Published online Feb 27, 2020. doi: 10.4240/wjgs.v12.i2.68
Peer-review started: September 1, 2019
First decision: September 25, 2019
Revised: November 13, 2019
Accepted: November 26, 2019
Article in press: November 26, 2019
Published online: February 27, 2020
Processing time: 137 Days and 8 Hours
A right-sided round ligament (RSRL) is a rare, congenital anomaly of the intrahepatic portal vein, with a reported frequency of 0.2%-1.2%. For patients with perihilar cholangiocarcinoma associated with an RSRL, an accurate understanding of the vascular and biliary anatomy is indispensable.
We report a 70-year-old male with perihilar cholangiocarcinoma associated with an RSRL. After percutaneous transhepatic embolization of the left and anterior portal branches, we conducted a left trisectionectomy of the liver with extrahepatic bile duct resection and hepaticojejunostomy. The postoperative course was uneventful, and R0 resection was achieved. When the liver volume of each section was compared between 7 patients with an RSRL and 20 patients with normal portal vein anatomy, the posterior section in RSRL patients was significantly larger than that in patients with normal portal vein anatomy (median: 457 mL vs 306 mL, P = 0.031). In patients with perihilar cholangiocarcinoma associated with an RSRL, left trisectionectomy has several surgical advantages: (1) The posterior branch of the portal vein often ramifies independently, and the division of the portal vein is easily conducted; (2) A relatively large amount of remnant liver can be retained; and (3) The anatomy of the posterior branch of the Glissonian pedicle is similar to that in patients with normal anatomy.
In patients with an RSRL and perihilar cholangiocarcinoma that does not involve the posterior section, left trisectionectomy may be a favorable choice.
Core tip: A right-sided round ligament (RSRL) is a rare, congenital portal vein anomaly. We present a case of perihilar cholangiocarcinoma in a 70-year-old male with an RSRL. Additionally, we reviewed the medical records of 7 patients with an RSRL who underwent hepatobiliary and pancreatic surgery at our hospital. Left trisectionectomy may be a favorable choice for resection in patients with an RSRL and perihilar cholangiocarcinoma because: (1) The posterior branch of the portal vein often ramifies independently; (2) The volume of the posterior section is relatively large; and (3) The anatomy of the posterior Glissonian pedicle in an RSRL is not very different from that in ordinary patients.
- Citation: Ishida T, Nara S, Akahoshi K, Takamoto T, Kishi Y, Esaki M, Hiraoka N, Shimada K. Left hepatic trisectionectomy for perihilar cholangiocarcinoma with a right-sided round ligament: A case report. World J Gastrointest Surg 2020; 12(2): 68-76
- URL: https://www.wjgnet.com/1948-9366/full/v12/i2/68.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i2.68
A right-sided round ligament (RSRL) is a rare, congenital portal vein (PV) anomaly with a reported frequency of 0.2%-1.2%, which has been mostly described in East Asian patients[1,2]. RSRLs have also been referred to as a “left-sided gallbladder” in several reports[3-5]. However, it is now clear that in this anomaly, the gallbladder is located in its normal position along the main portal fissure[5]. An RSRL arises as a result of the diminishment of the left umbilical vein in the prenatal period. Consequently, the right umbilical vein forms an umbilical portion (UP)[1]. RSRLs are classified into three types: Ppost-i, P-bifurcation, and P-trifurcation types[6]. In the Ppost-i type, the posterior branch of the PV (Ppost) ramifies independently from the main portal trunk. In the P-bifurcation type, the main PV ramifies to the right PV and the left PV (LPV), similar to the normal anatomy, and in the P-trifurcation type, the main PV ramifies to the left, anterior, and posterior branches.
In this report, we present a case of perihilar cholangiocarcinoma with an RSRL. Additionally, the volume of each liver section was compared among 7 patients who had an RSRL and underwent hepatobiliary or pancreatic surgery at National Cancer Center Hospital, Tokyo, Japan and 20 patients who were randomly selected with normal PV anatomy. The aim of this study was to analyze the characteristic differences in the liver volume in patients with an RSRL, and to investigate the appropriate surgical procedure for patients with an RSRL and perihilar cholangiocarcinoma.
Statistical analysis was performed by using a Mann-Whitney U test. The terminology of the liver anatomy was principally based on the Brisbane 2000 nomenclature[7]. The volumetric evaluation of each liver section was achieved manually by tracing each liver section on contrast-enhanced computed tomography (CT) or magnetic resonance imaging and determining the area. Two examiners (Ishida T and Nara S) independently performed the volumetry, and cases with marked discrepancy were resolved by discussion. A three-dimensional (3D) image analysis software system (Synapse Vincent ver2, Fujifilm, Japan) was used to confirm the results of liver volumetry.
Epigastric pain.
A 70-year-old male visited a nearby hospital with a 1-mo history of epigastric pain. Laboratory examinations revealed an elevation of liver enzymes and total bilirubin (T-Bil: 4.5 mg/dL). Contrast-enhanced CT revealed the wall thickening of the hilar bile duct and the bilateral dilatation of the intrahepatic bile ducts. Thus, perihilar cholangiocarcinoma was suspected, and the patient was referred to our hospital.
The patient received medical treatment for hypertension and gout.
The patient had no particular personal or family history.
The patient was afebrile. An abdominal examination revealed no distention or tenderness.
At our hospital, laboratory tests revealed that the levels of total bilirubin (T-Bil), serum aspartate transaminase, and alanine aminotransferase were all within the normal limits. However, biliary enzymes including serum alkaline phosphatase and γ-glutamyltransferase were elevated, with values of 461 IU/L (normal range, 106–322 IU/dL) and 118 IU/L (normal range, 13-64 IU/dL), respectively. The white blood cell counts were slightly elevated at 9.5 × 103/µL (normal range, 3.3-8.6 × 103/µL), but the serum C-reactive protein level was 0.05 mg/dL (normal range, ≤ 0.14 mg/dL). The levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were slightly elevated, with values of 7.7 ng/mL (normal range, 0.0-5.0 ng/mL) and 52 IU/mL (normal range, 0-37 IU/mL), respectively. The indocyanine green retention rate at 15 min after injection was 7.7% (normal range, ≤ 10%).
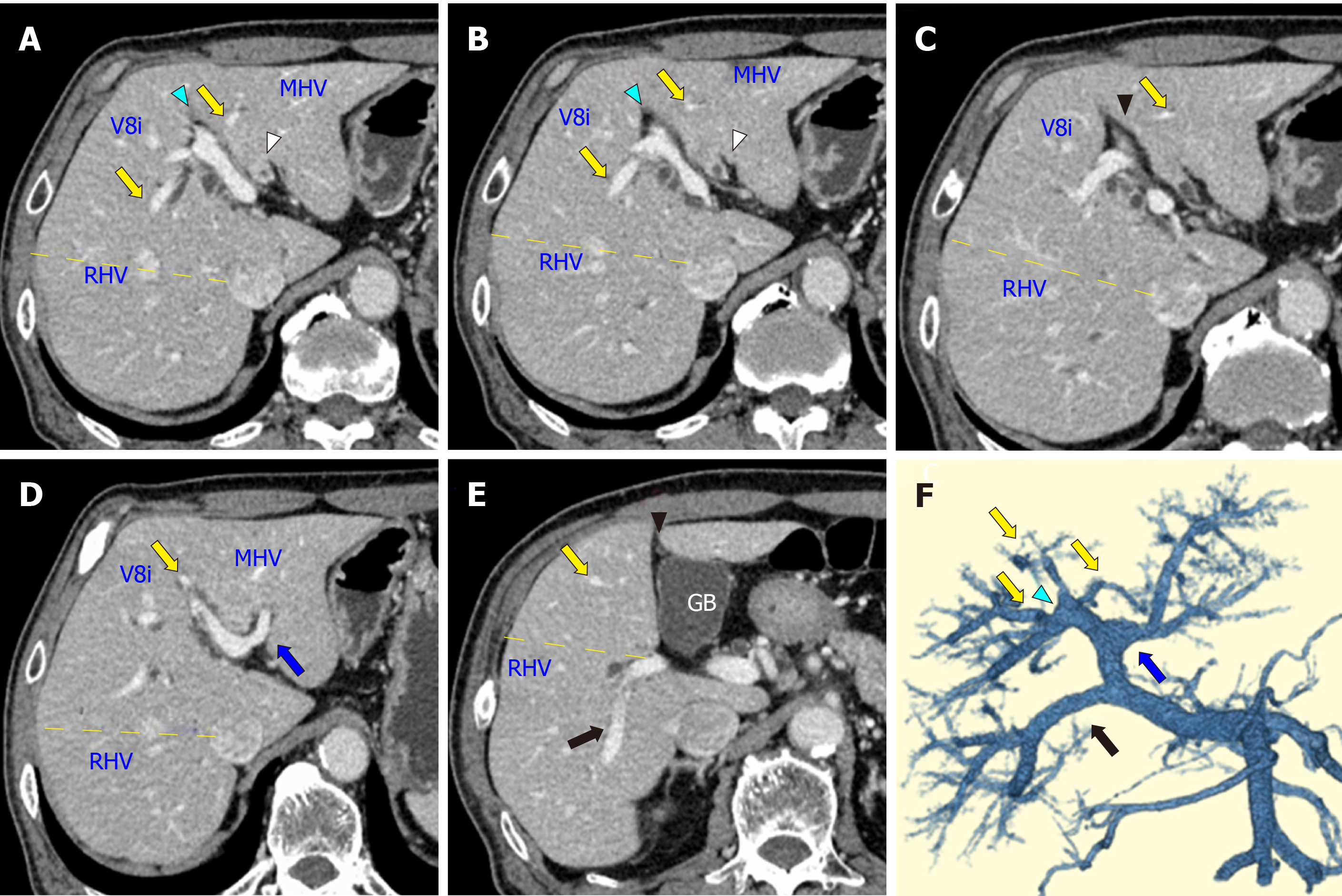
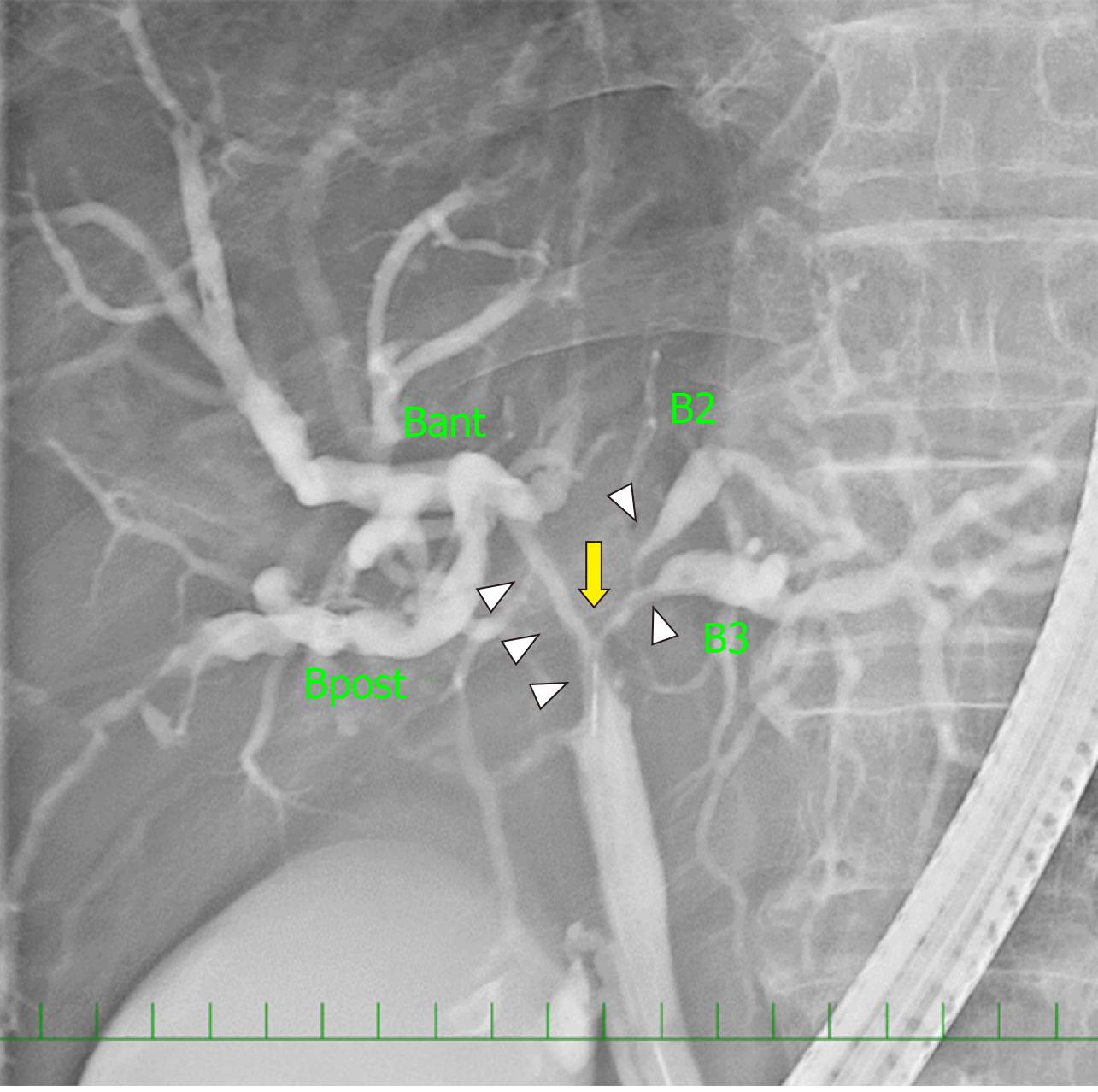
On contrast-enhanced CT images, the biliary wall was thickened at the confluence of the left and right hepatic ducts (Figure 1A and B, white arrowhead). The biliary intrahepatic bile ducts were moderately dilated. The posterior branch of the PV ramified independently (Ppost-i type, Figure 1E and F, black arrow), followed by the ramification of the anterior branch of the PV (Pant) and the LPV (Figure 1D and F, blue arrow). The Pant formed the right-sided UP (Figure 1A, B, and F, blue arrowhead) and connected to the round ligament (Figure 1C, black arrowhead). The PV branches to the anterior section diverged from the UP (Figure 1, yellow arrow). The round ligament was located on the right side of the gallbladder (Figure 1E). The 3D image of the PV clearly illustrated the independent ramification of the posterior branch (Ppost-i) and the subsequent bifurcation of the Pant and LPV (Figure 1F). Endoscopic retrograde cholangiography revealed the severe stenosis of the hilar bile duct (Figure 2, yellow arrow). The stenotic portion extended from the hepatic hilum to the right, B2, and B3 bile ducts (Figure 2, white arrowheads). According to CT volumetry, the posterior section of the liver was 501 mL (42% of the total liver volume). The biopsy of the stenotic bile ducts suggested a diagnosis of adenocarcinoma.
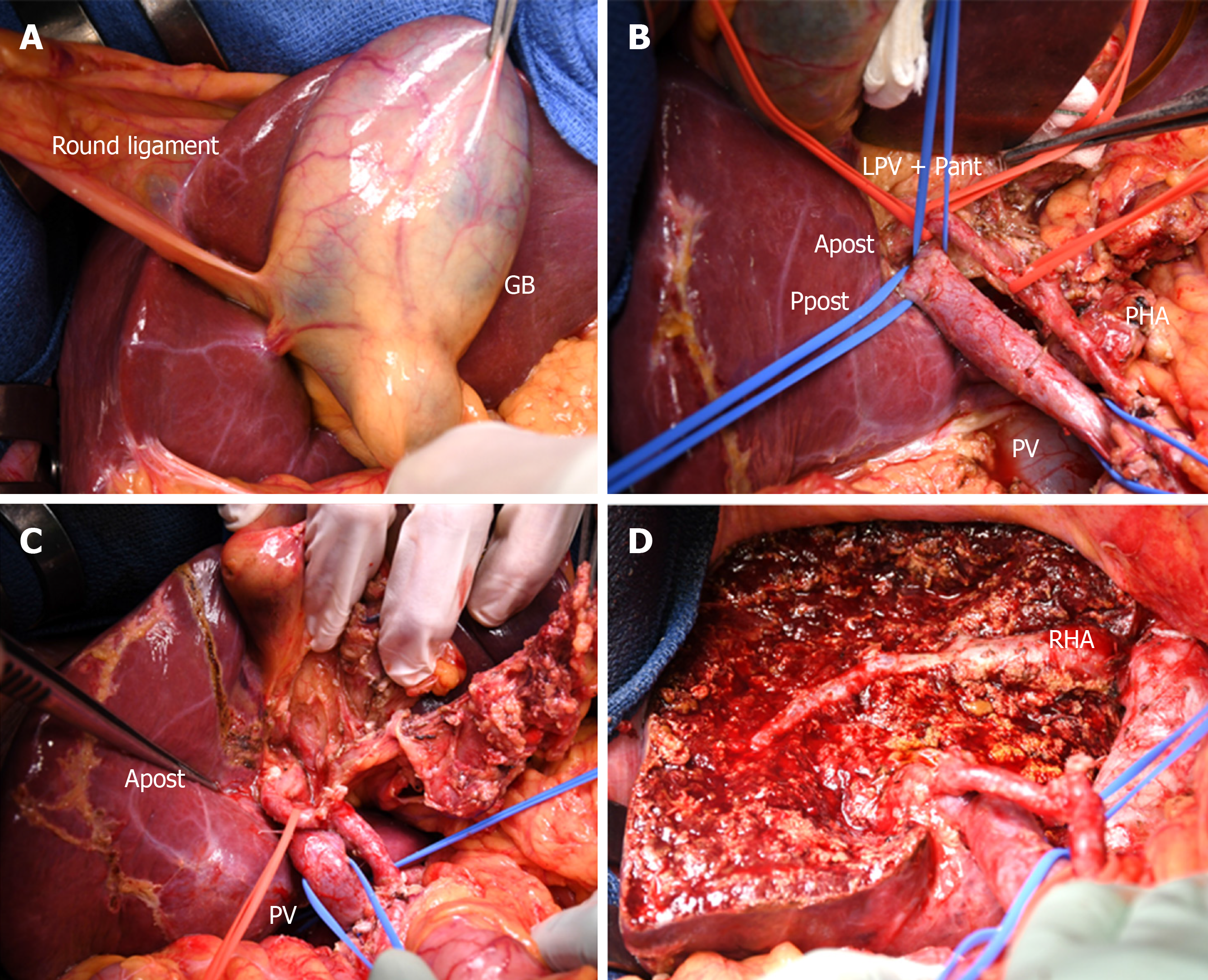
The patient underwent percutaneous transhepatic PV embolization (PTPE) of the left and anterior portal branches. Twenty days after PTPE, the volume of the posterior section increased up to 49%, and left trisectionectomy (including S1) with extrahepatic bile duct resection and hepaticojejunostomy was performed (Figure 3). After the sequential division of the left and right anterior hepatic arteries, the root of the LPV and Pant was ligated and divided. Hepatic parenchymal resection was conducted along the right hepatic vein, and the common trunk of the middle and the left hepatic veins was stapled and divided. Finally, the posterior branch of the bile duct (Bpost) was cut, and a specimen was removed. The frozen section analysis of the bile duct stump was negative for carcinoma (both the duodenal and hepatic sides). Biliary reconstruction was performed using the Roux-en-Y method. The operation time was 9 h and 40 min, and the intraoperative blood loss was 780 mL. The postoperative course was uneventful, and the patient was discharged on the 18th day after surgery.
Histologically, the tumor was diagnosed as a well-differentiated adenocarcinoma extending along the perihilar bile ducts that was 4.5 cm × 3.3 cm in size. The depth of invasion was confined to the subserosal layer.
There was no sign of recurrence 15 mo after surgery. Carbohydrate antigen 19-9 level decreased within normal range 1 mo after surgery. However, CEA level continued to show slight elevation of 6.0-7.0 ng/mL after surgery.
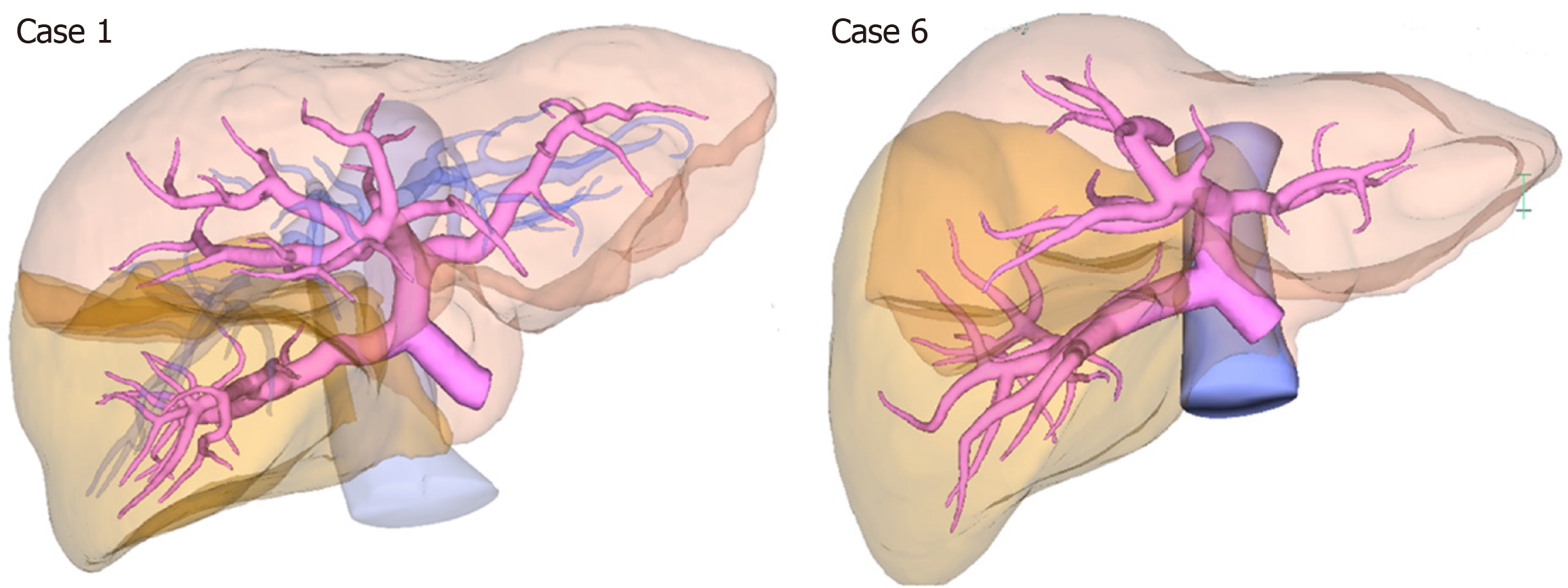
The clinicopathological characteristics of 7 patients, who had an RSRL and underwent hepatobiliary or pancreatic surgery at National Cancer Center Hospital, Tokyo, Japan, are shown in Table 1, Figure 4, and Supplementary Figures 1-6. Five patients were classified as the Ppost-i type, and the other two patients were classified as the P-bifurcation type.
| Case | Sex | Age | Diagnosis | Operation | Portal anatomy | Portal configuration[12] | Biliary architecture[11] | Volumetry of the posterior/anterior/left liver |
| 1 | M | 58 | Hepatocellular carcinoma | Partial hepatectomy | Ppost-i | Z shape | NA | 53%/32%/14% |
| 2 | M | 58 | Hepatocellular carcinoma | Partial hepatectomy | Ppost-i | Z shape | NA | 56%/28%/15% |
| 3 | F | 54 | Colorectal liver metastasis | Right hemihepatectomy and choledochojejunostomy | P-bifurcation | I shape | NA | 33%/30%/34% |
| 4 | M | 71 | Benign bile duct stenosis | Left trisectionectomy | Ppost-i | Z shape | Independent right lateral type | 44%/33%/21% |
| 5 | F | 41 | Pancreatic head cancer | Pancreaticoduodenectomy | Ppost-i | Z shape | Total left type | 41%/31%/27% |
| 6 | M | 70 | Perihilar cholangiocarcinoma | Left trisectionectomy | Ppost-i | Z shape | Total left type | 42%/35%/21% |
| 7 | M | 74 | Combined hepatocellular cholangiocarcinoma | Extended posterior sectionectomy | P-bifurcation | I shape | Symmetrical type | 21%/30%/44%1 |
The volume of each liver section was compared between patients with an RSRL (n = 7) and normal PV anatomy (n = 20). CT volumetry revealed that the median volumes of the posterior section in patients with an RSRL, Ppost-i type, and P-bifurcation type were 457 mL (42%), 501 mL (44%), and 241 mL (27%), respectively. In contrast, the median volume of the posterior section in patients with normal portal anatomy was 306 mL (31%) (Table 2). The volume of the posterior section was significantly larger in patients with an RSRL of the Ppost-i type than in patients with normal PV anatomy (P < 0.001).
| Liver section | RSRL, n = 7 | Ppost-i, n = 5 | P-bifurcation, n = 2 | Normal PV anatomy, n = 20 | P1 value | P2 value |
| Lateral section | 247 mL (19.6%) | 210 mL (17.5%) | 296 mL (32.2%) | 190 mL (18.5%) | 0.431 | 0.767 |
| Medial section | 13 mL (1.0%) | 12 mL (1.0%) | 69 mL (6.8%) | 102 mL (9.9%) | 0.002 | < 0.001 |
| Anterior section | 343 mL (30.8%) | 371 mL (31.6%) | 275 mL (29.9%) | 384 mL (36.1%) | 0.001 | 0.004 |
| Posterior section | 457 mL (41.8%) | 501 mL (43.9%) | 241 mL (27.2%) | 306 mL (31.0%) | 0.031 | < 0.001 |
| Caudate lobe (S1) | 24 mL (2.3%) | 23 mL (2.0%) | 36 mL (3.8%) | 17 mL (1.7%) | 0.092 | 0.447 |
| Total liver | 1115 mL | 1175 mL | 917 mL | 1032 mL | 0.607 | 0.272 |
During embryonic development of the liver, both right- and left-sided umbilical veins are present, but in the 8th wk of embryonic development, the umbilical vein on the right side atrophies, and the vein on the left side becomes dominant[10]. Therefore, the UP is usually located anatomically in the left liver. Conversely, in the case of an RSRL, the left umbilical vein is believed to atrophy and the right umbilical vein is believed to persist to form a right-sided UP and round ligament. Consequently, the gallbladder is located on the left side of the round ligament. Therefore, this type of anomaly has often been referred to as a “left-sided gallbladder” in the literature.
However, in 1997, Nagai et al[1] reported that in this anomaly, the location of the gallbladder is normal along the boundary of the left and right liver (Cantlie line), and it is the round ligament that is mispositioned. In RSRL cases, the careful estimation of the biliary and vascular structures is required before hepatectomy because both the biliary[11] and portal[12] anatomy around the hepatic hilum are different from that in normal cases. Strong et al[13] reported that many patients with an RSRL did not obtain a preoperative diagnosis of an RSRL and experienced postoperative morbidity.
Among the three patterns of PV branching, the Ppost-i type is characteristic of an RSRL, with a reported incidence of 48.6%[6]. Indeed, this pattern was the most common type in our patients [5 in our 7 patients (71%)]. The reported incidences of P-bifurcation and P-trifurcation types were 28.6% and 22.9%, respectively[6], and each pattern was observed in 2 (29%) and 0 of our 7 patients, respectively.
In RSRL cases, the anatomy of the PV differs from normal cases. However, the constitution of the liver section/segment and venous anatomy is reported to be the same as that in normal anatomy[14]. In summary: (1) The liver consists of four Couinaud sectors: The left lateral (S2), the left paramedian (S3+4), the right paramedian (S5+8), and the right lateral (S6+7) sectors; (2) The right, middle and left hepatic veins (RHV, MHV and LHV) run along the border of each sector; (3) The gallbladder is located along the MHV, and the gallbladder bed is along the border of the right and left liver; and (4) The RSRL separates the ventral and dorsal parts of the right paramedian sector in patients with an RSRL, similar to how the round ligament separates S3 and S4 in normal PV anatomy. Additionally, the umbilical fissure vein (UFV)[6] and intermediate vein for S8 (V8i)[15], both of which are located along the watersheds of the main venous trunks (i.e. the UFV between the tributaries of the LHV and MHV and the V8i between the tributaries of the MHV and RHV), are consistently found in patients with both an RSRL and normal PV anatomy. Indeed, the UFV and V8i were detected in all 7 of our patients.
However, the recognition of PV ramification in RSRL patients is sometimes difficult in clinical practice. Yamashita et al[12] classified the configuration of the PV into two types: I-shaped and Z-shaped. In the Z-shaped type, the PV between the branching point of the Ppost and the LPV was tilted to left or almost parallel to the vertical plane. In our patients, all 5 patients with a Ppost-I RSRL were classified as having the Z-shaped type, and 2 patients with the P-bifurcation type were classified as having the I-shaped type (Table 1). Even when the UP is not markedly tilted to the right side, we should still be aware that there is a possibility of an RSRL.
With respect to the anatomy of the hepatic artery in patients with an RSRL, Shindoh et al[6] noted that, in most instances, the hepatic arteries for the left hemiliver branched from the proper hepatic artery or from the left gastric artery. In our cases, 4 of 7 patients had this this type of configuration. No specific correlation between PV ramification and arterial branching was identified[6]. In addition, Nishitai et al[11] reported that four biliary confluence patterns were identified in patients with an RSRL, and no correlation was observed between the biliary confluence pattern and the portal ramification pattern. In our patients, the biliary confluence anatomy was obtained in only 4 patients, and no relationship was detected between the biliary anatomy and portal ramification (Table 1). According to developmental embryology, the intrahepatic biliary ducts and arteries are formed in the stages after PV formation[16]. Couinaud also noted that the arterial and biliary distributions did not always follow the preexisting portal ramification[17].
In patients with an RSRL, the proportion of each liver section is reportedly different from that in the normal liver. Shindoh et al[6] noted that in RSRL livers, either side of the lateral sectors [S2 and S6+7 (posterior section)] and the dorsal segment of the right paramedian sector were larger than those in patients with typical anatomy, whereas the left paramedian sector (S3+4) and the ventral segment of the right paramedian sector were significantly smaller[6]. The reason for this observation is unknown, but because the umbilical vein is connected to the right liver, the right liver may be shifted to the left side and become enlarged, while the left liver (especially S4) may become smaller. Additionally, in a population of normal (left-sided) round ligaments, Watanabe et al[18] reported that the posterior section of the Ppost-i type anatomy was significantly larger than that of the P-bifurcation anatomy (37.4 ± 6.1% vs 27.3 ± 5.1%), and 68.3% of patients with perihilar cholangiocarcinoma and Ppost-i type anatomy underwent left trisectionectomy[18]. In our patients, the posterior section in patients with an RSRL with the Ppost-i type, but not in patients with the P-bifurcation type, was larger than that in patients with normal liver anatomy (43.9% vs 31.0%), suggesting that the Ppost-i anatomy substantially contributes to the relatively large volume of the posterior section.
Based on these findings, left trisectionectomy has several surgical advantages, especially in patients with perihilar cholangiocarcinoma associated with a Ppost-i type RSRL. First, a large liver remnant volume can be retained. Second, the ligation and division of the Pant + LPV is easy. Third, bile duct division and reconstruction can be performed in the usual manner because the biliary anatomy at the root of posterior Glissonian pedicle is distant from the complicated anatomy at the hepatic hilum associated with an RSRL, and a similar approach is possible to that of patients with a typical PV anatomy. Although left trisectionectomy is a technically demanding operation with a relatively high mortality rate[19,20], on average, an additional 6.7 mm of bile duct can be resected compared with that in left hemihepatectomy in patients with a supraportal right posterior bile duct[21], and left trisectionectomy has now become one of the standard procedures for perihilar cholangiocarcinoma at high volume centers[22-24]. Therefore, left trisectionectomy may be a favorable choice for these patients, as long as there is no tumor extension to the posterior section.
A limitation of this study was the small number of patients. To validate the results of our study, further investigations based on a large number of RSRL cases are necessary.
We presented a case of perihilar cholangiocarcinoma associated with an RSRL and reviewed 7 cases with RSRL who underwent hepatobiliary or pancreatic surgery at our hospital. The volume of the posterior section was significantly larger in patients with a Ppost-i type RSRL than that in patients with normal PV anatomy. Left trisectionectomy may be a favorable choice for patients with a Ppost-i type RSRL and perihilar cholangiocarcinoma that does not involve the posterior section.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mastoraki A, Tchilikidi KY S-Editor: Yan JP L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Nagai M, Kubota K, Kawasaki S, Takayama T, BandaiY, Makuuchi M. Are left-sided gallbladders really located on the left side? Ann Surg. 1997;225:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Maetani Y, Itoh K, Kojima N, Tabuchi T, Shibata T, Asonuma K, Tanaka K, Konishi J. Portal vein anomaly associated with deviation of the ligamentum teres to the right and malposition of the gallbladder. Radiology. 1998;207:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Ogawa T, Ohwada S, Ikeya T, Shiozaki H, Aiba S, Morishita Y. Left-sided gallbladder with anomalies of the intrahepatic portal vein and anomalous junction of the pancreaticobiliary ductal system: a case report. Hepatogastroenterology. 1995;42:645-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Uesaka K, Yasui K, Morimoto T, Torii A, Kodera Y, Hirai T, Yamamura Y, Kato T, Kito T. Left-Sided gallbladder with intrahepatic portal venous anomalies. J Hepato-Bil-Pan Surg. 1995;2:425-430. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Kaneoka Y, Yamaguchi A, Isogai M, Harada T. Hepatectomy for cholangiocarcinoma complicated with right umbilical portion: anomalous configuration of the intrahepatic biliary tree. J Hepatobiliary Pancreat Surg. 2000;7:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Shindoh J, Akahane M, Satou S, Aoki T, Beck Y, Hasegawa K, Sugawara Y, Ohtomo K, Kokudo N. Vascular architecture in anomalous right-sided ligamentum teres: three-dimensional analyses in 35 patients. HPB (Oxford). 2012;14:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, Makuuchi M, Strong RW. The Brisbane 2000 Terminology of Liver Anatomy and Resections. Hpb. 2000;2:333-339. [DOI] [Full Text] |
| 8. | Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 512] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Brierley JD, Gospodarowicz MK, Witteking C. TNM Classifacation of Malignat Tumours. 8th ed. Wiley-Blackwell, 2017. |
| 10. | Sadler TW. Langman's Medical Embryology. Wolters Kluwer Health, 2018. |
| 11. | Nishitai R, Shindoh J, Yamaoka T, Akahane M, Kokudo N, Manaka D. Biliary architecture of livers exhibiting right-sided ligamentum teres: an indication for preoperative cholangiography prior to major hepatectomy. HPB (Oxford). 2016;18:929-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Yamashita R, Yamaoka T, Nishitai R, Isoda H, Taura K, Arizono S, Furuta A, Ohno T, Ono A, Togashi K. Portal vein branching order helps in the recognition of anomalous right-sided round ligament: common features and variations in portal vein anatomy. Abdom Radiol (NY). 2017;42:1832-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Strong RW, Fawcett J, Hatzifotis M, Hodgkinson P, Lynch S, O'Rourke T, Slater K, Yeung S. Surgical implications of a left-sided gallbladder. Am J Surg. 2013;206:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Shindoh J, Fukui Y, Hashimoto M. A Case of Hilar Cholangiocarcinoma with Right-sided Ligamentum Teres Hepatis. Nihon Rinsho Geka Gakkai Zasshi. 2015;76:374-381. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Cho A, Okazumi S, Makino H, Miura F, Ohira G, Yoshinaga Y, Toma T, Kudo H, Matsubara K, Ryu M, Ochiai T. Relation between hepatic and portal veins in the right paramedian sector: proposal for anatomical reclassification of the liver. World J Surg. 2004;28:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Skandalakis JE, Gray SW, Ricketts R, Skandalakis LJ. The liver. Embryology for surgeons. 2nd ed. Baltimore: Williams and Wilkins, 1994. |
| 17. | Couinaud C. Surgical anatomy of the liver revisited. 1989. Available from: URL: https://www.worldcat.org/title/surgical-anatomy-of-the-liver-revisited/oclc/461976990. |
| 18. | Watanabe N, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nagino M. Anatomic features of independent right posterior portal vein variants: Implications for left hepatic trisectionectomy. Surgery. 2017;161:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Kimura N, Young AL, Toyoki Y, Wyatt JI, Toogood GJ, Hidalgo E, Prasad KR, Kudo D, Ishido K, Hakamada K, Lodge JPA. Radical operation for hilar cholangiocarcinoma in comparable Eastern and Western centers: Outcome analysis and prognostic factors. Surgery. 2017;162:500-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Otsubo T, Kobayashi S, Sano K, Misawa T, Ota T, Katagiri S, Yanaga K, Yamaue H, Kokudo N, Unno M, Fujimoto J, Miura F, Miyazaki M, Yamamoto M. Safety-related outcomes of the Japanese Society of Hepato-Biliary-Pancreatic Surgery board certification system for expert surgeons. J Hepatobiliary Pancreat Sci. 2017;24:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Natsume S, Ebata T, Yokoyama Y, Igami T, Sugawara G, Shimoyama Y, Nagino M. Clinical significance of left trisectionectomy for perihilar cholangiocarcinoma: an appraisal and comparison with left hepatectomy. Ann Surg. 2012;255:754-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 499] [Article Influence: 41.6] [Reference Citation Analysis (1)] |
| 23. | Hosokawa I, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Miyazaki M. Surgical strategy for hilar cholangiocarcinoma of the left-side predominance: current role of left trisectionectomy. Ann Surg. 2014;259:1178-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Esaki M, Shimada K, Nara S, Kishi Y, Sakamoto Y, Kosuge T, Sano T. Left hepatic trisectionectomy for advanced perihilar cholangiocarcinoma. Br J Surg. 2013;100:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |