Published online Sep 27, 2020. doi: 10.4240/wjgs.v12.i9.397
Peer-review started: June 15, 2020
First decision: July 21, 2020
Revised: August 1, 2020
Accepted: August 31, 2020
Article in press: August 31, 2020
Published online: September 27, 2020
Processing time: 102 Days and 0.6 Hours
Survival rates in patients with esophageal cancer undergoing esophagectomy have improved, but the prevalence of gastric tube cancer (GTC) has also increased. Total resection of the gastric tube with lymph node dissection is considered a radical treatment, but GTC surgery is more invasive and involves a higher risk of severe complications or death, particularly in elderly patients.
We report an elderly patient with early GTC that had invaded the duodenum who was successfully treated with resection of the distal gastric tube and Roux-en-Y (R-Y) reconstruction. The tumor was a type 0-IIc lesion with ulcer scars surrounding the pyloric ring. Endoscopic submucosal resection was not indicated because the primary lesion was submucosally invasive, was undifferentiated type, surrounded the pyloric ring, and had invaded the duodenum. Resection of distal gastric tube with R-Y reconstruction was safely performed, with preservation of the right gastroepiploic artery (RGEA) and right gastric artery (RGA).
Distal resection of the gastric tube with preservation of the RGEA and RGA is a good treatment option for elderly patients with cT1bN0 GTC in the lower part of the gastric tube.
Core Tip: Surgical removal of the reconstructed gastric tube is invasive and carries a relatively high risk of postoperative morbidity and mortality, especially for elderly patients. We present the case of an 82-year-old man who underwent successful resection of distal gastric tube for early gastric tube cancer with duodenal invasion. The interesting features of this case include the advanced age of the patient, distal resection of a gastric tube reconstructed via the posterior mediastinal route, and preservation of the right gastroepiploic artery and right gastric artery. None of these features have been described in previous reports.
- Citation: Yura M, Koyanagi K, Adachi K, Hara A, Hayashi K, Tajima Y, Kaneko Y, Fujisaki H, Hirata A, Takano K, Hongo K, Yo K, Yoneyama K, Dehari R, Nakagawa M. Distal gastric tube resection with vascular preservation for gastric tube cancer: A case report and review of literature. World J Gastrointest Surg 2020; 12(9): 397-406
- URL: https://www.wjgnet.com/1948-9366/full/v12/i9/397.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i9.397
Gastric and head and neck cancers are the most frequent malignancies co-occurring with esophageal cancer[1]. Recent advances in the diagnosis and treatment of esophageal cancer have improved prognosis after esophagectomy but have also led to an increasing occurrence of gastric tube cancer (GTC). While the use of endoscopic resection for early GTC without lymph node (LN) metastasis has increased[2-4], total resection of the gastric tube with LN dissection is considered the standard radical treatment for GTC when endoscopic submucosal dissection (ESD) is not indicated. Surgical removal of a reconstructed gastric tube is an invasive procedure that involves a higher risk of postoperative morbidity and mortality[5]. In particular, the possibility of LN metastasis and operative mortality must be carefully considered in choosing the surgery. Less invasive surgery should be considered for elderly and other high-risk patients even if there is a risk of LN metastasis. Distal resection is one of the less invasive procedures for patients with early GTC located on the distal side of the gastric tube. Moreover, preservation of the main trunk of the right gastroepiploic artery (RGEA) and the right gastric artery (RGA) would retain the main blood supply to the reconstructed gastric tube, given that the other gastric vessels were divided during the previous surgery. Several reports have described successful partial resection for early GTC[6,7]. Here, we present a successful case of surgical treatment for GTC and provide details of the surgical procedure. The noteworthy features of this case include the advanced age of the patient (82 years; older than in any previous surgical reports), GTC invading the duodenum, previous reconstruction via the posterior mediastinum, and resection of the distal gastric tube while preserving the RGEA and RGA.
An 82-year-old man presented to the Department of Surgery of the Hiratsuka City Hospital (Japan). He had no complaints and had continued to visit the hospital for routine postoperative follow-up examinations after esophagectomy for esophageal cancer.
The patient had undergone subtotal esophagectomy with gastric tube reconstruction via the posterior mediastinal route for esophageal cancer 13 years earlier. No recurrence was detected, but gastric tube cancer was found during a routine follow-up 13 years after the original subtotal esophagectomy.
The patient had hypertension, chronic renal disease, and a history of subtotal esophagectomy with posterior mediastinal reconstruction for esophageal cancer 13 years earlier. Pathological examination of the resected esophageal cancer revealed a basaloid carcinoma with invasion of the submucosa, diagnosed as pT1bN0M0, p-stage I according to the Union for International Cancer Control Tumor Node Metastasis Classification of Malignant Tumors, 8th edition[8].
No noticeable physical findings other than the previous surgical incision were observed.
A blood analysis revealed mild anemia (hemoglobin; 12.3 g/dL) with normal white blood cell and platelet counts. Prothrombin and partial thromboplastin times were normal. Blood biochemistry revealed chronic renal disease (creatinine, 1.09 mg/dL: Creatinine clearance, 46.7 mL/min) and increased brain natriuretic peptide 128.5 pg/mL (normal range: < 18.0 pg/mL).
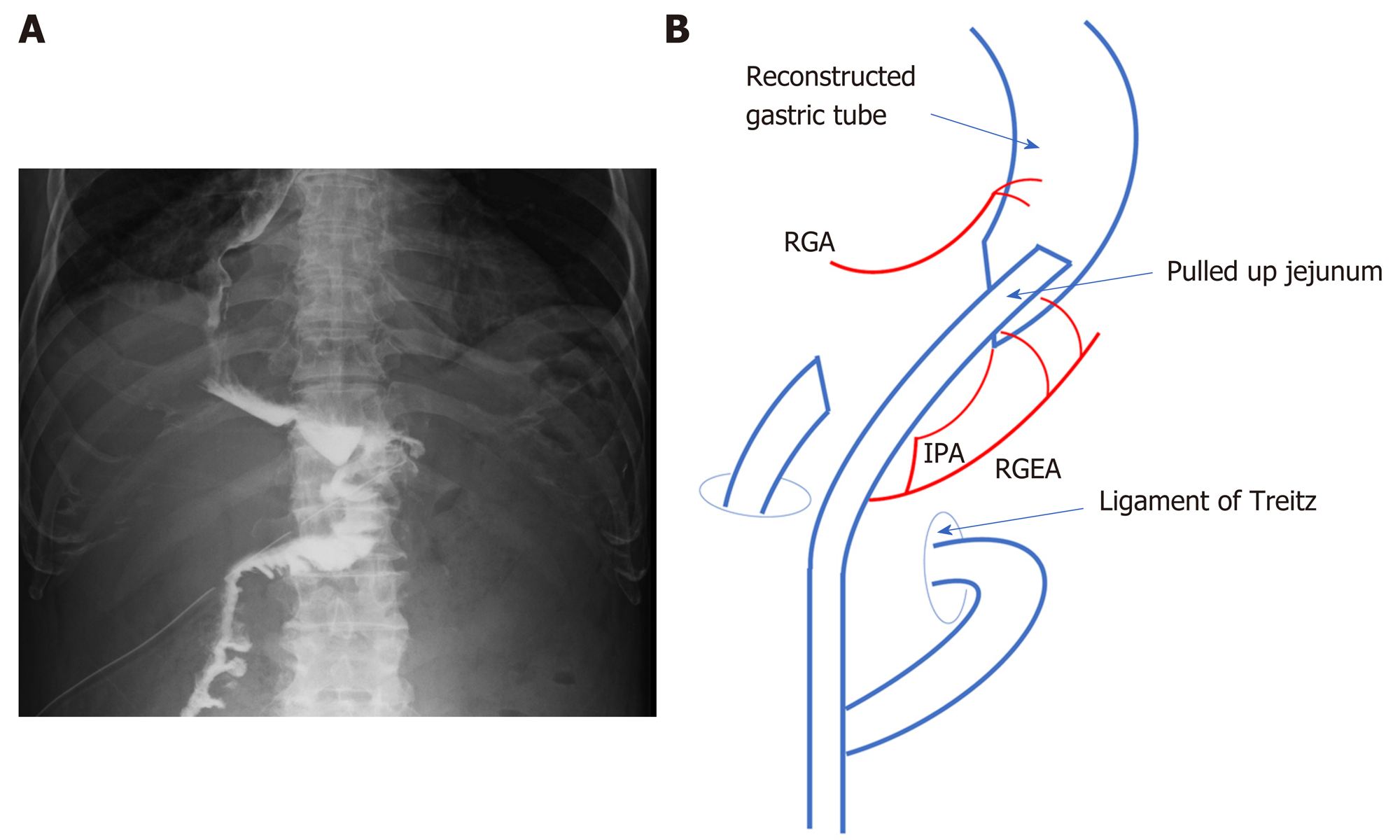
An electrocardiogram showed ST-T abnormalities on leads II, III, aVf, and V4-6. Echocardiography revealed almost normal cardiac function with mild aortic stenosis. A pulmonary function examination revealed the following: Vital capacity, 72.6% of predicted; and forced expiratory volume in 1 s, 67.7% of predicted (1.57 L). A chest X-ray showed the reconstructed gastric tube in the mediastinum.
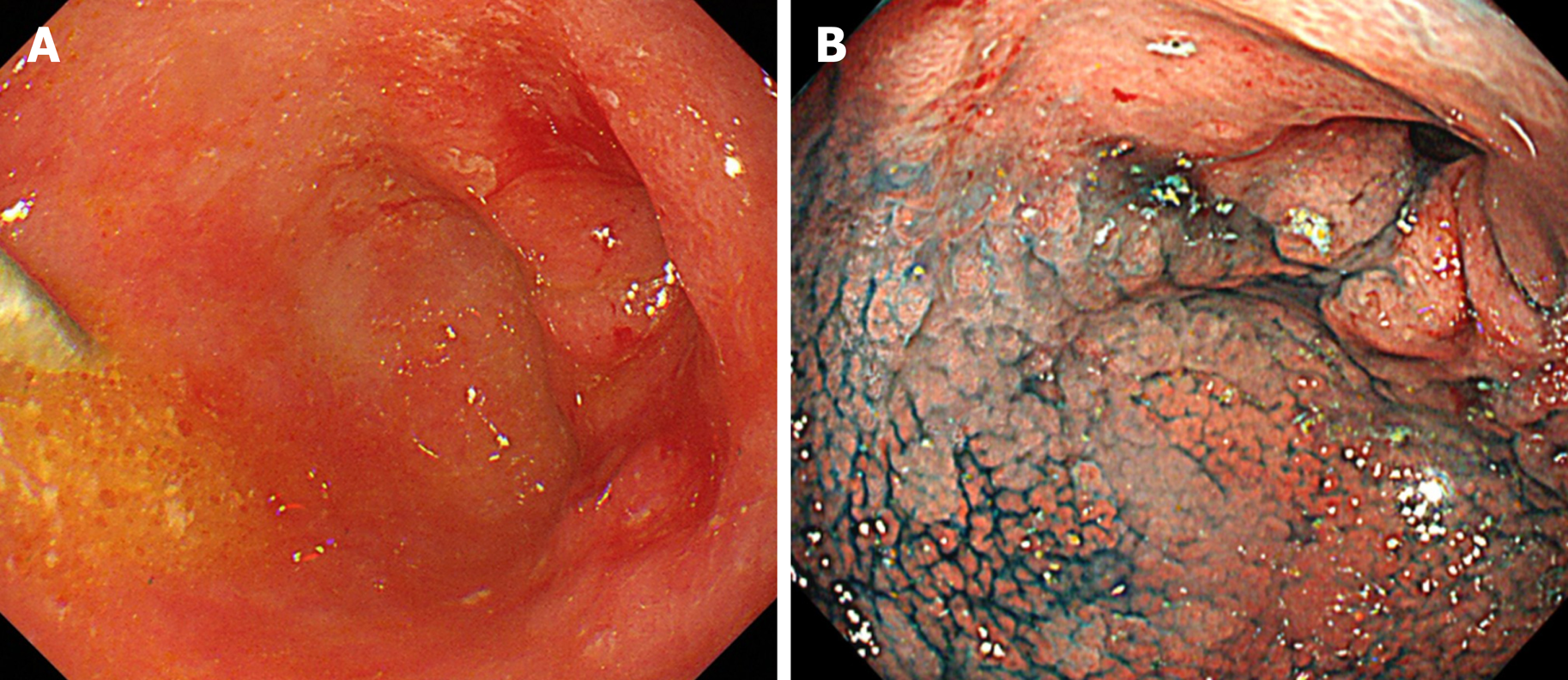
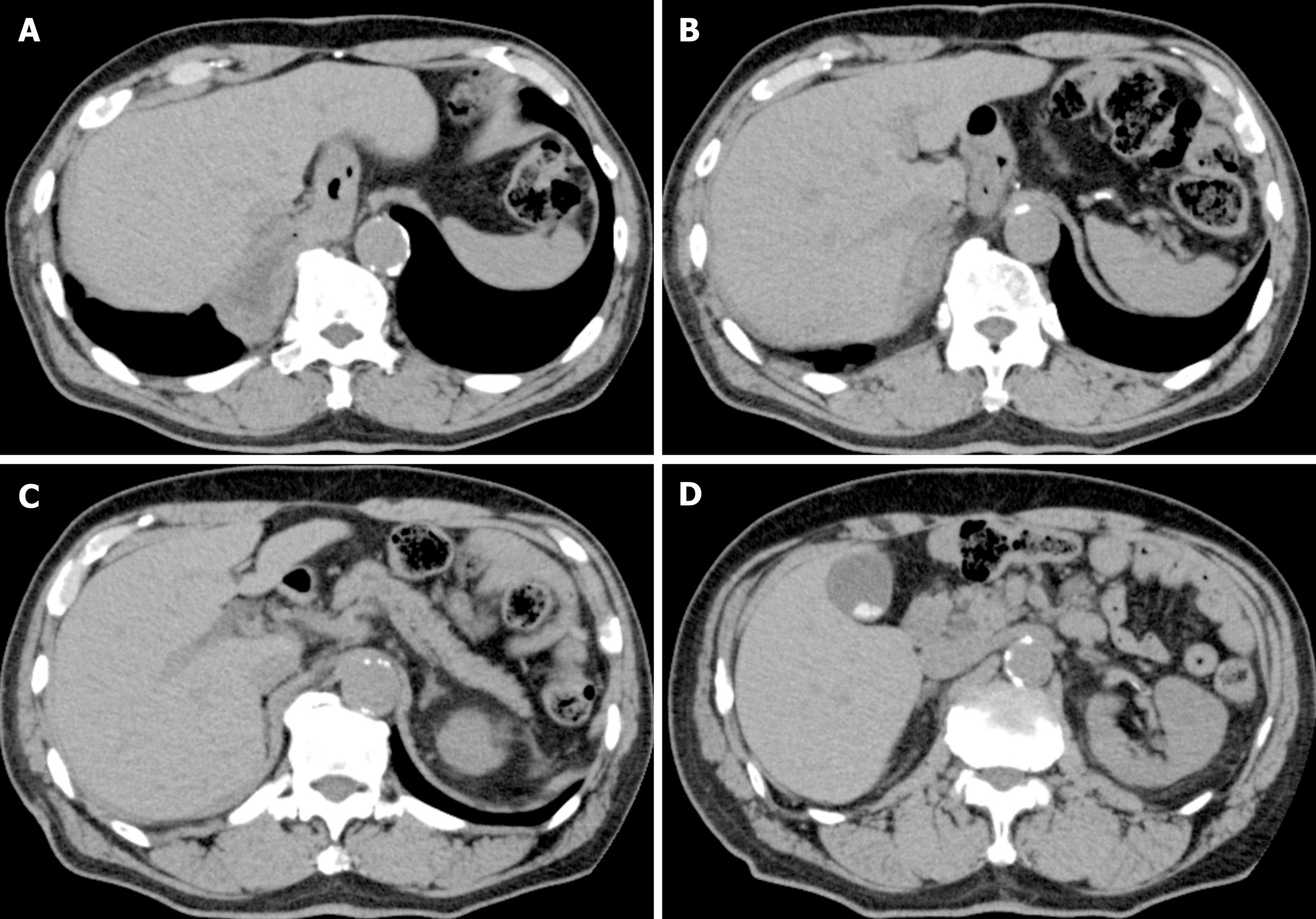
Endoscopic examination revealed a type 0-IIc tumor with an ulcer scar surrounding the pyloric ring and invading the duodenum. The lesion was not stained by indigo carmine (Figure 1A and B). Computed tomography was unable to detect the primary tumor, and no obvious nodal metastasis was observed (Figure 2A-D).
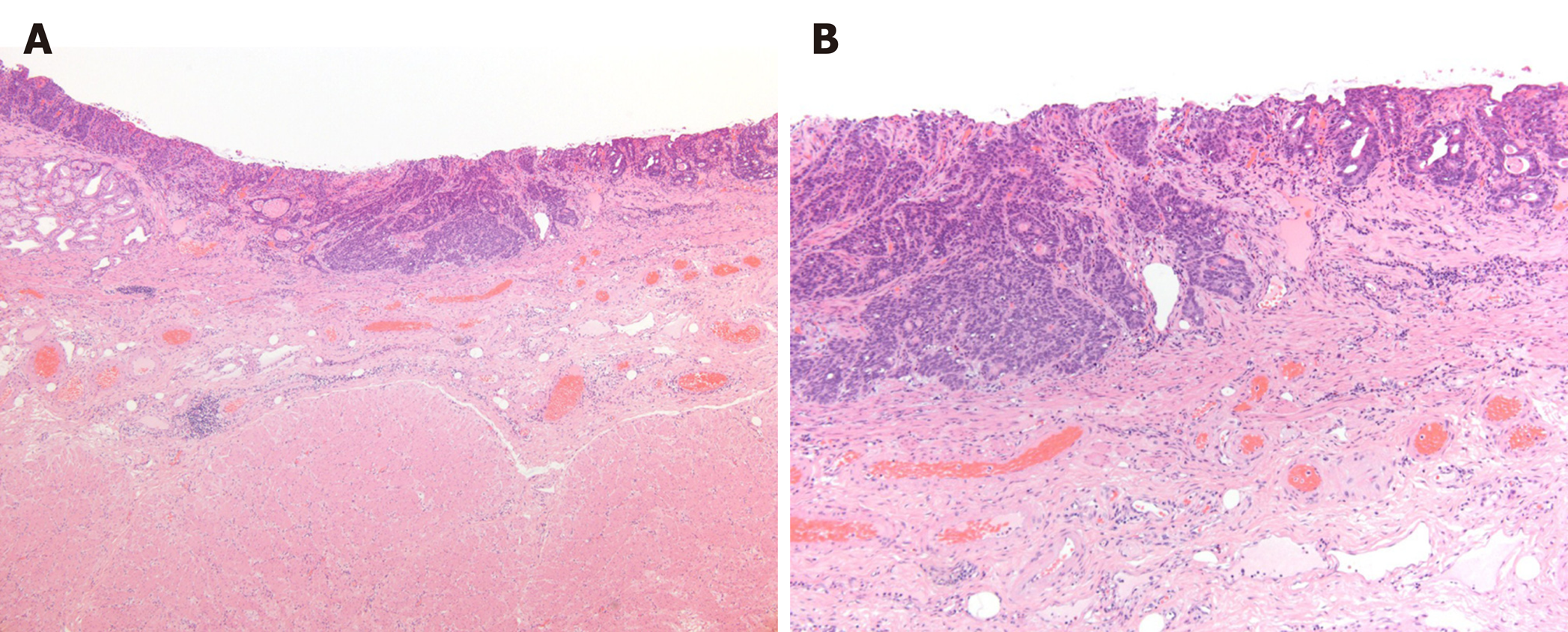
Pathological examination of the resected specimen revealed a moderately to poorly differentiated adenocarcinoma with submucosal invasion and ulcerative scars (UL-IIs–IIIs) without lymphovascular invasion (Figure 3A and B). The cancer had invaded the duodenum. The horizontal and vertical surgical margins were negative, and the #6LN was negative for metastasis. The pathological diagnosis was pT1bN0M0 pStage IA.
Biopsies were taken, and the histological examination led to a diagnosis of a moderately to poorly differentiated adenocarcinoma. The preoperative GTC stage was LD circ cT1bN0M0 cStage I according to the Japanese Classification of Gastric Carcinoma, 15th edition[9].
ESD was not indicated for the GTC based on guidelines for treating gastric cancer[10] because the lesion was diagnosed as an undifferentiated type with submucosal invasion. Additionally, the lesion surrounded the pyloric ring and had invaded the duodenum, so ESD would have been technically difficult. Surgical risk factors for this patient included his advanced age (82 years), poor pulmonary function, chronic renal disorder, hypertension, and history of subtotal esophagectomy with posterior mediastinal reconstruction and mild aortic stenosis. The therapeutic strategy was explained to the patient, who opted to undergo surgery.
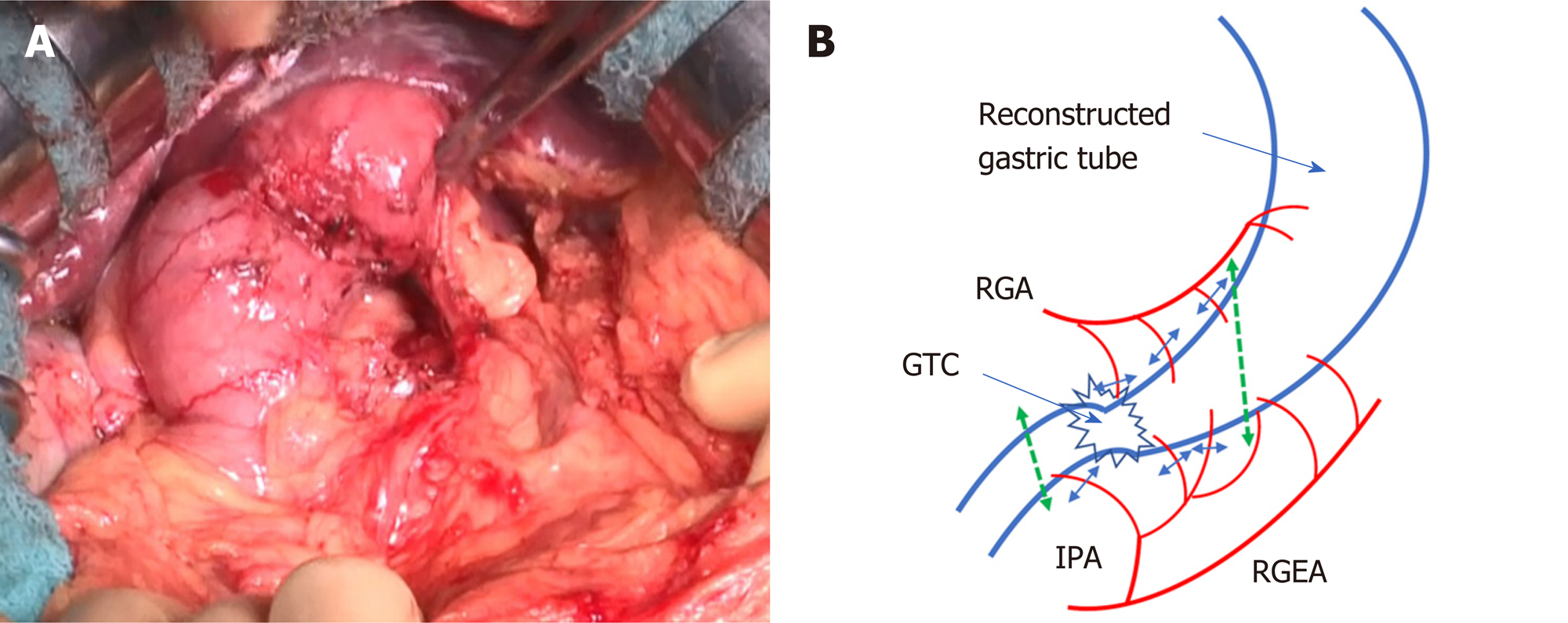
Delamination was performed between the omentum and abdominal wall along the previous surgical wound. The gastric tube was then mobilized by separating the anterior and posterior lobes of the mesocolon up to the vicinity of the root of the right gastroepiploic vein. After a Kocher mobilization, the RGA, RGEA, and infrapyloric artery supplying blood to the gastric tube were identified. To preserve the main trunks of the arteries, peripheral branches to the stomach wall were cut according to the area to be resected (Figure 4A and B). We also performed #6LN sampling because of swelling, but the sample was not submitted for an intraoperative pathological examination. The duodenum was cut as distally as possible to maintain an adequate surgical margin, using a 60-mm Endo GIA Reinforced Reload with Tri-Staple stapler (Covidien, Mansfield, MA, United States). Esophagogastroduodenoscopy was performed during surgery to determine the proximal cutting location line. The gastric tube was cut in the abdominal cavity using a 60-mm stapler. Gastrojejunostomy (GJ) was performed with the stapler, and the antecolic R-Y reconstruction was completed. GJ was performed on the anterior wall of the greater curvature to avoid vessel injury (Figure 5A and B). Resection of the distal gastric tube was successful, and the proximal side was preserved without the need for thoracotomy. Sufficient surgical margins were confirmed in the resected material. The total operation time was 4 h 48 min, and total blood loss was < 10 mL.
The patient was able to drink water on postoperative day 1, resumed eating on postoperative day 2, and was discharged on postoperative day 12 without any surgical complications (Clavien-Dindo grade > 3)[11]. The patient’s diet and activities of daily living were the same as before surgery.
We present the case of an 82-year-old man who underwent successful resection of distal gastric tube for early GTC with duodenal invasion. The interesting features of this case include the advanced age of the patient, distal resection of a gastric tube reconstructed via the posterior mediastinal route, and preservation of the RGEA and RGA. None of these features have been described in previous reports. In general, total resection of the gastric tube with LN dissection is considered the standard radical treatment for GTC for which ESD is not indicated. However, surgical removal of the reconstructed gastric tube is invasive and carries a relatively high risk of postoperative morbidity and mortality[5]. Furthermore, this procedure is complicated when the reconstruction route selected at the original esophagectomy was via the posterior mediastinum, because the gastric tube is surrounded by vital organs, including the heart, lungs, and aorta. We previously reported that the level of difficulty of the surgical procedure differs according to the route of reconstruction, and accounts for differences in the resection rate (50%, 77%, and 93% for the posterior mediastinal, retrosternal, and ante-thoracic routes, respectively)[12]. Because a gastric tube pulled up via the posterior mediastinal route exists in the deepest part of the abdomen, even partial resection will be difficult unless the lesion is located near the pyloric ring, as in the present case. However, in elderly and high-risk patients with early GTC without LN metastasis, distal resection of the gastric tube is considered to be a safer surgical technique than complete resection of the gastric tube.
Due to recent advances in the diagnosis and treatment of esophageal cancer, surgical outcomes have improved and the prevalence of GTC after esophagectomy has increased to 2.1%-3.5%[2,13]. The characteristics and treatments of 224 GTC patients reported in 29 studies (12 retrospective studies and 17 case reports) between 1998 and 2020 (Table 1)[3-6,12,14-37] showed that the mean age of patients at the time of GTC treatment was 67.2 years (range: 43-85 years), and 202 patients (90.2%) were male. According to the available data of these studies, the majority of GTCs were the differentiated type (77.3%; n = 160), followed by the undifferentiated type (19.8%; n = 41), and others (mixed type, neuroendocrine carcinoma, squamous cell carcinoma; n = 7). The majority of the lesions were located in the lower part of the gastric tube (59.4%; n = 107), while the middle third was the second most common location (32.2%; n = 58). Endoscopic mucosal resection and ESD were the most frequently used treatments (n = 138), followed by total or subtotal resection (n = 44), partial resection (n = 31), chemotherapy/chemoradiotherapy/palliative treatment (n = 19), and distal resection or anterectomy of the gastric tube (n = 5). The mean time from the original esophageal cancer to GTC was 60.9 mo (range: 4-236 mo).
| Ref. | Publication year | Journal | Study type | Number of patients |
| Horie et al[36] | 2020 | Asian J Endosc Surg | Case report | 1 |
| Yamana et al[35] | 2020 | Int J Surg Case Rep | Case report | 1 |
| Hara et al[34] | 2020 | Surg Case Rep | Case report | 1 |
| Hirayama et al[4] | 2019 | Esophagus | Retrospective study | 29 |
| Shirakawa et al[6] | 2018 | Esophagus | Retrospective study | 30 |
| Mukasa et al[33] | 2015 | World J Gastroenterol | Retrospective study | 11 |
| Lee et al[32] | 2014 | Eur J Cardiothorac Surg | Retrospective study | 18 |
| Tawaraya et al[3] | 2014 | Gastrointest Endosc | Retrospective study | 15 |
| Ho et al[31] | 2014 | Dis Esophagus | Case report | 4 |
| Kim et al[30] | 2012 | Korean J Thorac Cardiovasc Surg | Case report | 1 |
| Saito et al[29] | 2012 | J Surg Oncol | Case report | 2 |
| Jabłoński et al[28] | 2012 | World J Surg Oncol | Case report | 1 |
| Shiozaki et al[27] | 2012 | Surg Today | Case report | 1 |
| Oki et al[26] | 2011 | Surg Today | Retrospective study | 10 |
| Yoon et al[25] | 2011 | Eur J Cardiothorac Surg | Retrospective study | 10 |
| Bamba et al[24] | 2010 | Surg Endosc | Retrospective study | 25 |
| Zygoń et al[37] | 2010 | BMJ Case Rep | Case report | 1 |
| Osumi et al[23] | 2009 | Endoscopy | Retrospective study | 7 |
| Motoyama et al[22] | 2006 | Ann Thorac Surg | Case report | 2 |
| Yamashita et al[21] | 2006 | Dig Liver Dis | Case report | 1 |
| Atmani et al[20] | 2006 | Dis Esophagus | Case report | 2 |
| Okamoto et al[19] | 2004 | Ann Thorac Surg | Retrospective study | 8 |
| Akita et al[18] | 2004 | J Surg Oncol | Retrospective study | 5 |
| Ikeda et al[17] | 2003 | J Thorac Cardiovasc Surg | Case report | 1 |
| Lamblin et al[16] | 2003 | Dis Esophagus | Case report | 3 |
| Sugiura et al[5] | 2002 | J Am Coll Surg | Retrospective study | 26 |
| Shigemitsu et al[15] | 2002 | Jpn J Clin Oncol | Case report | 5 |
| Ben-nun et al[14] | 2000 | Dis Esophagus | Case report | 1 |
| Koyanagi et al[12] | 1998 | J Gastroenterol Hepatol | Case report | 2 |
In our patient, GTC was detected 13 years after esophagectomy. Thus, long-term follow-up (> 5 years) is necessary to detect GTC in the early stage after esophagectomy. Early detection allows less invasive and curative treatment via ESD, partial resection, or resection of the gastric tube, without the need for total gastric tube resection. The efficacy and safety of endoscopic resection for GTC have been demonstrated in several studies[2,4,33]. Hirayama et al[4] reported an 85% (28/33) curative resection rate using ESD for early GTC, and the 2- and 5-year overall survival rates were 73.3% and 64.1%, respectively. Nonaka et al[2] reported that the disease-specific 5-year survival rate in non-curative patients after ESD was 72.7%. Additional surgery was not performed in 15 of 16 (94%) non-curative resection patients at risk of LN metastasis. These survival rates were considered acceptable in the present case, because the 5-year survival rate of a general 82-year-old male cohort in Japan is 66.4%[38]. Nonetheless, even at an early stage of GTC, endoscopic resection is difficult when the lesion is located on the staple line or pyloric ring, as was the case in our patient, and it may require a surgical approach with a higher risk of postoperative complications. Suzuki et al[39] reported a high mortality rate (33%) for total resection of a reconstructed gastric tube after esophagectomy. Nonaka et al[2] also reported a high mortality rate (23.8%) even in a high-volume Japanese cancer center.
A previous study reported that the frequency of nodal metastasis in GTC was 0% for intramucosal tumors and 10% for submucosal tumors[24], such that LN dissection may be unnecessary for the vast majority of patients with early GTC. Generally, surgical gastrectomy with LN dissection is the standard treatment for early gastric cancer because there is a 1%-10% possibility of concurrent LN metastasis[10]. However, elderly people are at higher risk of surgery-related death than are non-elderly patients, and the benefits of minimizing the risk of recurrence and metastasis in this group may be less profound. Therefore, if the estimated LN metastasis rate is lower than the 5-year mortality rate of a general cohort of the same age, surgery without LN dissection should be considered. In the present case, the swollen #6LN was resected but not checked by intraoperative pathological examination, because we had no plans to perform radical LN dissection to prevent fatal complications (such as necrosis of the gastric tube) considering the status of the patient. Yagi et al[7] reported that sentinel node biopsy could help avoid unnecessary LN dissection. However, it cannot be performed at every institution, nor has the accuracy of sentinel node biopsy been confirmed in patients undergoing gastrointestinal tract reconstruction including the gastric tube.
Preserving the blood supply of the remnant gastric tube is necessary for any partial resection of reconstructed gastric tube. LN dissection with resection of the distal gastric tube requires ligation of the RGEA and RGA, which can injure these vessels and cause ischemia in the lower and middle parts of the gastric tube. Yoshida et al[40] reported two cases of resection of the distal part of the gastric tube, with dissection of the RGEA and RGEV, that required vascular reconstruction. Saito et al[29] also reported two cases of subtotal gastric tube resection in which the RGEA and RGA were sacrificed based on an intraoperative indocyanine green evaluation of blood flow. In those cases, blood supply was re-established from the cervical esophagus for 5 cm until the proximal region of the gastric tube. However, vascular charge was needed during GJ. Additionally, operation time and blood loss were 538-783 min and 1490-2855 mL, both of which are considered highly invasive in elderly and/or high-risk patients. Therefore, the RGEA and RGA should be preserved during resection of the distal gastric tube in older or high-risk patients to prevent invasive surgery and fatal complications.
Some limitations of this study should be discussed. First, the observation period after the surgery was only 5 mo, although we continued to check for recurrence during additional follow-up visits. Thus, only the feasibility of the procedure could be demonstrated in this report. Second, the acceptable morbidity and mortality rates for prophylactic LN dissection are not clear but will vary depending on the patient’s age, condition and attitudes about life and death, which will complicate surgical decision-making.
We suggest that resection of the distal gastric tube with preservation of the main trunk of the RGEA and RGA can serve as a safe surgical treatment for cT1bN0 GTC in elderly patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Han JG, Yu PF S-Editor: Huang P L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Nagasawa S, Onda M, Sasajima K, Takubo K, Miyashita M. Multiple primary malignant neoplasms in patients with esophageal cancer. Dis Esophagus. 2000;13:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Nonaka S, Oda I, Sato C, Abe S, Suzuki H, Yoshinaga S, Hokamura N, Igaki H, Tachimori Y, Taniguchi H, Kushima R, Saito Y. Endoscopic submucosal dissection for gastric tube cancer after esophagectomy. Gastrointest Endosc. 2014;79:260-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Tawaraya S, Jin M, Matsuhashi T, Suzuki Y, Sawaguchi M, Watanabe N, Onochi K, Koizumi S, Hatakeyama N, Ohba R, Mashima H, Ohnishi H. Advanced feasibility of endoscopic submucosal dissection for the treatment of gastric tube cancer after esophagectomy. Gastrointest Endosc. 2014;79:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Hirayama Y, Fujisaki J, Yoshimizu S, Horiuchi Y, Yoshio T, Ishiyama A, Hirasawa T, Imamura Y, Mine S, Watanabe M, Tsuchida T. Efficacy and safety of endoscopic resection for gastric tube cancer after surgical resection of esophageal squamous cell carcinoma. Esophagus. 2019;16:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Sugiura T, Kato H, Tachimori Y, Igaki H, Yamaguchi H, Nakanishi Y. Second primary carcinoma in the gastric tube constructed as an esophageal substitute after esophagectomy. J Am Coll Surg. 2002;194:578-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Shirakawa Y, Noma K, Maeda N, Ninomiya T, Tanabe S, Kikuchi S, Kuroda S, Nishizaki M, Kagawa S, Kawahara Y, Okada H, Fujiwara T. Clinical characteristics and management of gastric tube cancer after esophagectomy. Esophagus. 2018;15:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Yagi Y, Ii T, Tanaka S, Oguri H. Resection of distal gastric tube cancer with sentinel node biopsy: a case report and review of the literature. World J Surg Oncol. 2015;13:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors: International union against cancer. 8th ed. Oxford: Wiley; 2017. |
| 9. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, 15th ed. Tokyo: Kanehara Publisher, 2017. |
| 10. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1338] [Article Influence: 334.5] [Reference Citation Analysis (2)] |
| 11. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8627] [Article Influence: 539.2] [Reference Citation Analysis (0)] |
| 12. | Koyanagi K, Ozawa S, Ando N, Shih CH, Nakamura E, Takeuchi H, Hayashi K, Kitajima M. Case report: Metachronous early gastric carcinoma in a reconstructed gastric tube after radical operation for oesophageal carcinoma. J Gastroenterol Hepatol. 1998;13:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Motoyama S, Saito R, Kitamura M, Suzuki H, Nakamura M, Okuyama M, Imano H, Inoue Y, Ogawa J. Prospective endoscopic follow-up results of reconstructed gastric tube. Hepatogastroenterology. 2003;50:666-669. [PubMed] |
| 14. | Ben-nun A, Soudack M, Best LA. Gastric tube gastrectomy. Dis Esophagus. 2000;13:243-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Shigemitsu K, Naomoto Y, Shirakawa Y, Haisa M, Gunduz M, Tanaka N. Five cases of early gastric cancer in the reconstructed gastric tube after radical resection for esophageal cancer. Jpn J Clin Oncol. 2002;32:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Lamblin A, Mariette C, Triboulet JP. Adenocarcinoma in a gastric tube after esophagectomy for esophageal carcinoma. Dis Esophagus. 2003;16:158-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Ikeda Y, Tobari S, Niimi M, Kodaira S, Okinaga K. Second primary double carcinomas of the residual cervical esophagus and the gastric tube after thoracic esophagectomy. J Thorac Cardiovasc Surg. 2003;125:1561-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Akita H, Doki Y, Ishikawa O, Takachi K, Miyashiro I, Sasaki Y, Ohigashi H, Murata K, Noura S, Yamada T, Eguchi H, Imaoka S. Total removal of the posterior mediastinal gastric conduit due to gastric cancer after esophagectomy. J Surg Oncol. 2004;85:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Okamoto N, Ozawa S, Kitagawa Y, Shimizu Y, Kitajima M. Metachronous gastric carcinoma from a gastric tube after radical surgery for esophageal carcinoma. Ann Thorac Surg. 2004;77:1189-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Atmani A, Topart P, Vandenbroucke F, Louzi A, Ferrand L, Lozac'h P. Metachronous cancer of gastroplasty after esophagectomy. Dis Esophagus. 2006;19:512-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Yamashita H, Kitayama J, Ishigami H, Yamaguchi H, Souma D, Nagano R, Nagawa H. Multiple gastric tube carcinomas after curative oesophagectomy. Dig Liver Dis. 2006;38:214-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Motoyama S, Saito R, Okuyama M, Maruyama K, Ogawa J. Treating gastric tube cancer with distal gastrectomy preserving the gastroepiploic artery. Ann Thorac Surg. 2006;81:751-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Osumi W, Fujita Y, Hiramatsu M, Kawai M, Sumiyoshi K, Umegaki E, Tokioka S, Yoda Y, Egashira Y, Abe S, Higuchi K, Tanigawa N. Endoscopic submucosal dissection allows less-invasive curative resection for gastric tube cancer after esophagectomy - a case series. Endoscopy. 2009;41:777-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Bamba T, Kosugi S, Takeuchi M, Kobayashi M, Kanda T, Matsuki A, Hatakeyama K. Surveillance and treatment for second primary cancer in the gastric tube after radical esophagectomy. Surg Endosc. 2010;24:1310-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Yoon YS, Kim HK, Choi YS, Kim K, Kim J, Shim YM. Primary gastric cancer in an oesophageal gastric graft after oesophagectomy. Eur J Cardiothorac Surg. 2011;40:1181-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Oki E, Morita M, Toh Y, Kimura Y, Ohgaki K, Sadanaga N, Egashira A, Kakeji Y, Tsujitani S, Maehara Y. Gastric cancer in the reconstructed gastric tube after radical esophagectomy: a single-center experience. Surg Today. 2011;41:966-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Shiozaki A, Fujiwara H, Ichikawa D, Okamoto K, Komatsu S, Murayama Y, Ikoma H, Kuriu Y, Nakanishi M, Ochiai T, Kokuba Y, Sonoyama T, Otsuji E. Video-assisted surgery for gastric carcinoma arising in a gastric tube reconstructed retrosternally. Surg Today. 2012;42:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Jabłoński S, Piskorz L, Wawrzycki M. Gastric tube resection due to metachronic cancer and a recurrence in anastomosis after Ivor-Lewis esophagectomy--case report. World J Surg Oncol. 2012;10:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Saito T, Yano M, Motoori M, Kishi K, Fujiwara Y, Shingai T, Noura S, Ohue M, Ohigashi H, Ishikawa O. Subtotal gastrectomy for gastric tube cancer after esophagectomy: a safe procedure preserving the proximal part of gastric tube based on intraoperative ICG blood flow evaluation. J Surg Oncol. 2012;106:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Kim JJ, Park JK, Wang YP, Sung SW, Park HJ, Lee SI. Total gastrectomy in gastric conduit cancer. Korean J Thorac Cardiovasc Surg. 2012;45:53-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Ho C, Tong DK, Tsang JS, Law SY. Post-esophagectomy gastric conduit cancers: treatment experiences and literature review. Dis Esophagus. 2014;27:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Lee GD, Kim YH, Choi SH, Kim HR, Kim DK, Park SI. Gastric conduit cancer after oesophagectomy for oesophageal cancer: incidence and clinical implications. Eur J Cardiothorac Surg. 2014;45:899-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Mukasa M, Takedatsu H, Matsuo K, Sumie H, Yoshida H, Hinosaka A, Watanabe Y, Tsuruta O, Torimura T. Clinical characteristics and management of gastric tube cancer with endoscopic submucosal dissection. World J Gastroenterol. 2015;21:919-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Hara K, Matsunaga T, Fukumoto Y, Miyauchi W, Kono Y, Shishido Y, Hanaki T, Miyatani K, Watanabe J, Kihara K, Yamamoto M, Tokuyasu N, Takano S, Sakamoto T, Honjo S, Fujiwara Y. Successful preservation of the proximal stomach tube by evaluating blood flow using indocyanine green for gastric tube cancer: a case report. Surg Case Rep. 2020;6:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Yamana I, Murakami T, Ryu S, Ichikawa J, Shin Y, Koreeda N, Sannomiya H, Sato K, Okamoto T, Sakamoto Y, Yoshida Y, Yanagisawa J, Noritomi T, Hasegawa S. Subtotal gastrectomy for gastric tube cancer using intraoperative indocyanine green fluorescence method. Int J Surg Case Rep. 2020;71:290-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Horie K, Oshikiri T, Kitamura Y, Shimizu M, Yamazaki Y, Sakamoto H, Ishida S, Koterazawa Y, Ikeda T, Yamamoto M, Kanaji S, Matsuda Y, Yamashita K, Matsuda T, Nakamura T, Suzuki S, Kakeji Y. Thoracoscopic retrosternal gastric conduit resection in the supine position for gastric tube cancer. Asian J Endosc Surg. 2020;13:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Zygoń JI, Skokowski J, ZieliÅ„ski J, Drucis K, Golabek-Dropiewska K. Metachronous adenocarcinoma in a gastric tube after radical surgery for oesophageal cancer. BMJ Case Rep. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | National Cancer Center Japan. Center for Cancer Control and Information Services. Cohort Survival https://ganjohojp/reg_stat/statistics/qa_words/cohort01html. |
| 39. | Suzuki H, Kitamura M, Saito R, Motoyama S, Ogawa J. Cancer of the gastric tube reconstructed through the posterior mediastinal route after radical surgery for esophageal cancer. Jpn J Thorac Cardiovasc Surg. 2001;49:466-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Yoshida T, Nagahama T, Maruyama M, Ebuchi M. Endoscopic comparison of two cases: distal resection of reconstructed gastric tube. Hepatogastroenterology. 2002;49:371-374. [PubMed] |