Published online Aug 27, 2020. doi: 10.4240/wjgs.v12.i8.336
Peer-review started: February 29, 2020
First decision: May 24, 2020
Revised: June 12, 2020
Accepted: August 1, 2020
Article in press: August 1, 2020
Published online: August 27, 2020
Processing time: 168 Days and 17.4 Hours
Liver transplant (LT) is a complex procedure with frequent postoperative complications. In other surgical procedures such as gastrectomy, esophagectomy or resection of liver metastases, these complications are associated with poorer long-term survival. It is possible this happens in LT but there are not enough data to establish this relationship.
To analyze the possible influence of postoperative complications on long-term survival and the ability of the comprehensive complication index (CCI) to predict this.
Retrospective study in a tertiary-level university hospital. The 164 participants were all patients who received a LT from January 2012 to July 2019. The follow-up was done in the hospital until the end of the study or death. Comorbidity and risk after transplantation were calculated using the Charlson and balance of risk (BAR) scores, respectively. Postoperative complications were graded according to the Clavien-Dindo classification and the CCI. To assess the CCI cut-off value with greater prognostic accuracy a receiver operating characteristic (ROC) curve was built, with calculation of the area under the curve (AUC). Overall survival was estimated according to the Kaplan-Meier test and log-rank test. Groups were compared by the Mann-Whitney test. For the multivariable analysis the Cox regression was used.
The mean follow-up time of the cohort was 37.76 (SD = 24.5) mo. A ROC curve of CCI with 5-year survival was built. The AUC was 0.826 (0.730-0.922), P < 0.001. The cut-off was calculated by means of the Youden index with a result of 35.95. The sensitivity was 84.6% and the specificity 61.3%. Survival curves for comparison of patients with CCI score < 36 vs ≥ 36 were calculated. The estimated 5-year survival was 57.65 and 43.95 months, respectively (log-rank < 0.001). This suggests that patients with more severe complications exhibit worse long-term survival. Other cut-off values were analysed. Comparison between patients with CCI < 33.5 vs > 33.5 (33.5 = median CCI value) showed estimated 5-year survival was 57.4 and 45.71 months, respectively (log-rank < 0.0001). Dividing patients according to the mode CCI value (20.9) showed an estimated 5-year survival of 60 mo for a CCI below 20.9 vs 57 mo for a CCI above 20.9 (log-rank = 0.147). The univariate analysis did not show any association between individual complications and long-term survival. A multivariate analysis was carried out to analyse the possible influence of CCI, Charlson comorbidity index, BAR and hepatocellular carcinoma on survival. Only the CCI score showed significant influence on long-term survival.
A complicated postoperative period – well-defined by means of the CCI score – can influence not only short-term survival, but also long-term survival.
Core tip: It is not known whether postoperative complications after liver transplant (LT) are associated with poorer long-term survival. The objective of the present study is therefore to analyse the possible influence of postoperative complications on the long-term survival of LT patients. A retrospective study of 164 LT patients was conducted, analysing complications and grading them by means of the Clavien classification and the comprehensive complication index (CCI). We found that a complicated postoperative period – well-defined by means of the CCI score – can influence not only short-term survival, but also long-term survival.
- Citation: Castanedo S, Toledo E, Fernández-Santiago R, Castillo F, Echeverri J, Rodríguez-Sanjuán JC. Influence of postoperative complications on long-term survival in liver transplant patients. World J Gastrointest Surg 2020; 12(8): 336-345
- URL: https://www.wjgnet.com/1948-9366/full/v12/i8/336.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i8.336
Liver transplant (LT) is performed in the end stage of a chronic liver disease or acute liver failure. In spite of medical progress –skills and technology- LT is a complex procedure associated with many potential complications, such as bleeding, portal or arterial thrombosis, primary liver dysfunction or biliary leaks or stenosis[1-4]. Many complications require interventional procedures, reoperations, prolonged intensive care unit and hospital stays and some lead to death.
Several studies have investigated the possible influence of these postoperative complications on the long-term survival of patients treated because of gastric cancer[5,6], colo-rectal cancer[7], colo-rectal liver metastases[8], or squamous cell esophageal carcinoma[9]. However, there are no data concerning LT with the exception of papers dealing with graft damage after biliary or ischemic complications[10].
Postoperative complications were graded using the Dindo-Clavien classification[11], which comprises seven grades based on the therapy required to treat each complication. However, this score does not combine multiple complications in the same patient. The comprehensive complication index (CCI)[12] further develops the Dindo-Clavien classification, taking into account all complications to provide each patient with a combined morbidity score.
The objective of the present study is therefore to analyse the possible influence of postoperative complications on the long-term survival of LT patients.
Study design: Retrospective study in a tertiary university hospital in Santander (Spain). The participants were all the patients who received a LT from January 2012 to July 2019. All were adults (n = 164) and received a cadaveric transplant following donor brain death -153- or cardiac death -11-. A technique of cava preservation with piggy-back anastomosis was performed in every case. Follow-up was done in the hospital until the end of the study or death.
Demographic, clinical, surgical and pathological variables were recorded from hospital data bases.
Comorbidity was calculated using the Charlson index[13]. The risk after transplantation was calculated by means of the balance of risk (BAR) score[14], which includes donor age, recipient age, model for end-stage liver disease (MELD) score, retransplantation, pretransplant life support and cold ischemia time.
Postoperative complications were graded according to the Clavien-Dindo classification[11] -which grades the most severe complication -and the CCI[12], which calculates the sum of all the complications that are weighted for their severity. CCI was calculated according to the formula available at https://www.assessurgery.com/about_cci-calculator/, with a score between 0 –no complications- and 100 –death-.
The programme IBM SPSS Statistics version 21.0 (Chicago, EE. UU., 2012) was used. Values of P < 0.05 were considered significant. The Kolmogorov–Smirnov test was used to assess the distribution of the continuous variables. To assess the CCI cut-off value with greatest prognostic accuracy a receiver operating characteristic (ROC) curve was built, with calculation of the area under the curve (AUC). The highest Youden index (sensitivity + specificity -1) was calculated.
For survival analysis, patients who died in the postoperative period before discharge from hospital were excluded. Overall survival was estimated according to the Kaplan-Meier test and distribution comparison with the log-rank test. Groups were compared with the Mann-Whitney test.
To investigate whether other factors such as Charlson comorbidity index, BAR score or hepatocellular carcinoma also influenced survival, a multivariable analysis using the Cox regression was performed. The statistical review of the study was performed by a biomedical statistician.
One hundred and sixty-four patients, 130 men (79.3%) and 34 women (20.7%) with a mean age of 55.3 years (SD = 9.5) were analysed. The most frequent indications for transplant were hepatocellular carcinoma (36%) and alcoholic liver disease (26.7%).
The main variables of donor and recipient are shown in Table 1.
| Recipient variables | |
| Gender, n (%) | |
| Men | 130 (79.3) |
| Women | 34 (20.7) |
| Child | |
| A | 60 (36.8) |
| B | 65 (39.9) |
| C | 38 (23.3) |
| Indication, n (%) | |
| OH | 43 (26.7) |
| HPC | 58 (36) |
| VHC | 13 (8.1) |
| Retransplantation | 13 (8.1) |
| Other | 34 (20.7) |
| Age, mean ± SD | 55.34 ± 9.55 |
| BMI, mean ± SD | 26.34 ± 4.27 |
| Charlson index, mean ± SD | 5.96 ± 2.06 |
| MELD, mean ± SD | 15.45 ± 6.93 |
| BAR score, mean ± SD | 6.14 ± 3.73 |
| CCI score, mean ± SD | 42.43 ± 25.01 |
| Donor variables, mean ± SD | |
| Age | 61.59 ± 16.02 |
| Cold ischemia (min) | 327.03 ± 119.2 |
| Donor type | |
| Brain death | 93.3% |
| Cardiac death | 6.7% |
| Death cause cerebrovascular | 80% |
| Trauma | 12.7% |
| Other | 7.3% |
All donors were cadaveric, 153 from brain death and 11 from controlled cardiac death (Maastricht type III), with premortem cannulation and normothermic regional perfusion using extracorporeal membrane oxygenation.
Thirteen patients died in hospital (7.9%): 4 due to primary liver dysfunction, 4 due to biliary complications, 3 because of portal thrombosis and 2 because of arterial bleeding.
The most frequent complications (Table 2) were biliary leaks -23.2%-, biliary strictures –9.1%-, wound infection -13.4%- and hepatic artery thrombosis -10.4%-. The treatment consisted of re-transplantation in 6 patients (3.6%) due to ischemic cholangiopathy -2-, hepatic artery thrombosis -2-, hyperacute rejection -1- and portal thrombosis -1-.
| Complications | 5-yr Survival | Log rank | ||
| Acute rejection | Yes | 10 (6.1) | 55.1 | 0.857 |
| No | 154 (93.3) | 55.8 | ||
| Arterial thrombosis | Yes | 17 (10.4) | 48.3 | 0.057 |
| No | 147 (89.6) | 56.5 | ||
| Portal thrombosis | Yes | 15 (9.1) | 51.1 | 0.722 |
| No | 149 (90.9) | 54.5 | ||
| Biliary stricture | Yes | 15 (9.1) | 54.7 | 0.898 |
| No | 149 (90.9) | 55.7 | ||
| Biliary leak | Yes | 38 (23.2) | 55.4 | 0.574 |
| No | 126 (76.8) | 56.9 | ||
| Wound infection | Yes | 22 (13.4) | 55.3 | 0.568 |
| No | 142 (86.6) | 58.8 | ||
| Acute renal failure | No | 89 (54.3) | 58 | 0.237 |
| AKIN I | 40 (24.4) | 52.1 | ||
| AKIN II | 18 (11) | 53.2 | ||
| AKIN III | 17 (10.4) | 53.1 | ||
| Death | Yes | 13 (7.9) | ||
| No | 151 (92.1) | |||
The patients were graded according to the Dindo-Clavien classification considering the most severe complications (Table 3).
| Clavien | Number | Description (n) |
| 0 | 9 (5.5) | |
| I | 4 (2.4) | Surgical wound hematoma (3) |
| Postoperative ileus (1) | ||
| II | 73 (44.5) | Portal thrombosis (5) |
| Vena cava thrombosis (1) | ||
| Intraabdominal hematoma (6) | ||
| Intraabdominal abscess (2) | ||
| Biliary leak (4) | ||
| Surgical wound infection (7) | ||
| Bacteriemia (2) | ||
| Respiratory complication (8) | ||
| Acute renal failure (19) | ||
| Urinary tract infection (4) | ||
| Acute rejection (3) | ||
| Thrombocytopenia (1) | ||
| Neurological alteration (5) | ||
| Fever without a source (5) | ||
| Hypocalcemia (1) | ||
| IIIa | 22 (13.4) | Intraabdominal abscess (3) |
| Biliary leak (12) | ||
| Biliary stricture (6) | ||
| Perforated diverticulitis (1) | ||
| IIIb | 26 (15.9) | Arterial thrombosis (7) |
| Portal thrombosis (2) | ||
| Biliary leak (6) | ||
| Biliary stricture (1) | ||
| Hemoperitoneum (7) | ||
| Vena cava leak (1) | ||
| Abdominal hernia (1) | ||
| Abdominal compartment syndrome (1) | ||
| IVa | 16 (9.8) | Arterial thrombosis (4) |
| Portal thrombosis (1) | ||
| Biliary leak (1) | ||
| Hemoperitoneum (7) | ||
| Vena cava leak (2) | ||
| Primary graft dysfunction (1) | ||
| IVb | 1 (0.6) | Primary graft dysfunction (1) |
| V | 13 (7.9) | Arterial thrombosis (4) |
| Portal thrombosis (2) | ||
| Hemoperitoneum (4) | ||
| Ischemic cholangiopathy (1) | ||
| Biliary stricture (1) | ||
| Bilateral pneumonia (1) | ||
| Total | 164 (100) |
Hepatic artery thrombosis happened in 17 patients and was treated as follows: Surgical revascularization in 8, anticoagulation or antiaggregation in 7 and retransplantation in the 2 above-mentioned cases. Portal thrombosis happened in 15 patients and was treated with anticoagulation in 14 and in one by the above-mentioned retransplantation. All patients with biliary leaks or strictures were initially treated by means of endoscopically-placed stents, although one later needed hepatico-jejunostomy.
The mean value of CCI was 4243 (SD = 2501).
Fourteen patients died during follow-up. The causes were: 3 septic complications not directly related with the transplant, 4 biliary complications, 1 bleed due to hepatic artery pseudoaneurism, 2 due to spread of hepatocellular carcinoma, 2 cases of humoral rejection, 1 necrotizing pancreatitis and one death of unknown etiology.
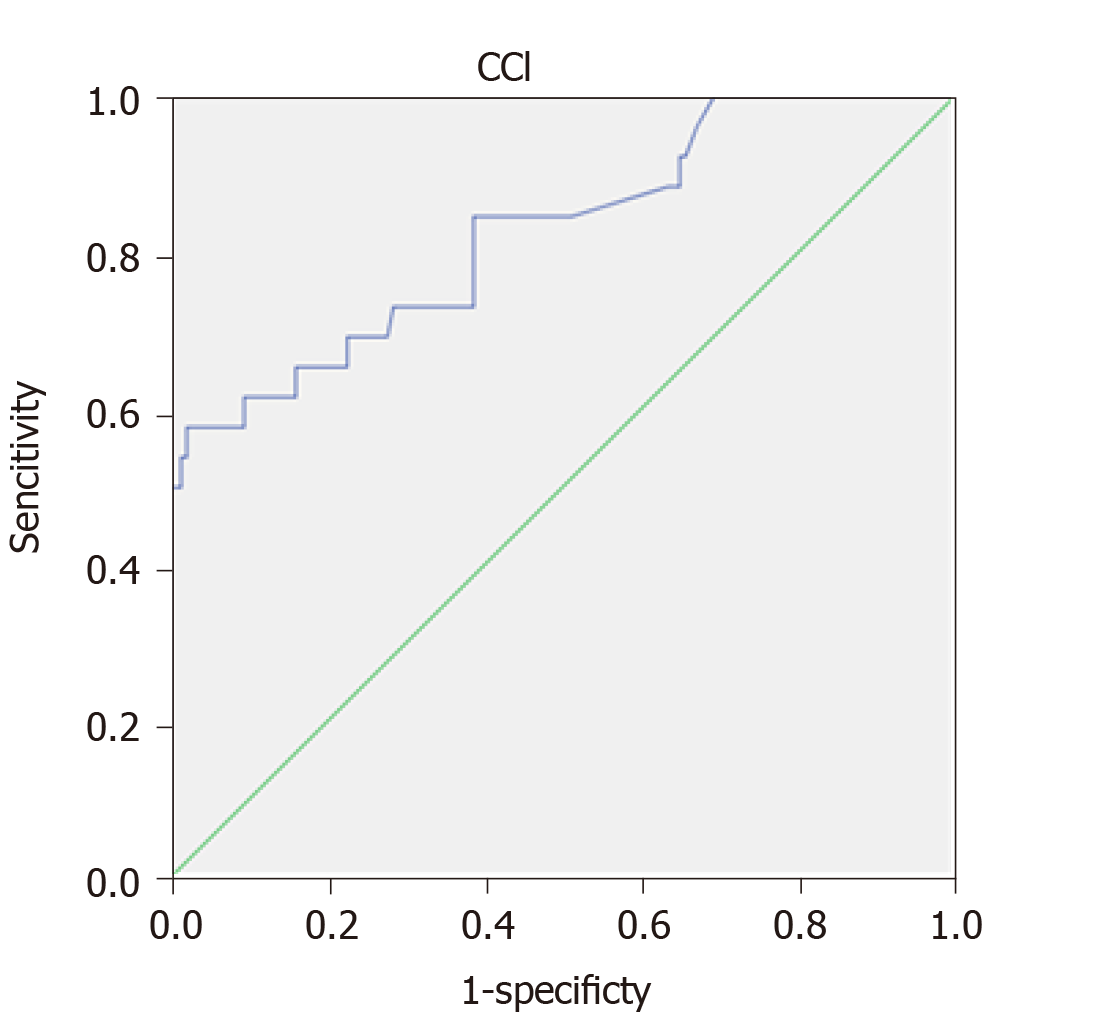
The mean follow-up time of the cohort was 37.76 (SD = 24.5) mo. A ROC curve of CCI with 5-year survival was built. The AUC was 0.826 (0.730-0.922), P < 0.001. The cut-off was calculated by means of the Youden index with a result of 35.95. The sensitivity was 84.6% and the specificity 61.3% (Figure 1, Table 4).
| CCI | 5-yr Survival (mo) | Log-rank | |
| < 20.9 | 12 (7.3) | 60.0 | 0.167 |
| ≥ 20.9 | 152 (92.7) | 50.7 | |
| < 33.5 | 78 (47.6) | 57.4 | < 0.001 |
| ≥ 33.5 | 86 (52.4) | 45.7 | |
| < 36 | 88 (53.7) | 57.6 | < 0.001 |
| ≥ 36 | 76 (46.3) | 43.9 | |
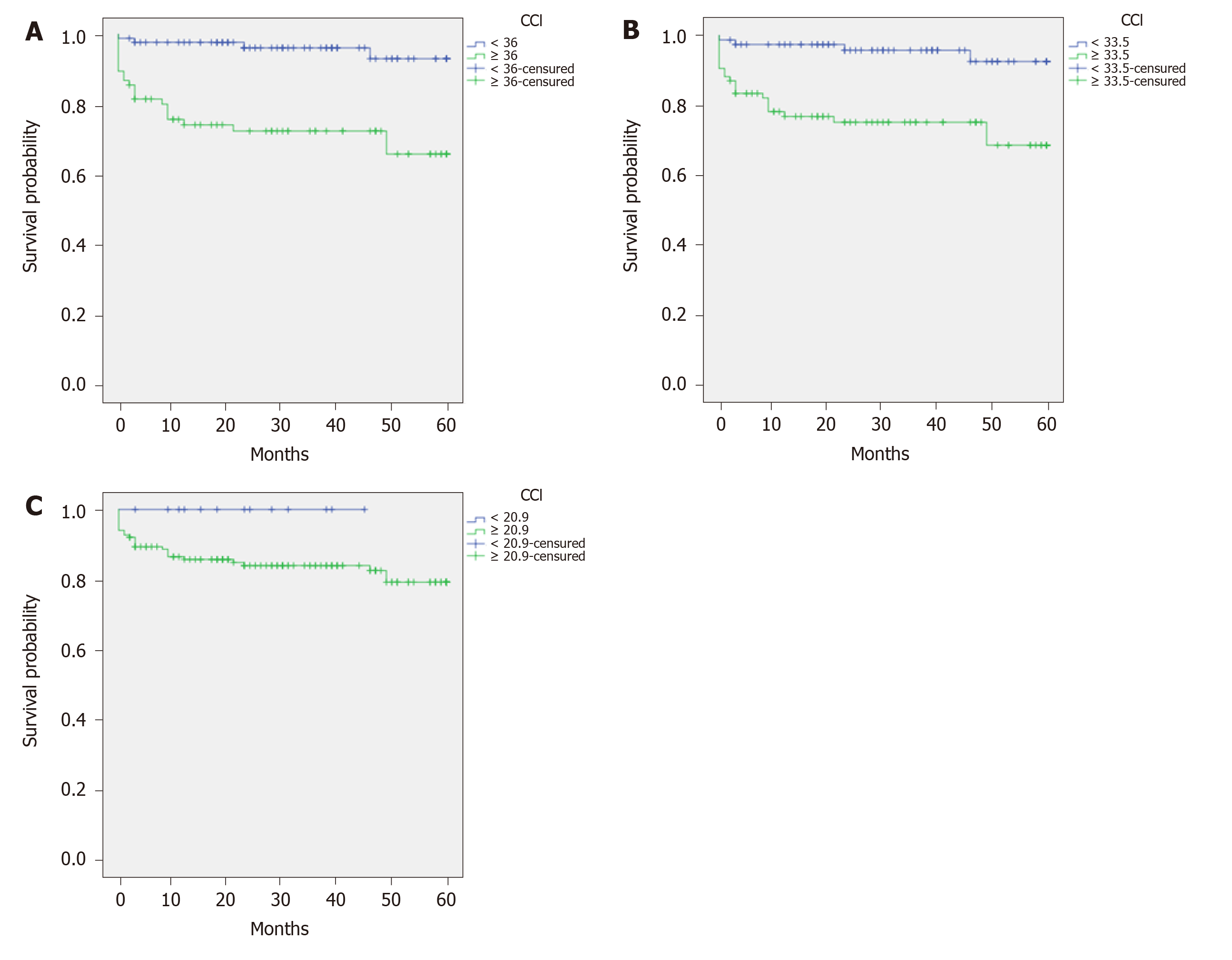
Survival curves for comparison of patients with CCI scores < 36 vs ≥ 36 were calculated. The estimated 5-year survival was 57.65 and 43.95 months, respectively (P < 0.001) (Figure 2A).
Other cut-off values were analysed. Comparison between patients with CCI < 33.5 vs > 33.5 (33.5 = median CCI value) showed estimated 5-year survival was 57.4 and 45.71 mo, respectively (P < 0.0001) (Figure 2B). Comparison between patients with CCI < 20.9 vs > 20.9 (20.9 = mode CCI value) showed estimated 5-year survival was 60 and 57 months, respectively (P = 0.147) (Figure 2C).
The univariate analysis did not show any association between individual complications and long-term survival (Table 2).
The multivariate analysis to investigate the possible influence of other factors on survival is shown in Table 5. Only the CCI score showed significant influence on long-term survival.
| Variables | P value | HR (95%CI) |
| BAR | 0.101 | 0.922 (0.797-1.016) |
| Charlson index | 0.58 | 0.764 (0.463-1.007) |
| CCI | < 0.001 | 0.941 (0.922-0.96) |
| HPC | 0.311 | 0.277 ( 0.028-1.402) |
Postoperative complications are frequent and often severe after liver transplantation. Many of them lead to intervention, reoperation, retransplantation and even death[15]. The postoperative mortality of the present study was 7.9%, similar to other European series -8-20%-[16,17] and according to the standard of the Spanish Society of LT[18].
Several studies have investigated the possible influence of complications on long-term survival in other abdominal surgical diseases. In colorectal cancer liver metastases, researchers have hypothesized[19,20] that postoperative morbidity prolongs systemic inflammatory response and induces changes that worsen long-term survival as observed in some studies[21,22].
Gastric cancer investigations[5,6] have also observed association between postoperative complications and lower cancer-specific survival. The authors hypothesize that complications could inhibit immune response to spreading tumor cells leading to decreased survival.
Studies in colorectal cancer[7] have found an association between postoperative morbidity due to exclusively infectious complications –mainly severe - and lower long-term survival.
The same association has been found with esophageal squamous cell carcinoma, especially with pulmonary complications and anastomotic leaks[9].
However, there is no data concerning LT with the exception of papers dealing with graft damage after biliary or ischemic complications[10].
In this study, LT patients with more postoperative complications –estimated by the CCI score – exhibit a significantly lower survival than patients with fewer complications. No single complication was associated with worse long-term survival, although this could be due to the sample size. Nevertheless, the CCI – as a measure of overall morbidity – was shown to be an independent negative predictive factor of long-term survival.
The multivariable analysis was performed to rule out the influence of other variables on long-term survival. MELD was not included because it was already weighted within the BAR variable. Patients with greater preoperative comorbidity could be expected to suffer more complications after surgery, and therefore to have lower long-term survival. However, we found long-term survival was not influenced by other pre-transplant factors such as the Charlson and BAR scores or the presence of hepatocellular carcinoma. Only the CCI score showed significant influence on long-term survival.
Of note, according to the analysed cut-offs, not many complications are needed to enter in the high-risk zone: Only one complication requiring interventional treatment under general anesthesia or two treated without general anesthesia are enough. As a result, enhancing postoperative care is extremely important not only to minimize postoperative mortality but also to improve long-term survival.
The association between complications and poorer survival is not clear. Many of the delayed deaths were related with surgical aspects such as vascular or biliary problems[23], but they seemed more aggressive in those patients with complications in the immediate postoperative period. The main hypothesis is that an increased and prolonged inflammatory systemic response produces deleterious effects. In addition, these patients receive high doses of immunosuppressive drugs in the immediate postoperative period and prolonged treatment thereafter. The role of this treatment is unknown.
This study has several limitations, such as its retrospective and unicentric design, the relatively small number of patients and the limited follow-up of the patients. Prospective multicentric studies with more patients are needed to validate our results.
In conclusion, according to our results, a complicated postoperative period –well defined by means of the CCI score- can influence not only short-term survival, but also long-term survival in LT recipients.
In surgical procedures such as gastrectomy, esophagectomy or resection of liver metastases, postoperative complications are associated with poorer long-term survival. It is possible this happens in liver transplant (LT) but there are not enough data to establish this relationship.
To define whether long-term prognosis is influenced by postoperative complications after LT.
To analyze the possible influence of postoperative complications on long-term survival and the ability of the comprehensive complication index (CCI) to predict this.
Retrospective study of 164 LT patients. The medical records concerning postoperative complications and long-term survival were analyzed. Univariate and multivariable tests were performed for statistical analysis.
A ROC curve of CCI with 5-year survival was built. Survival curves for comparison of patients with CCI cut-off values of 36 and 33.5 showed significant statistical differences, suggesting that patients with more severe complications exhibit worse long-term survival. A multivariate analysis was carried out to analyze the possible influence of CCI, Charlson comorbidity index, BAR and hepatocellular carcinoma on survival. Only the CCI score showed significant influence on long-term survival.
A complicated postoperative period – well-defined by means of the CCI score – can influence not only short-term survival, but also long-term survival of LT patients.
Refinement and surgical technique and postoperative care are mandatory to improve short-term result but this also influence long-term survival.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cimen SG, Gonzalez FM, Moschovi MA, Uhlmann D, Zheng H S-Editor: Ma YJ L-Editor: A P-Editor: Li JH
| 1. | Parrilla P, Ramírez P, Bueno F, Robles R, Rodríguez JM, Luján J, Hernández Q, Acosta F. Complications in liver transplant surgery. Cir Esp. 2001;69:259-296. |
| 2. | Chen XB, Xu MQ. Primary graft dysfunction after liver transplantation. Hepatobiliary Pancreat Dis Int. 2014;13:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Astarcıoglu I, Egeli T, Gulcu A, Ozbilgin M, Agalar C, Cesmeli EB, Kaya E, Karademir S, Unek T. Vascular Complications After Liver Transplantation. Exp Clin Transplant. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Koffron A, Stein JA. Liver transplantation: indications, pretransplant evaluation, surgery, and posttransplant complications. Med Clin North Am. 2008;92:861-888, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Tu RH, Lin JX, Li P, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Zheng CH, Huang CM. Comprehensive Complication Index Predicts Cancer-Specific Survival of Patients with Postoperative Complications after Curative Resection of Gastric Cancer. Gastroenterol Res Pract. 2018;2018:4396018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Kubota T, Hiki N, Sano T, Nomura S, Nunobe S, Kumagai K, Aikou S, Watanabe R, Kosuga T, Yamaguchi T. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol. 2014;21:891-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 7. | Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg. 2015;261:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 8. | Yamashita S, Sheth RA, Niekamp AS, Aloia TA, Chun YS, Lee JE, Vauthey JN, Conrad C. Comprehensive Complication Index Predicts Cancer-specific Survival After Resection of Colorectal Metastases Independent of RAS Mutational Status. Ann Surg. 2017;266:1045-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Saeki H, Tsutsumi S, Tajiri H, Yukaya T, Tsutsumi R, Nishimura S, Nakaji Y, Kudou K, Akiyama S, Kasagi Y, Nakanishi R, Nakashima Y, Sugiyama M, Ohgaki K, Sonoda H, Oki E, Maehara Y. Prognostic Significance of Postoperative Complications After Curative Resection for Patients With Esophageal Squamous Cell Carcinoma. Ann Surg. 2017;265:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Kienlein S, Schoening W, Andert A, Kroy D, Neumann UP, Schmeding M. Biliary complications in liver transplantation: Impact of anastomotic technique and ischemic time on short- and long-term outcome. World J Transplant. 2015;5:300-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24657] [Article Influence: 1174.1] [Reference Citation Analysis (0)] |
| 12. | Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1272] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 13. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 38075] [Article Influence: 1002.0] [Reference Citation Analysis (0)] |
| 14. | Dutkowski P, Oberkofler CE, Slankamenac K, Puhan MA, Schadde E, Müllhaupt B, Geier A, Clavien PA. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011;254:745-53; discussion 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 337] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 15. | Pischke S, Lege MC, von Wulffen M, Galante A, Otto B, Wehmeyer MH, Herden U, Fischer L, Nashan B, Lohse AW, Sterneck M. Factors associated with long-term survival after liver transplantation: A retrospective cohort study. World J Hepatol. 2017;9:427-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Adam R, Karam V, Cailliez V, O Grady JG, Mirza D, Cherqui D, Klempnauer J, Salizzoni M, Pratschke J, Jamieson N, Hidalgo E, Paul A, Andujar RL, Lerut J, Fisher L, Boudjema K, Fondevila C, Soubrane O, Bachellier P, Pinna AD, Berlakovich G, Bennet W, Pinzani M, Schemmer P, Zieniewicz K, Romero CJ, De Simone P, Ericzon BG, Schneeberger S, Wigmore SJ, Prous JF, Colledan M, Porte RJ, Yilmaz S, Azoulay D, Pirenne J, Line PD, Trunecka P, Navarro F, Lopez AV, De Carlis L, Pena SR, Kochs E, Duvoux C; all the other 126 contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). 2018 Annual Report of the European Liver Transplant Registry (ELTR) - 50-year evolution of liver transplantation. Transpl Int. 2018;31:1293-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 326] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 17. | Neuberger J. Liver transplantation in the United Kingdom. Liver Transpl. 2016;22:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Herrero JI; Sociedad Española de Trasplante Hepático. [III Consensus Meeting of the Spanish Society of Liver Transplantation. Hepatitis C, living-donor liver transplantation, quality of liver grafts and of liver transplantation programs]. Gastroenterol Hepatol. 2011;34:641-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Ito H, Are C, Gonen M, D'Angelica M, Dematteo RP, Kemeny NE, Fong Y, Blumgart LH, Jarnagin WR. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Tanaka K, Kumamoto T, Nojiri K, Matsuyama R, Takeda K, Endo I. Impact of Postoperative Morbidity on Long-Term Survival After Resection for Colorectal Liver Metastases. Ann Surg Oncol. 2016;23:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Shibutani M, Maeda K, Nagahara H, Ohtani H, Iseki Y, Ikeya T, Sugano K, Hirakawa K. The prognostic significance of a postoperative systemic inflammatory response in patients with colorectal cancer. World J Surg Oncol. 2015;13:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Lahiri R, Derwa Y, Bashir Z, Giles E, Torrance HD, Owen HC, O'Dwyer MJ, O'Brien A, Stagg AJ, Bhattacharya S, Foster GR, Alazawi W. Systemic Inflammatory Response Syndrome After Major Abdominal Surgery Predicted by Early Upregulation of TLR4 and TLR5. Ann Surg. 2016;263:1028-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Zhang YC, Zhang Q, Li H, Zhang J, Wang GS, Xu C, Yi SH, Yi HM, Cai CJ, Lu MQ, Yang Y, Chen GH. Prognostic factors for late mortality after liver transplantation for benign end-stage liver disease. Chin Med J (Engl). 2011;124:4229-4235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |