cystenterostomy
Acute pancreatitis is common, and endoscopic drainage through a transoral route is rarely possible outside of dedicated centers. Large, well-formed pancreatic pseudocysts can interfere in recovery from acute pancreatitis, causing pain and poor oral intake. In these delicate patients, an open operation can cause considerable physiologic stress and delayed healing. A cyst posterior to the stomach can be an excellent opportunity to enlist laparoscopy. The abdomen is explored laparoscopically and an anterior gastrostomy created with diathermy, attending to hemostasis as varices may be present if the splenic vein is thrombosed. If ultrasonography is available, an optimal location for cystogastrostomy can be determined by placing the probe on the posterior gastric wall anterior to the cyst. Otherwise, the cyst is punctured at a point of bulging through the posterior gastric wall to confirm cyst location. Again, attending to hemostasis as varices may be present, the posterior gastric wall is incised with diathermy approximately 2-4 cm until the inside of the cyst can be visualized. Then using endovascular staplers with staple height depending on wall thickness, a stapled, hemostatic anastomosis is created between the posterior gastric and anterior cyst walls. Any debris should be aspirated. The anterior wall of the stomach is stapled closed. In the absence of staplers, the cysto-gastrostomy anastomosis and anterior gastrotomy can be sewn. For large symptomatic cysts not aligned with the stomach, a Roux-en-Y cyst-enterostomy can be created by anastomosing the drainage limb to the pseudocyst. This can be done with staplers or suture, but clearly requires more advanced laparoscopic skills than that for cysto-gastrostomy.
Drainage procedures for chronic pancreatitis–Frey, Puestow
Similarly, the drainage procedures involve a pancreatic anastomosis to a Roux limb of jejunum. The conditions necessitating such procedures often render the gland very firm and fibrotic–delightful to manipulate, but challenging for suture placement with the laparoscopic needle drivers. The length of the instrument is mechanically not favorable to grab on to the needle if too much force is required to pass it through tissue. Little experience is reported on these cases.
CONCLUSION
With minimally invasive pancreatic surgery, one should aim for a better operation that results in less morbidity and improved survival with lesser importance to the abdominal access method or shortening the hospital stay. That being said, more and broader experience with minimally invasive techniques in pancreatic surgery will determine the future of this modality. Despite low pancreatic case volume in the Caribbean, and financial barriers to MIS in general, laparoscopic distal pancreatectomy, enucleation and cystogastrostomy are feasible operations to integrate in to a resource-limited healthcare environment. This is because they can be performed with minimal to no consumables and require an intermediate MIS skillset to complement an open pancreatic surgeon’s peri-operative experience.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Caribbean Chapter AHPBA.
Specialty type: Surgery
Country/Territory of origin: Trinidad and Tobago
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Samiullah S, Wang Z S-Editor: Zhang H L-Editor: A P-Editor: Li X