Published online Apr 27, 2020. doi: 10.4240/wjgs.v12.i4.138
Peer-review started: December 21, 2019
First decision: January 15, 2020
Revised: January 21, 2020
Accepted: March 25, 2020
Article in press: March 25, 2020
Published online: April 27, 2020
Processing time: 123 Days and 19.5 Hours
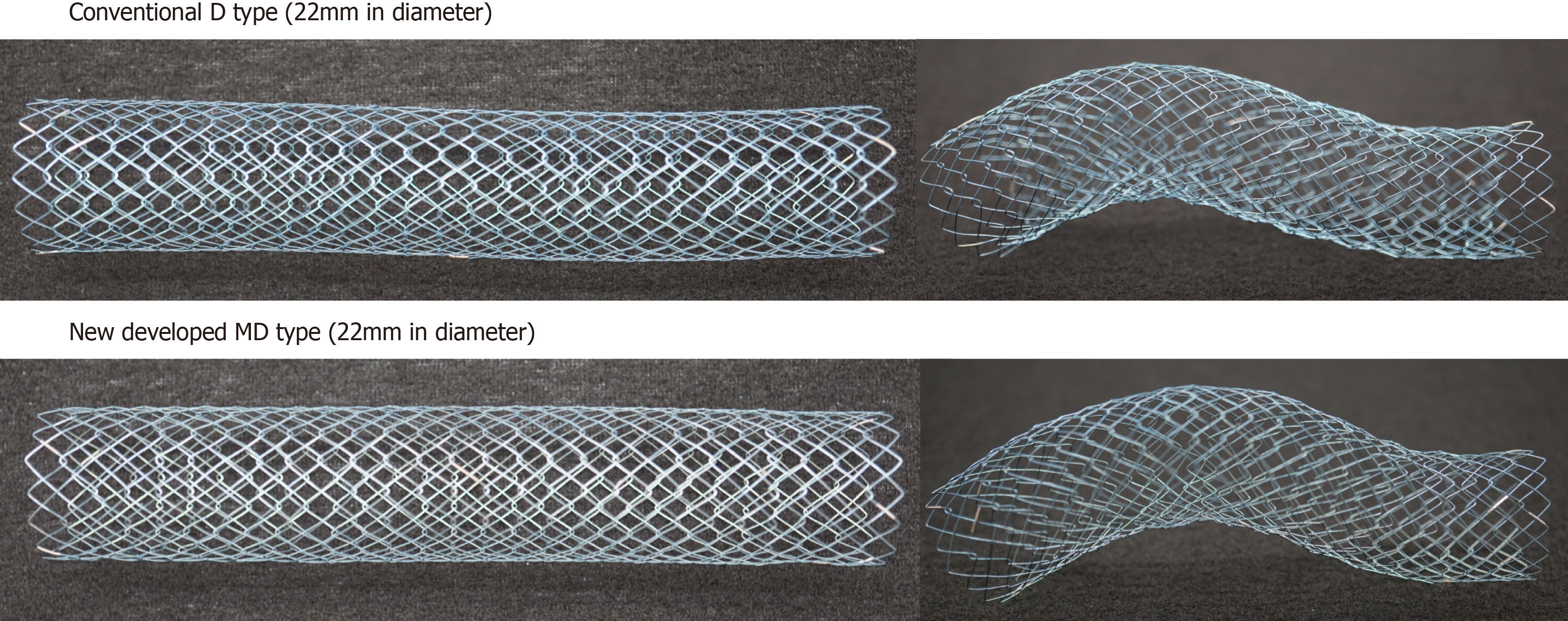
Colonic stents are increasingly used to treat acute malignant colonic obstructions. The WallFlex and Niti-S D type stents are the commonly used self-expandable metallic stents available in Japan since 2012. WallFlex stent has a risk of stent-related perforation because of its axial force, while the Niti-S D type stent has a risk of obstructive colitis because of its weaker radial force. Niti-S MD type stents not only overcome these limitations but also permit delivery through highly flexible-tipped smaller-caliber colonoscopes.
To compare the efficacy and safety of the newly developed Niti-S MD type colonic stents.
This single-center retrospective observational study included 110 patients with endoscopic self-expandable metallic stents placed between November 2011 and December 2018: WallFlex (Group W, n = 37), Niti-S D type (Group N, n = 53), and Niti-S MD type (Group MD, n = 20). The primary outcome was clinical success, defined as a resolution of obstructive colonic symptoms, confirmed by clinical and radiological assessment within 48 h. The secondary outcome was technical success, defined as accurate stent placement with adequate stricture coverage on the first attempt without complications.
The technical success rate was 100% in Groups W, N, and MD, and the overall clinical success rate was 89.2% (33/37), 96.2% (51/53), and 100% (20/20) in Groups W, N, and MD, respectively. Early adverse events included pain (3/37, 8.1%), poor expansion (1/37, 2.7%), and fever (1/37, 2.6%) in Group W and perforation due to obstructive colitis (2/53, 3.8%) in Group N (likely due to poor expansion). Late adverse events (after 7 d) included stent-related perforations (4/36, 11.1%) and stent occlusion (1/36, 2.8%) in Group W and stent occlusion (2/51, 3.9%) in Group N. The stent-related perforation rate in Group W was significantly higher than that in Group N (P < 0.05). No adverse event was observed in Group MD.
In our early and limited experience, the newly developed Niti-S MD type colonic stent was effective and safe for treating acute malignant colonic obstruction.
Core tip: We developed a new self-expandable metallic stent, the Niti-S MD colonic stent (with a diameter of 22 mm), that can be deployed using the 9-Fr delivery system. The stent not only increased the radial force while maintaining the stent structure and low axial force but also permitted delivery through highly flexible-tipped smaller-caliber colonoscope with a working channel of 3.2 mm. In this study, the technical and clinical success rate of the Niti-S MD type was 100%, and its perforation rate was 0%. It was safe and effective for treating acute malignant colonic obstruction.
- Citation: Miyasako Y, Kuwai T, Ishaq S, Tao K, Konishi H, Miura R, Sumida Y, Kuroki K, Tamaru Y, Kusunoki R, Yamaguchi A, Kouno H, Kohno H. Newly developed self-expandable Niti-S MD colonic metal stent for malignant colonic obstruction. World J Gastrointest Surg 2020; 12(4): 138-148
- URL: https://www.wjgnet.com/1948-9366/full/v12/i4/138.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i4.138
Colorectal cancer is ranked third in the United States. Acute colonic obstruction is one of the symptoms seen among patients with colorectal cancer, requiring urgent decompression. Endoscopic stenting with self-expandable metallic stents (SEMS) has become one of the standard treatments for symptomatic malignant colonic obstruction (MCO). SEMS insertion for palliative decompression of MCO was first reported by Dohmoto[1] in 1991; nowadays, SEMS offers an effective alternative option for palliative (PAL) surgery and act as a bridge-to-surgery (BTS)[2]. The incidence of adverse events of SEMS for MCO is considered low; however, the serious complication (such as perforation) -related mortality rate could increase to 50%[3].
Lee et al[3] reported the risk factors for perforation and proposed that the axial force, radial force, and shape of the stent, including those of the tip, can be factors for perforation. The WallFlex colonic stent (Enteral Colonic Uncovered Stent; Boston Scientific, Corp., Natick, MA, United States) and Niti-S D type colonic stent (Enteral Colonic Uncovered Stent; Taewoong Medical Co., Gimpo, South Korea) are widely used in Japan. The WallFlex stent is knitted in a spiral shape with a proximal flared end, whereas the Niti-S D type stent is hand-knitted in a net shape and does not have a flared proximal end. With its design and spiral knitting construction, the WallFlex stent has stronger axial force than the Niti-S D type stent, resulting in recoil of the WallFlex stent to a straight position after deployment. This may increase the risk of the stent-related perforation when compared with the Niti-S D type stent[4-6]. On the other hand, the Niti-S D type stent has lower radial force, resulting in weaker horizontal expansion of the radius of the stent to overcome tumor obstruction that can cause obstructive colitis and perforation.
Stents are deployed using a standard colonoscope, which can pose a challenge while overcoming sharp angles. Smaller caliber colonoscopes are designed for passive bending and easy maneuverability, facilitating scope advancement and cecal intubation where the standard colonoscope has failed[7]. A smaller-caliber colonoscope can be ideal for stent deployment, but the main drawback is its channel of 9.2 mm, that would only allow 9Fr delivery catheter (available only with stents of diameter 18 mm that have less radial force than 22 mm stents that require larger scope channels).
To overcome the above, we developed a new SEMS, the Niti-S MD colonic stent (with diameter of 22 mm), that can be deployed using a 9-Fr delivery system. Although this 22-mm Niti-S MD stent has stronger radial force than the conventional 18-mm Niti-S D type, it maintains a low axial force that facilitates maintenance of the shape of the stent when deployed.
This observational study aimed to evaluate the efficacy and safety of the newly developed Niti-S MD type colonic stent and to retrospectively compare it with conventional colonic stents.
This single-center retrospective observational study was conducted to evaluate the efficacy, safety, and feasibility of the newly developed Niti-S MD type colonic stent. Additionally, retrospective comparison was carried out with the conventional WallFlex and Niti-S D type colonic stents. Data were collected and analyzed from 105 consecutive patients (110 lesions; male/female, 58/47; average age, 73.5 years), who underwent endoscopic SEMS placement for MCO between November 2011 and December 2018 at the Kure Medical Center and Chugoku Cancer Center.
This study was carried out in accordance with the principles of the Declaration of Helsinki in compliance with good clinical practice and with local regulations. The nature of the procedure was explained, and informed consent for the procedure and data collection was obtained from all patients. The study was approved by the Institutional Review Board Ethics Committees of the National Hospital Organization Kure Medical Center and Chugoku Cancer Center.
The WallFlex colonic stent was used in 35 consecutive patients (37 lesions: Group W) between November 2011 and September 2013. In 2013, the Niti-S D type colonic stent became available in Japan and was used in 51 consecutive patients (53 lesions: Group N) between October 2013 and December 2017. We developed a new stent (Niti-S MD type) in 2018 and used it to treat 20 consecutive patients (20 lesions: Group MD) between January 2018 and December 2018. Data in all cases were analyzed.
The WallFlex stent is a SEMS made from knitted nitinol wire in a spiral shape with flared oral end (proximal side) and a loop anal end (distal end). Because of the spiral structure, the stent extends when pulled on the long axis. In addition, as the axial force is strong, the stent is easy to linearize. The WallFlex stent is available in three sizes (6 cm, 9 cm, and 12 cm) and two diameters (22 mm and 25 mm).
The Niti-S D type stent is a SEMS made from hand-knitted nitinol wire mesh that has neither the flare nor the loop end. Weak axial force enables it to adapt well even in the bent position. This stent is available in two diameters (18 mm and 22 mm) and four sizes (6 cm, 8 cm, 10 cm, and 12 cm).
The newly developed stent, Niti-S MD type, is a 22-mm diameter stent mounted onto a 9-Fr delivery system that maintains the shape and axial force of the Niti-S D type stent but provides additional expansion radial force. Since this stent can be inserted through a working channel, 3.2 mm in diameter, it allows the use of a smaller caliber colonoscope. It is available in four sizes (6 cm, 8 cm, 10 cm, and 12 cm) (Figure 1).
The inclusion criteria for colonic SEMS placement were as follows: Patients presenting with acute colonic obstruction and radiological features (as observed by computed tomography) consistent with a carcinoma.
The exclusion criteria were as follows: Suspected bowel perforation, multiple sites of small bowel or colonic obstruction due to peritoneal dissemination, severe inflammatory changes around the tumor, and contraindication to endoscopic treatment.
Procedures were performed using CF-HQ290ZI (Olympus Optical Co., Tokyo, Japan), PCF-H290I (smaller-caliber; Olympus Optical Co., Tokyo, Japan), or PCF-Q260AZI (smaller-caliber; Olympus Optical Co., Tokyo, Japan) colonoscopes. A certified endoscopist experienced in stenting performed all the procedures. Combined endoscopic and fluoroscopic approaches were used to deploy the stent.
Glycerin enema was used to prepare and clean the colon distal to the stenosis to improve endoscopic views. After identifying the obstruction site, the length of the stricture was measured under fluoroscopy by a contrast agent using an endoscopic retrograde cholangiopancreatography catheter. The stricture site was marked with clips to identify the location prior to stent placement. The guidewire was then advanced through the stenosis to cover the entire length of the stenosis (using the scope method). After accurate positioning, the stent was deployed from the oral (proximal) to the anal (distal) side by releasing the sheath from the stent catheter. Proper positioning and expansion of the stent were confirmed both with radiological images and endoscopic views. In addition, abdominal radiographs were obtained at 24 and 48 h to rule out stent migration and poor or failed expansion.
The primary outcome was clinical success, defined as resolution of the obstructive symptoms confirmed by clinical and radiological assessment within 48 h. Clinical success was based on the ColoRectal Obstruction Scoring System (CROSS) score[8]. Adler et al[9,10] constructed this score to establish a scoring system similar to that used for assessing the condition of patients with malignant gastric outlet obstruction, and to assess oral intake and abdominal symptoms before and after treatment. CROSS is scored by oral intake ability and abdominal symptoms as follows: (1) Requiring continuous decompressive procedure, 0; (2) No oral intake, 1; (3) Liquid or enteral nutrient, 2; (4) Soft solids, low residue, 3; and (5) Full diet without symptoms of stricture, 4.
The secondary outcome was technical success, which was defined as accurate stent placement with adequate stricture coverage on the first attempt without any adverse events. Procedure-related adverse events recorded were as follows: Perforation, re-obstruction, stent migration, infection/fever, abdominal pain, and tenesmus. Adverse events that developed within and after 7 d, including the day of stenting, were defined as early and late adverse events. respectively[11].
Data are presented as mean ± SD or median (range). Fisher’s exact test was used to compare qualitative variables, and Wilcoxon rank sum test was used to compare quantitative variables. A P value of < 0.05 was considered statistically significant. All statistical analyses were performed using JMP software (SAS Institute, Inc., Cary, NC, United States).
Figure 2 shows a flowchart of the patient allocation. The study participants included 105 patients (male/female: 58/47) with 110 lesions. No patients were excluded from the study during the study period. Among these, 35 patients (37 lesions) were treated with WallFlex colonic stents (Group W), 51 patients (53 lesions) with Niti-S D type colonic stents (Group N), and 19 patients (20 lesions) with the newly developed “Niti-S MD type” colonic stent (Group MD). In Group W, a SEMS was placed in 19 lesions (51.4%) as BTS and in 18 lesions (48.6%) as PAL; in Group N, a SEMS was placed in 32 lesions (60%) as BTS and in 21 lesions (40%) as PAL; and in Group MD, a SEMS was placed in 10 lesions (50%) as BTS and in 10 lesions (50%) as PAL.
Table 1 shows a summary of the clinical characteristics of the patients and tumors in this study. Among them, 18 men (48.6%) and 19 women (51.4%) comprised the Group W, while 28 men (52.8%) and 25 women (47.2%) comprised the Group N.
| Group W (n = 37) | Group N (n = 53) | Group MD (n = 20) | Total (n = 110) | |
| Patients’ characteristics | ||||
| Age (yr, mean ± SD) | 71.4 ± 11.8 | 74.3 ± 13.6 | 73.7 ± 9.6 | 73.5 ± 12.5 |
| Male/Female | 18/19 | 28/25 | 15/5 | 61/49 |
| PS score (mean ± SD) | 1.6 ± 1.2 | 1.9 ± 1.2 | 2.3 ± 1.0 | 1.9 ± 1.2 |
| Therapeutic intent | ||||
| BTS | 19/37 (51.4%) | 32/53 (60.4%) | 10/20 (50%) | 61/110 (55.5%) |
| PAL | 18/37 (48.6%) | 21/53 (39.6%) | 10/20 (50%) | 49/110 (44.5%) |
| Tumor characteristics | ||||
| Obstruction/tumor site | ||||
| Right colon | 8/37 (21.6%) | 14/53 (26.4%) | 6/20 (30%) | 28/110 (25.4%) |
| Left colon | 27/37 (73.0%) | 38/53 (71.7%) | 10/20 (50%) | 75/110 (68.2%) |
| Rectum | 2/37 (5.4%) | 1/53 (1.9%) | 4/20 (20%) | 7/110 (6.4%) |
| Etiology of colorectal obstruction | ||||
| Primary colorectal cancer | 30/37 (81.1%) | 49/53 (92.5%) | 13/20 (65%) | 92/110 (83.6%) |
| Metastatic lesion | 7/37 (18.9%) | 4/53 (7.5%) | 7/20 (35%) | 18/110 (16.4%) |
| Noncancerous stenosis | 0/37 (0%) | 0/53 (0%) | 0/20 (0%) | 0/110 (0%) |
| Stenosis length [cm, median (range)] | 5.0 (2.0-13.0) | 5.0 (2.0-11.0) | 6.2 (2.5-11.5) | 5.0 (2.0-13.0) |
The mean patient age was 71.4 years ± 11.8 years in Group W (n = 37) and 74.3 years ± 13.6 years in Group N (n = 53). The stricture was located in the right colon (ileocecal, ascending colon, hepatic flexure, and transverse colon) in 8/37 (21.6%) of cases, in the left colon (rectosigmoid junction, sigmoid and descending colon, splenic flexure) in 27/37 (73.0%), and in the rectum in 2/37 (5.4%) in Group W vs 14/53 (26.4%), 38/53 (71.7%), and 1/53 (1.9%) in Group N, respectively. The stenosis was due to the primary tumor in 30/37 (81.1%) of cases and due to metastatic lesion in 7/37 (18.9%) in Group W vs 49/53 (92.5%) and 4/53 (7.5%) in Group N, respectively. The median length of the stenosis was 5.0 cm (range 2.0–13.0 cm) in Group W and 5.0 cm (range 2.0–11.0 cm) in Group N.
Group MD was composed of 15 (75%) men and 5 (25%) women, and the mean patient age was 73.7 years ± 9.6 years. The stricture was located in the right colon in 6/20 (30%), in the left colon in 10/20 (50%), and in the rectum in 4/20 (20%). The stenosis was due to the primary tumor in 13/20 (65%) of cases and 7/20 (35%) due to a metastatic lesion. The median length of the stenosis was 6.2 cm (range 2.5–11.5 cm).
Table 2 shows outcomes in each stent group and a summary of stent types with their sizes and diameters. Stents were placed successfully in all patients. The clinical success rate in Group MD was 100% (20/20). The average procedure time (± SD) was 31.3 min ± 11.2 min, and the mean CROSS score before/after stenting was 1.4 ± 0.9/3.8 ± 0.5.
| Group W | Group N | Group MD | Total | |
| Technical success rate | 37/37 (100%) | 53/53 (100%) | 20/20 (100%) | 110/110 (100%) |
| Stent length | ||||
| 6 cm | 24/37 (64.9%) | 12/53 (22.6%) | 3/20 (15%) | 39/110 (35.5%) |
| 8 cm | NA | 17/53 (32.1%) | 7/20 (35%) | 24/110 (21.8%) |
| 9 cm | 11/37 (29.7%) | NA | NA | 11/110 (10.0%) |
| 10 cm | NA | 18/53 (34.0%) | 6/20 (30%) | 24/110 (21.8%) |
| 12 cm | 2/37 (5.4%) | 6/53 (11.3%) | 4/20 (20%) | 12/110 (10.9%) |
| Stent diameter | ||||
| 18 mm | NA | 6/53 (11.3%) | NA | 6/110 (5.5%) |
| 22 mm | 30/37 (81.1%) | 47/53 (88.7%) | 20/20 (100%) | 97/110 (88.2%) |
| 25 mm | 7/37 (18.9%) | NA | NA | 7/110 (6.3%) |
| Procedure time (min, mean ± SD) | 32.6 ± 14.0 | 33.6 ± 23.7 | 31.3 ± 11.2 | 33.0 ± 19.1 |
| Clinical success rate | 33/37 (89.2%) | 51/53 (96.2%) | 20/20 (100%) | 104/110 (94.5%) |
| BTS | 19/19 (100%) | 31/32 (96.9%) | 10/10 (100%) | 60/61 (98.4%) |
| PAL | 14/18 (77.8%) | 20/21 (95.2%) | 10/10 (100%) | 44/49 (89.8%) |
| CROSS before stent placement (mean ± SD) | 1.6 ± 1.3 | 1.2 ± 1.0 | 1.4 ± 0.9 | 1.4 ± 1.1 |
| 0 | 12/37 (32.5%) | 10/53 (18.9%) | 3/20 (15%) | 25/110 (22.7%) |
| 1 | 8/37 (21.6%) | 32/53 (60.4%) | 9/20 (45%) | 49/110(44.6%) |
| 2 | 3/37 (8.1%) | 4/53 (7.5%) | 5/20 (25%) | 12/110 (10.9%) |
| 3 | 13/37 (35.1%) | 5/53 (9.4 %) | 3/20 (15%) | 21/110 (19.1%) |
| 4 | 1/37 (2.7%) | 2/53 (3.8%) | 0/20 (0%) | 3/110 (2.7%) |
| CROSS after stent placement (mean ± SD) | 3.8 ± 0.8a | 3.8 ± 0.7a | 3.8 ± 0.5a | 3.8 ± 0.7a |
| 0 | 0/37 (0%) | 0/53 (0%) | 0/20 (0%) | 0/110 (0%) |
| 1 | 3/37 (8.1%) | 2/53 (3.8%) | 0/20 (0%) | 5/110(4.6%) |
| 2 | 0/37 (0%) | 2/53 (3.8%) | 1/20 (5.0%) | 3/110 (2.7%) |
| 3 | 0/37 (0%) | 0/53 (0 %) | 2/20 (10%) | 2/110 (1.8%) |
| 4 | 34/37 (91.9%) | 49/53 (92.4%) | 17/20 (85%) | 110/110 (90.9%) |
The clinical success rate was 89.2% (33/37) in Group W and 96.2% (51/53) in Group N. The clinical success rate for BTS and PAL in Group W were 100% (19/19) and 77.8% (14/18), while 96.9% (31/32) and 95.2 % (20/21) in Group N, respectively. The average procedure times were 32.6 min ± 14.0 min and 33.6 min ± 23.7 min in Groups W and N, respectively. The mean CROSS score before/after stenting was 1.6 ± 1.2/3.8 ± 0.8 and 1.2 ± 1.0/3.8 ± 0.7 in Groups W and N, respectively. No significant difference in outcomes was found between Group MD and the other groups.
Early and late adverse events are compared in Table 3. Despite the small number of cases, no adverse events occurred in the early or late stage in Group MD.
| Group W | Group N | Group MD | Total | |
| Early (≤ 7 d) | ||||
| Perforations | 0/37 (0%) | 2/53 (3.8%)1 | 0/20 (0%) | 2/110 (1.8%) |
| Bleeding | 0/37 (0%) | 0/53 (0%) | 0/20 (0%) | 0/110 (0%) |
| Poor expansion | 1/37 (2.7%) | 0/53 (0%) | 0/20 (0%) | 1/110 (0.9%) |
| Abdominal pain | 3/37 (8.1%) | 0/53 (0%) | 0/20 (0%) | 3/110 (2.7%) |
| Stent occlusion | 0/37 (0%) | 0/53 (0%) | 0/20 (0%) | 0/110 (0%) |
| Fever | 1/37 (2.7%) | 0/53 (0%) | 0/20 (0%) | 1/110 (0.9%) |
| Late (> 7 d) | ||||
| Perforations | 4/36 (11.1%)a2 | 0/51 (0%)a | 0/20 (0%) | 4/107 (3.7%) |
| Bleeding | 0/36 (0%) | 0/51 (0%) | 0/20 (0%) | 0/107 (0%) |
| Stent migration | 0/36 (0%) | 0/51 (0%) | 0/20 (0%) | 0/107 (0%) |
| Abdominal pain | 0/36 (0%) | 0/51 (0%) | 0/20 (0%) | 0/107 (0%) |
| Stent occlusion | 1/36 (2.8%) | 2/51 (3.9%) | 0/20 (0%) | 3/107 (2.8%) |
Early adverse events in Group W included abdominal pain (3/37, 8.1%, BTS 2/PAL 1), poor expansion (1/37, 2.7%, PAL 1), and fever (1/37, 2.7%, BTS 1), and late adverse events included stent-related perforations (4/36, 11.1%, PAL 4) and stent occlusion (1/36, 2.8%, PAL 1). On the contrary, the only early adverse event in Group N was perforation (2/53, 3.8%, BTS 1/PAL 1) caused by obstructive colitis, which was defined as “proximal ulceration related to unresolved colonic obstruction,” and late adverse events included stent occlusion (2/51, 3.9%, PAL 2).
Although the proportion with each adverse event was not significantly different between Group MD and other groups, the stent-related perforation rate in Group W was significantly higher than that in Group N (P < 0.05), and perforation likely occurred because of obstructive colitis (due to unresolved obstruction) in Group N compared with Group W.
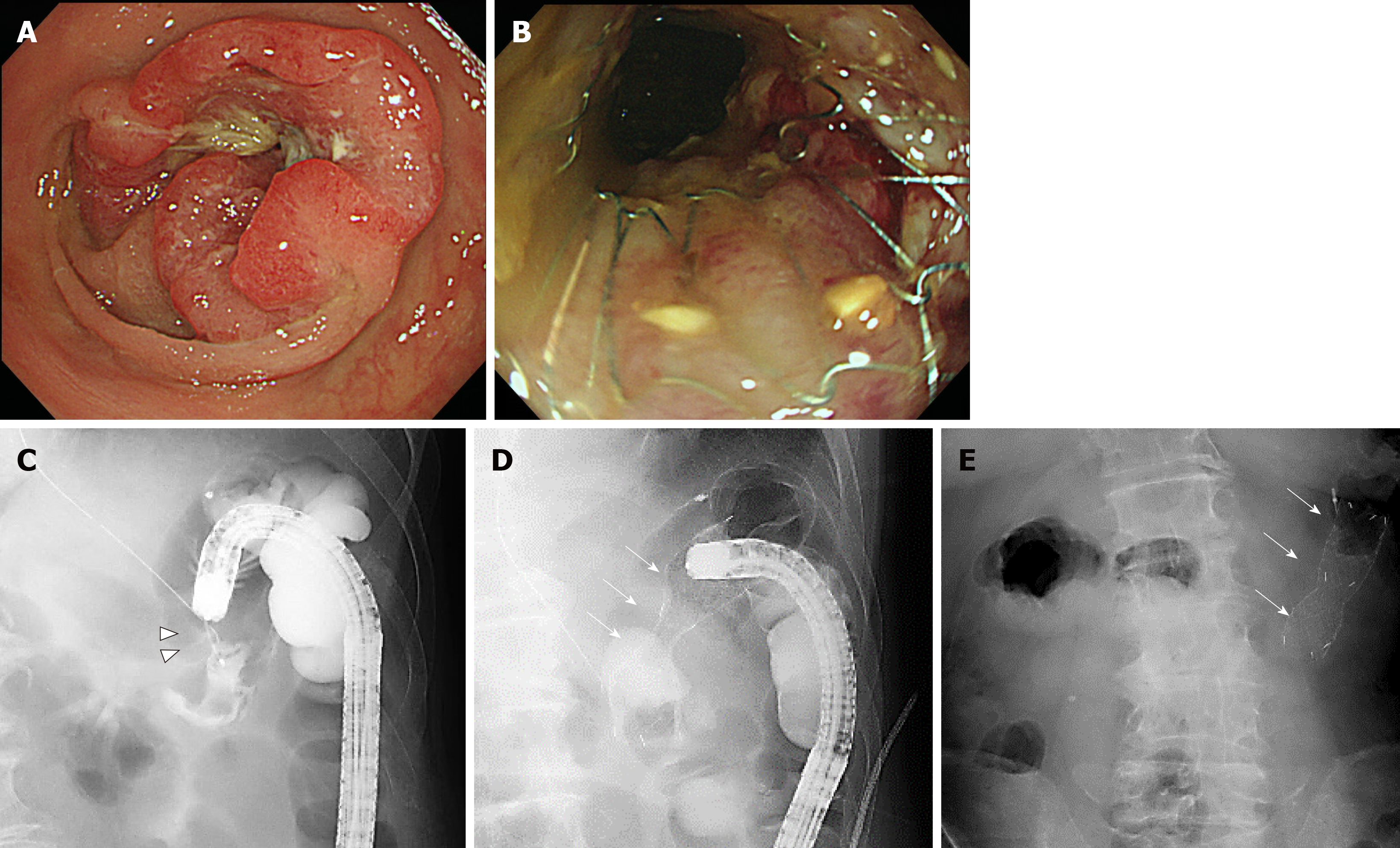
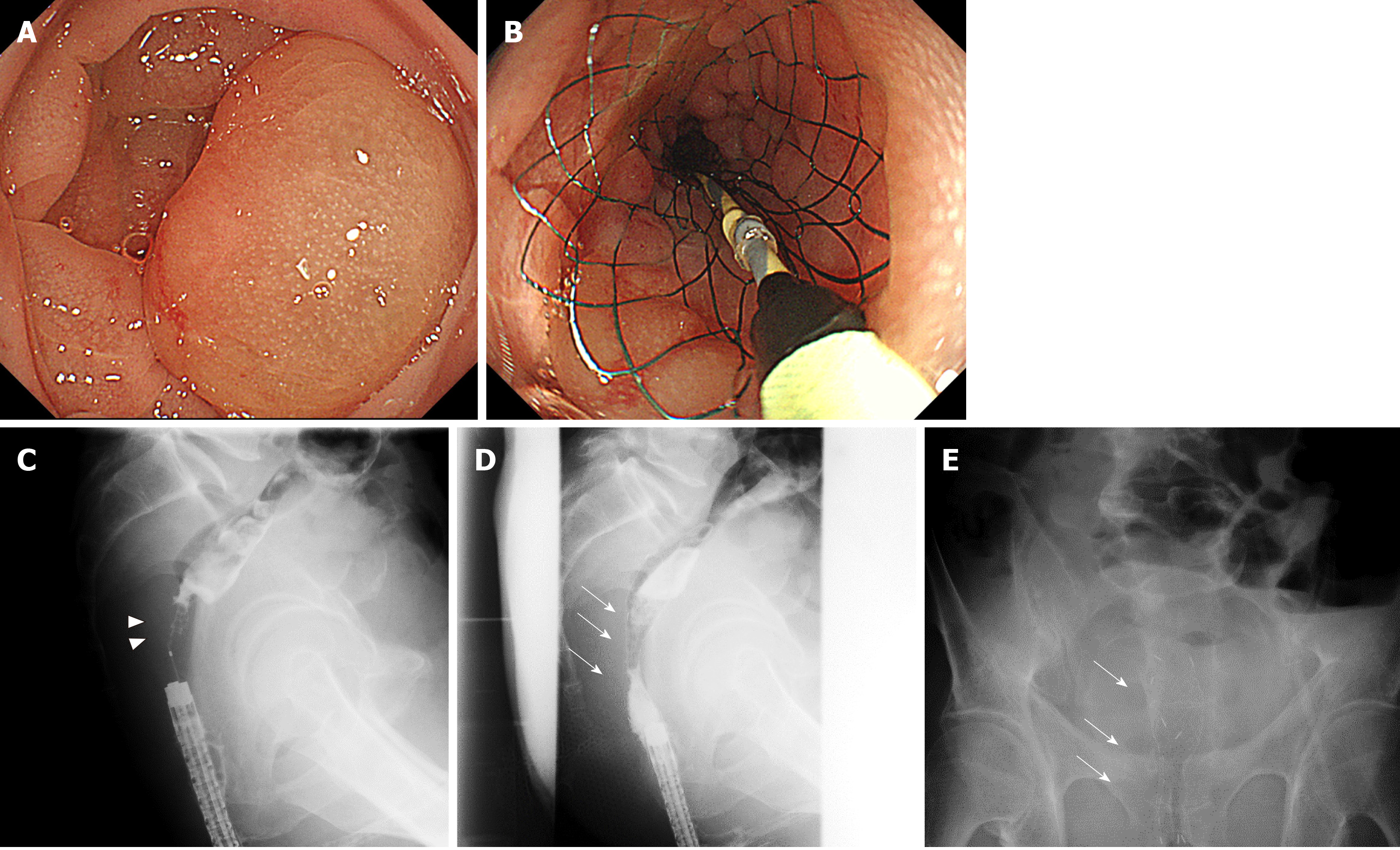
One case of BTS and one case of PAL are shown in Figures 3 and 4, respectively.
In our study, overall, endoscopic colorectal stenting was relatively safe and had a low incidence of complications, but the rate of stent-related perforation was significantly higher with the WallFlex stent than that with the Niti-S D type stent. We believe that this is likely caused by the lower axial force of the Niti-S D type stent. The newly designed “Niti-S MD type” stent, with a 22 mm diameter, mounted to a 9-Fr delivery system not only allows increased radial force while maintaining the stent structure and low axial force but also permits delivery through highly flexible-tipped smaller-caliber colonoscope with a working channel of 3.2 mm. In this study, the technical and clinical success rate of the Niti-S MD type was 100%, and its perforation rate was 0%.
The real advantage of our newly designed “Niti-S MD type” stent is that it maintains the structure and low axial force of the conventional Niti-S D type, but its 22-mm diameter provides additional radial force. Another advantage is that it allows use of flexible smaller-caliber colonoscope as it has a 9-Fr catheter delivery system. It is the first 22-mm diameter colonic stent with 9-Fr delivery system that causes less damage on the intestinal wall and could reduce the risk of stent-related perforation. Cheung et al[12] reported the results of a multicenter randomized prospective trial of WallFlex and Niti-S D type stents. They reported a technical success rate of 100% in both groups, while the perforation rate with WallFlex was 3.6% (1/28) vs 0% (0/30) with the Niti-S D type in the PAL group. The clinical success rate was 86.0% and 90.1% in the WallFlex and Niti-S D type, respectively, and the perforation rate was 6.9% (3/43) and 4.5% (1/22) in the WallFlex and Niti-S D type in the BTS group, respectively.
In addition, in a multicenter prospective study (n = 513) in Japan, the technical success rates, clinical success rates, and perforation rates of WallFlex at 7 d were 97.9%, 95.5%, and 2.1%, respectively[10]. On the other hands in a multicenter study using Niti-S D type (n = 200) from the same group in Japan, the technical success rate at 7 d, the clinical success rate, and the perforation rates were 98.0%, 96.5%, and 0%, respectively[13], that considered to be lower than that of WallFlex. These perforation rates of the two studies were considerably lower than those in studies performed outside Japan. This probably occurred because the safety procedure was established and shared before the study, so the perforation rates during the procedure were low[10]. Therefore, this value was considered to represent the original rate of perforation by the stent itself, not including perforation during the procedure.
In our study, the technical success rate in both WallFlex and Niti-S stents was 100%, which was similar to the previously reported results[14-17]. The clinical success rates were 89.2% and 96.2% for WallFlex and Niti-S D type stents, respectively, which were also similar to previously reported data[14-17]. These previous studies, including our present study suggested that the Niti-S D type stent had a lower tendency to cause perforation than did the WallFlex stent. This raises the possibility of differences in stent characteristics: WallFlex stent has about three times stronger radial force and about two times stronger axial force than Niti-S D type stent[12], which may have influenced the perforation rate. Indeed, Yamao et al[18] proposed that perforation was more likely to occur when the gastroduodenal stent has higher axial force.
Our newly designed Niti-S MD type stent has another advantage, i.e., it could be deployed with a smaller-caliber colonoscope using the through-the-scope technique, because it is the first 22-mm diameter colonic stent in the 9-Fr delivery system. In our previous study, we reported that risk factors related to prolonged and difficult SEMS placement were peritoneal carcinomatosis, CROSS score of 0, or extensive strictures[19]. These challenging situations could be overcome with higher scope operability by using a smaller-caliber colonoscope, such as a PCF colonoscope[7,20].
Despite the advantages of our newly designed Niti-S MD type stent, we encountered a few limitations. First, the visibility of the newly developed stent was not as good as that under fluoroscopy. Second, the Niti-S MD stent tended to be pulled toward the oral side during deployment; hence, determining the exact length of the stent compared with that of the WallFlex stent was difficult. Hence, the commonly used stent length was 6 cm for the WallFlex stent, whereas that for the Niti-S MD stent was 10 cm. Further improvement in design to overcome this weakness will improve the performance of this new stent.
This study has several limitations. First, this was a retrospective study from a single center. However, we included all cases to reduce the confounding factors. Second, as the Niti-S MD type stent was recently developed, the number of cases using this new stent was small. Thus, we think that a prospective study with a large number of cases is necessary to validate our results. Third, each stent was used sequentially. The WallFlex colonic stent was the first stent used from November 2011 to September 2013, followed by Niti-S D type stent since 2013 (became available since 2013 in Japan). We developed the new Niti-S MD type stent in 2018 and treated 19 consecutive patients from January 2018 to December 2018. This potentially introduces time bias, as the expertise of the operator may have improved over time. A good technical success rate of the WallFlex stent possibly negate any significant time bias. Lastly, we only focused on the important factors linked with perforation, such as axial and radial forces, and did not consider other factors, such as stenosis size and characteristics of stenosis.
In conclusion, our preliminary data suggested that the new “Niti-S MD type” stent with increased radial force while maintaining low axial force was feasible and safe with a lower perforation rate. Despite the small number of cases in our study, the clinical success in all cases with no perforation was promising; however, larger prospective studies and randomized comparison trials are required to completely evaluate and compare this new stent with other conventional colonic stents.
The most serious adverse event of colonic stenting is perforation. The Niti-S D type stent could be ideal to reduce risk of perforation due to its structure with weaker axial force. Stents are deployed using a standard colonoscope, which can pose a challenge while overcoming sharp angles. Smaller caliber colonoscopes could be ideal for easy maneuverability, facilitating scope advancement and cecal intubation where the standard colonoscope has failed. The main drawback of using small caliber colonoscope is its small channel of 9.2 mm, that would only allow 9Fr delivery catheter available only with stents of diameter 18 mm that has less radial force to overcome obstruction. Stents with greater radial force are 22 mm that require larger channel standard colonoscope.
We would like to develop a new colonic stent that maintains the structure with low axial force of the conventional Niti-S D type and takes additional radial force with 22-mm diameter, but that requires 9Fr delivery system, hence can be deployed using smaller caliber colonoscope.
We evaluated the efficacy and safety of the newly developed “Niti-S MD type” colonic stent.
This single-center retrospective observational study with endoscopic self-expandable metallic stents placed between November 2011 and December 2018, and we evaluated the short-term outcomes including success rates and adverse events.
The technical and clinical success rate of the Niti-S MD type was 100%, and its perforation rate was 0%.
Our preliminary data suggested that the newly developed “Niti-S MD type” colonic stent was feasible and safe.
The stent might have a potential to be an ideal one that offers high radial force and can be deployed with small caliber colonoscope. Larger prospective studies and randomized comparison trials are warranted to evaluate and compare this new stent with available conventional colonic stents.
The authors wish to thank Naoko Matsumoto for data collection assistance and administrative support.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Suzuki Y S-Editor: Wang YQ L-Editor: A E-Editor: Xing YX
| 1. | Dohmoto M. New method-endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig. 1991;3:1507-1512. |
| 2. | Nagula S, Ishill N, Nash C, Markowitz AJ, Schattner MA, Temple L, Weiser MR, Thaler HT, Zauber A, Gerdes H. Quality of life and symptom control after stent placement or surgical palliation of malignant colorectal obstruction. J Am Coll Surg. 2010;210:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Lee YJ, Yoon JY, Park JJ, Park SJ, Kim JH, Youn YH, Kim TI, Park H, Kim WH, Cheon JH. Clinical outcomes and factors related to colonic perforations in patients receiving self-expandable metal stent insertion for malignant colorectal obstruction. Gastrointest Endosc. 2018;87:1548-1557.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Sato T, Hara K, Mizuno N, Hijioka S, Imaoka H, Niwa Y, Tajika M, Tanaka T, Ishihara M, Shimizu Y, Bhatia V, Kobayashi N, Endo I, Maeda S, Nakajima A, Kubota K, Yamao K. Gastroduodenal stenting with Niti-S stent: long-term benefits and additional stent intervention. Dig Endosc. 2015;27:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | van Hooft JE, Fockens P, Marinelli AW, Bossuyt PM, Bemelman WA; Dutch Stent-In study group. Premature closure of the Dutch Stent-in I study. Lancet. 2006;368:1573-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | van Hooft JE, Fockens P, Marinelli AW, Timmer R, van Berkel AM, Bossuyt PM, Bemelman WA; Dutch Colorectal Stent Group. Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy. 2008;40:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Sofi AA, Nawras A, Khan MA, Howden CW, Lee WM. Meta-analysis of the performance of ultrathin vs. standard colonoscopes. Endoscopy. 2017;49:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Japan Colonic Stent Safe Procedure Research Group. Mini-Guidelines for Safe Placement of Colonic Stents: safe placement of colonic stents at a glance. Available from: URL: https://colon-stent.com/001_mainpage_en.html. |
| 9. | Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002;97:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 383] [Article Influence: 16.7] [Reference Citation Analysis (6)] |
| 10. | Matsuzawa T, Ishida H, Yoshida S, Isayama H, Kuwai T, Maetani I, Shimada M, Yamada T, Saito S, Tomita M, Koizumi K, Hirata N, Sasaki T, Enomoto T, Saida Y. A Japanese prospective multicenter study of self-expandable metal stent placement for malignant colorectal obstruction: short-term safety and efficacy within 7 days of stent procedure in 513 cases. Gastrointest Endosc. 2015;82:697-707.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Small AJ, Baron TH. Comparison of Wallstent and Ultraflex stents for palliation of malignant left-sided colon obstruction: a retrospective, case-matched analysis. Gastrointest Endosc. 2008;67:478-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Cheung DY, Kim JY, Hong SP, Jung MK, Ye BD, Kim SG, Kim JH, Lee KM, Kim KH, Baik GH, Kim HG, Eun CS, Kim TI, Kim SW, Kim CD, Yang CH. Outcome and safety of self-expandable metallic stents for malignant colon obstruction: a Korean multicenter randomized prospective study. Surg Endosc. 2012;26:3106-3113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | 23rd UEG Week 2015: Barcelona, Spain, October 2015. United European Gastroenterol J. 2015;3:i. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Jiménez-Pérez J, Casellas J, García-Cano J, Vandervoort J, García-Escribano OR, Barcenilla J, Delgado AA, Goldberg P, Gonzalez-Huix F, Vázquez-Astray E, Meisner S. Colonic stenting as a bridge to surgery in malignant large-bowel obstruction: a report from two large multinational registries. Am J Gastroenterol. 2011;106:2174-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Meisner S, González-Huix F, Vandervoort JG, Repici A, Xinopoulos D, Grund KE, Goldberg P, Registry Group TW. Self-Expanding Metal Stenting for Palliation of Patients with Malignant Colonic Obstruction: Effectiveness and Efficacy on 255 Patients with 12-Month's Follow-up. Gastroenterol Res Pract. 2012;2012:296347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Saito S, Yoshida S, Isayama H, Matsuzawa T, Kuwai T, Maetani I, Shimada M, Yamada T, Tomita M, Koizumi K, Hirata N, Kanazawa H, Enomoto T, Sekido H, Saida Y. A prospective multicenter study on self-expandable metallic stents as a bridge to surgery for malignant colorectal obstruction in Japan: efficacy and safety in 312 patients. Surg Endosc. 2016;30:3976-3986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Yoshida S, Watabe H, Isayama H, Kogure H, Nakai Y, Yamamoto N, Sasaki T, Kawakubo K, Hamada T, Ito Y, Yashima Y, Sasahira N, Hirano K, Yamaji Y, Tada M, Omata M, Koike K. Feasibility of a new self-expandable metallic stent for patients with malignant colorectal obstruction. Dig Endosc. 2013;25:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Yamao K, Kitano M, Kayahara T, Ishida E, Yamamoto H, Minaga K, Yamashita Y, Nakajima J, Asada M, Okabe Y, Osaki Y, Chiba Y, Imai H, Kudo M. Factors predicting through-the-scope gastroduodenal stenting outcomes in patients with gastric outlet obstruction: a large multicenter retrospective study in West Japan. Gastrointest Endosc. 2016;84:757-763.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Kuwai T, Yamaguchi T, Imagawa H, Yoshida S, Isayama H, Matsuzawa T, Yamada T, Saito S, Shimada M, Hirata N, Sasaki T, Koizumi K, Maetani I, Saida Y. Factors related to difficult self-expandable metallic stent placement for malignant colonic obstruction: A post-hoc analysis of a multicenter study across Japan. Dig Endosc. 2019;31:51-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Sato K, Shigiyama F, Ito S, Kitagawa T, Tominaga K, Suzuki T, Maetani I. Colonoscopy using a small-caliber colonoscope with passive-bending after incomplete colonoscopy due to sharp angulation or pain. Surg Endosc. 2013;27:4171-4176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |