Published online Sep 27, 2020. doi: 10.4240/wjgs.v12.i9.377
Peer-review started: June 3, 2020
First decision: June 15, 2020
Revised: July 2, 2020
Accepted: September 8, 2020
Article in press: September 8, 2020
Published online: September 27, 2020
Processing time: 114 Days and 9.2 Hours
Palliative therapy has been associated with improved overall survival (OS) in several tumor types. Not all patients with metastatic esophageal cancer receive palliative chemotherapy, and the roles of other palliative therapies in these patients are limited.
To investigate the impact of other palliative therapies in patients with metastatic esophageal cancer not receiving chemotherapy.
The National Cancer Database was used to identify patients between 2004-2015. Patients with M1 disease who declined chemotherapy and had known palliative therapy status [palliative therapies were defined as surgery, radiotherapy (RT), pain management, or any combination thereof] were included. Cases with unknown chemotherapy, RT, or nonprimary surgery status were excluded. Kaplan-Meier estimates of OS were calculated. Cox proportional hazards regression models were employed to examine factors influencing survival.
Among 140234 esophageal cancer cases, we identified 1493 patients who did not receive chemotherapy and had complete data. Median age was 70 years, most (66.3%) had a Charlson Comorbidity Index (CCI) of 0, and 37.1% were treated at an academic center. The majority (72.7%) did not receive other palliative therapies. On both univariate and multivariable analyses, there was no difference in OS between those receiving other palliative therapy (median 2.83 mo, 95%CI: 2.53-3.12) vs no palliative therapy (2.37 no, 95%CI: 2.2-2.56; multivariable P = 0.290). On univariate, but not multivariable analysis, treatment at an academic center was predictive of improved OS [Hazard ratio (HR) 0.90, 95%CI: 0.80-1.00; P = 0.047]. On multivariable analysis, female sex (HR 0.81, 95%CI: 0.71-0.92) and non-black, other race compared to white race (HR 0.72, 95%CI: 0.56-0.93) were associated with reduced mortality, while South geographic region relative to West region (HR 1.23, 95%CI: 1.04-1.46) and CCI of 1 relative to CCI of 0 (HR 1.17, 95%CI: 1.03-1.32) were associated with increased mortality. Higher histologic grade and T-stage were also associated with worse OS (P < 0.05).
Palliative therapies other than chemotherapy conferred a numerically higher, but not statistically significant difference in OS among patients with metastatic esophageal cancer not receiving chemotherapy. Quality of life metrics, inpatient status, and subgroup analyses are important for examining the role of palliative therapies other than chemotherapy in metastatic esophageal cancer and future studies are warranted.
Core Tip: We evaluated the impact of non-chemotherapy-based palliative treatments in patients with metastatic esophageal cancer not receiving chemotherapy. A remarkably small fraction of these patients does not receive any palliative therapy. These findings merit further investigation to identify those at greatest risk who may benefit from risk-tailored management approaches. There was a numerically higher but not statistically significant difference in overall survival among those who received other palliative therapies vs those who did not (median overall survival 2.83 mo vs 2.37 mo). Our analysis was limited by lack of ability to account for patients at different stages of presentation or severity of disease.
- Citation: Kim S, DiPeri TP, Guan M, Placencio-Hickok VR, Kim H, Liu JY, Hendifar A, Klempner SJ, Nipp R, Gangi A, Burch M, Waters K, Cho M, Chao J, Atkins K, Kamrava M, Tuli R, Gong J. Impact of palliative therapies in metastatic esophageal cancer patients not receiving chemotherapy. World J Gastrointest Surg 2020; 12(9): 377-389
- URL: https://www.wjgnet.com/1948-9366/full/v12/i9/377.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i9.377
Esophageal cancer is the 8th most common cause of cancer worldwide and a majority of Western patients present with advanced disease at the time of diagnosis[1]. In 2014 alone, the total esophageal cancer-related deaths were estimated to be greater than 15000 in the United States[2]. The two primary subtypes of esophageal cancer are adenocarcinoma and squamous cell carcinoma, with adenocarcinoma representing the most common pathologic subtype in the Western world[3]. Most patients present with advanced disease, and many of those who are initially eligible for surgical resection ultimately have disease recurrence[4]. Nearly 50%-60% of patients present with locally advanced disease with invasion into adjacent structures (T4b), extensive nodal disease, or distant metastatic disease (M1) which preclude upfront surgical management[3]. In patients who are not considered for surgical therapy, other treatment options such as palliative chemotherapy, palliative radiation, and supportive care for symptoms are available[5].
Seminal studies have shown that early integration of palliative care can improve patient outcomes, including quality of life, mood, and potentially overall survival (OS)[6-8]. These enhanced patient outcomes occurred despite patients receiving less aggressive care[9]. Based on these findings from important studies in palliative medicine, practice guidelines have been developed that recommend all patients with advanced cancer should receive dedicated palliative care services early in the disease course and concurrent with active treatment[10]. However, data are lacking regarding the optimal pathway that would allow for integration of palliative care into the metastatic esophageal cancer patient treatment algorithm[5].
The systemic toxicity of cytotoxic chemotherapy in patients with metastatic gastroesophageal cancer is high, with an estimated median survival of less than one year in this population[3]. Despite the high rates of advanced disease and considerable symptom burden in this population, data are lacking about how to best integrate palliative therapies into the care of patients with advanced esophageal cancer[11]. In a recent series of advanced gastroesophageal cancer patients, only 18% of patients received chemotherapy whereas the most common treatment choice was supportive care alone (21%)[12]. In this study, even among those who agreed to palliative chemotherapy, considerable heterogeneity existed regarding the choice of first-line chemotherapy, as fluorouracil and oxaliplatin represented the only regimen exceeding 10% utilization in the first-line setting[13]. These results suggest that a remarkable proportion of patients with metastatic esophageal cancer do not receive palliative chemotherapy, and the role of other forms of palliative therapy in this population are also poorly described.
The goal of the current study was to describe outcomes among patients with metastatic esophageal cancer who did not receive chemotherapy. We sought to compare patient characteristics and outcomes between those who did or did not receive other palliative therapy. Specifically, we placed an emphasis on patient and disease factors that were independently associated with OS with a comparison of survival between those with metastatic esophageal cancer declining chemotherapy who received palliative therapy and those declining chemotherapy who did not receive any palliative therapy.
The National Cancer Database (NCDB) was queried for patients with esophageal cancer (n = 140234) between 2004-2015 and the subgroup of patients with M1 disease who were not receiving chemotherapy and had known palliative therapy status (n = 1493 patients) were included in this retrospective analysis. Data were extracted and defined using the existing NCDB data dictionary (http://ncdbpuf.facs.org/node/259?q=print-pdf-all). In particular, the Charlson Comorbidity Index (CCI) is a weighted score of comorbid conditions modified by Deyo et al[14] from the original Charlson score that has been validated to independently predict for patient outcomes (e.g., mortality) as based on International Classification of Diseases codes found in administrative data such as hospital abstracts data[15]. Palliative therapy was defined as surgery, radiation therapy (RT), and/or other pain management therapy provided to prolong the patient's life by controlling symptoms, to alleviate pain, or to make the patient comfortable as per the NCDB data dictionary. Cases with unknown chemotherapy, RT, or nonprimary surgery status were excluded (n = 138741).
Missing data patterns for variables with missing values such as treatment site (missing rate: 0.67%), geographic location (0.67%), race (0.47%), insurance type (1.81%), income (3.48%), education level (3.48%), residence area type (2.88%), grade (24.38%), American Joint Committee on Cancer (AJCC) clinical T stage (50.90%) and N stage (24.25%) were examined using the method proposed by Little[16] and was not missing completely at random. To reduce the chance of bias from missing data, missing values were imputed using fully conditional specification implemented by the multivariate imputation by chained equations algorithm under the missing at random assumption[17,18]. We generated thirty complete data sets, which were analyzed separately and then the results were combined using the formula in Rubin[19].
OS was calculated from diagnosis to the date of death or censored at the date of last follow-up. Baseline characteristics in patients who did and did not receive palliative treatment were compared using Wilcoxon rank-sum test for continuous variables and χ2 test for categorical variables. Median follow-up was calculated using the reverse Kaplan-Meier method[20]. Survival functions were estimated by the Kaplan-Meier method and compared using a log-rank test[21]. Univariate and multivariable survival analyses were carried out using Cox proportional hazards regression models[22]. Multivariable analyses were performed using a stepwise variable selection procedure based on Akaike Information Criterion (AIC) while receipt of palliative treatment was forced into the models[23]. Final multivariable models were returned by the lowest AIC value. The proportional hazards assumption was assessed graphically and analytically with scaled Schoenfeld residuals[24]. Variables included in the multivariable model in Table 1 were common to all 30 models fitted to the 30 imputed data sets. Each multivariable model had between 0-3 additional variables. Likelihood ratio tests were carried out to compare each full model to the reduced model and the results were not statistically significant. Possibility of multi-collinearity was assessed by tolerance and the variance inflation factor. Analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina) and R package version 3.5.3 with two-sided tests at a significance level of 0.05.
| Variable | n | Univariate | Multivariable | ||
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Treatment received | |||||
| No chemotherapy/received palliative therapy | 407 | 0.94 (0.84-1.05) | 0.288 | 0.94 (0.83-1.06) | 0.290 |
| No chemotherapy/no palliative therapy | 1086 | 1 (Reference) | 1 (Reference) | ||
| Age (yr) | 1493 | 1.00 (1.00-1.00) | 0.773 | 2 | |
| Gender | |||||
| Female | 340 | 0.82 (0.73-0.93) | 0.002 | 0.81 (0.71-0.92) | 0.002 |
| Male | 1153 | 1 (Reference) | 1 (Reference) | ||
| Race1 | |||||
| Black | 142 | 0.87 (0.73-1.04) | 0.136 | 0.87 (0.72-1.05) | 0.136 |
| Other | 82 | 0.76 (0.60-0.97) | 0.026 | 0.72 (0.56-0.93) | 0.011 |
| White | 1270 | 1 (Reference) | 1 (Reference) | ||
| Insurance type1 | |||||
| Medicaid | 134 | 1.00 (0.81-1.24) | 0.977 | 2 | |
| Medicare | 944 | 1.04 (0.91-1.18) | 0.605 | ||
| Not insured | 89 | 0.99 (0.77-1.28) | 0.963 | ||
| Other government | 21 | 1.01 (0.63-1.64) | 0.956 | ||
| Private | 305 | 1 (Reference) | |||
| Income quartiles for place of residence1 | |||||
| $30000-$34999 | 291 | 1.03 (0.86-1.24) | 0.723 | 1.04 (0.86-1.25) | 0.710 |
| $35000-$45999 | 452 | 1.19 (1.01-1.41) | 0.034 | 1.21 (1.01-1.44) | 0.035 |
| $46000+ | 514 | 1.04 (0.89-1.23) | 0.593 | 1.06 (0.89-1.26) | 0.518 |
| Less than $30000 | 236 | 1 (Reference) | 1 (Reference) | ||
| Education level1,3 | |||||
| 14%-19.9% | 372 | 1.03 (0.90-1.18) | 0.679 | 2 | |
| 20%-28.9% | 369 | 1.01 (0.88-1.16) | 0.871 | ||
| 29% or more | 263 | 0.96 (0.82-1.12) | 0.580 | ||
| Less than 14% | 489 | 1 (Reference) | |||
| Treatment site1 | |||||
| Academic | 554 | 0.90 (0.80-1.00) | 0.047 | 2 | |
| Non-Academic | 939 | 1 (Reference) | |||
| Geographic location in United States1 | |||||
| Midwest | 487 | 1.10 (0.93-1.29) | 0.259 | 1.10 (0.94-1.30) | 0.232 |
| Northeast | 341 | 1.12 (0.94-1.32) | 0.203 | 1.17 (0.98-1.39) | 0.076 |
| South | 431 | 1.20 (1.02-1.42) | 0.026 | 1.23 (1.04-1.46) | 0.017 |
| West | 234 | 1 (Reference) | 1 (Reference) | ||
| Residence area type1 | |||||
| Metro | 1183 | 1.06 (0.92-1.21) | 0.431 | 2 | |
| Rural | 31 | 1.07 (0.73-1.57) | 0.719 | ||
| Urban | 279 | 1 (Reference) | |||
| Number of comorbidities4 | |||||
| 1 | 351 | 1.17 (1.03-1.32) | 0.014 | 1.17 (1.03-1.32) | 0.018 |
| ≥ 2 | 152 | 1.20 (1.00-1.42) | 0.044 | 1.12 (0.94-1.35) | 0.200 |
| 0 | 990 | 1 (Reference) | 1 (Reference) | ||
| Year of diagnosis | |||||
| 2010-2014 | 889 | 1.06 (0.96-1.18) | 0.249 | 2 | |
| 2004-2009 | 604 | 1 (Reference) | |||
| Grade1,5 | |||||
| 1 | 50 | 0.64 (0.37-1.09) | 0.101 | 0.58 (0.33-1.01) | 0.054 |
| 2 | 513 | 0.60 (0.38-0.93) | 0.023 | 0.58 (0.37-0.92) | 0.020 |
| 3 | 901 | 0.73 (0.47-1.13) | 0.153 | 0.71 (0.45-1.11) | 0.133 |
| 4 | 29 | 1 (Reference) | 1 (Reference) | ||
| AJCC T stage1 | |||||
| T0 | 17 | 0.69 (0.35-1.35) | 0.280 | 0.64 (0.33-1.25) | 0.192 |
| T1 | 335 | 0.89 (0.74-1.07) | 0.199 | 0.89 (0.73-1.08) | 0.223 |
| T2 | 162 | 0.73 (0.59-0.91) | 0.005 | 0.72 (0.58-0.90) | 0.003 |
| T3 | 499 | 0.77 (0.66-0.89) | < 0.001 | 0.76 (0.66-0.89) | < 0.001 |
| T4 | 480 | 1 (Reference) | 1 (Reference) | ||
| AJCC N stage1 | |||||
| Positive | 1083 | 0.92 (0.81-1.04) | 0.187 | 2 | |
| Negative | 410 | 1 (Reference) | |||
Among the 140234 cases of esophageal cancer screened from the NCDB between 2004-2015, we identified a final 1493 patients with metastatic disease who declined chemotherapy and had complete data. In these patients, the median follow-up was 40.71 mo (95%CI: 33.81-59.47). A total of 407 patients with metastatic esophageal cancer did not receive chemotherapy but received some form of palliative therapy, while 1086 (73%) did not receive chemotherapy or any palliative therapy. We examined the baseline characteristics of patients who did or did not receive any palliative treatments (Table 2). In all patients, the median age was 70 years (interquartile range 62-79) and the majority were white (85%) and male (77%). In the overall cohort, the majority of cases had Medicare for insurance (63%), were treated at non-academic sites (63%) as defined by the Commission on Cancer Accreditation program, and resided in a metropolitan county (79%) as defined by population size by the U.S. Department of Agriculture Economic Research Service. Higher CCI scores (≥ 2) have been shown to be poor prognostic indicators in esophageal cancer patients undergoing curative intent esophagectomy[25]. In our cohort of metastatic esophageal cancer patients, most (66%) had a CCI of 0.
| Variable | All patients (n = 1493) | No chemotherapy/received palliative therapy (n = 407) | No chemotherapy/no palliative therapy (n = 1086) | P value | |
| Age (yr) | 0.775 | ||||
| Median (IQR) | 70 (62-79) | 71 (62-79) | 70 (62-79) | ||
| Gender | 0.748 | ||||
| Female | 340 (22.77) | 95 (23.34) | 245 (22.56) | ||
| Male | 1153 (77.23) | 312 (76.66) | 841 (77.44) | ||
| Race1 | 0.727 | ||||
| Black | 142 (9.50) | 38 (9.36) | 104 (9.58) | ||
| Other | 82 (5.46) | 19 (4.71) | 62 (5.71) | ||
| White | 1270 (85.04) | 350 (85.93) | 920 (84.71) | ||
| Insurance type1 | 0.398 | ||||
| Medicaid | 134 (8.98) | 33 (8.11) | 101 (9.30) | ||
| Medicare | 944 (63.30) | 255 (62.65) | 689 (63.45) | ||
| Not insured | 89 (5.96) | 19 (4.67) | 70 (6.44) | ||
| Other government | 21 (1.41) | 7 (1.72) | 14 (1.29) | ||
| Private | 305 (20.43) | 93 (22.85) | 212 (19.52) | ||
| Income quartiles for place of residence1 | 0.282 | ||||
| Less than $30000 | 236 (15.79) | 70 (17.20) | 166 (15.29) | ||
| $30000-$34999 | 291 (19.51) | 87 (21.38) | 204 (18.78) | ||
| $35000-$45999 | 452 (30.29) | 125 (30.71) | 327 (30.11) | ||
| $46000+ | 514 (34.41) | 125 (30.71) | 389 (35.82) | ||
| Education level1,2 | 0.971 | ||||
| Less than 14% | 489 (32.73) | 134 (32.92) | 355 (32.69) | ||
| 14%-19.9% | 372 (24.94) | 101 (24.82) | 271 (24.95) | ||
| 20%-28.9% | 369 (24.73) | 97 (23.83) | 272 (25.05) | ||
| 29% or more | 263 (17.60) | 75 (18.43) | 188 (17.31) | ||
| Treatment site1 | 0.118 | ||||
| Academic | 554 (37.11) | 164 (40.29) | 390 (35.91) | ||
| Non-Academic | 939 (62.89) | 243 (59.71) | 696 (64.09) | ||
| Geographic location in United States1 | 0.003 | ||||
| Midwest | 487 (32.62) | 128 (31.45) | 359 (33.05) | ||
| Northeast | 341 (22.84) | 116 (28.50) | 225 (20.72) | ||
| South | 431 (28.87) | 96 (23.59) | 335 (30.85) | ||
| West | 234 (15.67) | 67 (16.46) | 167 (15.38) | ||
| Residence area type1 | 0.637 | ||||
| Metro | 1183 (79.25) | 316 (77.61) | 867 (79.83) | ||
| Rural | 31 (2.07) | 9 (2.28) | 22 (2.02) | ||
| Urban | 279 (18.68) | 82 (20.11) | 197 (18.15) | ||
| Number of comorbidities3 | 0.759 | ||||
| 0 | 990 (66.31) | 266 (65.36) | 724 (66.67) | ||
| 1 | 351 (23.51) | 101 (24.82) | 250 (23.02) | ||
| ≥ 2 | 152 (10.18) | 40 (9.83) | 112 (10.31) | ||
| Year of diagnosis | 0.753 | ||||
| 2004-2009 | 604 (40.46) | 162 (39.8) | 442 (40.7) | ||
| 2010-2014 | 889 (59.54) | 245 (60.2) | 644 (59.3) | ||
| Grade1,4 | 0.395 | ||||
| 1 | 50 (3.35) | 16 (3.93) | 34 (3.13) | ||
| 2 | 513 (34.36) | 127 (31.20) | 386 (35.54) | ||
| 3 | 901 (60.35) | 254 (62.41) | 647 (59.58) | ||
| 4 | 29 (1.94) | 10 (2.46) | 19 (1.75) | ||
| AJCC T stage1 | 0.091 | ||||
| T0 | 17 (1.14) | 3 (0.74) | 14 (1.29) | ||
| T1 | 335 (22.44) | 80 (19.65) | 255 (23.48) | ||
| T2 | 162 (10.85) | 37 (9.09) | 125 (11.51) | ||
| T3 | 499 (33.42) | 160 (39.31) | 339 (31.21) | ||
| T4 | 480 (32.20) | 127 (31.20) | 353 (32.50) | ||
| AJCC N stage1 | 0.006 | ||||
| Positive | 1083 (72.54) | 319 (78.38) | 764 (70.35) | ||
| Negative | 410 (27.46) | 88 (21.62) | 322 (29.65) | ||
Across all patients and those not receiving palliative chemotherapy with or without other palliative therapies, there were no statistically significant differences by age, gender, race, insurance type, income quartiles, education level, treatment site (academic vs non-academic), residence area type, CCI, year of diagnosis, grade, and AJCC T stage (Table 2). Notably, a higher proportion of cases from U.S. South geographic region (31%) not receiving chemotherapy and palliative therapy vs 24% who were not receiving chemotherapy but did receive palliative therapy (P = 0.003). The only other clinical variable showing a statistically significant difference was AJCC node positivity whereby 78% of node-positive patients did not receive chemotherapy but did receive palliative therapy compared with 70% of node-positive patients who did not receive either chemotherapy or palliative therapy (P = 0.006).
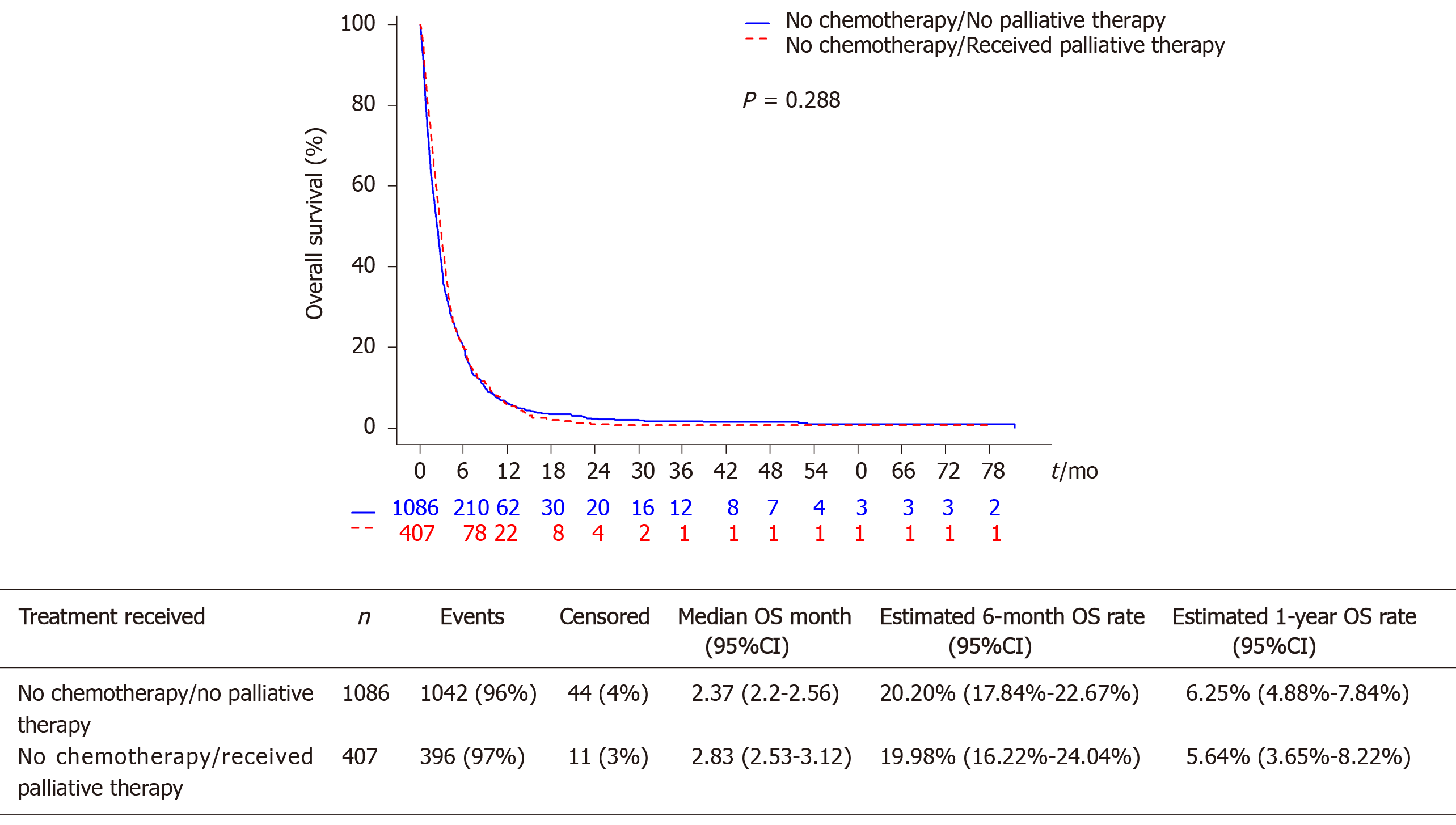
The median OS was 2.53 mo (95%CI: 2.33-2.66) in our overall cohort of patients with advanced esophageal cancer not receiving palliative chemotherapy. There was no statistically significant difference in OS between those receiving other palliative therapies (median OS 2.83 mo, 95%CI: 2.53-3.12) vs those who did not receive other palliative therapies (median OS 2.37 mo, 95%CI: 2.2-2.56, P = 0.288, Figure 1). The 6-mo and 12-mo OS rates were also similar in patients declining chemotherapy who received and did not receive palliative therapy.
We next performed univariate analyses of these same patient and clinicopathologic variables using Cox proportional hazards regression models for OS (Table 1). In metastatic esophageal cancer patients not receiving chemotherapy, there was no difference in OS between those who received and those who did not receive other palliative therapies [Hazard ratio (HR) 0.94, 95%CI: 0.84-1.05, P = 0.288]. On univariate analyses, female gender (HR 0.82, 95%CI: 0.73-0.93, P = 0.002), non-black, other race relative to white race (HR 0.76, 95%CI: 0.60-0.97, P = 0.026), treatment at an academic site (HR 0.90, 95%CI: 0.80-1.00, P = 0.047), and grade 2 histology relative to grade 4 (HR 0.60, 95%CI: 0.38-0.93, P = 0.023) were all significantly associated with reduced risk of death (Table 1). An income quartile of $35000-$45999 annually (HR 1.19, 95%CI: 1.01-1.41, P = 0.034), residing in the South compared to the West (HR 1.20, 95%CI: 1.02-1.42, P = 0.026), and higher CCI ≥ 2 (HR 1.20, 95%CI: 1.00-1.42, P = 0.044) were associated with poor OS. Compared to AJCC T4 stage, T3 (HR 0.77, 95%CI: 0.66-0.89, P < 0.001) and T2 (HR 0.73, 95%CI: 0.59-0.91, P = 0.005) were associated with significantly improved OS. No other variables were predictive of OS on univariate analyses (Table 1).
On multivariable analyses, in metastatic esophageal cancer patients not receiving chemotherapy, receipt of other palliative therapies remained not associated with OS (HR 0.94, 95%CI: 0.83-1.06, P = 0.290; Table 1). Female gender (HR 0.81, 95%CI: 0.71-0.92, P = 0.002) and non-black, other race compared to white race (HR 0.72, 95%CI: 0.56-0.93, P = 0.011) independently predicted for reduced risk of death. Compared to grade 4, grade 2 histology (HR 0.58, 95%CI: 0.37-0.92) predicted for improved OS (P = 0.020). Compared to T4 stage, T3 (HR 0.76, 95%CI: 0.66-0.89, P < 0.001) and T2 (HR 0.72, 95%CI: 0.58-0.90, P = 0.003) were significantly associated with reduced risk of death. Compared to a CCI of 0, a score of 1 (HR 1.17, 95%CI: 1.03-1.32) predicted for increased mortality (P = 0.018). Residing in the South (HR 1.23, 95%CI: 1.04-1.46, P = 0.017) and an income quartile of $35000-$45999 annually (HR 1.21, 95%CI: 1.01-1.44, P = 0.035) predicted for worse OS. All other variables dropped out of the multivariable model (Table 1).
In patients diagnosed with advanced esophageal cancer, a clinically significant proportion of patients decline did not receive chemotherapy and often opt for best supportive care[12]. Palliative chemotherapy in this population has been positively associated with an OS benefit, but grade 3-5 toxicity rates have been shown to be a relevant 33%-48% with platinum-based doublet regimens[26,27]. Other palliative modalities for esophageal cancer have historically included surgery, radiation therapy, nutritional optimization, relief of obstruction, pain control, or a combination of the above[28]. However, in patients with metastatic esophageal cancer who do not receive chemotherapy, our understanding of disease related outcomes is fairly limited. We are among the first groups to specifically look at patient and disease factors associated with treatment with palliative therapy and OS in those with metastatic esophageal cancer not receiving chemotherapy.
From a large retrospective cohort of 1493 patients with metastatic esophageal cancer who did not receive chemotherapy, we identified a surprisingly high 72.7% of cases who also did not receive any other palliative therapies. This is quite surprising given that a majority (66.7%) of these patients not receiving chemotherapy or any other palliative therapy had a no comorbidities, suggesting that this is a group who might be able to tolerate certain treatments. In another large cohort of 11,242 patients with gastrointestinal (GI) cancers (17% who had esophageal cancer), 22% did not receive outpatient palliative care, which is largely discordant with our findings[29]. It is worthwhile to note that this study included a variety of GI cancers and evaluated outpatient palliative care encounters only.
Importantly, our survival analysis showed that patients with metastatic esophageal cancer who received any palliative therapy but no chemotherapy had a numerically higher, but not statistically significant OS compared to those who did not receive chemotherapy or any other palliative therapies (Table 1). These findings are important, as they suggest that other palliative therapies do not provide significant survival benefits to patients who are not receiving chemotherapy. Data is lacking on whether these palliative therapies influence other important factors, such as symptom burden and quality of life. Additionally, although the use of formal palliative care consultation was not directly investigated, integration of palliative care along with usual oncologic care is now a widely recommended approach across national practice guidelines in oncology[10]. Early initiation of palliative care and beyond the outpatient setting is important as nearly 40% of patients with gastroesophageal cancer die within the first 6 months of presentation, reflective of a population with aggressive tumors or a disease state that is too advanced for curative therapy[30]. In advanced esophageal cancer, greater implementation of this practice is certainly warranted. One manner to increase widespread implementation could involve more multidisciplinary discussions, e.g. tumor boards, as multidisciplinary discussions of gastroesophageal cancer patients resulted in more referrals for treatment with palliative therapies including radiation therapy and chemotherapy compared to those cases not discussed in a multidisciplinary setting[31]. Furthermore, application of palliative care needs to come from an integrative approach encompassing a comprehensive assessment of biological, psychological, social, and spiritual concerns, communication and decision-making domains, physical domains including pain, fatigue, nausea, and other symptoms, and ethical domains including advanced care planning[32].
Several patient-related factors were associated with OS. For example, treatment at an academic center (HR 0.90 on univariate, P = 0.047), non-black, other race compared to white race (HR 0.72 on multivariable, P = 0.011), and female gender (HR 0.81 on multivariable, P = 0.002) were significantly associated with decreased mortality in our cohort. We found sex disparities in our current study, which contrasts findings from a retrospective series of esophageal cancer patients referred to a specialist UK cancer center by 6 National Health Service sites and multiple primary care referral centers whereby there were no statistically significant differences in survival between men and women[30]. However, advancing age and socioeconomic deprivation was impaired to poorer OS in this study. A worse prognosis has been associated with black individuals with esophageal cancer, but this has not held true when adjusting for socioeconomic status, while blacks, Asians, and Hispanics have been shown to undergo lower rates of surgery, when compared to whites, in localized esophageal cancer[33]. In addition, there were more patients from the geographic Southern region who did not receive any palliative therapy (30.9%) compared to those who received palliative therapy (23.6%, P = 0.003), and notably being from the South was significantly associated with a worse OS on both univariate and multivariable analyses (Table 1). Not surprisingly, having a higher T stage, histologic grade, and CCI were predictive of worse OS and were statistically significant on multivariable analysis (Table 1).
Although not statistically significant, having an income quartile of > $46000 was associated with a lower HR of 1.06, while having an income quartile of $35000-$45999 annually was significantly associated with poorer survival (HR 1.21, Table 1) on multivariable analysis. It has been shown that the presence of modifiable risk factors such as smoking, poor diet, impaired physical activity, and increased BMI are more commonly found in socioeconomically deprived groups, which can attribute to reduced survival outcomes in multiple studies on cancer[30]. In other studies of esophageal cancer, lower socioeconomic status has been associated with poorer prognosis, while the incidence and mortality rates for esophageal cancer were higher in rural areas compared to urban areas across multiple nations[33].
Our study has several limitations. It is worthwhile to mention that our retrospective analysis limited our ability to attribute differences in OS across demographic factors as we cannot account for delays in diagnosis and treatment or access to treatment, which can all contribute significantly to patient outcomes[34]. However, our fairly large sample size and restriction to metastatic esophageal cancer patients who are not receiving chemotherapy offers an initial glimpse into potential socioeconomic, racial, and sex disparities that exist and can factor into prognosis in this population. Further investigation may help identify those with metastatic esophageal cancer who have declined or unable to receive chemotherapy having these demographic factors associated with poorer prognosis in need of other modalities (e.g., palliative therapy) to improve upon outcomes and not necessarily OS. Also, our analysis does not distinguish across palliative therapy offered in the outpatient vs inpatient and early vs late referral settings whereby a difference in survival of the advanced stage patient is possible. Furthermore, palliative care entails a multidisciplinary approach to improve quality of life beyond palliative-intent therapies and our study does not account for referrals to palliative care. Additionally, in patients with incurable, end-stage esophageal cancer, survival may not be the appropriate outcome measure, whereas symptom burden, psychological distress, prognostic understanding, and quality of life may be more relevant.
In this retrospective analysis of 1493 patients with metastatic esophageal cancer who were not receiving chemotherapy, we identified a relatively high percentage of patients who did not receive any other palliative therapies. Several socioeconomic and clinicopathologic factors were predictive of OS and receipt of palliative therapies in these patients who did not receive chemotherapy. We found a numerical, but not statistically significant difference in OS associated with the receipt of palliative therapies when comparing patients with metastatic esophageal cancer not receiving chemotherapy. Collectively, our findings underscore that for populations at risk for worse survival outcomes, such as those with metastatic esophageal cancer, additional research is needed to prospectively study the impact of palliative therapies on patient outcomes, including not just survival, but also symptom burden, psychological distress, prognostic understanding, and quality of life.
Palliative chemotherapy has been associated with improved overall survival (OS) in metastatic esophageal cancer, but the role of other palliative therapies in this population is poorly understood.
Palliative therapies in patients with metastatic esophageal cancer who do not receive chemotherapy, defined as surgery, radiation therapy (RT), and/or other pain management therapy provided to prolong the patient's life by controlling symptoms, to alleviate pain, or to make the patient comfortable may offer an improvement in OS as well.
The objectives of this study were to investigate the patient and disease characteristics associated with receipt of other palliative therapies in metastatic esophageal cancer patients not receiving palliative chemotherapy. We also investigated the association of receiving other palliative therapies vs not receiving other palliative therapies with OS in these patients who did not receive chemotherapy.
The National Cancer Database was used to identify patients between 2004-2015. Patients with M1 disease who did not receive chemotherapy but had been confirmed to receive other palliative therapies or not were included. Cases with unknown chemotherapy, RT, or nonprimary surgery status were excluded. Kaplan-Meier estimates of OS were calculated. Cox proportional hazards regression models were employed to examine factors influencing survival.
Out of 1493 patients who did not receive chemotherapy and had complete data, the majority (72.7%) did not receive other palliative therapies. There was no statistically significant difference in OS between those receiving other palliative therapies vs no palliative therapy. Several factors including treatment at an academic center, female sex, non-black, other race (compared to white race) were associated with improved OS, while South geographic region relative to West region and higher Charlson Comorbidity Index, histologic grade, and T-stage were associated with worse OS.
Palliative therapies other than chemotherapy conferred a numerically higher, but not statistically significant difference in OS among patients with metastatic esophageal cancer not receiving chemotherapy. Several socioeconomic and clinicopathologic factors were predictive of OS and receipt of other palliative therapies in these patients who did not receive chemotherapy
Additional research is needed to prospectively study the impact of other palliative therapies on patient outcomes that OS may not capture in metastatic esophageal cancer. Quality of life metrics, inpatient status, and subgroup analyses are important for examining the role of palliative therapies other than chemotherapy in metastatic esophageal cancer and future studies are warranted.
The data used in the study are derived from a de-identified National Cancer Database file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sommariva A S-Editor: Wang JL L-Editor: A P-Editor: Li JH
| 1. | Janmaat VT, Steyerberg EW, van der Gaast A, Mathijssen RH, Bruno MJ, Peppelenbosch MP, Kuipers EJ, Spaander MC. Palliative chemotherapy and targeted therapies for esophageal and gastroesophageal junction cancer. Cochrane Database Syst Rev. 2017;11:CD004063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Paul S, Altorki N. Outcomes in the management of esophageal cancer. J Surg Oncol. 2014;110:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | van Rossum PSN, Mohammad NH, Vleggaar FP, van Hillegersberg R. Treatment for unresectable or metastatic oesophageal cancer: current evidence and trends. Nat Rev Gastroenterol Hepatol. 2018;15:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, Cunningham D. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 737] [Article Influence: 92.1] [Reference Citation Analysis (2)] |
| 5. | Haj Mohammad N, Bernards N, van Putten M, Lemmens VEPP, van Oijen MGH, van Laarhoven HWM. Volume-outcome relation in palliative systemic treatment of metastatic oesophagogastric cancer. Eur J Cancer. 2017;78:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5013] [Article Influence: 334.2] [Reference Citation Analysis (0)] |
| 7. | Bakitas MA, Tosteson TD, Li Z, Lyons KD, Hull JG, Li Z, Dionne-Odom JN, Frost J, Dragnev KH, Hegel MT, Azuero A, Ahles TA. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J Clin Oncol. 2015;33:1438-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 839] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 8. | Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, Moore M, Rydall A, Rodin G, Tannock I, Donner A, Lo C. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383:1721-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1241] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 9. | Greer JA, Pirl WF, Jackson VA, Muzikansky A, Lennes IT, Heist RS, Gallagher ER, Temel JS. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 412] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 10. | Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, Firn JI, Paice JA, Peppercorn JM, Phillips T, Stovall EL, Zimmermann C, Smith TJ. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:96-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 1400] [Article Influence: 155.6] [Reference Citation Analysis (0)] |
| 11. | Gupta V, Coburn N, Kidane B, Hess KR, Compton C, Ringash J, Darling G, Mahar AL. Survival prediction tools for esophageal and gastroesophageal junction cancer: A systematic review. J Thorac Cardiovasc Surg. 2018;156:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Opstelten JL, de Wijkerslooth LR, Leenders M, Bac DJ, Brink MA, Loffeld BC, Meijnen-Bult MJ, Minderhoud IM, Verhagen MA, van Oijen MG, Siersema PD. Variation in palliative care of esophageal cancer in clinical practice: factors associated with treatment decisions. Dis Esophagus. 2017;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Abrams T, Hess LM, Zhu YE, Schelman W, Liepa AM, Fuchs C. Predictors of heterogeneity in the first-line treatment of patients with advanced/metastatic gastric cancer in the U.S. Gastric Cancer. 2018;21:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7532] [Cited by in RCA: 8646] [Article Influence: 262.0] [Reference Citation Analysis (0)] |
| 15. | Mnatzaganian G, Ryan P, Norman PE, Hiller JE. Accuracy of hospital morbidity data and the performance of comorbidity scores as predictors of mortality. J Clin Epidemiol. 2012;65:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Little R. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83:1198-1202. [DOI] [Full Text] |
| 17. | van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1-67. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3242] [Cited by in RCA: 3278] [Article Influence: 234.1] [Reference Citation Analysis (0)] |
| 18. | van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1635] [Cited by in RCA: 1927] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 19. | Rubin D. Multiple imputation for nonresponse in surveys. New York: John Wiley Sons Inc., 1987: Chapter 3. |
| 20. | Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 2149] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 21. | Kalbfleisch J, Prentice R. The statistical analysis of failure time data. Wiley series in probability and mathematical statistics. New York: John Wiley Sons Inc., 1980. |
| 22. | Cox D. Regression Models and Life Tables. J Royal Stat Society. 1972;B34:187-220. |
| 23. | Yamashita T, Yamashita K, Kamimura R. A Stepwise AIC Method for Variable Selection in Linear Regression. Commun Stat Theory Methods. 2007;36:2395-2403. [RCA] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515-526. [DOI] [Full Text] |
| 25. | Yamashita K, Watanabe M, Mine S, Fukudome I, Okamura A, Yuda M, Hayami M, Imamura Y. The impact of the Charlson comorbidity index on the prognosis of esophageal cancer patients who underwent esophagectomy with curative intent. Surg Today. 2018;48:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Baumgartner R, Taghizadeh H, Jomrich G, Schoppmann SF, Preusser M, Ilhan-Mutlu A. Utilization and Efficacy of Palliative Chemotherapy for Locally Advanced or Metastatic Gastroesophageal Carcinoma. Anticancer Res. 2020;40:965-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Dijksterhuis WPM, Verhoeven RHA, Slingerland M, Haj Mohammad N, de Vos-Geelen J, Beerepoot LV, van Voorthuizen T, Creemers GJ, van Oijen MGH, van Laarhoven HWM. Heterogeneity of first-line palliative systemic treatment in synchronous metastatic esophagogastric cancer patients: A real-world evidence study. Int J Cancer. 2020;146:1889-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Freeman RK, Ascioti AJ, Mahidhara RJ. Palliative therapy for patients with unresectable esophageal carcinoma. Surg Clin North Am. 2012;92:1337-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Merchant SJ, Brogly SB, Booth CM, Goldie C, Nanji S, Patel SV, Lajkosz K, Baxter NN. Palliative Care and Symptom Burden in the Last Year of Life: A Population-Based Study of Patients with Gastrointestinal Cancer. Ann Surg Oncol. 2019;26:2336-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Lee A, Khulusi S, Watson R. Gastroesophageal cancer patients need earlier palliative intervention - Using data to inform appropriate care. Eur J Oncol Nurs. 2019;40:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Vermeulen BD, Bruggeman L, Bac DJ, Schrauwen RWM, Epping LSM, Scheffer RCH, Tan ACITL, Groenen MJM, Verhoeven RHA, Siersema PD. Impact of multidisciplinary tumor board discussion on palliation of patients with esophageal or gastro-esophageal junction cancer: a population-based study. Acta Oncol. 2020;59:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Dy SM, Isenberg SR, Al Hamayel NA. Palliative Care for Cancer Survivors. Med Clin North Am. 2017;101:1181-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Xie SH, Lagergren J. Social group disparities in the incidence and prognosis of oesophageal cancer. United European Gastroenterol J. 2018;6:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Khorana AA, Tullio K, Elson P, Pennell NA, Grobmyer SR, Kalady MF, Raymond D, Abraham J, Klein EA, Walsh RM, Monteleone EE, Wei W, Hobbs B, Bolwell BJ. Time to initial cancer treatment in the United States and association with survival over time: An observational study. PLoS One. 2019;14:e0213209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |