Published online Dec 27, 2020. doi: 10.4240/wjgs.v12.i12.555
Peer-review started: September 21, 2020
First decision: October 27, 2020
Revised: November 3, 2020
Accepted: November 10, 2020
Article in press: November 10, 2020
Published online: December 27, 2020
Processing time: 87 Days and 3.6 Hours
Gastric cancer (GC) with bone metastasis is rare, and rib metastasis is even less common. The clinical prognosis of GC with bone metastasis is poor given the lack of an effective treatment.
A 70 year old man was referred to Shaoxing People’s Hospital with left chest pain and slight dyspnea. Chest computed tomography (CT) revealed a metastatic lesion in the left 3rd rib. Esophagogastroduodenoscopy revealed several ulcers in the angle and antrum of the stomach, and tumor biomarkers including CEA and CA-199 were clearly increased. In addition, lymph node metastasis in the lesser curvature of the stomach was identified by positron emission tomography/CT scanning. Further pathological examination confirmed metastatic adenocarcinoma in the rib and medium-low differentiated adenocarcinoma in the gastric space. The patient had GC with rib metastasis, and was clinically staged as T3NxM1 (IVB). Based on multidisciplinary team opinions, the patient received five courses of chemotherapy (CAPOX plus aptinib), and then underwent rib resection and laparoscopic radical distal gastrectomy. The patient started four courses of chemotherapy after surgery, and then capecitabine and aptinib were administered orally for 3 mo. Follow-up was performed on an outpatient basis using abdominal/chest CT and tumor biomarkers. The patient exhibited an overall survival greater than 2 years, and the disease-free survival was approximately 18 mo. His adverse events were tolerable.
The incidence of GC with rib metastases is extremely low, and patients can obtain more benefits from individualized treatment formulated by multidisciplinary team. Chemotherapy plus surgery might represent an alternative option for GC with rib metastasis.
Core Tip: Gastric cancer (GC) with bone metastasis is uncommon, and rib metastasis is even rarer. The prognosis of patients suffering from GC with rib metastasis is extremely poor. We treated a patient with rib oligometastasis of GC individually and surgically, which led to a long-term tumor-free survival. This case revealed a new idea for the treatment of GC with rib metastasis or other bone metastases.
- Citation: Zhang Y, Zhang ZX, Lu ZX, Liu F, Hu GY, Tao F, Ye MF. Individualized treatment for gastric cancer with rib metastasis: A case report. World J Gastrointest Surg 2020; 12(12): 555-563
- URL: https://www.wjgnet.com/1948-9366/full/v12/i12/555.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i12.555
Gastric cancer (GC) with bone metastasis is infrequent, and rib metastasis is even less common. The clinical prognosis of GC with bone metastasis is poor given lack of an effective treatment[1,2]. It has been reported that the incidence of bone metastasis in GC is 0.9%-2.1%, and the incidence of bone metastasis in postoperative GC is approximately 0.9%-1.8%[3-6]. As a special bone metastasis in GC, rib metastasis is relatively rare clinically, and its treatment options are limited. To our knowledge, there is still no large retrospective report about the incidence of rib metastasis in GC. Only a few cases have been mentioned in clinical analysis. Mikami et al[7] reported ten patients who were radiologically diagnosed as having GC with rib metastasis between January 2010 and December 2015. Wen et al[8] reported 31 GC cases with rib metastasis that were identified from a retrospectively conducted gastric cancer database in their institute between April 2008 and April 2018. Thus, there are a variety of rib metastasis patients who were included in the reports about the incidence of GC with bone metastasis. Correspondingly, there is no specialized report about the treatment for GC with rib metastasis. We present a successful treatment case of GC with rib metastasis, via chemotherapy and surgery under the guidance of multidisciplinary team (MDT) that led to a long-term tumor-free survival in the patient.
A 70yearold man was referred to Shaoxing People’s Hospital with left chest pain and slight dyspnea but without significant loss of body weight.
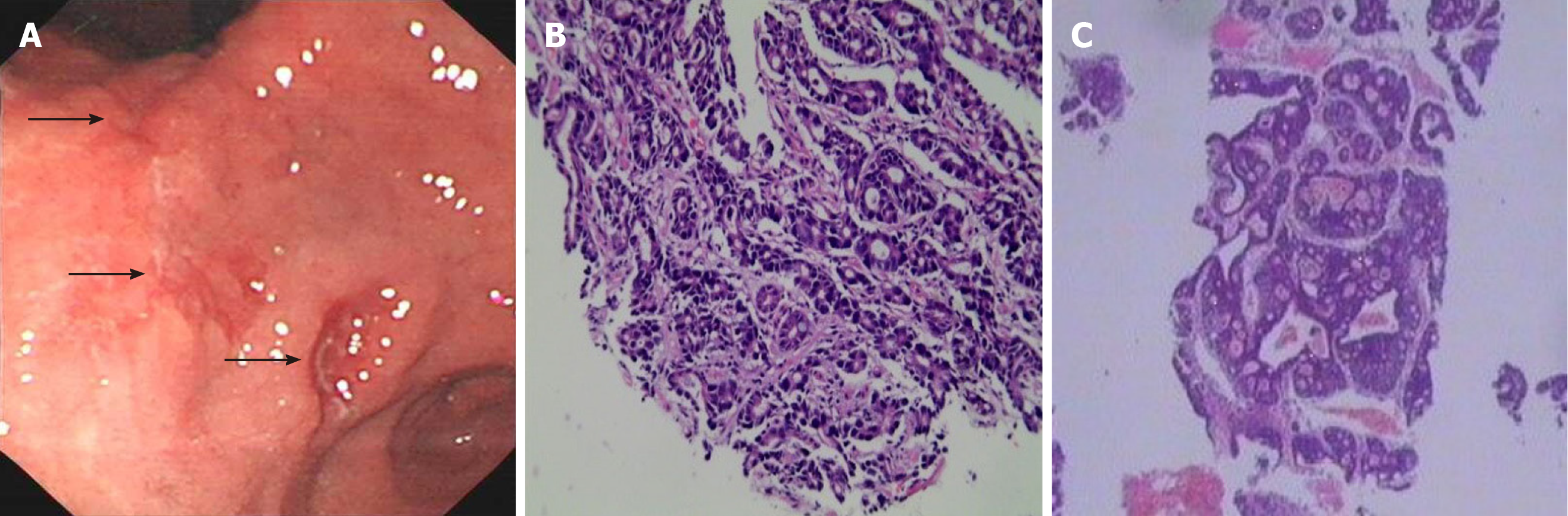
On September 6, 2018, the patient was admitted to our hospital with outpatient chest CT indicating a lump of approximately 10.0 cm × 5.2 cm in the left 3rd rib with an unclear surrounding boundary, mainly growing into the pleural cavity. Positron emission tomography (PET)/CT examination showed that the maximum standardized uptake values (SUV) in the gastric antrum and left 3rd rib were abnormally increased. Further esophagogastroduodenoscopy confirmed several ulcers in the angle and antrum of the stomach, and pathology suggested HER-2(-) low-grade adenocarcinoma. Puncture biopsy of the rib was performed, which confirmed metastatic adenoca-rcinoma by pathology (Figure 1). CAPOX pus apatinib were selected as the chemotherapy regimen for five courses. Left 3rd rib resection and laparoscopic distal gastrectomy D2 lymph node dissection were performed. The patient recovered well, and was administered four courses of the same chemotherapy half a month after the operation. Then capecitabine and aptinib were given orally for 3 mo.
The patient suffered from hypertension, asthma, and chronic bronchitis for years.
No family history was identified.
Physical examination revealed mild tenderness in the left chest.
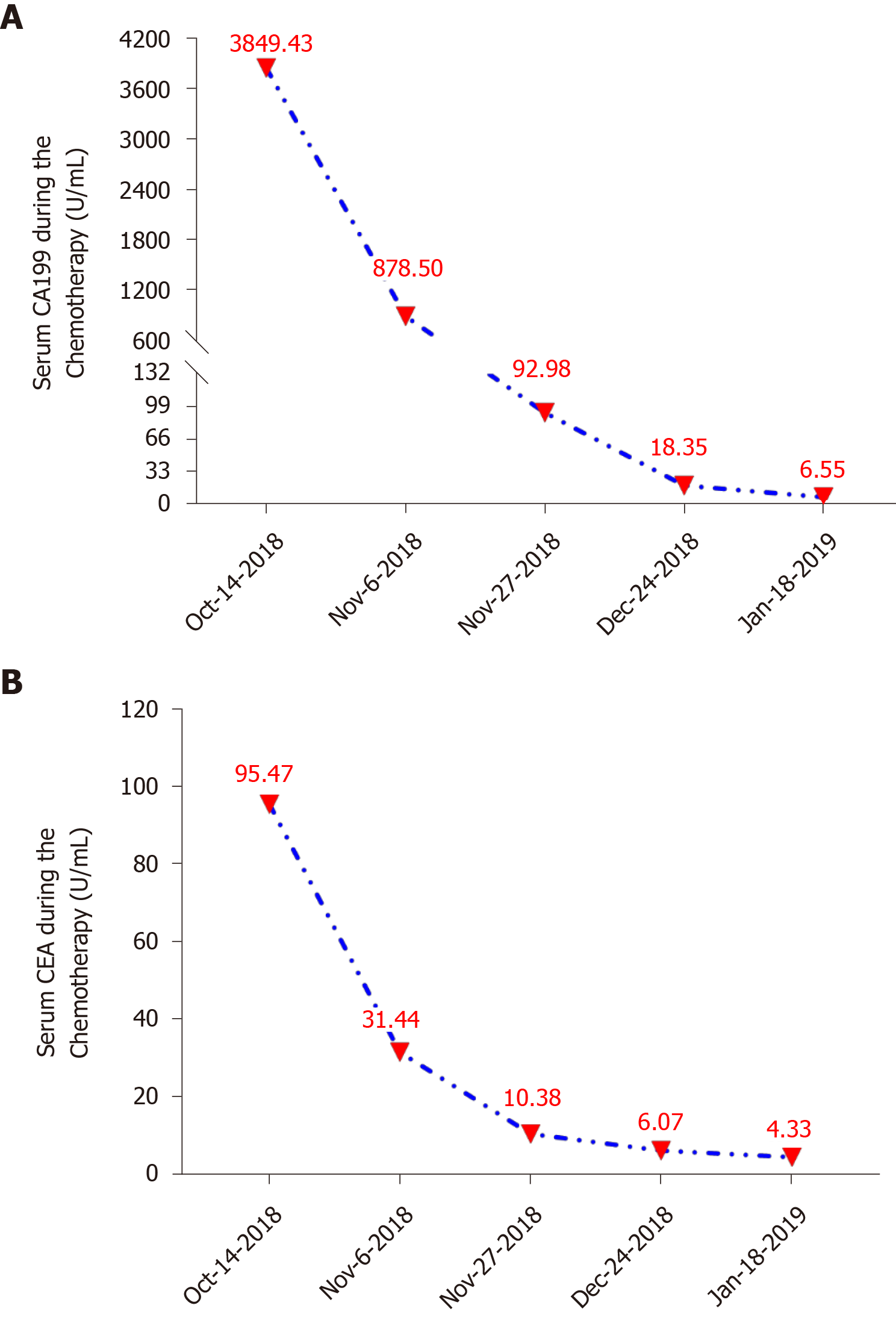
Routine blood and biochemical examinations showed no obvious abnormalities. Tumor biomarkers were present at the following levels: CEA = 60.51 ng/mL, CA199 = 2773.4 U/mL, CA242 > 300 IU/mL, and CA50 > 500 IU/mL. Then, these levels gradually returned to normal levels with chemotherapy (Figure 2).
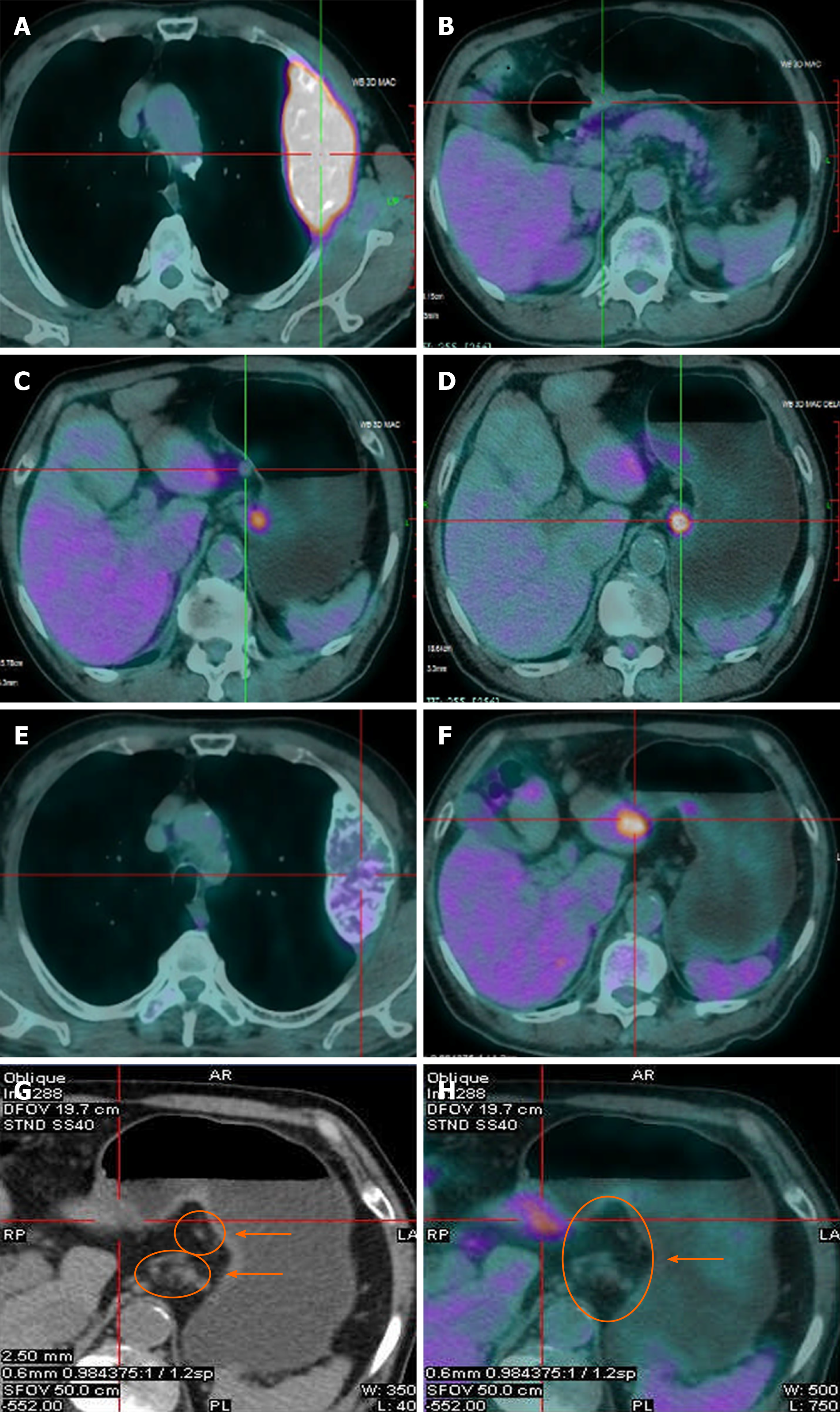
PET/CT examination showed that the maximum SUV in the gastric antrum (SUVmax = 3.6) and the left 3rd rib (SUVmax = 8.05) were abnormally increased. After five courses of chemotherapy, PET/CT suggested that the activity of gastric lesions was moderately reduced, whereas metastatic lymph nodes were devitalized. The activity of metastatic tumors in the rib was significantly reduced (Figure 3).
The preoperative diagnosis was GC with left 3rd rib metastasis, and the tumor was clinically staged as T3NxM1 (IVB). The final pathologic report was ypT1N1M0 (IB).
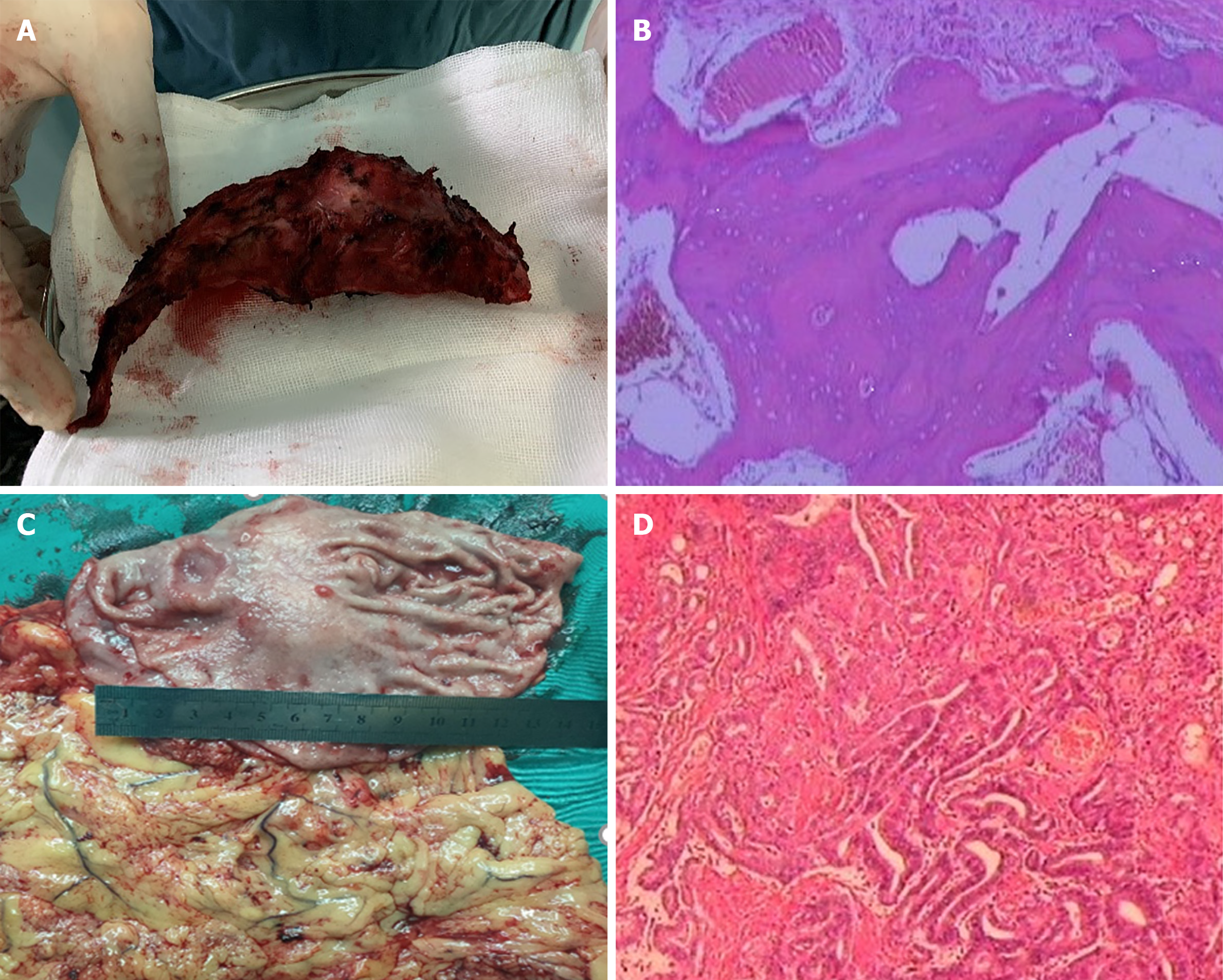
CAPOX pus apatinib was selected as the chemotherapy regimen. Capecitabine (1500 mg/m2 per day) was orally administered twice daily for the first 2 wk of a 3 wk course, and aptinib (started with 500 mg/body per day, then decreased to 250 mg/body per day) was administered orally throughout the 3-wk course. Oxaliplatin was given as an intravenous infusion of 130 mg/m2 per day on day 1 of each course. Zoletriphosphate was used to alleviate chest pain, and chest and abdominal CT were used to evaluate changes in the size of primary and metastatic lesions after every two courses of chemotherapy. After five courses of chemotherapy, left 3rd rib resection was performed on February 4, 2019, and postoperative pathology suggested complete regression of rib metastasis. Laparoscopic distal gastrectomy and D2 lymph node dissection were performed on March 4, 2019, and postoperative pathology suggested ulcerative gastric adenocarcinoma with moderate differentiation (Figure 4). The patient started the same chemotherapy as previously noted for four courses half a month after the operation. Then, capecitabine and aptinib were administered orally for 3 mo.
The patient’s overall survival was > 2 years, and the disease-free survival was approximately 18 mo. The patient has been monitored regularly in the outpatient department and no tumor recurrence has been noted to date.
Currently, the most effective treatment for GC is radical gastrectomy with D2 lymph node dissection. Once GC transfers to bone tissues, the prognosis is extremely poor. The survival time is typically less than half a year because GC with bone metastasis is mostly found in those types with poor pathological differentiation, a large number of lymph node metastases, and late T stage. Studies have reported that the 8-mo and 1-year survival rates of patients with GC with bone metastasis were 18.3% and 3.3%, respectively. A survival rate of greater than 2 years has not been reported[9,10]. In recent years, the diagnosis and treatment of GC with bone metastasis have greatly improved. It has been reported that the survival of GC with bone metastasis patients can be improved by systemic chemotherapy[11]. S-1 plus oxaliplatin, docetaxel plus S-1, and FOLFOX were the most common regimens for first-line chemotherapy. Case reports from Japan revealed good therapeutic effects of S-1 plus cisplatin for bone metastasis[12,13]. In addition to chemoradiotherapy, the available treatment options for GC with bone metastasis can also be combined with other treatment methods, such as targeted therapy, biotherapy, and immunotherapy. Therefore, for GC with bone metastasis, individualized treatment formulated according to the actual situation can obtain more benefit and maximize the survival time.
For GC patients with metastasis, radical resection of primary and metastatic lesions can also improve overall survival. A retrospective study enrolled 414 patients with GC associated with a single distant metastasis. In total, 333 patients only received palliative chemotherapy, and 81 patients underwent gastrectomy + D2 lymph node dissection after conversion chemotherapy. The study demonstrated that patients with liver metastasis may benefit from aggressive surgery[14]. In this patient, the metastatic lesion was simple and localized to one rib compared to spinal, pelvis, skull, and femur metastases, and the rib was looser and easily subject to radical resection. The primary lesion and metastasis were both potentially resectable when viewed separately. However, radiography examination revealed that the primary lesion had perigastric lymph node metastasis, whereas rib metastasis boundary with surrounding tissue was unclear. Thus, radical resection was difficult. In patients with liver metastases from GC who underwent resection for primary cancer and metastasis, 55 research centers in 17 European countries and Japan conducted a questionnaire survey and found that most centers recommend that patients undergo resection of the primary lesion and metastasis after preoperative chemotherapy[15]. Based on these experiences, MDT first suggested neoadjuvant chemotherapy.
Fluorouracil, platinum, and paclitaxel are the primary chemotherapeutic agents for advanced GC. The first-line chemotherapy is usually a two- or three-drug regimen based on fluorouracil, combined with platinum and/or paclitaxel[16-19]. Evidence in the literature has confirmed that chemotherapy with a two-drug combination is routinely recommended for advanced GC in first-line treatment[20-23]. Meanwhile, oral fluorouracil (S-1, capecitabine) is tantamount to intravenous 5-FU and oxaliplatin can substitute for cisplatin. In China, the priority for platinum is oxaliplatin due to better toleration in patients and the real-world clinical application[24,25]. Therefore, we selected a two-drug combination of oxaliplatin and capecitabine. The patient was simulta-neously administered apatinib to improve the effect of chemotherapy based on some studies by Chinese scholars, which demonstrated that apatinib can improve the therapeutic effect of advanced GC. Peng et al[26] reported a real-world study of apatinib for GC in first-line therapy and suggested that the median PFS was 3.33 mo and 5.03 mo for apatinib alone vs apatinib plus chemotherapy, respectively, which was statistically significant. Cheng et al[27] conducted a phase II clinical study (Ahead-G325) and showed that patients with unresectable advanced GC could benefit from conversion therapy with apatinib in combination with S-1/paclitaxel/cisplatin/5-FU. The median-survival time was 30.2 mo in the surgical group compared to 8.9 mo in the nonsurgical group.
After five courses of chemotherapy, the evaluation suggested that chemotherapy was effective. Both the primary tumor and metastatic tumor exhibited a partial response, indicating the possibility for surgical resection, and the patient smoothly received radical distal gastrectomy and left third rib resection. Postoperative pathology demonstrated that the rib metastasis had a complete response and the gastric lesion showed a partial response, suggesting that CAPOX plus aptinib was highly effective in this patient.
On the other hand, based on the data from the phase III study, apatinib was approved in October 2014 by the China Food and Drug Administration for patients with metastatic gastric or gastroesophageal junction adenocarcinoma after second-line chemotherapy[28]. We must note that the use of apatinib in GC with rib metastasis in first-line treatment is currently controversial because the benefit from apatinib is unclear statistically.
We provide a very special case of GC with simultaneous rib metastasis. Fortunately, the patient had a very good outcome after MDT-guided individualized treatment. Chemotherapy plus surgery represents a potential alternative option for GC with rib metastasis, but the role of apatinib in this type of treatment needs to be further investigated.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe S S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Li JH
| 1. | Yoshikawa K, Kitaoka H. Bone metastasis of gastric cancer. Jpn J Surg. 1983;13:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Turkoz FP, Solak M, Kilickap S, Ulas A, Esbah O, Oksuzoglu B, Yalcin S. Bone metastasis from gastric cancer: the incidence, clinicopathological features, and influence on survival. J Gastric Cancer. 2014;14:164-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Park HS, Rha SY, Kim HS, Hyung WJ, Park JS, Chung HC, Noh SH, Jeung HC. A prognostic model to predict clinical outcome in gastric cancer patients with bone metastasis. Oncology. 2011;80:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Ahn JB, Ha TK, Kwon SJ. Bone metastasis in gastric cancer patients. J Gastric Cancer. 2011;11:38-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Park JM, Song KY, O JH, Kim WC, Choi MG, Park CH. Bone recurrence after curative resection of gastric cancer. Gastric Cancer. 2013;16:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Silvestris N, Pantano F, Ibrahim T, Gamucci T, De Vita F, Di Palma T, Pedrazzoli P, Barni S, Bernardo A, Febbraro A, Satolli MA, Bertocchi P, Catalano V, Giommoni E, Comandone A, Maiello E, Riccardi F, Ferrara R, Trogu A, Berardi R, Leo S, Bertolini A, Angelini F, Cinieri S, Russo A, Pisconti S, Brunetti AE, Azzariti A, Santini D. Natural history of malignant bone disease in gastric cancer: final results of a multicenter bone metastasis survey. PLoS One. 2013;8:e74402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Mikami J, Kimura Y, Makari Y, Fujita J, Kishimoto T, Sawada G, Nakahira S, Nakata K, Tsujie M, Ohzato H. Clinical outcomes and prognostic factors for gastric cancer patients with bone metastasis. World J Surg Oncol. 2017;15:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Wen L, Li YZ, Zhang J, Zhou C, Yang HN, Chen XZ, Xu LW, Kong SN, Wang XW, Zhang HM. Clinical analysis of bone metastasis of gastric cancer: incidence, clinicopathological features and survival. Future Oncol. 2019;15:2241-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Kim YJ, Kim SH, Kim JW, Lee JO, Kim JH, Bang SM, Lee JS, Lee KW. Gastric cancer with initial bone metastasis: a distinct group of diseases with poor prognosis. Eur J Cancer. 2014;50:2810-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | D'Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 488] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 11. | Sudo H, Takagi Y, Katayanagi S, Hoshino S, Suda T, Hibi Y, Ito K, Tsutida A, Aoki T. [Bone metastasis of gastric cancer]. Gan To Kagaku Ryoho. 2006;33:1058-1060. [PubMed] |
| 12. | Migita K, Watanabe A, Sakamoto C, Nakamura T, Ohyama T, Ishikawa H, Yamamoto K. [A case of multiple bone metastases from gastric cancer treated with combination chemotherapy of S-1 and CDDP]. Gan To Kagaku Ryoho. 2007;34:929-931. [PubMed] |
| 13. | Fujishima Y, Yoneda R, Iwai M, Fukunaga H, Miura M, Koide M, Fukuda T. [A case of advanced gastric cancer with disseminated carcinomatosis of bone marrow treated by S-1 and CDDP]. Gan To Kagaku Ryoho. 2009;36:2653-2655. [PubMed] |
| 14. | Wang Y, Yu YY, Li W, Feng Y, Hou J, Ji Y, Sun YH, Shen KT, Shen ZB, Qin XY, Liu TS. A phase II trial of Xeloda and oxaliplatin (XELOX) neo-adjuvant chemotherapy followed by surgery for advanced gastric cancer patients with para-aortic lymph node metastasis. Cancer Chemother Pharmacol. 2014;73:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Kataoka K, Kinoshita T, Moehler M, Mauer M, Shitara K, Wagner AD, Schrauwen S, Yoshikawa T, Roviello F, Tokunaga M, Boku N, Ducreux M, Terashima M, Lordick F; EORTC GITCG Group and JCOG SCGC Group. Current management of liver metastases from gastric cancer: what is common practice? Gastric Cancer. 2017;20:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, Philco-Salas M, Suarez T, Santamaria J, Forster G, McCloud PI. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 591] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 17. | Lu Z, Zhang X, Liu W, Liu T, Hu B, Li W, Fan Q, Xu J, Xu N, Bai Y, Pan Y, Xu Q, Bai W, Xia L, Gao Y, Wang W, Shu Y, Shen L. A multicenter, randomized trial comparing efficacy and safety of paclitaxel/capecitabine and cisplatin/capecitabine in advanced gastric cancer. Gastric Cancer. 2018;21:782-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Wang J, Xu R, Li J, Bai Y, Liu T, Jiao S, Dai G, Xu J, Liu Y, Fan N, Shu Y, Ba Y, Ma D, Qin S, Zheng L, Chen W, Shen L. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer. 2016;19:234-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, Nasu J, Ohtsu A; Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 20. | Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G, Homann N, Wilhelm G, Schuch G, Stoehlmacher J, Derigs HG, Hegewisch-Becker S, Grossmann J, Pauligk C, Atmaca A, Bokemeyer C, Knuth A, Jäger E; Arbeitsgemeinschaft Internistische Onkologie. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 575] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 21. | Luo HY, Xu RH, Wang F, Qiu MZ, Li YH, Li FH, Zhou ZW, Chen XQ. Phase II trial of XELOX as first-line treatment for patients with advanced gastric cancer. Chemotherapy. 2010;56:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, Tsuji A, Imamura H, Tsuda M, Yasui H, Fujii H, Yamaguchi K, Yasui H, Hironaka S, Shimada K, Miwa H, Hamada C, Hyodo I. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 398] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 23. | Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 431] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 24. | Hall PS, Swinson DE, Lord S, Marshall H, Ruddock S, Allmark C, Cairns D, Waters J, Wadsley J, Falk S, Roy R, Joseph M, Nicoll J, Kamposioras KV, Tillett T, Cummins S, Grumett S, Stokes Z, Seymour M. Optimizing chemotherapy for frail and elderly patients (pts) with advanced gastroesophageal cancer (aGOAC): The GO2 phase III trial. J Clin Oncol. 2019;37 suppl 15:4006. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA; V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1458] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 26. | Peng W, Zhang F, Wang Z, Li D, He Y, Ning Z, Sheng L, Wang J, Xia X, Yu C, Wang Z, Zhao Y, Liang H, Hu B, Sun C, Wang D, Cheng Y, Pan M, Xia L, Guo X, Zhang Y, Hu Z, Li X, Lu L, Zhang J, Qian H, Xie H, Sun G. Large Scale, Multicenter, Prospective Study of Apatinib in Advanced Gastric Cancer: A Real-World Study from China. Cancer Manag Res. 2020;12:6977-6985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Cheng XD, Xu ZY, Du YA, Ping Hu P, Hu CG. Phase II study of conversion therapy using S1/paclitaxel chemotherapy plus apatinib in unresectable gastric cancer (Ahead-G325 trial). Journal of Clinical Oncology. 2017;35 suppl 4:53-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 732] [Article Influence: 81.3] [Reference Citation Analysis (1)] |