Published online Aug 27, 2020. doi: 10.4240/wjgs.v12.i8.355
Peer-review started: April 7, 2020
First decision: May 5, 2020
Revised: May 8, 2020
Accepted: July 19, 2020
Article in press: July 19, 2020
Published online: August 27, 2020
Processing time: 136 Days and 1.7 Hours
Drug-eluting beads transarterial chemoem-bolization (DEB-TACE) has the advantages of slow and steady release, high local concentration, and low incidence of adverse drug reactions compared to the traditional TACE. DEB-TACE combined with sequentially ultrasound-guided radiofrequency ablation (RFA) therapy has strong anti-cancer effects and little side effects, but there are fewer related long-term studies until now.
To explore the outcome of DEB-TACE sequentially combined with RFA for patients with primary hepatocellular carcinoma (HCC).
Seventy-six patients with primary HCC who underwent DEB-TACE sequentially combined with RFA were recruited. Forty patients with untreated HCC were included in Group A, and 36 patients with recurrent HCC were included in Group B. In addition, 40 patients with untreated HCC who were treated with hepatectomy were included in Group C. The serological examination, preoperative magnetic resonance imaging examination, and post-treatment computed tomography enhanced examination were performed for all patients. The efficacy was graded as complete remission (CR), partial remission (PR), stable disease and progressive disease at the 3rd, 6th, and 9th. All patients were followed up for 3 years and their overall survival (OS), disease-free survival (DFS) were assessed.
The efficacy of Group A and Group C was similar (P > 0.05), but the alanine aminotransferase, aspartate aminotransferase and total bilirubin of Group A were lower than those of Group C (all P < 0.05). The proportions of CR (32.5%), PR (37.5%) were slightly higher than Group A (CR: 27.5%, PR: 35%), but the difference was not statistically significant (χ2 = 0.701, P = 0.873). No operational-related deaths occurred in Group A and Group C. The OS (97.5%, 84.7%, and 66.1%) and the DFS (75.0%, 51.7%, and 35.4%) of Group A at the 1st, 2nd, and 3rd year after treatment were similar with those of Group C (OS: 90.0%, 79.7%, and 63.8%; DFS: 80.0%, 59.7%, and 48.6%; P > 0.05). The OS rates in Group A and Group B (90%, 82.3%, and 66.4%) were similar (P > 0.05). The DFS rates in Group B (50%, 31.6%, and 17.2%) were lower than that of Group A (P = 0.013).
The efficacy of DEA-TACE combined with RFA for untreated HCC is similar with hepatectomy. Patients with recurrent HCC could get a longer survival time through the combined treatment.
Core tip: Drug-eluting beads transarterial chemoembolization (DEB-TACE) can continuously and slowly release chemotherapeutic drugs compared to traditional TACE. It can maintain higher local concentrations meanwhile reducing the adverse drug reactions. DEB-TACE combined with ultrasound-guided radiofrequency ablation (RFA) has a strong anti-cancer effect, but due to its expensive price, there are fewer clinical studies. This study explored the outcome of DEA-TACE combined with RFA in the treatment of primary and recurrent hepatocellular carcinoma (HCC). The results indicated that the outcome of combined treatment for untreated HCC was comparable to hepatectomy, with less bleeding, faster recovery, and less damage to liver function. More importantly, the combination therapy has a positive effect on the treatment of recurrent HCC with fewer complications and can prolong the survival time of patients.
- Citation: Zhang Y, Zhang MW, Fan XX, Mao DF, Ding QH, Zhuang LH, Lv SY. Drug-eluting beads transarterial chemoembolization sequentially combined with radiofrequency ablation in the treatment of untreated and recurrent hepatocellular carcinoma. World J Gastrointest Surg 2020; 12(8): 355-368
- URL: https://www.wjgnet.com/1948-9366/full/v12/i8/355.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i8.355
Ultrasound-guided radiofrequency ablation (RFA) is one of the most effective treatments for primary hepatocellular carcinoma (HCC)[1]. However, due to the limitation of local tumor control range, RFA is difficult to completely cover tumors with a diameter of more than 3 cm[2]. The combined use of transarterial che-moembolization (TACE)[3-5] and RFA is one of the strategies to obtain a larger ablation coverage volume[6-8]. Nevertheless, some concentrated chemotherapy drugs are easily to release into the peripheral blood after TACE, which will result in some adverse reactions (e.g. liver injury)[9]. Drug-eluting beads TACE (DEB-TACE) is a new treatment method for replacing traditional embolic agents with drug-loaded microspheres[10]. Because of its good deformability, the microsphere can adhere to the blood vessel to achieve complete embolization, and avoid aggregation at the proximal or distal end of the blood vessel[11-14]. Zhang et al[15] found through the animal experiments that DEB-TACE can slowly release chemotherapy drugs and achieve a long-term blood drug concentration with less liver injury. Clinically, Yamakado et al[16] found that DEB-TACE combined with RFA is more effective in controlling tumor development for patients with liver metastases from colon cancer. The post-treatment efficacy and safety are better than traditional TACE combined with RFA. However, due to the high cost of drug-loaded microspheres, there are limited reports of DEB-TACE combined with RFA in patients with primary HCC. The aim of this study is to explore the possible benefits of DEB-TACE sequentially combined with ultrasound-guided RFA by analyzing the liver function and clinical efficacy of patients with untreated and recurrent HCC.
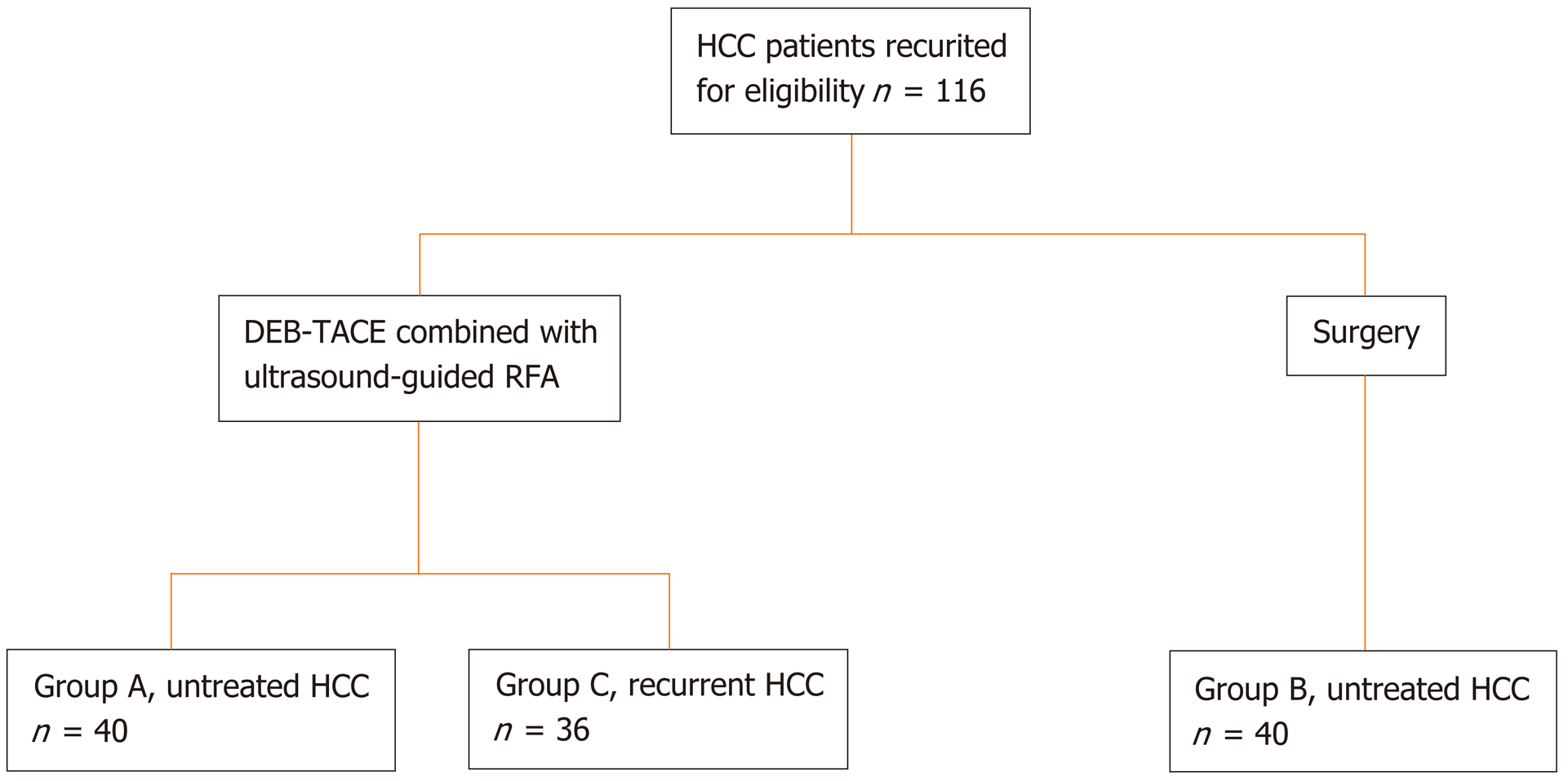
From March 2014 to March 2016, patients with primary HCC who underwent DEB-TACE sequentially combined with RFA were recruited. Forty patients with untreated HCC were included in Group A, while 36 patients with recurrent HCC were included in Group B. Besides, another 40 patients with untreated HCC who were treated with hepatectomy were included in Group C with a 1:1 match using a propensity score matching (PSM) (Figure 1). The inclusion criteria of primary HCC were as follows: (1) Age 18-75 years; (2) Diagnosis of primary HCC according to the medical gui-delines[17,18]; (3) Invasion of large blood vessel branches and extrahepatic metastases found in imaging examination; (4) Maximum diameter ≤ 7 cm for single tumor, maximum diameter ≤ 3 cm and tumor number ≤ 3 for multiple tumors; and (5) Child-Pugh grade A or B. The exclusion criteria were as follows: (1) Severe coagulopathy; (2) Combined liver decompensation symptoms such as refractory ascites, esophageal varices bleeding or hepatic encephalopathy; and (3) Allergic to contrast agents or chemotherapy drugs. All patients or their families signed an informed consent before treatment, and the study was approved by the ethics committee of Hwa Mei Hospital, University of Chinese Academy of Sciences.
Fasting elbow vein blood was collected from all participants. The separated serum was divided into 2 portions, one for alpha-fetoprotein (AFP) levels (Microparticle Enzyme Immunoassay AxSYM Abbott) and the other for liver function determination such as serum albumin (ALB), serum total bilirubin (TBIL), serum alanine aminotransferase (ALT), and serum aspartate Aminotransferase (AST) by an autoanalyzer (ADVIA® 2400 Clinical Chemistry System, Siemens, Tarrytown, NY). In the measurement process, the relevant operational specifications and quality requirements were strictly followed to ensure the measurement results.
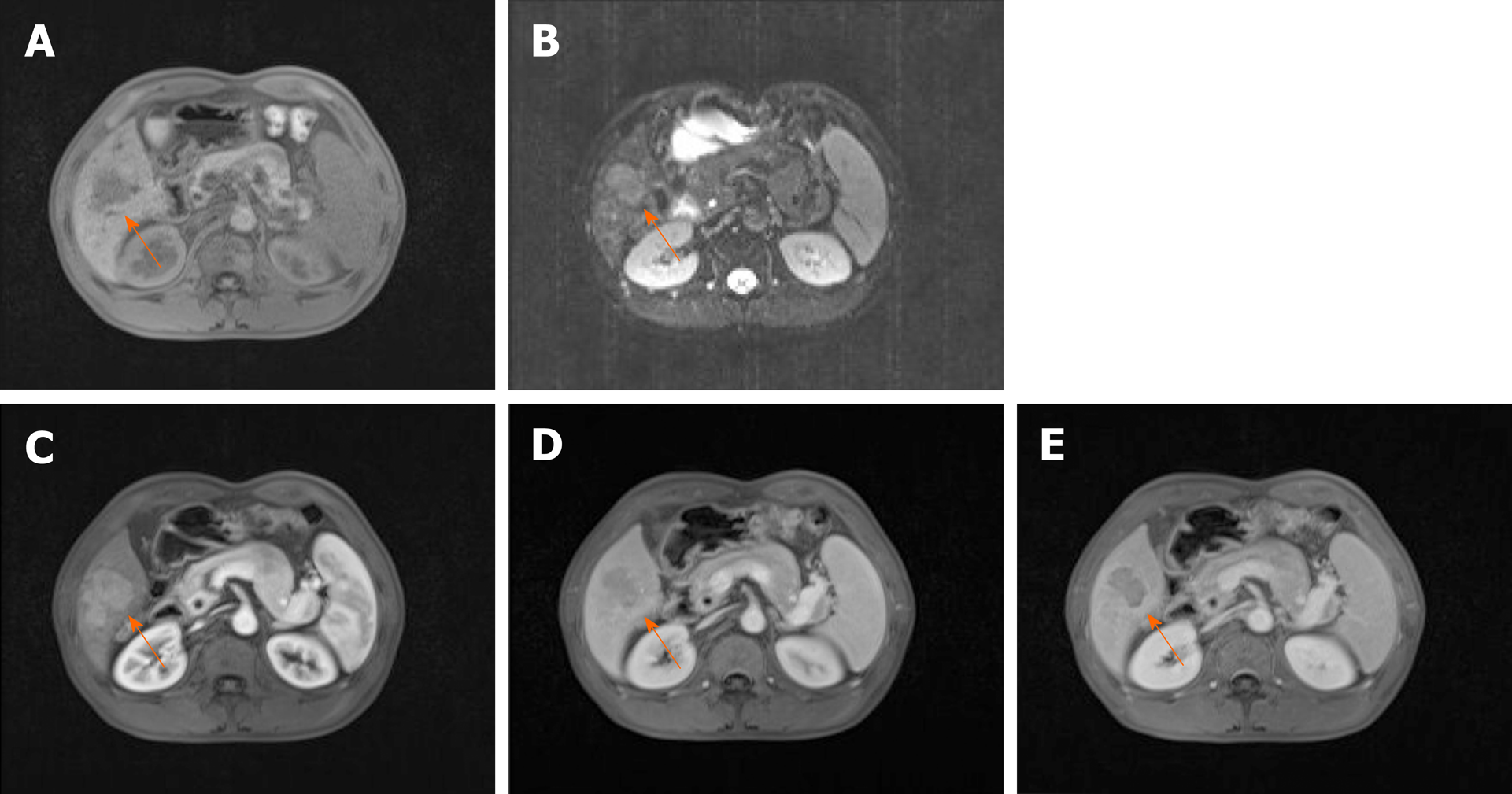
Preoperative magnetic resonance imaging (MRI) examination: Plain and enhanced scans of the upper abdomen were performed using a Siemens 1.5T superconducting MRI system (Siemens Healthcare, Erlangen, Germany). Conventional acquisition of axial T1-weighted image (T1WI) (Figure 2A) and T2-weighted image (T2WI) (Figure 2B) were required. The contrast agent was gadolinium-diethylene-triamine penta-acetic acid (Gd-DTPA) at a dose of 0.1 mmol/kg and an injection rate of 4 ml/s. The dynamic enhanced scan images of the hepatic arterial phase (Figure 2C), portal vein phase (Figure 2D), and parenchymal phase (Figure 2E) were obtained
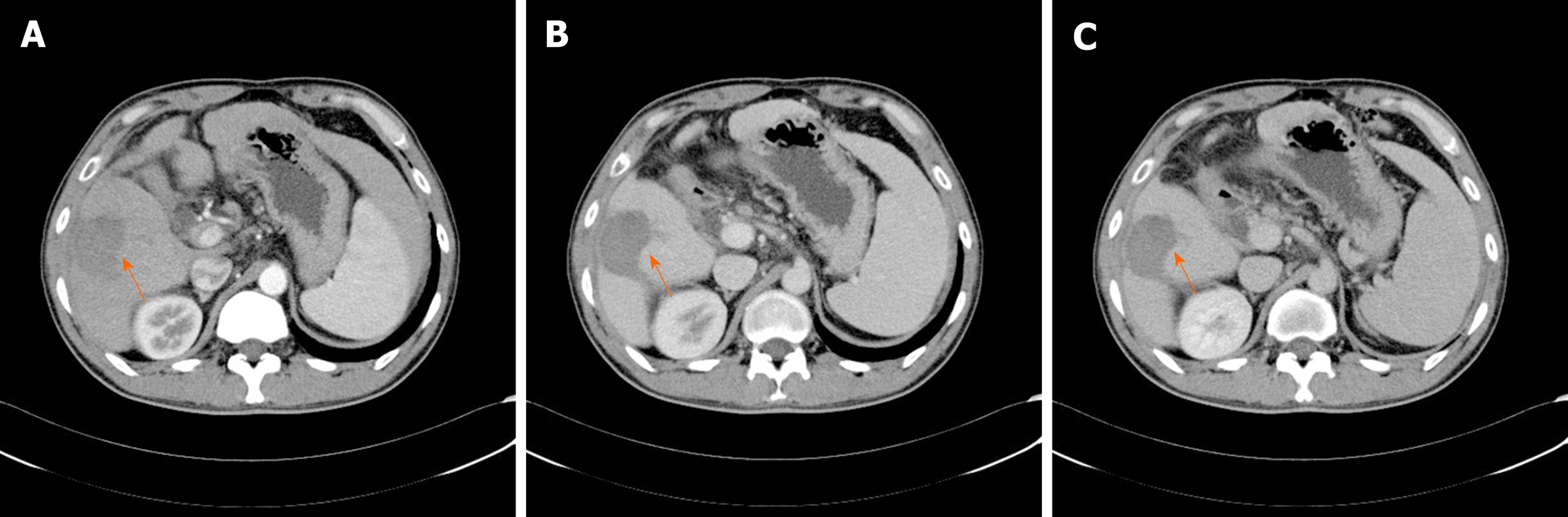
Post-treatment computed tomography (CT) enhanced examination: A CT-enhanced scan was performed using a Siemens SOMATOM Difinition 64-segment dual-source spiral CT scanner (Siemens, Germany). The patient was fasted for 6-8 h before the examination, and the supine position was taken. The nonionic contrast agent iohexol (General Electric Healthcare, Waukesha, Wisconsin, USA) with 0.9% sodium chloride injection was injected. A dynamic contrast-enhanced CT scan of the upper abdomen was performed and the dynamic enhanced examinations of hepatic arterial phase (Figure 3A), portal vein phase (Figure 3B), and parenchymal phase (Figure 3C) were obtained.
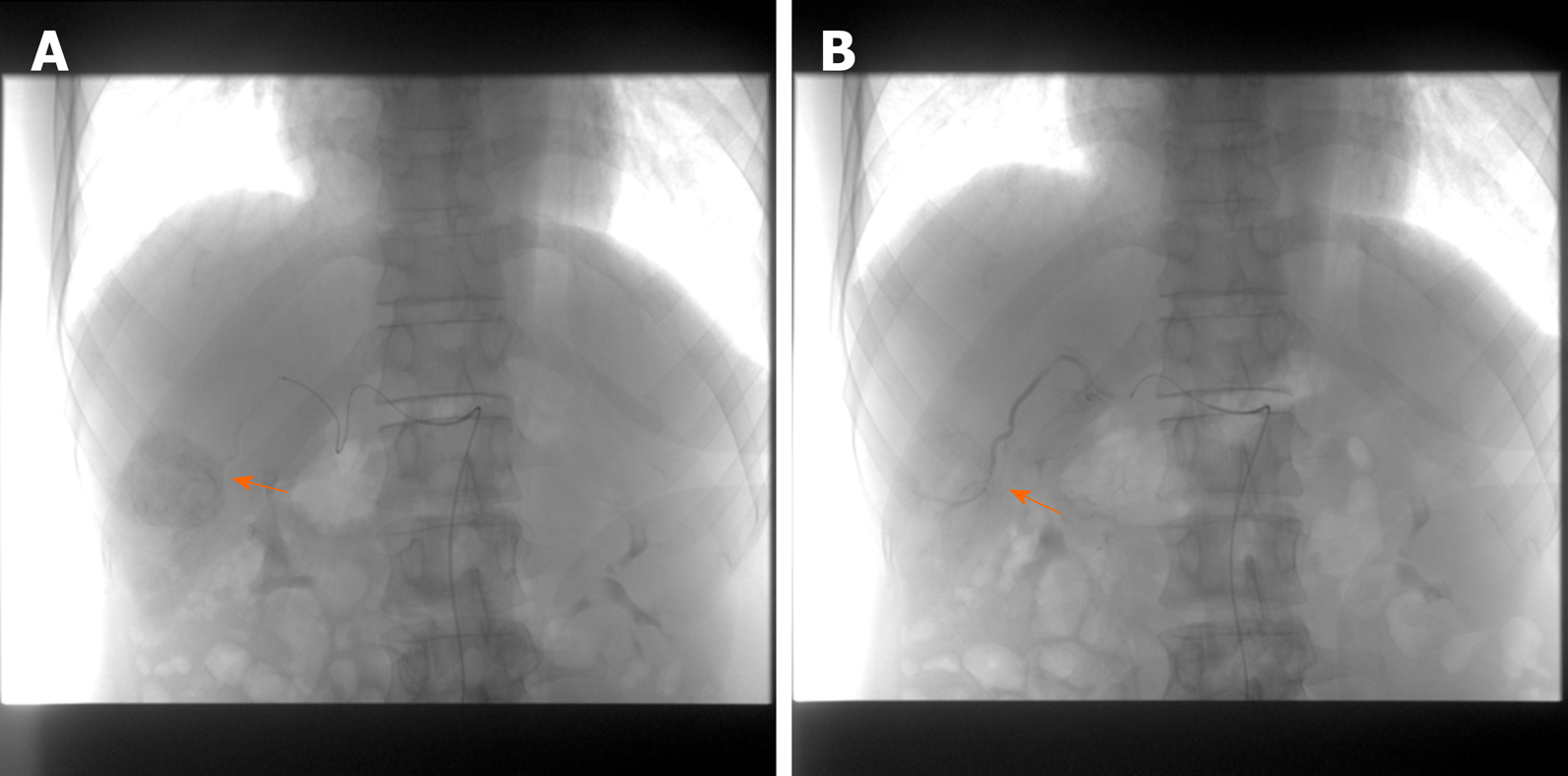
DEB-TACE sequentially combined with RFA: Patients in Group A and group B received DEB-TACE sequentially combined with RFA treatment. The Allura X-per FD20 digital substraction angiography (Philips Medical Systems, Madison, WI, USA) was used to perform hepatic angiography to determine the location, shape, size, and number of tumors, and then to select the tumor-feeding arteries. Epirubicin was loaded into 100-300 um of CalliSpheres drug-loaded microspheres (Heng Rui Galison, Suzhou, China) for TACE. The injection was continued until the contrast flow rate was very slow. The embolization results were confirmed by angiography, and the vascular sheath and catheter were withdrawn after the satisfied embolization (Figure 4).
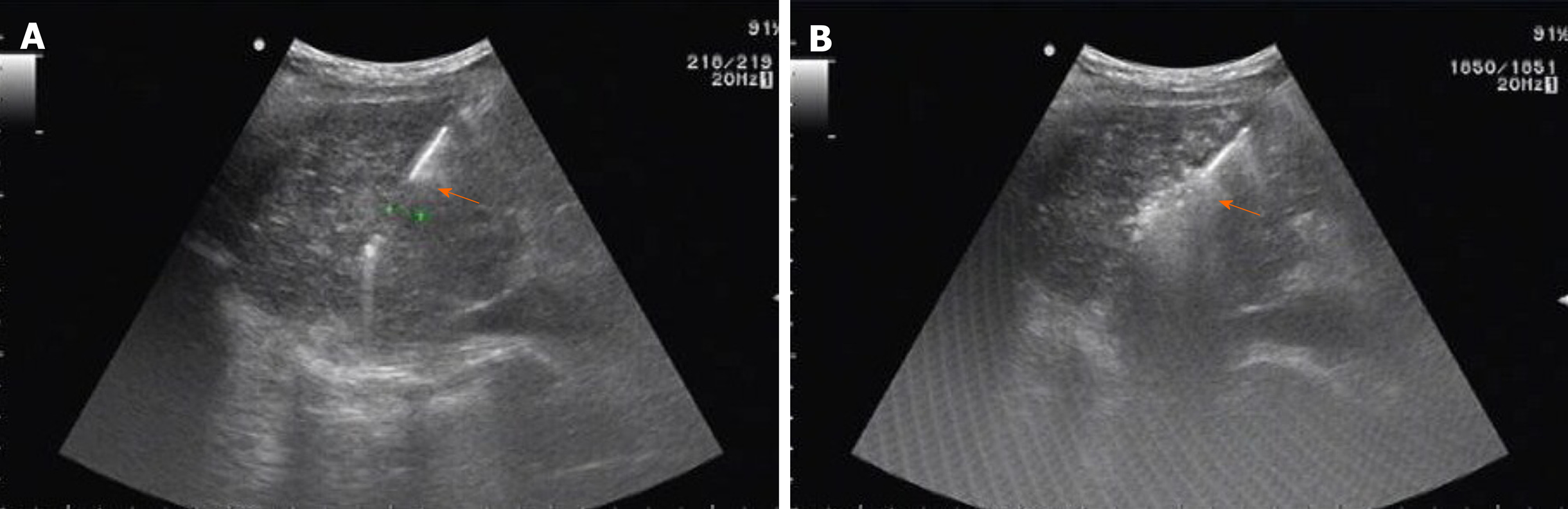
After DEB-TACE, was performed for 1-2 wk, and RFA was performed after the liver function recovered. RFA was performed using a 17-gauge internally cooled electrode (Cool-TipTM, Valleylab, Boulder, CO, USA) under the guidance of ALOKA Prosound Alpha 7 ultrasound system (Hitachi AlokaMedical Systems, Tokyo, Japan). The electrode needle was punctured into the tumor nodule, and the needle was placed according to the location and size of the tumor. The ablation range covered the entire cancerous foci and the surrounding 0.5-1 cm of liver tissue. When the ablation was started, the needle tip gradually warmed up and maintained for 6-12 min after reaching the target temperature (105°C). After the ablation was completed, the ablation apparatus was set to the needle channel ablation mode, and then gradually retracted (Figure 5).
Hepatectomy: Hepatectomy was performed for patients in Group C after observing the size, number, location, and surrounding organs in detail. After the operation was completed, the bleeding at the surgical incision site was carefully observed and the abdominal cavity was closed layer by layer.
Liver protection treatment: All groups were given diammonium glycyrrhizin enteric-coated capsules or tiopronin liver-protective therapy, and lamivudine or adefovir dipivoxil were administered orally for more than 6 mo.
Liver function tests were performed at the 1st week and 1st month after treatment to assess liver damage. The enhanced CT was scanned at the 3rd, 6th, and 9th month after treatment and the efficacy was graded according to modified response evaluation criteria in solid tumors[19] [complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD)]. All patients who participated in the study were followed up for 3 years, and serum AFP levels and liver CT were reviewed every 3 month. The endpoint event was defined as tumor recurrence, local tumor progression (enlarged foci on contrast-enhanced CT images around the ablated tumor), distant metastasis, and death. The patient's refusal to visit and the exit is defined as loss to follow-up. Overall survival (OS) and disease-free survival (DFS) were calculated 3 years after treatment.
The statistical analysis was performed using SPSS (Chicago, IL, USA) software (version 22.0). PSM was used to avoid the effects of selective bias and confounding factors on treatment outcomes[20]. Age, tumor size, number of tumors, Child-Pugh classification, AFP and liver function indicators were used as covariates. A logistic regression model was included to estimate the probability of the subjects being assigned to Group A and Group C, and then individuals with similar probabilities were enrolled from the two groups for pairing to achieve randomization criteria. In this study, Group A and Group C were matched by 1:1, and the caliper value was 0.2. The numerical data were expressed as mean ± SD and t-test was used for the comparison between the two groups. The categorical variables were expressed as number and percentage and the comparison between the two groups was performed by the χ2 test. The Kaplan Meier curve was used to analyze the prognosis of the patient. Statistical significance was defined as 2-tailed P < 0.05 for all tests.
Comparison of baseline data between Group A and Group C is shown in Table 1. The gender, age, tumor size, number of tumors, Child-Pugh classification, AFP and liver function indexes were similar in the two groups. The differences were not statistically significant (all P > 0.05).
| Group A (n = 40) | Group C (n = 40) | t/χ2 value | P value | |
| Gender (Male) | 29 | 31 | 0.267 | 0.606 |
| Age (yr) | 62.82 ± 12.93 | 61.38 ± 10.29 | 0.551 | 0.583 |
| Tumor size (cm) | 3.75 ± 1.21 | 3.68 ± 1.17 | 0.263 | 0.793 |
| Number of tumors | 0.050 | 0.823 | ||
| Single | 22 | 21 | ||
| 2-3 | 18 | 19 | ||
| Child-Pugh classification | 0.054 | 0.816 | ||
| A | 26 | 25 | ||
| B | 14 | 15 | ||
| TBiL (μmol/L) | 18.28 ± 5.28 | 17.83 ± 5.16 | 0.583 | 0.562 |
| AFP (ng/ml) | 612.29 ± 127.38 | 625.93 ± 139.27 | 0.457 | 0.649 |
| ALB (g/L) | 37.19 ± 5.39 | 36.93 ± 5.33 | 0.217 | 0.829 |
| ALT (U/L) | 35.27 ± 15.14 | 36.42 ± 17.46 | 0.315 | 0.754 |
| AST (U/L) | 38.63 ± 15.13 | 40.41 ± 16.52 | 0.503 | 0.617 |
At the 3rd month after treatment, in the patients of Group A, the number of CR was 11 (27.5%), PR was 14 (35%), and the treatment efficiency was 62.5%. In the patients of Group C, the number of CR was 13 (32.5%), PR was 15 (37.5%), and the treatment efficiency was 70%. It was slightly higher than Group A, but the difference was not statistically significant (χ2 = 0.701, P = 0.873). At the 6th and 9th month after treatment, the SD of the two groups decreased slightly, and the PD increased. However, the clinical efficacy between the two groups was still similar, and the differences were not statistically significant (both P > 0.05, Table 2).
| 3nd month | 6th month | 9th month | ||||
| Group A | Group C | Group A | Group C | Group A | Group C | |
| CR | 11 (27.5) | 13 (32.5) | 11 (27.5) | 13 (32.5) | 11 (27.5) | 13 (32.5) |
| PR | 14 (35.0) | 15 (37.5) | 14 (35.0) | 15 (37.5) | 14 (35.0) | 15 (37.5) |
| SD | 13 (32.5) | 11 (27.5) | 12 (30.0) | 10 (25.0) | 10 (25.0) | 10 (25.0) |
| PD | 2 (5.0) | 1 (2.5) | 3 (7.5) | 2 (5.0) | 4 (10.0) | 2 (5.0) |
| Efficiency | 25 (62.5) | 28 (70.0) | 25 (62.5) | 28 (70.0) | 25 (62.5) | 28 (70.0) |
| χ2 value | 0.701 | 0.583 | 0.855 | |||
| P value | 0.873 | 0.900 | 0.836 | |||
Transient liver damage occurred in both groups at the 1st week after treatment. Among them, patients in Group A had lower ALT, AST and TBiL compared with Group C (all P < 0.05, Table 3). Liver function-related indicators were improved in both groups at the 1st month after treatment.
| Group A | Group C | t/χ2 value | P value | |
| ALB (g/L) | 32.82 ± 5.93 | 31.32 ± 5.28 | 1.195 | 0.236 |
| ALT (U/L) | 48.29 ± 15.39 | 67.29 ± 22.93 | 4.351 | 0.000 |
| AST (U/L) | 51.73 ± 14.92 | 79.28 ± 20.28 | 6.921 | 0.000 |
| TBiL (μmol/L) | 23.93 ± 8.38 | 31.39 ± 9.29 | 3.771 | 0.000 |
In Group A, 14 patients started to have fever at the 2nd or 3rd day after treatment, and 4 of them had a body temperature of more than 38.5 °C. They were given anti-infective and antipyretic treatments, and improved in 3-5 d. Eleven patients had different degrees of post-embolic syndrome, 10 patients hepatic pain, 18 patients elevated transaminase, and 3 patients had ascites. All of them were effectively relieved after symptomatic treatment and liver protection treatment for one week. Serious complications such as needle-free transfer, peripheral organ damage, and biliary fistula were not found. In Group C, 21 patients developed infection, including 11 wound infections, 6 pulmonary infections, and 4 intestinal infections. Six patients developed ascites. They were effectively relieved after the corresponding anti-infective treatment and liver protection treatment. No operational-related deaths occurred in Group A and Group C.
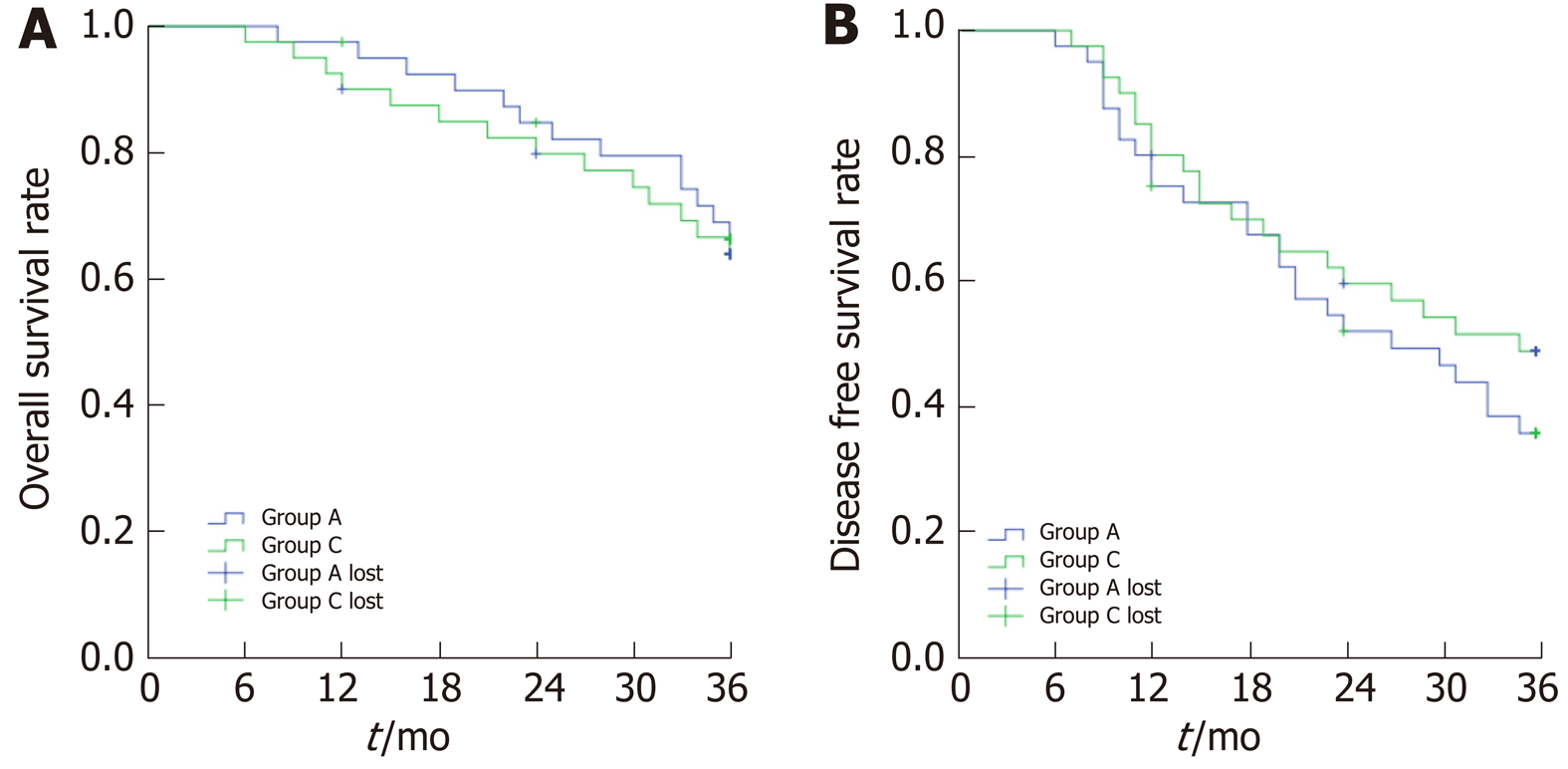
Two patients in Group A were lost to follow-up. The average follow-up time was 30.9 ± 1.5 mo. The OS rates at the 1st, 2nd, and 3rd year after treatment was 97.5%, 84.7%, and 66.1%, respectively. The DFS rates were 75.0%, 51.7%, and 35.4%. A total of 13 patients died, including 6 cases of liver failure, 4 cases of hepatorenal syndrome, and 3 cases of upper gastrointestinal bleeding. In Group C, 3 patients were lost to follow-up. The average follow-up time was 32.3 ± 1.2 mo. The OS rates were 90.0%, 79.7%, and 63.8%, and the DFS rates were 80.0%, 59, 7%, and 48.6%. A total of 14 patients died, including 8 patients with liver failure, 5 patients with hepatorenal syndrome, and 1 patient with upper gastrointestinal bleeding. Kaplan-Meier survival analysis revealed that the OS and DFS were similar between the two groups, and the differences were not statistically significant (OS: Log Rank = 0.121, P = 0.728; DFS: Log Rank = 1.042, P = 0.307; Figure 6).
The tumor size of Group B was less than Group A (P < 0.05). The gender, age, number of tumors, Child-Pugh classification, AFP and liver function indexes were similar in the two groups. The differences were not statistically significant (all P > 0.05, Table 4).
| Group A (n = 40) | Group B (n = 36) | t/χ2 value | P value | |
| Gender (Male) | 29 | 26 | 0.001 | 0.978 |
| Age (yr) | 62.82 ± 12.93 | 64.82 ± 11.82 | 0.722 | 0.472 |
| Tumor size (cm) | 3.75 ± 1.21 | 3.13 ± 0.82 | 2.683 | 0.009 |
| Number of tumors | 0.002 | 0.961 | ||
| Single | 22 | |||
| 2-3 | 18 | 16 | ||
| Child-Pugh classification | 0.023 | 0.878 | ||
| A | 26 | 24 | ||
| B | 14 | 12 | ||
| TBiL (μmol/L) | 18.28 ± 5.28 | 17.38 ± 5.62 | 0.738 | 0.463 |
| AFP (ng/ml) | 612.29 ± 127.38 | 567.28 ± 92.39 | 1.746 | 0.085 |
| ALB (g/L) | 37.19 ± 5.39 | 34.94 ± 4.53 | 1.958 | 0.054 |
| ALT (U/L) | 35.27 ± 15.14 | 36.49 ± 12.31 | 0.383 | 0.703 |
| AST (U/L) | 38.63 ± 15.13 | 36.22 ± 13.26 | 0.735 | 0.465 |
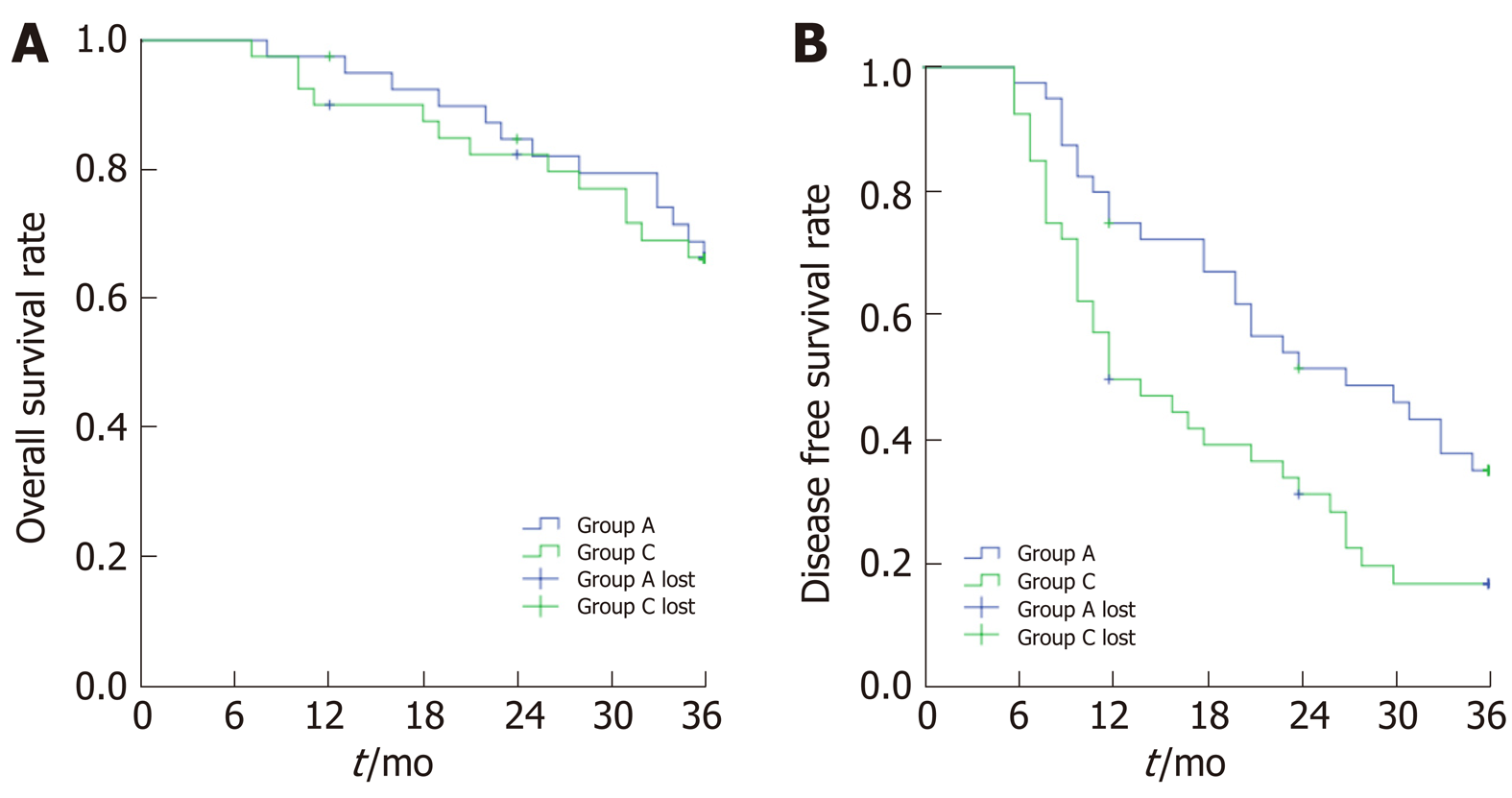
Two patients in Group B were lost to follow-up. The average follow-up time was 31.2 ± 1.4 mo. The OS rates at the 1st, 2nd, and 3rd year after treatment was 90%, 82.3%, and 66.4%, respectively. The OS rates in Group A and Group B were similar, and the difference was not statistically significant (Log Rank = 0.017, P = 0.897). The DFS rates were 50%, 31.6%, and 17.2%, respectively, which was lower than that of Group A (Log Rank = 6.123, P = 0.013, Figure 7). A total of 13 patients died, including 8 patients with liver failure and 5 patients with hepatorenal syndrome.
Due to the hidden onset of HCC, only 30% of patients have the chance of hepatectomy[21]. The treatment after recurrence is also a major problem since the recurrence rate of HCC is higher[22]. Ultrasound-guided RFA is widely used as an effective minimally invasive interventional treatment[23]. It is simple, reproducible, and has few serious post-treatment complications[24]. However, since most RF needles can only ablate 4-5 cm spherical necrosis areas, the ablation effect is satisfied only if the tumor diameter is less than 3 cm. The difficulty of RFA will be significantly increased if the tumor diameter increases, and multiple needles and points must be required for complete ablation[25]. Mazzaferro et al[26] indicated that the advantages of hepatectomy over RFA are mainly focus on tumors with a diameter of 3-5 cm. This is because RFA is more likely to leave local tumor lesions during tumor ablation of 3-5 cm in diameter.
TACE before RFA can effectively reduce the residual of active tissue after RFA[27]. The study by Kim et al[28] revealed that when RFA combined with TACE was used in the treatment of HCC with a maximum diameter of 3-5 cm, the local tumor progression rate was significantly lower than that of RFA alone. Iezzi et al[29] reported that for patients with HCC > 3 cm, the long-term survival of RFA combined with TACE was better than that of TACE or RFA alone. It is worth noting that the traditional emulsion has poor stability, and the chemotherapeutic drugs are easily diffused into the patient's peripheral circulatory system, resulting in an uncontrolled local release of the drug, and easily reducing the local treatment effect and aggravating the systemic adverse reactions of the chemotherapeutic drugs[30].
The development of DEB-TACE solves these two problems[31]. Compared with traditional TACE embolic material (lipiodol), DEB-TACE has the advantage of sustained and slow release. After the drug-loaded microspheres enter the nourishing artery, the loaded drug can be released for up to two weeks, which allows it to maintain a high local concentration in tumor cells and prolong the effect of chemotherapy drugs on tumor cells[32]. In addition, the good deformation ability of the drug-loaded microsphere makes it possible to adhere to the blood vessels to achieve a complete embolization. Meanwhile, the accumulation of chemotherapeutic drugs at the proximal or distal end of the blood vessel is avoided, reducing the incidence of adverse drug reactions[33-35]. Moreover, the drug dose carried by the microspheres is much larger than that of lipiodol, so DEB-TACE can reach higher chemotherapeutic drug concentrations in tumor tissues, while ensuring lower concentrations in the systemic circulation[36]. Compared with traditional TACE, DEB-TACE has higher disease control rate and overall survival rate[37]. The study by Xiang et al[38] reported that DEB-TACE treatment does not aggravate liver toxicity in patients compared to traditional TACE treatment. Song et al[39] found that DEB-TACE had less common adverse reactions than traditional TACE. They indicated that HCC patients have better tolerance to DEB-TACE treatment.
Studies have shown that the synergistic effect of DEB-TACE and RFA has a powerful anti-cancer effect[40]. It is demonstrated by the studies of patients with liver metastases from colorectal cancer from Lee et al[41] and Ahmed et al[42]. Ahmed et al[43] found that RFA can increase the permeability of capillaries by dilating the nutritional blood vessels of the tumor, which is conducive to the residual chemotherapy drugs acting on tumor tissue. Zhu et al[44] found that the combined application of DEB-TACE and RFA improved the efficacy and survival time. However, combined treatment will increase the cost and prolong the hospital stay. Therefore, there are fewer long-term studies on DEB-TACE combined with RFA in the treatment of HCC, and its advantages need to be verified by prospective studies. In this study, the long-term follow-up of HCC patients who received the combination of DEB-TACE and RFA was performed to explore the application value of the combined treatment of DEB-TACE and RFA.
This study showed that the effective rates of Group A with DEB-TACE combined with RFA and Group C with surgery were similar at the 3rd, 6th, and 9th month. It indicated that DEB-TACE combined with RFA can achieve the same short-term effects as surgical treatment. In terms of liver damage, the results implied that the treatment of DEA-TACE combined with RFA had less damage to the liver than surgical treatment, which is similar to the results of the Pan et al[45]. In terms of complications, it indicated that patients treated with DEB-TACE combined with RFA showed better tolerance.
In terms of long-term efficacy, the treatment of DEB-TACE combined with ultrasound-guided RFA can achieve long-term effects similar to surgical treatment. It is worth noting that the average tumor diameter of Group A was 3.75 ± 1.21 cm, of which 16 patients had a tumor diameter > 3 cm. It revealed that the long-term effect of DEB-TACE combined with RFA on > 3 cm tumors is no different from the curative effect of surgical treatment, thus overcoming the limitation of RFA treatment on the tumor volume.
After comparing the short-term and long-term effects of the two groups, we believe that DEB-TACE combined with RFA is safe for patients. Compared with surgery, combined treatment has less trauma, less liver damage, faster post-treatment recovery, and is more suitable for patients with multiple HCC or cirrhosis. In addition, in clinical practice, we found that if the tumor is located around the liver, it is easy to be surgically removed, while RFA is not suitable because it needs to prevent puncture to cause tumor rupture and prevent normal tissues around burns. However, if the tumor is located in the center of the liver parenchyma, RFA has more advantages. Surgical resection requires more normal tissues to be sacrificed and it is easy to cause cancer cell metastasis due to surgical compression.
HCC recurrence is the main factor limiting the effectiveness of HCC treatment. Although reoperation is the preferred treatment for recurrent HCC, there are fewer opportunities for reoperation due to surgical adhesions, liver dysfunction, and insufficient residual liver capacity[46,47]. Therefore, DEB-TACE combined with ultrasound-guided RFA is expected to be an effective treatment for patients with recurrent HCC. This study compared the clinical characteristics of patients with untreated and recurrent HCC and found that the clinical characteristics of patients with untreated and recurrent HCC were similar. However, the tumor diameter of patients with recurrent HCC was smaller than that of patients with untreated HCC. It is because HCC patients would be followed up after treatment. Recurrent patients will be detected early by imaging. In terms of long-term efficacy, 13 patients with recurrent HCC died, and their DFS in 1-3 years was lower than that in patients with untreated HCC. The reason why untreated HCC is more likely to recur than untreated HCC may be because liver volume decreases and liver reserve decreases after liver cancer resection. However, it is worth noting that this study found the OS of recurrent HCC patients in 1-3 years was similar to that of untreated HCC patients, and the difference was not statistically significant. It indicated that in the treatment of recurrent HCC, TACE combined with RFA could well control local tumors and prolong the survival time of patients, and can obtain the same effect as reoperation.
In conclusion, DEB-TACE combined with ultrasound-guided RFA provides a new method for the treatment of HCC. Its short-term effect is comparable to traditional surgical treatment, and it has less bleeding, quicker recovery during treatment, and less damage to liver function. More importantly, DEB-TACE combined with ultrasound-guided RFA in the treatment of recurrent HCC can prolong the survival time of patients. However, this study is still a single-center study, and the sample size is limited. It is necessary to conduct a further study of a larger sample.
Ultrasound-guided radiofrequency ablation (RFA) is one of the most effective treatments for early hepatocellular carcinoma (HCC). However, due to the limitation of local tumor control ability, RFA is difficult to completely cover tumors with a diameter of more than 3 cm. Transarterial chemoembolization (TACE) can significantly reduce the volume of hepatic carcinoma. Hence the combined use of TACE and RFA can obtain a larger ablation coverage volume.
Drug-eluting beads TACE (DEB-TACE) has the advantages of sustained slow release, maintaining a high local concentration, and reducing the incidence of adverse drug reactions compared to traditional TACE. DEB-TACE combined with ultrasound-guided RFA therapy has strong anti-cancer effects and little side effects, but there are fewer related long-term studies in clinical setting.
The aim of our study was to explore the possible benefits of DEB-TACE combined with RFA by analyzing the liver function and clinical efficacy of patients with primary HCC. It is hopeful to help the management of HCC.
Seventy-six patients with primary HCC who underwent DEB-TACE combined with ultrasound-guided RFA were recruited. Among them, 40 patients with untreated HCC were defined as Group A, 36 patients with recurrent HCC were defined as Group B, and 40 patients with untreated HCC who were treated with surgery were defined as Group C. All patients underwent serological examination and recorded alpha-fetoprotein and liver function. Liver function tests were performed at the 1st week and 1st month after treatment to assess liver damage. Efficacy was assessed at the 3rd, 6th, and 9th month after treatment. All patients were followed up for 3 years and their overall survival (OS), disease-free survival (DFS) were calculated.
After 3 mo of treatment, the effective rate of Group A and C was similar. Among them, group A has less damage to liver function during treatment. The OS and DFS were similar in the two groups. It indicated that the efficacy of DEA-TACE combined with ultrasound-guided RFA in the treatment of primary HCC is comparable to traditional surgical treatment. It has faster recovery, and less damage to liver function during treatment. The OS of Group B were similar to Group A, and the DFS of Group B were lower than Group A. It indicated that the efficacy of DEA-TACE combined with ultrasound-guided RFA in the treatment of recurrent HCC is positive, with fewer complications, and it can prolong the survival time.
The efficacy of DEA-TACE combined with ultrasound-guided RFA in the treatment of primary HCC is comparable to that of traditional surgical treatment. Moreover, it has less bleeding, faster recovery, and less damage to liver function during treatment. Its efficacy in the treatment of recurrent HCC is positive, with fewer complications, and it can prolong the survival time of patients.
DEB-TACE combined with ultrasound-guided RFA in the treatment of recurrent HCC has a positive effect, fewer complications, and can prolong the survival time of patients. However, this study is still a single-center study, and the sample size is limited. This study will perform a larger sample and more detailed research with big data services to get more accurate conclusions.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Armellini E, Musquer N, Tanimine N S-Editor: Wang JL L-Editor: A P-Editor: Zhang YL
| 1. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 2. | Dhir M, Melin AA, Douaiher J, Lin C, Zhen WK, Hussain SM, Geschwind JF, Doyle MB, Abou-Alfa GK, Are C. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann Surg. 2016;263:1112-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 3. | Galle PR, Tovoli F, Foerster F, Wörns MA, Cucchetti A, Bolondi L. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol. 2017;67:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Grumme J, Werncke T, Meine TC, Becker LS, Kloeckner R, Maschke SK, Kirstein MM, Vogel A, Wacker FK, Meyer BC, Hinrichs JB, Rodt T. Transarterial chemoembolization for hepatocellular carcinoma: quality of life, tumour response, safety and survival comparing two types of drug-eluting beads. Abdom Radiol (NY). 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262:689-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Liu F, Chen M, Mei J, Xu L, Guo R, Lin X, Zhang Y, Peng Z. Transarterial Chemoembolization Combined with Radiofrequency Ablation in the Treatment of Stage B1 Intermediate Hepatocellular Carcinoma. J Oncol. 2019;2019:6298502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, Russo M, Beecroft R, Ghanekar A, Bhat M, Brierley J, Greig PD, Knox JJ, Dawson LA, Grant DR. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 217] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 8. | Xu Z, Xie H, Zhou L, Chen X, Zheng S. The Combination Strategy of Transarterial Chemoembolization and Radiofrequency Ablation or Microwave Ablation against Hepatocellular Carcinoma. Anal Cell Pathol (Amst). 2019;2019:8619096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Wu B, Zhou J, Ling G, Zhu D, Long Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol. 2018;16:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Zou JH, Zhang L, Ren ZG, Ye SL. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis. 2016;17:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Xing M, Kokabi N, Prajapati HJ, Close O, Ludwig JM, Kim HS. Survival in unresectable AJCC stage I and II HCC and the effect of DEB-TACE: SEER versus tertiary cancer center cohort study. J Comp Eff Res. 2016;5:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Xie ZB, Wang XB, Peng YC, Zhu SL, Ma L, Xiang BD, Gong WF, Chen J, You XM, Jiang JH, Li LQ, Zhong JH. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res. 2015;45:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Ou HY, Cheng YF, Chuang YH, Hsu HW, Chen CL, Lazo MZ, Weng CC, Yu CY, Tsang LL, Huang TL, Tong YS. Quantification of Functional MR Predicts Early Response in Post-doxorubicin Drug-Eluting Beads Chemoembolization for Hepatocellular Carcinoma. Dig Dis Sci. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Zhang X, Lin X, Qiu H, Peng Z. An investigation of efficacy, safety, and prognostic factors of drug-eluting beads-transarterial chemoembolization operation with CalliSpheres® Microspheres in treating Chinese hepatocellular carcinoma patients. J Clin Lab Anal. 2019;33:e22975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Correction to: Zhang etal. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv. 2017;24:1927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Yamakado K, Inaba Y, Sato Y, Yasumoto T, Hayashi S, Yamanaka T, Nobata K, Takaki H, Nakatsuka A. Radiofrequency Ablation Combined with Hepatic Arterial Chemoembolization Using Degradable Starch Microsphere Mixed with Mitomycin C for the Treatment of Liver Metastasis from Colorectal Cancer: A Prospective Multicenter Study. Cardiovasc Intervent Radiol. 2017;40:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3000] [Article Influence: 428.6] [Reference Citation Analysis (3)] |
| 18. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 5990] [Article Influence: 855.7] [Reference Citation Analysis (3)] |
| 19. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2583] [Cited by in RCA: 3277] [Article Influence: 218.5] [Reference Citation Analysis (36)] |
| 20. | D'Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 21. | Ni CY, Yang Y, Chang YQ, Cai H, Xu B, Yang F, Lau WY, Wang ZH, Zhou WP. Fast-track surgery improves postoperative recovery in patients undergoing partial hepatectomy for primary liver cancer: A prospective randomized controlled trial. Eur J Surg Oncol. 2013;39:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Gabrielson A, Wu Y, Wang H, Jiang J, Kallakury B, Gatalica Z, Reddy S, Kleiner D, Fishbein T, Johnson L, Island E, Satoskar R, Banovac F, Jha R, Kachhela J, Feng P, Zhang T, Tesfaye A, Prins P, Loffredo C, Marshall J, Weiner L, Atkins M, He AR. Intratumoral CD3 and CD8 T-cell Densities Associated with Relapse-Free Survival in HCC. Cancer Immunol Res. 2016;4:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 243] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 23. | Zhang W, Luo E, Gan J, Song X, Bao Z, Zhang H, Chen M. Long-term survival of hepatocellular carcinoma after percutaneous radiofrequency ablation guided by ultrasound. World J Surg Oncol. 2017;15:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Patidar Y, Singhal P, Gupta S, Mukund A, Sarin SK. Radiofrequency ablation of surface v/s intraparenchymal hepatocellular carcinoma in cirrhotic patients. Indian J Radiol Imaging. 2017;27:496-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Pompili M, Mirante VG, Rondinara G, Fassati LR, Piscaglia F, Agnes S, Covino M, Ravaioli M, Fagiuoli S, Gasbarrini G, Rapaccini GL. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: Assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, Sarli D, Schiavo M, Garbagnati F, Marchianò A, Spreafico C, Camerini T, Mariani L, Miceli R, Andreola S. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 379] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 27. | Kong QF, Jiao JB, Chen QQ, Li L, Wang DG, Lv B. Comparative effectiveness of radiofrequency ablation with or without transarterial chemoembolization for hepatocellular carcinoma. Tumour Biol. 2014;35:2655-2659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Kim W, Cho SK, Shin SW, Hyun D, Lee MW, Rhim H. Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma: comparison with TACE or RFA monotherapy. Abdom Radiol (NY). 2019;44:2283-2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 29. | Iezzi R, Cesario V, Siciliani L, Campanale M, De Gaetano AM, Siciliano M, Agnes S, Giuliante F, Grieco A, Pompili M, Rapaccini GL, Gasbarrini A, Bonomo L, HepatoCATT Group for the Multidisciplinary Management of HCC. Single-step multimodal locoregional treatment for unresectable hepatocellular carcinoma: balloon-occluded percutaneous radiofrequency thermal ablation (BO-RFA) plus transcatheter arterial chemoembolization (TACE). Radiol Med. 2013;118:555-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Nhu QM, Knowles H, Pockros PJ, Frenette CT. Pulmonary complications of transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Respirol. 2016;6:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Pesapane F, Nezami N, Patella F, Geschwind JF. New concepts in embolotherapy of HCC. Med Oncol. 2017;34:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Wáng YX, De Baere T, Idée JM, Ballet S. Transcatheter embolization therapy in liver cancer: an update of clinical evidences. Chin J Cancer Res. 2015;27:96-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 33. | Facciorusso A, Licinio R, Muscatiello N, Di Leo A, Barone M. Transarterial chemoembolization: Evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol. 2015;7:2009-2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Melchiorre F, Patella F, Pescatori L, Pesapane F, Fumarola E, Biondetti P, Brambillasca P, Monaco C, Ierardi AM, Franceschelli G, Carrafiello G. DEB-TACE: a standard review. Future Oncol. 2018;14:2969-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 35. | Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: Current state of the art. World J Gastroenterol. 2018;24:161-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 36. | Zhao C, Ma SPZCY. Comparison of treatment response, survival and safety between drug-eluting bead transarterial chemoembolization with CalliSpheres® microspheres versus conventional transarterial chemoembolization in treating hepatocellular carcinoma. J BUON. 2019;24:1150-1166. [PubMed] |
| 37. | Huang K, Zhou Q, Wang R, Cheng D, Ma Y. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:920-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Xiang H, Long L, Yao Y, Fang Z, Zhang Z, Zhang Y. CalliSpheres Drug-Eluting Bead Transcatheter Arterial Chemoembolization Presents With Better Efficacy and Equal Safety Compared to Conventional TACE in Treating Patients With Hepatocellular Carcinoma. Technol Cancer Res Treat. 2019;18:1533033819830751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Song JE, Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol. 2017;9:808-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 40. | Vasnani R, Ginsburg M, Ahmed O, Doshi T, Hart J, Te H, Van Ha TG. Radiofrequency and microwave ablation in combination with transarterial chemoembolization induce equivalent histopathologic coagulation necrosis in hepatocellular carcinoma patients bridged to liver transplantation. Hepatobiliary Surg Nutr. 2016;5:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Lee H, Heo JS, Cho YB, Yun SH, Kim HC, Lee WY, Choi SH, Choi DW. Hepatectomy vs radiofrequency ablation for colorectal liver metastasis: a propensity score analysis. World J Gastroenterol. 2015;21:3300-3307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Ahmed M; Technology Assessment Committee of the Society of Interventional Radiology. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014;25:1706-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 43. | Ahmed M, Moussa M, Goldberg SN. Synergy in cancer treatment between liposomal chemotherapeutics and thermal ablation. Chem Phys Lipids. 2012;165:424-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Zhu D, Yuan D, Wang Z, Chen S. Efficacy of drug-eluting bead transarterial chemoembolization (DEB-TACE) combined with radiofrequency ablation versus DEB-TACE alone in Chinese hepatocellular carcinoma patients. Medicine (Baltimore). 2019;98:e15682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Pan T, Mu LW, Wu C, Wu XQ, Xie QK, Li XS, Lyu N, Li SL, Deng HJ, Jiang ZB, Lin AH, Zhao M. Comparison of Combined Transcatheter Arterial Chemoembolization and CT-guided Radiofrequency Ablation with Surgical Resection in Patients with Hepatocellular Carcinoma within the Up-to-seven Criteria: A Multicenter Case-matched Study. J Cancer. 2017;8:3506-3513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Nakada S, Allard MA, Lewin M, Awad S, Dahbi N, Nitta H, Cunha AS, Castaing D, Vibert E, Cherqui D, Miyazaki M, Ohtsuka M, Adam R. Ischemic Cholangiopathy Following Transcatheter Arterial Chemoembolization for Recurrent Hepatocellular Carcinoma After Hepatectomy: an Underestimated and Devastating Complication. J Gastrointest Surg. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Midorikawa Y, Takayama T, Moriguchi M, Yagi R, Yamagishi S, Nakayama H, Aramaki O, Yamazaki S, Tsuji S, Higaki T. Liver Resection Versus Embolization for Recurrent Hepatocellular Carcinoma. World J Surg. 2020;44:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |