Published online Dec 27, 2020. doi: 10.4240/wjgs.v12.i12.468
Peer-review started: October 14, 2020
First decision: December 1, 2020
Revised: December 6, 2020
Accepted: December 11, 2020
Article in press: December 11, 2020
Published online: December 27, 2020
Processing time: 68 Days and 4.8 Hours
Carbohydrate antigen 19-9 (CA 19-9) is a cell surface glycoprotein complex most commonly associated with pancreatic ductal adenocarcinoma (PDAC). Koprowski first described it in 1979 using a mouse monoclonal antibody in a colorectal carcinoma cell line. Historically, it is one of the most commonly used tumor markers for diagnosing, managing, and prognosticating PDAC. Additionally, elevated CA 19-9 levels are used as an indication for surgery in suspected benign pancreatic conditions. Another common application of CA 19-9 in the biliary tract includes its use as an adjunct in diagnosing cholangiocarcinoma. However, its clinical value is not limited to the hepatopancreatobiliary system. The reality is that the advancing literature has broadened the clinical value of CA 19-9. The potential value of CA 19-9 in patients' workup extends its reach to gastrointestinal cancers – such as colorectal and oesophageal cancer – and further beyond the gastrointestinal tract - including urological, gynecological, pulmonary, and thyroid pathologies. Apart from its role in investigations, CA 19-9 presents a potential therapeutic target in PDAC and acute pancreatitis. In a bid to consolidate its broad utility, we appraised and reviewed the biomarker’s current utility and limitations in investigations and management, while discussing the potential applications for CA 19-9 in the works for the future.
Core Tip: Carbohydrate antigen 19-9 (CA 19-9) is the “go-to” tumor marker when one discusses the diagnosis, prognosis, and recurrence of pancreatic ductal adenocarcinoma. However, its utility in screening special population groups and determining management for pancreatic ductal adenocarcinoma is more obscure, with many interpretations of its utility in the literature. Additionally, CA 19-9 is useful beyond pancreatic pathologies, with current studies pointing towards its potential use in gastrointestinal, urological, pulmonary, uterine, ovarian, thyroid, and salivary gland diseases. We provide an in-depth analysis of the current practices of CA 19-9 and its potential applications in the future.
- Citation: Lee T, Teng TZJ, Shelat VG. Carbohydrate antigen 19-9 — tumor marker: Past, present, and future. World J Gastrointest Surg 2020; 12(12): 468-490
- URL: https://www.wjgnet.com/1948-9366/full/v12/i12/468.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i12.468
Carbohydrate antigen 19-9 (CA 19-9), also known as Sialyl Lewis-a, is a cell surface glycoprotein complex. It was first described in 1979 using a mouse monoclonal antibody (1116-NS-19-9) in a colorectal carcinoma cell line (SW1116)[1]. Structurally, it is a tetrasaccharide carbohydrate with a transmembrane protein skeleton and extensively glycosylated extracellular oligosaccharide chains. CA 19-9 expression requires the Lewis gene product, 1,4-fucosyltransferase, found only in patients with Le (a-b+) or Le (a+b-) blood groups. Roughly 6% and 22% of the Caucasian and non-Caucasian population are genotypically Lea-b- and hence are perpetual CA 19-9 non-producers[1].
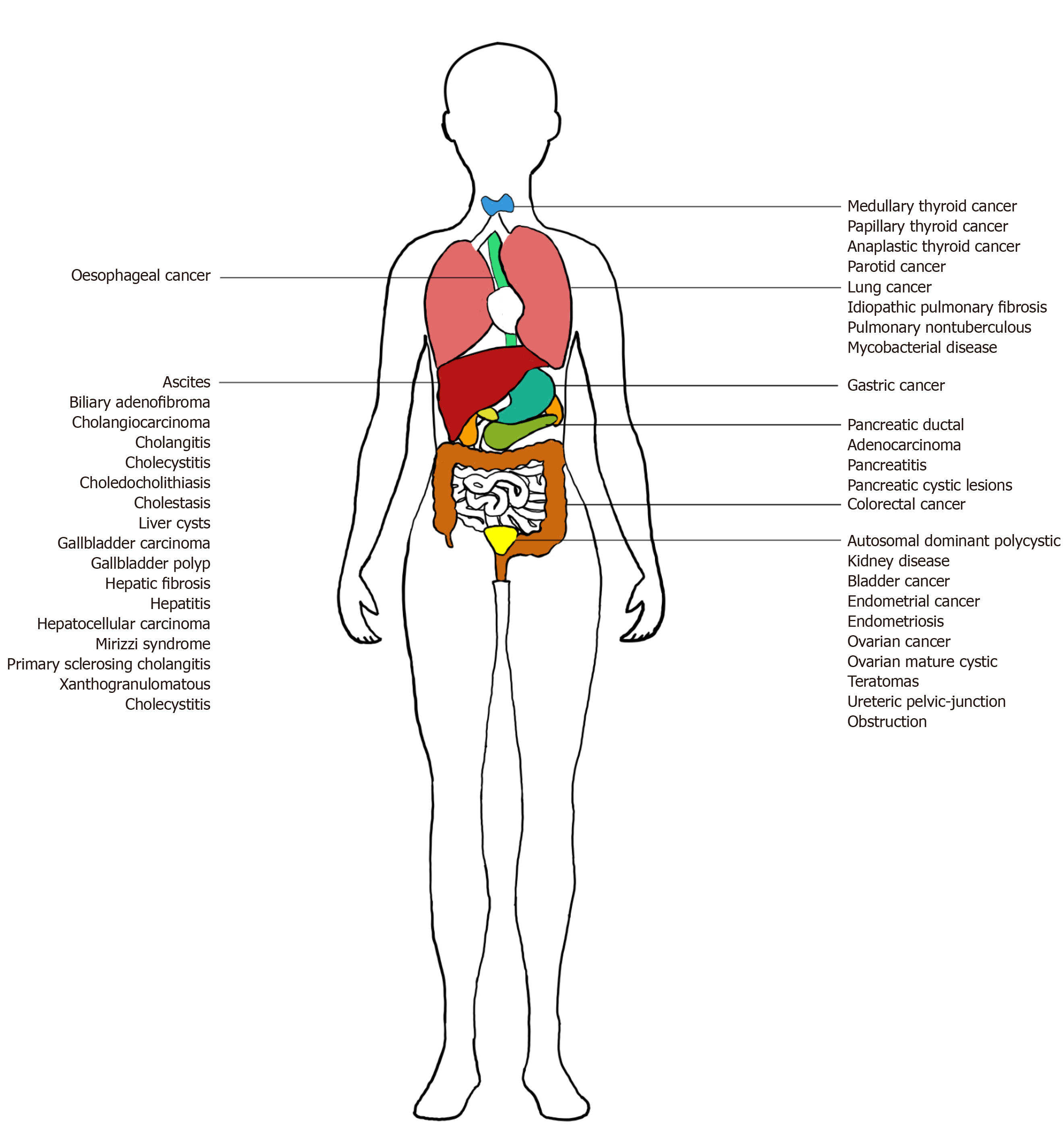
CA 19-9 is produced by ductal cells in the pancreas, biliary system, and epithelial cells in the stomach, colon, uterus, and salivary glands. While its key implication is in pancreatic ductal adenocarcinoma (PDAC), CA 19-9 is also overexpressed in a wide gamut of benign and malignant, gastrointestinal, and extra-gastrointestinal diseases. Benign conditions include pancreatitis, pancreatic cysts, diabetes mellitus (DM), liver fibrosis, benign cholestatic diseases, and other urological, pulmonary, and gynecological diseases (Figure 1)[2-4]. Inflammation or obstruction of biliary and respiratory tracts lead to elevated CA 19-9[2]. Malignancies include pancreatic, biliary, hepatocellular, gastrointestinal, urological, pulmonary, gynecological, thyroid, and salivary gland cancers[2,5,6]. During carcinogenesis, epigenetic silencing of the gene for 2->6 sialyltransferase, responsible for the attachment of an extra sialic acid residue in Disialyl Lewis-a, occurs. This results in the predominance of Sialyl Lewis-a, instead of Disialyl Lewis-a, which are expressed in normal epithelial cells. Sialyl Lewis-a’s role as a ligand for endothelial E-selectin facilitates hematogenous metastasis[7].
This article will discuss the current utility and future applications of CA 19-9 from serum, saliva, pancreatic cysts, ascitic fluid, pleural fluid, bronchoalveolar lavage (BAL), antibodies, and immunohistochemical staining. Mentions of CA 19-9 in this article refer to serum CA 19-9, which is the most common source of CA 19-9 unless otherwise stated.
An electronic search of PubMed was conducted in August 2020 for literature published in English. The following terms were used, and relevant articles were considered: ("Ca-19-9 antigen" [MeSH Terms]), ("pancreatic diseases" [MeSH Terms]), ("liver diseases" [MeSH Terms]), ("biliary tract diseases" [MeSH Terms]), ("gastrointestinal diseases" [MeSH Terms]), ("urologic diseases" [MeSH Terms]), ("lung diseases" [MeSH Terms]), ("uterine diseases" [MeSH Terms]), ("ovarian diseases" [MeSH Terms]), ("thyroid diseases" [MeSH Terms]) and ("salivary gland diseases" [MeSH Terms]). Since the main application of CA19-9 is in PDAC, we discuss it first.
PDAC carries poor prognosis due to a paucity of early detection methods, advanced presentations, limited effective treatment options, and high recurrence rates after curative resection[8]. Despite the development of numerous PDAC biomarkers, CA 19-9 remains a crucial biomarker in everyday clinical use. This section discusses the current uses of serum CA 19-9 in the screening, diagnosis, staging, management, prognostication, and prediction of recurrence in PDAC.
In asymptomatic and symptomatic populations, it is well-established that CA 19-9 is an ineffective screening tool due to low positive predictive value (PPV) and high negative predictive value (NPV). A landmark study by Kim et al[9] involving 70940 asymptomatic individuals reported a low PPV (0.9%), despite high sensitivity (100%) and specificity (98.5%)[9]. Patients presenting with symptoms suspicious for PDAC (e.g., abdominal pain, jaundice, weight loss) have low PPV (0.5%-0.9%)[7].
CA 19-9 proves to have limited screening utility even in high-risk populations, such as patients with familial PDAC or Peutz-Jeghers syndrome. In these populations, CA 19-9 levels were normal even when imaging revealed preinvasive lesions[1]. However, in a study for 546 patients, Zubarik et al[10] identified the potential in the combined use of conventional imaging modalities (e.g., endoscopic ultrasonography) in conjunction with CA 19-9 increase screening accuracy, but this protocol has yet to be validated[10].
The role of CA 19-9 in the screening of special population groups, such as patients with DM, remains ambiguous and evolving. A different threshold for suspicion of PDAC and elevated CA 19-9 applies in people with diabetes. New-onset (< 2 years) DM is a possible symptom of PDAC, and people with diabetes are susceptible to PDAC[8]. However, CA 19-9 levels are often raised in DM as part of the natural disease process due to hyperglycemia or acute metabolic situations like ketoacidosis and hyperosmolarity[11]. Therefore, it is essential to distinguish whether elevated CA 19-9 is due to the natural disease process or underlying PDAC.
Uncorrected CA 19-9 measurements have better potential as a screening tool in DM patients (PPV of 10.7%)[11] than in the general population (PPV of 0.9%)[9]. Furthermore, a reduction in fasting plasma glucose correlated with CA19-9 downtrending[12]. Thus, there is growing interest in using CA 19-9 cut-off values individually adjusted to blood glucose levels, instead of using a blanket cut-off value. This may increase the accuracy of CA 19-9 in screening DM patients for PDAC[11]. Recently, Kim et al[2] postulated that CA 19-9 values should only be considered as a screening marker for PDAC in a radiologically-normal patient whose CA 19-9 remains persistently elevated after intensive blood sugar control and normalization of HbA1c[2]. These algorithms need prospective validation.
Threshold levels for elevated CA 19-9, specifically for its diagnostic value in PDAC, are largely standardized at > 37-40 U/mL. A systematic review by Goonetilleke et al[13] involving 2283 symptomatic patients reported sensitivity (79.0%), specificity (82.0%), PPV (72.0%) and NPV (81.0%)[13]. Nevertheless, international guidelines like the European Group on Tumor Marker and the National Academy of Clinical Biochemistry (United States) do not recommend using CA 19-9 levels alone due to its suboptimal PPV, NPV, specificity, and sensitivity. For diagnostic purposes, these guidelines recommend CA 19-9 as an adjunct to radiological investigations such as the pancreas protocol computed tomography (CT), which is the current gold standard[14].
CA 19-9 levels decrease with poorer degrees of differentiation in neoplasms[1]. 10%-50% of benign pancreatic conditions (e.g., pancreatitis) and precursor lesions (e.g., intraductal pancreatic mucinous neoplasms (IPMNs), pancreatic intraepithelial neoplasia) have resultant raised CA 19-9 levels. Thus, standalone CA 19-9 levels cannot differentiate these from true PDACs. Multiple studies have established that median preoperative CA 19-9 levels increase with tumor size, burden, American Joint Committee on Cancer stage, and pathological stage[15]. In a study by Ferrone et al[16] involving 176 patients, it was revealed that 80%-90% of Stage III-IV PDAC patients have CA 19-9 levels >100 U/mL[7], patients with lower tumor (T) stage have lower median CA 19-9 levels (41 U/mL vs 162 U/mL for T1/2 and T3 disease respectively) and absence of nodal spread also have lower CA19-9 levels (90 vs 164 U/mL)[16].
Surgical resection remains the only potentially curative treatment in PDAC[17]. Resectability is determined by clear fat planes around major arteries and the absence of distant metastases[18]. Despite improvements in preoperative imaging, up to 25% of radiologically resectable patients are unresectable upon exploration[19]. Morbidity from non-therapeutic exploration leads to potential delays in initiating palliative treatment. Multiple studies have determined that elevated preoperative CA 19-9 values predict unresectability. However, unlike the clear threshold CA 19-9 value used in diagnosis, there is significant variability in optimal cut-off values used to determine unresectable disease, ranging from 37-1000 U/mL (sensitivity 69%-93% and specificity 78%-98%)[19].
Staging laparoscopy is an established tool to determine resectability in many gastrointestinal cancers, and it could avoid the morbidity of surgical exploration[20]. The pancreas is a retroperitoneal organ, and thus, extended laparoscopy is warranted. Further, with improvements in imaging technology, routine use of staging laparoscopy is not established. Staging laparoscopy criteria include CA 19-9 levels. De Rosa et al[21] designed criteria based on five large studies (n = 159-262), involving preoperative CA 19-9 ≥ 150 U/mL and tumor size > 3 cm in patients with the radiologically resectable disease[21]. The 2018 French intergroup guidelines proposed performing staging laparoscopy instead when preoperative CA 19-9 > 130-400 U/mL[22]. Thus, elevated CA 19-9 levels can guide decisions for staging laparoscopy.
Neoadjuvant chemotherapy is evolving in PDAC management. CA 19-9 has a role in monitoring treatment response by predicting resectability and prognosis. Regarding resectability, a study by Heger et al[23] involving 103 patients receiving neoadjuvant FOLFIRINOX therapy showed that patients with post-neoadjuvant CA 19-9 ≥ 91.8 U/mL did not benefit from resection (75% sensitivity and 76.9% specificity)[23]. In another report involving 78 patients by Boone et al[24], CA 19-9 response > 50% after neoadjuvant gemcitabine-based or FOLFIRINOX therapy predicted R0 resection in 40 borderline resectable patients[24]. Regarding prognosis, a study by Macedo et al[25] involving 274 patients that received neoadjuvant FOLFIRINOX or Gemcitabine/nab-paclitaxel therapy and curative-intent resection showed that a ≥ 50% decrease in CA 19-9 related to increased overall survival (OS) (42.3 vs 24.3 mo), local recurrence-free survival (RFS) (27.3 mo vs 14.1 mo) and metastasis-free survival (29.3 mo vs 13 mo) compared to patients with a < 50% decrease[25]. However, a recent study by Tsai et al[26] involving 98 localised PDAC patients found that the magnitude of change in CA 19-9 after neoadjuvant therapy itself, if without normalization, does not predict OS (P = 0.77). In contrast, the failure of post-neoadjuvant CA 19-9 to normalize predicted significantly lower OS (P = 0.02)[26]. Hence, different CA 19-9 parameters can act as surrogate markers of treatment response to better inform subsequent management.
CA 19-9 is evaluated for the utility to guide adjuvant therapy. In a study involving 260 patients, Humphris et al[27] found that patients with postoperative CA 19-9 ≥ 90 U/mL, those who underwent adjuvant chemotherapy, did not have significantly higher median survival than patients who did not (16.2 vs 9 mo respectively, P = 0.719). This contrasts with patients with postoperative CA 19-9 < 90 U/mL, were those who received adjuvant chemotherapy benefited significantly compared to those without (median survival 26.0 mo vs 16.7 mo, P = 0.0108)[27]. This highlights the potential of postoperative CA 19-9 in making decisions to start adjuvant chemotherapy.
Both pre- and post-treatment CA 19-9 values are valuable prognostic markers in PDAC patients undergoing curative resection or upfront chemotherapy.
For patients with resectable disease, elevated preoperative CA 19-9 levels predict poorer survival post-resection. A retrospective study by Berger et al[28] on 129 patients who underwent surgical resection showed that patients with preoperative CA 19-9 levels < 37 U/mL had the best median survival (35 mo), followed by those with CA 19-9 levels between 38-200 U/mL (22 mo), and lowest survival (16 mo) in patients with CA 19-9 levels > 200 U/mL[28]. Other studies combined preoperative CA 19-9 with carcinoembryonic antigen (CEA) values for prognostication. Distler et al[29] offered from his study of 264 patients that elevated CA 19-9 > 75 U/mL, and CEA > 3 ng/mL were associated with lower survival[29].
It is reported that the normalization or a downtrending of CA 19-9 levels predicts better survival, as it suggests a lower probability of residual local disease or occult metastasis. Despite various authors using different postoperative cut-off values for elevated CA 19-9 (> 37-180 U/mL), all studies have consistently reported significantly lower median survival when CA 19-9 is elevated[30].
Both pre- and post-chemotherapy CA 19-9 levels are useful markers for prognostication regardless if given as neoadjuvant, adjuvant, and upfront therapy. Hence, they are used as surrogate markers to measure the response to neoadjuvant chemotherapy and guide adjuvant chemotherapy, as mentioned above[25-27]. Even in patients with advanced PDAC who were offered upfront chemotherapy, a study of 247 patients by Reni et al[31] shows lower pre-chemotherapy CA 19-9 levels predicted better median survival (15.5 mo vs 11.9 mo vs 8 mo for CA 19-9 levels < 37, 38-1167, > 1167 U/mL respectively)[31]. However, a wide range of parameters has been adopted in existing literature with no current consensus on the best parameter, such as whether CA 19-9 decreases by > 50% within two months of treatment, log (CA 19-9) kinetics, and CA 19-9 velocity defined as the change in CA 19-9 values over four weeks[30].
The failure to normalize or the elevation of postoperative CA 19-9 levels has been recognized to predict the recurrence of PDAC.
More importantly, a key area for research is using CA 19-9 alone to predict recurrences post-resection. CA 19-9 elevations have been shown to precede clinical or radiological recurrence by 2-6 mo[7]. Given the difficulty in interpreting imaging post-resection due to post-surgical changes or artifacts, CA 19-9 potentially surpasses current imaging techniques to detect PDAC recurrence and expedite salvage therapy initiation. However, even with the monitoring of CA 19-9 during postoperative surveillance, current guidelines require radiologic evidence for confirmation of recurrence and initiation of therapy. This prohibits the use of CA 19-9 in detecting truly occult disease. A study involving 80 patients, Li et al[32] demonstrated improved disease-free survival (DFS) and OS in 26 patients with elevated postoperative CA 19-9 who received chemotherapy before radiologic evidence of recurrence[32]. In a study involving 93 patients, Azizian et al[15] advocate initiating treatment for assumed recurrence based solely on a 2.45 times increase in CA 19-9 on the merits of its 90% PPV[15]. Given the significant implications on survival rates, there is a pressing need for further studies to confirm the benefit of initiating palliative therapy based solely on CA 19-9 elevation without the need for conventional radiologic correlation.
Current literature has established the roles of CA 19-9 in diagnosis, management, and prognostication of PDAC. A caveat is that there is no validation of standardized cut-off values, except for CA 19-9 levels > 37-40 U/mL used in diagnosis[13]. This has prevented research findings from being translated into clear clinical guidelines, especially in criteria used to predict resectability, the need for staging laparoscopy, management sequence in resectable patients, and recurrence surveillance post-resection[19,30]. Further randomized controlled studies are needed to develop threshold values to guide clinical practice.
CA 19-9 is evaluated for its role in pancreatic cystic lesions and pancreatitis, but has limited utility. This section discusses the current uses of serum and pancreatic cyst fluid CA 19-9 in these benign pancreatic diseases.
Pancreatic cystic lesions are classified into pseudocysts, simple retention cysts and pancreatic cystic neoplasms (PCNs). PCNs comprise a spectrum of conditions with low malignant potential (such as serous cystic adenomas) or premalignant (commonly mucinous lesions such as mucinous cystic neoplasms and IPMNs).
Both serum CA 19-9 and cyst fluid CA 19-9 obtained from endoscopic ultrasonography-guided fine-needle aspiration have been evaluated for its ability to differentiate between various pancreatic cystic lesions.
Regarding serum CA 19-9, a meta-analysis by Cao et al[33] involving 1437 patients in 13 studies found that serum CA 19-9 alone is ineffective in distinguishing malignant PCNs (47% sensitivity and 88 % specificity), but useful when complementary to other diagnostic techniques, such as imaging or cyst size > 3 cm[33]. Serum CA 19-9 levels > 37 U/mL are validated as a relative indication for IPMN resection per the 2018 European evidence-based guidelines on pancreatic cystic neoplasms[34].
Cyst fluid CA 19-9 is less accurate than other cyst fluid tumour markers such as CEA and CA 125 in differentiating between different pancreatic cystic lesions. Nevertheless, cyst fluid CA 19-9 may have supplementary roles in IPMNs and pseudocysts.
A multicenter prospective study by Brugge et al[35] involving 341 patients demonstrated that cyst fluid CA 19-9 (cut-off of 2900 ng/mL) alone has limited ability in distinguishing between mucinous and non-mucinous cystic lesions [Area under the curve (AUC) = 0.665], as it is less accurate than cyst fluid CEA (cut-off of 192 ng/mL) alone (AUC = 0.793)[35]. Similarly, van der Waaij et al[36] demonstrated amongst 450 patients across 12 studies that while cyst fluid CA 19-9 is less accurate than CEA, CA 19-9 < 37 U/mL distinguishes pseudocysts and serous cystic adenomas from other pancreatic cystic lesions with specificity (98.0%) and sensitivity (19.0%)[36]. Furthermore, Nagashio et al[37] demonstrated amongst 68 patients that cyst fluid CA 19-9 could differentiate mucinous cystic neoplasms from IPMNs (AUC = 0.792), though less accurately than CA 125 (AUC = 0.960)[37].
In IPMNs, cyst fluid CA 19-9 can be used after initial analysis with CEA to further differentiate mucinous cyst subtypes. In a study by Snozek et al[38] involving 387 patients, for cysts presumed to be mucinous as cyst fluid CEA > 30 ng/mL, cyst fluid CA 19-9 < 8000 U/mL distinguished 71% of IPMNs from other mucinous cyst subtypes[38]. However, cyst fluid CA 19-9 is less effective in differentiating between benign and malignant IPMNs. A study by Maire et al[39] involving 41 patients revealed that cyst fluid CA 19-9 > 1000 U/mL (PPV = 0.360, NPV = 0.920) is less effective compared to CEA > 200 ng/mL (PPV = 0.500, NPV = 0.960) and CA 72-4 > 40 U/mL (PPV = 0.470, NPV = 0.960)[39]. Thus, the role of cyst fluid CA 19-9 remains supplementary to other tumour markers in IPMNs.
Regarding pseudocysts, low cyst fluid CA 19-9 is used together with raised amylase, low CEA, and mucin levels to distinguish pseudocysts from the pancreas' cystic lesions in ambiguous cases[3]. While clinical, biochemical, and radiological features suggesting pancreatitis with pseudocyst formation can accurately diagnose most pancreatic pseudocysts, certain PCNs can mimic these presentations well. Given that pseudocysts can be managed conservatively while PCNs have malignant potential, this distinction using cyst fluid analysis is useful[40].
Autoimmune pancreatitis is a subset of chronic pancreatitis that is often misdiagnosed as a PDAC. Serum CA 19-9 alone has limited diagnostic value in distinguishing autoimmune pancreatitis, but combinations with different biochemical markers (such as IgG4, hemoglobin, eosinophil, and globulin) yield good diagnostic value[41].
Besides its use in pancreatic diseases, CA 19-9 is currently selectively used in evaluating certain gastrointestinal cancers. This section discusses the uses and limitations of serum CA 19-9 in the diagnosis, prognostication, and management in cholangiocarcinoma (CCA), oesophageal, gastric, and colorectal cancers (CRC).
CA 19-9 is an adjunct tumor marker used in the diagnosis of CCA. CA 19-9 > 100 U/mL on a malignant biliary stricture background without bacterial cholangitis suggests perihilar CCA[42]. Its diagnostic value has also been evaluated in patients with Primary Sclerosing Cholangitis, which is a significant risk factor for CCA. In patients with Primary Sclerosing Cholangitis, a cut-off value of 129 U/mL had a sensitivity (78.6%), specificity (98.5%), PPV (56.6%) and NPV (99.4%) in predicting CCA[43]. Another study reported a cut-off value of 100 U/L had a sensitivity (53.0%) and NPV (92%) in predicting CCA[44]. Separately, CA 19-9 is used to distinguish intrahepatic CCA from hepatocellular carcinoma (HCC) with moderate accuracy (63%-67%)[45].
CA 19-9 has limited use in oesophagogastric cancer. Some studies have reported its accuracy in predicting unresectability and prognosis, with greater accuracy in the adenocarcinoma histotype. A systematic review by Acharya et al[46], involving 20223 patients with oesophagogastric cancers, showed that CA 19-9 had low accuracy in diagnosis (AUC = 0.663), prediction of recurrence (AUC = 0.478), and metastasis (AUC = 0.606)[46]. Elevated CA 19-9 was shown to be an accurate predictor of unresectability but a poor discriminator between R0 and R1/R2 resections[47]. The implication is that while unresectable patients would be refused surgical management upfront, CA 19-9 still cannot help detect advanced occult cancer seen in R1/2 resections preoperatively, subjecting these patients to inappropriate oesophagectomies. This same study also found that CA 19-9 is a better predictor in adenocarcinoma than squamous cell histotype. Tokunaga et al[48] demonstrated in their study of 211 patients that elevated preoperative CA 19-9 can predict poorer prognosis in patients with oesophagogastric junction adenocarcinoma, which may identify patients less likely to benefit from aggressive management[48].
CA 19-9 also predicts clinicopathological status, recurrence, and prognosis of gastric cancer. Raised CA 19-9 levels also correlated with higher tumor stage, lymph node and distant metastasis, and vessel invasion[49]. CA 19-9 levels alone are not recommended for staging gastric cancer but should be considered along with CEA and CA 72-4[50]. This same study showed that elevated preoperative CA 19-9 predicted hematogenous recurrence and should be considered in the decision for initiating adjuvant chemotherapy, whereas postoperative CA 19-9 elevations could predict peritoneal recurrence. A multi-center study by Suenaga et al[51] involving 998 stage II/III gastric cancer patients demonstrated that elevated preoperative and postoperative CA 19-9 (> 37 U/mL) and CEA (> 5 ng/mL) levels promisingly predict poorer DFS and OS, with postoperative levels being more accurate[51].
CEA remains the primary tumor marker implicated in CRC. However, more studies are evaluating CA 19-9 as an adjunct to CEA in CRC. An adjunct tumor marker is essential as 15%-40% of CRC patients have perpetually non-elevated CEA levels[52]. Specifically, CA 19-9 may be useful in staging, prognostication of CRC, and management of metastatic CRC (mCRC).
CA 19-9 is not useful in the diagnosis of CRC. A systematic review by Acharya et al[46] involving 156 studies demonstrated its low sensitivity (0.471) despite adequate specificity (0.924)[46]. Comparatively, CA 19-9 fares poorer than CEA (0.533 and 0.864 for sensitivity and specificity, respectively)[46], in which CEA has already been established to be ineffective for diagnosing CRC[53].
However, CA 19-9 may have a role in staging as higher Dukes classification correlated with raised CA 19-9[53]. In a 300 patients study, Stojkovic Lalosevic et al[54] reported that CA19-9 levels could also predict regional lymph node involvement and liver metastases, with proposed threshold values of 7.5 U/mL and 5.5 U/mL, respectively[54].
In a meta-analysis involving 6434 patients, Yu et al[55] reported that elevated pre-treatment CA19-9 predicted poor OS, DFS, and RFS[55]. A study by Stiksma et al[53] involving 150 patients further endorses CA 19-9 as an adjunct marker to CEA in prognostication. 7.3% of patients had elevated CA 19-9 levels without raised CEA levels. These patients had a significantly lower 5-year survival at 28.8%, compared to a higher 5-year survival of 41.1% in patients with raised CEA levels only[53]. Thus, given the limited utility of trending CEA in patients with limited CEA secretion[52] and the significantly poorer prognosis of raised CA 19-9 in isolation, it is crucial to consider CA 19-9 trending in the follow-up of CRC patients.
CA 19-9 has shown the potential to guide management, particularly in mCRC patients. Bevacizumab is a monoclonal antibody against vascular endothelial growth factor that has improved patient outcomes in mCRC when added to chemotherapy regimens[56]. In a study by Narita et al[57] involving 150 mCRC patients, only patients with elevated CA 19-9 benefited from Bevacizumab therapy. Among patients with raised CA 19-9 levels, patients receiving Bevacizumab have a significantly longer median OS than those without (27.8 vs 15.3 mo, P = 0.00190). This contrasts with patients with normal CA 19-9 levels, where those receiving Bevacizumab did not have significantly longer median OS than those without (36.5 mo vs 38.0 mo, P = 0.952)[57]. Given Bevacizumab's limited accessibility, CA 19-9 can guide more judicious use of Bevacizumab in mCRC.
Furthermore, CA 19-9 could select mCRC patients with likely poorer survival outcomes for more intensive management. Additionally, elevated CA 19-9 levels identified a particularly aggressive BRAF-mutant subtype of mCRC patients[58]. BRAF mutations in mCRC are associated with a poorer prognosis, but even within this group of patients, survival outcomes are heterogeneous. Thus, the ability to further stratify these patients based on disease's aggressiveness can better select patients for more intensive chemotherapy regimens.
Benign hepatic and biliary diseases are known causes of elevated CA 19-9 levels. This section discusses the current uses of serum CA 19-9 in these benign conditions.
Incidents of elevated CA 19-9 are reported in a range of benign hepatic diseases, such as liver cysts and hepatitis secondary to alcohol abuse, drugs, autoimmune diseases, and acute and chronic viral infections[2].
Pearce et al[59] reported a case of possible drug-induced CA 19-9 elevation in a 50-year-old man with alcohol-related chronic pancreatitis and recent dothiepin use. PDAC was initially suspected as he presented with anorexia, pale stools, transaminitis and elevated serum CA 19-9 at 2690 U/mL. However, CA 19-9 decreased on pancreatic enzyme replacement and dothiepin cessation[59]. While the cause for the elevated CA 19-9 is likely multifactorial (including alcohol-induced pancreatitis and hepatitis) and since dothiepin can cause cholestasis[60], the possibility of dothiepin-induced cholestasis causing CA 19-9 elevations cannot be excluded.
In these reports, CA 19-9 levels were measured in patients with clinical or biochemical indices of liver dysfunction, purely to exclude cancers[2]. Despite the association with elevated CA 19-9, the current clinical use of CA 19-9 in the further evaluation of benign hepatic conditions remains limited. Nevertheless, these case reports reinforce the need for cautious interpretation of elevated CA 19-9 in patients with hepatic disease.
The primary use of CA 19-9 in benign biliary diseases is to rule out underlying cancer. Benign biliary diseases that cause benign cholestasis and obstructive jaundice are known causes of elevated serum CA 19-9, but these elevations are generally mild, except for cholangitis[61]. Current literature on CA 19-9 in benign biliary diseases is mostly limited to case reports on patients with extraordinarily high CA 19-9 levels without cancer evidence. These are seen more commonly in patients with choledocholithiasis and acute cholangitis and Mirizzi Syndrome, biliary adenofibroma, and cholecystitis[62]. Amongst patients with cholangitis, CA 19-9 normalization after therapeutic intervention occurred within two months[63]. In contrast, most patients without cholangitis had normalized CA 19-9 levels within three weeks[62]. Several authors have recognized that such marked elevations are rare, and CA 19-9 levels differentiate between benign and malignant biliary conditions poorly in patients with obstructive jaundice.
Despite its first use being described close to 40 years ago, there remain many questions regarding the translation of CA 19-9 into clinical use. This section discusses the future applications of CA 19-9 in acute pancreatitis, hepatic, biliary tract, urological, pulmonary, gynecological, thyroid, and salivary gland diseases. In addition to serum CA 19-9, future applications explore the use of CA 19-9-targeted antibodies and CA 19-9 from other sources like urine, saliva, ascites, BAL and pleural fluid, and immunohistochemistry staining.
This section discusses (1) serum CA 19-9 kinetics and CA 19-9 velocity in PDAC, (2) the role of serum CA 19-9 in association with other adjunctive tumor markers in PDAC, and (3) the use of CA 19-9-targeted antibodies as therapeutic targets in PDAC and acute pancreatitis.
Future studies can first evaluate dynamic CA 19-9 parameters in different preoperative and postoperative uses in PDAC. Current studies have predominantly focused on single static CA 19-9 values, despite some studies highlighting the value of postoperative CA 19-9 kinetics in prognostication of PDAC[30] and the current clinical use of preoperative tumor marker kinetics in other cancers besides PDAC (e.g., PSA in prostate cancer)[64]. The use of the rate of change in CA 19-9 levels instead of an absolute value seems logical as a surrogate of aggressiveness of PDAC, given that cancer itself is a growth process. Additionally, it can be particularly useful when baseline CA 19-9 levels are unavailable. It also gives a better understanding of each individual’s unique disease process over time and could account for a wide range of optimal CA 19-9 threshold values quoted in literature.
In the preoperative setting, the value of dynamic preoperative CA 19-9 kinetics is less well-understood. In a retrospective review involving 72 patients, Brown et al[19] investigated the use of dynamic CA 19-9 kinetics in predicting resectability. They identified that the rate of change ≥ 1 U/mL/day predicted unresectability better than the absolute CA 19-9 (≥ 50 U/mL) value[19]. This study included a small sample (n = 72), and the results need to be validated.
In the postoperative setting, there is potential in other CA 19-9 parameters beyond simple baseline values. This can be done by examining dynamic CA 19-9 postoperative velocity in predicting recurrence, CA 19-9 patterns beyond the 6-mo postoperative period for prognostication, and multiple parameters in measuring chemotherapy response.
Most studies have proven the accuracy of predicting PDAC recurrence post-resection using single CA 19-9 values. An exception is a randomized control trial involving 96 patients by Hernandez et al[65] that showed that dynamic CA 19-9 postoperative velocity of > 95 U/mL/4 wk (rate of change in CA 19-9 levels over four weeks) predicted recurrence and OS better than baseline CA 19-9 levels[65]. This use of CA 19-9 velocity in recurrence prediction requires further validation in larger sample sizes. However, determining CA 19-9 velocity will demand more frequent CA 19-9 testing at the expense of cost and inconvenience to patients, which therein requires further studies to determine the optimum frequency of CA 19-9 testing.
For prognostication, few studies have trended CA 19-9 past six months postoperatively to understand its prognostic significance. One study by Rieser et al[66] involving 525 patients specifically studied the patterns of postoperative CA 19-9 levels of those who underwent pancreatectomy over a mean follow-up period of 35.9 mo. In concordance with current literature, postoperative CA 19-9 values that were persistently normal and elevated were associated with the longest and shortest OS. Interestingly, patients with postoperative CA 19-9 that fluctuated between normal and elevated during the surveillance period did not have statistically significant OS differences than patients with perpetually normal CA 19-9 levels[66]. This study highlights the need to better characterize the various dynamic patterns of CA 19-9 behavior beyond the 6-mo postoperative period for its impact on postoperative prognostication, instead of relying on data from a single time point. The use of patterns instead of stringent cut-off values would allow the data to be personalized and account for variability amongst individuals, especially given the numerous confounders affecting CA 19-9 values.
For measuring response to chemotherapy, current studies have explored multiple parameters, ranging from the log (CA 19-9 kinetics) to CA 19-9 velocity[30]. Future studies can compare the accuracy and feasibility of parameters used to forge a consensus on the most optimal parameters for clinical practice.
Secondly, given the limitations of CA 19-9 in PDAC, a growing number of biomarkers based on serum, tissue, and the pancreatic juice are researched in recent years. These range across serum proteins (CEA, Mucins, DU-PAN-2, MIC-1, REG-4), serum ratios (Neutrophil-to-Lymphocyte Ratio, Platelet-Lymphocyte Ratio), genetic mutations in key genes (K-ras, SMAD, BRCA2), miRNA, and estrogen receptors[67,68]. However, none have had adequate sensitivity and specificity or large-scale validation to be used as standalone markers. CA 19-9 remains the current clinical gold standard tumor marker in PDAC, although a growing body of research has shown increased accuracy when combined with other tumor markers[1]. Future research can focus on determining optimal combinations of tumor markers for use in PDAC.
Thirdly, antibodies targeting CA 19-9 can be useful immunotherapy in PDAC and acute pancreatitis.
In PDAC, current animal and human immunotherapy studies are in the preliminary stages of investigating its ability to reduce recurrences and metastatic progression. In mouse models, CA 19-9-targeted human antibodies reduced the progression of metastatic PDAC[69]. Antibodies are used to show potential for translation into clinical use as they have been well-tolerated in phase I clinical trials. Furthermore, in human studies, a cytotoxic human IgG1 antibody targeting CA 19-9 (MVT-5873) decreased serum CA 19-9, prevented tumor progression, and was well-tolerated by metastatic PDAC patients. MVT-5873 is currently entering a prospective phase II trial in patients undergoing resections for PDAC, CCA, or mCRC to the liver. This is to investigate its ability to eliminate micrometastases, particularly in the immediate perioperative period, to prolong RFS and OS[70]. A well-tolerated targeted treatment in the immediate perioperative period is significant as resections are postulated to trigger micrometastases' growth, and standard adjunct chemotherapy can only begin around 6-12 wk after surgery due to interferences with recovery[70]. Thus, if proven successful in human trials, antibodies targeting CA 19-9 may be promising adjuncts to standard neoadjuvant and adjuvant therapies.
Acute pancreatitis is a common cause of abdominal pain with varied clinical courses, and despite improvements in outcomes driven by advances in critical care, there remains an unmet need to develop therapeutic targets[71]. Furthermore, patients with recurrent pancreatitis or hereditary pancreatitis are at increased risk of developing PDAC. The current management of acute pancreatitis is mostly supportive. Currently, little can be done to expedite recovery, prevent a recurrence, or prevent episodes in routine procedures that carry the risk of pancreatitis. CA 19-9-targeted antibodies have potential in the treatment of acute pancreatitis. A mouse model study by Engle et al[72] identified two forms of CA 19-9-targeted antibody 5B1 that lowered serum amylase levels and restored pancreatic histology to mitigate pancreatitis[72]. While studies are needed to validate these findings in humans, this study is particularly promising as fully human CA 19-9-targeted antibodies have been approved through phase 1A clinical trials. Furthermore, the role of CA19-9 in the diagnosis of drug-induced acute pancreatitis is not reported but could be explored[73].
This section discusses the potential uses of serum CA 19-9 in evaluating HCC, CCA, and gallbladder cancer (GBC).
Hepatocellular carcinoma: Alpha-fetoprotein (AFP) is the predominant HCC tumor marker, but about 30%-40% of HCC patients are reported to be AFP-negative (AFP < 25 ng/mL)[74]. With other molecular markers currently being in the cell study or experimental model phase[75], there is a need to evaluate a tumor marker already in clinical use in these AFP-negative patients. Raised CA 19-9 levels have clinical utility in AFP-negative HCC patients. In a study by Lu et al[76] involving 750 AFP-negative HCC patients who underwent surgical resection, a preoperative CA 19-9 value > 32.6 U/mL (AUC = 0.640) predicted poor prognosis, with a 5-year OS lower in patients with elevated CA 19-9 (OS = 57.0%) than those with normal CA 19-9 (OS = 79.9%)[76]. More data is needed to evaluate the role of elevated CA 19-9 levels in AFP-negative patients.
Cholangiocarcinoma: Elevated preoperative serum CA 19-9 levels may be a useful predictor of poor prognosis in CCA. A meta-analysis by Liu et al[77] involving 953 patients in nine studies showed that elevated CA 19-9 levels were associated with 1.28 times higher mortality rate[77]. These results, however, cannot be applied to hilar CCA as it was not studied in the nine papers, and that subgroup analysis to stratify prognosis based on the location of the tumor (intra- and extra-hepatic) could not be performed due to the small sample size. These limitations are significant to note as biological behaviors, and thus the prognostic value of CA 19-9 may differ depending on the location of CCA.
CA 19-9 could be further evaluated in its ability to predict resectability across the different CCA subtypes. At present, 10%-45% of patients deemed to be resectable preoperatively are found to be unresectable during exploratory laparotomy[42]. Thus far, preoperative CA 19-9 levels have been evaluated in hilar CCA. In a study by Hu et al[78] involving 471 patients, it was reported that the threshold value of 204 U/mL predicted resectability with sensitivity (83.7%), specificity (80.0%), PPV (91.1%), and NPV (66.7%)[78]. Accurate preoperative markers complementing imaging can thus significantly reduce unnecessary surgery and delays in starting palliative treatment.
Furthermore, the utility of CA 19-9 in combined hepatocellular-cholangiocarcinoma (CHC) remains unclear. CHC is a mixed primary liver cancer between HCC and CCA, with different management and prognosis[79]. Accurate non-invasive preoperative diagnosis of CHC from HCC or CCA is crucial yet difficult due to overlaps in clinical and imaging features. Studies proposed that elevated CA 19-9, AFP, and discordant imaging features could suggest CHC[80]. Thus in a study by Huang et al[79] involving 40 CHC, CCA, and HCC patients, a diagnostic criterion was developed to distinguish CHC from HCC and CCA. It involved AFP (20 ng/mL threshold) and CA 19-9 (100 U/mL threshold), and contrast-enhanced ultrasonography findings. It however had poor sensitivity (32.5%) despite specificity (93.8%), PPV (72.2%) and NPV (73.5%)[79]. Thus, more studies using different imaging modalities are needed to ascertain its discriminatory value.
Gallbladder cancer: The current use of CA 19-9 in GBC is limited. Guidelines like the National Comprehensive Cancer Network do not currently recommend using CA 19-9 alone for confirming the diagnosis or prognosis of GBC[81]. However, CA 19-9 has shown potential as an adjunct in the diagnosis, prediction of resectability, and GBC[82].
CA 19-9 alone detects cancers poorly, for instance, in gallbladder polyps[83]. However, different combinations of CA 19-9 with tumor markers or biochemical markers have shown better diagnostic accuracies. A combination of tumor markers CA 19-9, CA 242, and CA 125 yielded a sensitivity (69.2%), specificity (100%), and PPV (100%)[82]. The use of CA 19-9 with Neutrophil-to-Lymphocyte Ratio displayed sensitivity (74.8%) and specificity (89.7%) in differentiating GBC from benign lesions like gallbladder adenomas and gallbladder polyps[84]. However, a caveat to CA 19-9 in GBC diagnostics lies in xanthogranulomatous cholecystitis (XGC). XGC is a rare benign inflammatory gallbladder disease often misdiagnosed as GBC based on preoperative radiological imaging. Different studies have reported similar elevations in CA 19-9 in XGC and GBC[85,86]. They concluded that CA 19-9 is not useful in differentiating XGC from GBC and may even push more strongly towards GBC's misdiagnosis. Thus, despite improved diagnostic accuracy when CA 19-9 is combined with other markers, the concern regarding XGC remains.
It has not been conclusively proven that CA 19-9 can predict the resectability of GBC. A study by Liu et al[87] involving 292 patients showed that preoperative CA 19-9 levels of ≥ 98.9 U/mL independently predicted unresectability with sensitivity (76.3%), specificity (70.8%), PPV (85.7%), and NPV (56.5%)[87]. This cut-off value, however, has yet to be validated prospectively in multi-center studies. It is further unclear how conflicting conclusions from preoperative imaging and CA 19-9 values can be reconciled.
The prognostic utility of CA 19-9 has also been evaluated in patients with resectable and unresectable GBC. Prognostication is challenging as imaging can miss metastases in 20%-40% of cases[81], and there lacks effective prognostication methods preoperatively[81]. For resectable GBC, different combinations of preoperative CA 19-9 with other markers such as CEA[88] or plasma fibrinogen[89] predicted survival preoperatively. For unresectable GBC patients on palliative chemotherapy, greater CA 19-9 kinetics predicted lower progression-free survival and OS[90]. Further studies are needed to prove the prognostic value of CA 19-9 in GBC.
This section discusses the potential uses of serum and ascites fluid CA 19-9 in evaluating benign hepatic and biliary diseases.
Hepatic diseases: Serum and ascites fluid CA 19-9 are potentially useful markers in hepatic fibrosis and ascites, respectively.
Recently, serum CA 19-9 is an indirect marker of the severity of hepatic fibrosis and viral hepatitis. While liver biopsies are traditionally the gold standard for diagnosing hepatic fibrosis, this is not routinely done due to its invasive nature and the development of less invasive serum markers and imaging modalities[91]. A study by Bertino et al[92] involving 60 patients demonstrated that those infected with hepatitis C and had an elevated CA 19-9 (mean 76.8 U/mL), 0%, 43.3%, and 56.7% of patients had METAVIR score F0, F1-3, F4 (indicating cirrhosis) respectively. Cirrhotic patients also had a significantly higher (P < 0.05) CA 19-9 Level than patients with chronic hepatitis[92]. This study thus shows a correlation between CA 19-9 and severity of fibrosis, but falls short of determining an optimal threshold for elevated CA 19-9 and determining its sensitivity and specificity in predicting the severity of hepatic fibrosis. Future studies can compare these sensitivities and specificities against other established serum markers such as liver transaminases or platelet counts[91]. Given that current serum markers and the gold standard liver biopsy have been useful only in excluding advanced stages of the disease[91], serum CA 19-9 can also be evaluated for its ability to distinguish between the early and intermediate phases of fibrosis. Studies have also attempted to evaluate the utility of CA 19-9 in ascites. Ascites fluid CA 19-9, combined with other ascitic tumor markers CEA and CA 153, is being proposed as an adjunctive method in differentiating malignant from benign causes of ascites, specifically in patients with suspected cancers but inconclusive cytology findings[93]. Currently, malignant ascites is diagnosed via ascites fluid cytology or laparoscopic peritoneal biopsies. However, positive cytology results are only seen in 40%-60% of patients with known malignant ascites, and biopsies are highly invasive and not widely accessible[93].
Currently, ascites fluid tumor analysis is not used in clinical practice as it had only been evaluated retrospectively on small sample populations, of which conflicting low sensitivities and specificities of the markers were reported[94]. This poor performance may be attributable to heterogeneity of the types of cancers evaluated. Not all cancers secrete the same tumor markers, and improvements in specificity and sensitivity were reported in subgroup analysis where tumor markers were correlated with specific cancers pleural fluid analysis[95]. A study by Liu et al[93] involving 437 patients demonstrated that if a threshold value of 200 U/mL was used, ascitic CA 19-9 has better diagnostic efficacy than serum CA 19-9, but were not substitutive of cytology in terms of sensitivity (58.5% vs 39.1% vs 56.8% for ascitic CA 19-9, serum CA 19-9 and cytology respectively), specificity (99.1% vs 96.8% vs 100%), PPV (98.4% vs 92.1% vs 100%) and NPV (71.8% vs 62.9% vs 51.3%). Amongst cytologically negative patients with malignant ascites, the diagnostic efficacy of ascitic CA 19-9 was good enough alone but even better when combined with CEA and CA 153, in terms of sensitivity (62.2% vs 85.9%), specificity (99.1% vs 97.3%), PPV (96.6% vs 92.9%) and NPV (86.6% vs 94.4%)[93]. Taken together, there is a potential for ascitic CA 19-9 for diagnostic use in cytologically negative patients.
Benign biliary diseases: Minor uses of CA 19-9 have been suggested in diseases like choledocholithiasis. A study by Gu et al[96] involving 135 patients proposed using serum CA 19-9 levels to preempt the risk of developing acute cholangitis secondary to choledocholithiasis early before disease progression[96]. This is significant as there are currently few reliable diagnostic indices for acute cholangitis in the early stages[96].
Currently, CA 19-9 is not used clinically in non-gastrointestinal or non-pancreatic diseases. Thus, this section will discuss potential applications of CA 19-9, particularly in urological, pulmonary, gynecological, thyroid, and salivary gland diseases.
Urological diseases: Serum and urinary CA 19-9 have been evaluated in various urological diseases, namely bladder cancer, ureteropelvic junction obstruction (UPJO), and autosomal dominant polycystic kidney disease.
Malignant urological diseases: In bladder cancer, serum and urinary CA 19-9 were studied for diagnostic, staging, and prognostic roles. The diagnosis of bladder cancer currently relies on radiologic imaging, cystoscopy, and urine cytology. Early detection of bladder cancer is challenging since cystoscopy is invasive and less accessible, and urine cytology has a significant false-negative rate, especially in low-grade carcinoma[6]. Serum CA 19-9 has poor diagnostic value in bladder cancer[97,98]; however, urinary CA 19-9 may be useful as a diagnostic adjunct to urine cytology. In a study by Roy et al[6] involving 55 patients where a urinary cut-off of 114.5 IU/L was used, CA 19-9 had better sensitivity than urine cytology, especially in low-grade transitional cell carcinoma patients[6]. Another study by Pal et al[99] involving 47 patients found elevated urinary CA 19-9 values despite negative urine cytology in 6 and 28 histopathologically confirmed high and low-grade transitional cell carcinomas, respectively[99]. Future studies can validate the role of urinary CA 19-9 in larger sample sizes, compare its diagnostic accuracy against cystoscopy and radiologic imaging and assess how urinary CA 19-9 should be interpreted based on contradicting imaging, cystoscopy, and cytology results.
The association of serum CA 19-9 with tumor stage and grade is controversial. Some studies find a correlation with both stage and grade[97], while others with stage only[98], with differences purportedly attributable to sample size and study methodology.
Lastly, serum CA 19-9 has potential as a prognostic marker. In a study involving 144 bladder cancer patients, elevated serum CA 19-9 levels were associated with 2.54 times higher risk of death[98]. On the contrary, the literature on the prognostic value of urinary CA 19-9 is scarce.
Benign urological diseases: Similarly, CA 19-9 may have potential use in UPJO and autosomal dominant polycystic kidney disease.
In UPJO, serum and urinary CA 19-9 are evaluated for their value in diagnosis and management. There are three main challenges in UPJO – inadequate diagnostic accuracy and accessibility of diagnostic investigations, and lack of consensus on which patients require early surgical management and postoperative monitoring[100].
Most studies have focused on UPJO in children. A study by Nabavizadeh et al[101] involving 27 pediatric UPJO patients proposed urinary CA 19-9 with a 30.6 U/mL threshold as a reliable diagnostic adjunct, while serum CA 19-9 was deemed less useful[4]. For optimizing management strategies, elevated urinary CA 19-9 independently predicted the failure of conservative management[101]. For postoperative monitoring, downtrending urinary CA 19-9 was proposed as a surrogate marker for successful surgical resolution of renal damage[101]. In another study on postoperative monitoring, the urinary CA 19-9/creatinine ratio fared better than urinary CA 19-9[102].
In contrast, research on CA 19-9 in UPJO in adults is limited. One study by Miranda et al[103] involving 47 adult UPJO patients demonstrated expected elevations and reductions in urinary CA 19-9 before and after pyeloplasty. However, it fell short of correlating the changes in CA 19-9 with differential renal function[103], which is one parameter currently used to select patients for surgical management[100]. On the other hand, another study by Banerjee et al[104] involving 24 adult UPJO patients found a significant inverse correlation between preoperative urinary CA 19-9 and renal function markers like GFR and split renal function[104]. Taken together, the potential of urinary CA 19-9 is promising but requires validation in larger scale studies across both adult and pediatric populations.
Next, the value of serum CA 19-9 in diagnosing liver cyst infections in autosomal dominant polycystic kidney disease patients is controversial. Liver cysts infections are rare but life-threatening complications of autosomal dominant polycystic kidney disease. Diagnosis is challenging due to unspecific symptoms and no standardized diagnostic criteria[105]. A case report by Kanaan et al[106] on three autosomal dominant polycystic kidney disease patients reported marked elevations in serum CA 19-9 from already elevated baselines during liver cyst infections (LCI)[106]. However, a subsequent case report by Neuville et al[105] in 2019 on five autosomal dominant polycystic kidney disease patients found no significant increases in absolute values and changes in baseline serum CA 19-9 during LCI[105].
Pulmonary diseases: Studies have evaluated serum, BAL, and pleural fluid and immunohistochemistry staining for CA 19-9 in lung cancer and benign pulmonary diseases such as idiopathic pulmonary fibrosis and pulmonary nontuberculous mycobacterial disease.
Malignant pulmonary diseases: Amongst lung cancer subtypes, CA 19-9 has potential in diagnosis, staging, prognostication, and prediction for complications and recurrences of Non-Small Cell Lung Cancer (NSCLC) specifically. However, a caveat is that only a minority of NSCLC patients express elevated CA 19-9 levels[107,108].
While CA 19-9 appears to have diagnostic value, the optimal combination and source of CA 19-9 (serum or BAL fluid) remain ambiguous. A study by Wang et al[109] involving 150 patients showed that a combination of Serum CA 19-9, CEA, neuron-specific enolase, and cytokeratin 19 fragment (CYFRA 21-1) had a 90.2% PPV in diagnosing lung cancer[109]. Another study by Ghosh et al[110] involving 90 patients demonstrated that BAL fluid CA 19-9 was more accurate than serum CA 19-9 and other BAL tumor markers. Hence, a combination of BAL fluid CA 19-9, CEA, CA 125, and serum CA 15-3 was proposed[110].
The prognostic role of CA 19-9 is controversial. A study by Sato et al[108] involving 246 Stage IIIB and Stage IV lung adenocarcinoma patients demonstrated that serum CA 19-9 was an independent prognostic marker in lung adenocarcinoma[108]. In contrast, another study by Tsoukalas et al[107] involving 100 NSCLC patients, who were not stratified by stage, showed that elevated CA 19-9 levels correlated with shorter OS, but could not prove CA 19-9 to be an independent prognostic factor[107]. Sato et al[108] also reported that stronger immunohistochemistry staining for CA 19-9 correlated with shorter RFS in 116 clinical stage I lung adenocarcinoma patients[108]. However, Ma et al[111]’ s study involving 164 patients showed that CA 19-9 had limited prognostic value in Stage 1 NSCLC[111]. The varying conclusions suggest that the prognostic value of CA 19-9 may depend on the subtype and stage of lung cancer and the source of CA 19-9 sample.
Specifically, in the adenocarcinoma subtype, serum and pleural fluid CA 19-9 were evaluated to predict recurrences, metastases, and lung cancer complications like cancer-associated stroke and malignant pleural effusion. One study by Isaksson et al[112] involving 107 stage I-III resectable lung adenocarcinoma patients suggested that elevated preoperative CA 19-9 predicts disease recurrence[112]. A study by Ren et al[113] showed that serum CA 19-9 could predict metastases with 92.0% specificity[113]. Regarding complications, a study by Xie et al[114] involving 102 patients showed that every 1 U/mL increase in serum CA 19-9 increased the risk of lung cancer-associated stroke by 2.1%[114]. These findings could expedite stroke-prevention therapy in lung cancer patients, given these patients are at increased risk of stroke within six months of diagnosis compared to the average population[114]. Furthermore, Feng et al[115]’ s meta-analysis involving 177 patients demonstrated that a combination of pleural fluid CA 19-9, CEA, and CYFRA 21-1 could accurately distinguish lung adenocarcinoma-associated malignant pleural effusion from benign effusion[115]. Detection of malignant pleural effusion has prognostic relevance as patients would be upstaged to stage IV disease.
Benign pulmonary diseases: CA 19-9 is also being studied in benign pulmonary diseases, like idiopathic pulmonary fibrosis and pulmonary nontuberculous mycobacterial disease.
CA 19-9 could assess disease progression in idiopathic pulmonary fibrosis, as it is a surrogate for epithelial damage. In a study by Maher et al[116] involving 106 patients, a significantly higher CA 19-9 value (53.7 U/mL vs 22.2 U/mL, P < 0.0001) was reported in patients with progressive disease compared to those with stable disease[116].
CA 19-9 could monitor therapeutic responses in pulmonary nontuberculous mycobacterial disease. In a study by Hong et al[117] involving 24 patients, CA 19-9 levels decreased significantly amongst all 17 patients who responded to treatment clinically but remained constant in 7 patients without treatment response[117]. Effective biomarkers are needed due to the increasing burden of pulmonary nontuberculous mycobacterial disease and the current difficulty in measuring treatment response using microbiological, radiologic, and quality-of-life parameters[117].
Gynecological diseases: Serum CA 19-9 has been studied in uterine and ovarian diseases. This section discusses its potential roles in endometrial cancer, endometriosis, benign, and malignant ovarian tumors.
Malignant and benign uterine diseases: Serum CA 19-9 may be useful as an adjunct in diagnosis and prognostication of endometrial cancer. No serum marker has been internationally validated in endometrial cancer. In a study by Bian et al[118] involving 105 patients, preoperative serum CA 19-9 alone had limited diagnostic accuracy. However, a combination of CA 19-9, HE4, CA 125, and CA 72-4 showed good sensitivity (59.1%), PPV (0.88), and NPV (0.90) in the diagnosis of endometrial cancer. Elevated CA 19-9 correlated with higher International Federation of Gynecology and Obstetrics stage and lymph node involvement, both of which are important factors in endometrial cancer prognosis[118].
In contrast, serum CA 19-9 has no role in the diagnosis of endometriosis. In a meta-analysis by Nisenblat et al[119] involving 309 patients across three studies, an inadequate sensitivity (0.36) and specificity (0.87) was reported[119]. Thus, serum CA 19-9 proved neither helpful in supplementing nor replacing diagnostic laparoscopy.
Malignant and benign ovarian diseases: CA 19-9 has potential as an adjunct in diagnosing and predicting complications in Ovarian Mature Cystic Teratomas (OMCT). OMCTs are common benign ovarian tumors that are often asymptomatic except when exceptionally enlarged, which results in torsion, rupture, or compression of surrounding structures. It is often detected incidentally on ultrasonography (US), but the diagnosis is histological. Particularly in premenopausal women where preserving fertility is crucial, the higher preoperative diagnostic accuracy of adnexal masses is needed. Preoperative CA 19-9 has potential diagnostic utility, if supplementary to imaging[120] and other tumor markers. In a study by Cho et al[121] involving 322 patients, OMCTs were associated with isolated elevation of CA 19-9, whereas cancers were associated more with a simultaneous elevation of CA 19-9 and CA 125[121]. Elevated CA 19-9 can also predict complications and guide management. A meta-analysis by Prodromidou et al[120] involving 995 patients in nine studies demonstrated a correlation between elevated CA 19-9 levels and significantly larger mean size of OMCT and ovarian torsion[120]. These studies, however, did not screen for concomitant conditions causing raised CA 19-9.
CA 19-9 is not useful in diagnosing ovarian cancer. CA 19-9 levels differentiate benign and malignant adnexal masses poorly, despite combination with other tumor markers such as AFP, CEA, CA 15-36, and CA 125. This is due to its poor sensitivity (18.4%), specificity (93.0%), PPV (43.7%) and NPV (79.6%)[122]. However, CA 19-9 can potentially differentiate between subtypes of ovarian cancer, which have vastly different prognoses. Zhang et al[123] showed in a study involving 183 patients that Ovarian Serous Carcinoma had lower mean CA 19-9 levels than Ovarian Malignant Epithelial Cancer, with the former having a better prognosis[123]. In the same study, the addition of CA 19-9 levels increased the sensitivity (81.0%) and specificity (73.0%) compared to the contrast-enhanced US alone (79.0% and 65.0%, respectively) in differentiating the two subtypes. Thus, a combination of US, CA 19-9, and CA 125 had the highest sensitivity (89.0%) and specificity (95%)[123].
Thyroid diseases: Serum and immunohistochemistry staining for CA 19-9 may have prognostic value in thyroid cancers, particularly in medullary thyroid cancers. Firstly, serum CA 19-9 can be an adjunct prognostic marker to calcitonin doubling time currently used[124]. Elevated CA 19-9 levels predict more aggressive forms of medullary thyroid cancers and significantly shorter OS[125], independently from calcitonin doubling time[124]. A cut-off value of 18.3 U/mL (with 83% sensitivity, 91% specificity) for CA 19-9 elevation was calculated, which is lower than the threshold typically associated with CA 19-9 in PDAC[125]. Secondly, a recent study by Vargas et al[126] involving 70 patients suggested that a diffuse pattern of immunohistochemistry staining for CA 19-9 in medullary thyroid cancer tissue could predict metastatic potential even at initial diagnosis[126], corroborating an earlier pilot study[127]. These findings will have crucial therapeutic implications if validated as metastatic potential cannot currently be determined at disease presentation and can only be evaluated after the initial treatment of medullary thyroid cancers using calcitonin and CEA[127].
In contrast, recent studies on CA 19-9 in papillary thyroid cancers have been scarcer. These are limited to case reports that suggest using CA 19-9 for monitoring disease progression. Firstly, serum CA 19-9 may detect anaplastic transformation of thyroid cancers[128]. Secondly, serum CA 19-9 could monitor recurrence and metastases more effectively than serum thyroglobulin, especially in patients with raised anti-thyroglobulin antibodies or after transformation to undifferentiated cancers[129]. Nevertheless, these conclusions are based on individual patients and need to be studied further in larger scale studies.
Salivary gland diseases: Little has been studied regarding CA 19-9 in salivary gland diseases. A study by Dyckhoff et al[5] involving 28 patients evaluated the diagnostic value of salivary CA 19-9 in parotid cancer. A threshold of 370 kU/L predicted parotid cancer[5]. Of note, no tumors that showed elevated salivary CA 19-9 displayed classical signs of cancer, showing that salivary CA 19-9 may have a role in asymptomatic patients. Furthermore, the current best preoperative investigation (fine-needle aspiration cytology) is highly operator-dependent. Hence, it may be worthwhile to prove further the diagnostic ability of salivary CA 19-9 in larger populations.
One major limitation in the clinical utility of CA 19-9 would be false-negative results in genotypically Lewisa-b- patients whose CA 19-9 levels will be perpetually undetectable. However, a recent study by Luo et al[130] involving 1482 patients identified raised CA 19-9 levels (> 37 U/mL) in 27.4% of Lewis-negative patients, suggesting counterintuitively that not all Lewis-negative patients are CA 19-9 non-producers[130]. Nevertheless, a simultaneous Lewis and secretor genotyping and measurement of disialyl Lewis A would still be useful in guiding the use of CA 19-9 in individuals[62]. Another limitation is false-positive elevations in obstructive jaundice, due to the blockage of biliary excretion. CA 19-9 measurements should be repeated after the relief of jaundice, in which the same cut-off values can be applied[13].
Furthermore, CA 19-9 is not a cancer-specific marker due to its non-specific expression in those mentioned above benign and malignant pathologies. Clinicians must be cautious in evaluating for cancers in patients with other concomitant benign diseases. Routine CA 19-9 testing is generally not recommended. However, as slightly elevated CA 19-9 Level without evidence of cancer or pancreatobiliary diseases remains a common dilemma in clinical practice, recommendations investigations for the work-up include Chest X-ray or CT, serum glucose, and HbA1c, liver function test, thyroid function test, and abdominopelvic CT for gynecological diseases. A benign causative disease is likely if changes in CA 19-9 correspond with the course and treatment of disease. Suspicion for cancer should be raised if there is marked elevation throughout a 3-mo follow-up period or baseline CA 19-9 ≥ 80 U/mL[2]. Measurements of monosialyl/disialyl Lewis-a ratios could be used to distinguish malignant from benign conditions, as sialyl Lewis-a expression is upregulated during carcinogenesis[1].
Lastly, accurate interpretation of CA 19-9 depends on assay type and absence of interference. Firstly, there lacks standardization in CA 19-9-measuring methods used across different assays in the market. While different assays have similar diagnostic accuracies, these discrepancies render their results incomparable across assays. The implication is that patients whose CA 19-9 values are being serially trended must be monitored consistently using one assay method. Secondly, while assays' interferences are uncommon, two key interferences (rheumatoid factor and heterophilic antibodies) must be purposefully excluded[1].
The utility of CA 19-9 extends beyond the conventional idea of it being used almost exclusively in pancreatic pathologies. In an attempt to summarize the vast current literature surrounding CA 19-9, we present the current consensus in its use in pancreatic pathologies and the potential applications of it in extra-gastrointestinal pathologies and beyond. Avenues for potential use in gastrointestinal, urological, lung, uterine, ovarian, salivary gland, and thyroid diseases bring forth excitement and anticipation for more innovation from this historic 40-year old marker.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soresi M S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Scarà S, Bottoni P, Scatena R. CA 19-9: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:247-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 221] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 2. | Kim S, Park BK, Seo JH, Choi J, Choi JW, Lee CK, Chung JB, Park Y, Kim DW. Carbohydrate antigen 19-9 elevation without evidence of malignant or pancreatobiliary diseases. Sci Rep. 2020;10:8820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (2)] |
| 3. | Goh BK, Tan YM, Thng CH, Cheow PC, Chung YF, Chow PK, Wong WK, Ooi LL. How useful are clinical, biochemical, and cross-sectional imaging features in predicting potentially malignant or malignant cystic lesions of the pancreas? J Am Coll Surg. 2008;206:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Kajbafzadeh AM, Elmi A, Talab SS, Emami H, Esfahani SA, Saeedi P. Urinary and serum carbohydrate antigen 19-9 as a biomarker in ureteropelvic junction obstruction in children. J Urol. 2010;183:2353-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 5. | Dyckhoff G, Warta R, Gonnermann A, Plinkert PK, Flechtenmacher C, Volkmann M. Carbohydrate antigen 19-9 in saliva: possible preoperative marker of malignancy in parotid tumors. Otolaryngol Head Neck Surg. 2011;145:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Roy S, Dasgupta A, Kar K. Comparison of urinary and serum CA 19-9 as markers of early stage urothelial carcinoma. Int Braz J Urol. 2013;39:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 7. | Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3:105-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 351] [Reference Citation Analysis (3)] |
| 8. | Chia CL, Lee AY, Shelat VG, Ahmed S, Junnarkar SP, Woon WW, Low JK. Does diabetes mellitus affect presentation, stage and survival in operable pancreatic cancer? Hepatobiliary Surg Nutr. 2016;5:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Kim JE, Lee KT, Lee JK, Paik SW, Rhee JC, Choi KW. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 2004;19:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Zubarik R, Gordon SR, Lidofsky SD, Anderson SR, Pipas JM, Badger G, Ganguly E, Vecchio J. Screening for pancreatic cancer in a high-risk population with serum CA 19-9 and targeted EUS: a feasibility study. Gastrointest Endosc. 2011;74:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Choe JW, Kim HJ, Kim JS, Cha J, Joo MK, Lee BJ, Park JJ, Bak YT. Usefulness of CA 19-9 for pancreatic cancer screening in patients with new-onset diabetes. Hepatobiliary Pancreat Dis Int. 2018;17:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Murai J, Soga S, Saito H, Otsuki M, Kitada T, Saisho Y, Nakamura H, Kasayama S, Koga M. Study on the mechanism causing elevation of serum CA19-9 levels in diabetic patients. Endocr J. 2013;60:885-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 611] [Article Influence: 32.2] [Reference Citation Analysis (1)] |
| 14. | Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R, Nicolini A, Topolcan O, Heinemann V. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010;21:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 15. | Azizian A, Rühlmann F, Krause T, Bernhardt M, Jo P, König A, Kleiß M, Leha A, Ghadimi M, Gaedcke J. CA19-9 for detecting recurrence of pancreatic cancer. Sci Rep. 2020;10:1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 16. | Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 414] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 17. | Shamali A, Shelat V, Jaber B, Wardak A, Ahmed M, Fontana M, Armstrong T, Abu Hilal M. Impact of obesity on short and long term results following a pancreatico-duodenectomy. Int J Surg. 2017;42:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Krishna K, Bekaii-Saab T. CA 19-9 as a Serum Biomarker in Cancer. In: Biomarkers in Cancer, V.R. Preedy, V.B. Patel, Editors. Springer Netherlands: Dordrecht. 2015: 179-201. |
| 19. | Brown EG, Canter RJ, Bold RJ. Preoperative CA 19-9 kinetics as a prognostic variable in radiographically resectable pancreatic adenocarcinoma. J Surg Oncol. 2015;111:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Shelat VG, Thong JF, Seah M, Lim KH. Role of staging laparoscopy in gastric malignancies - our institutional experience. World J Gastrointest Surg. 2012;4:214-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | De Rosa A, Cameron IC, Gomez D. Indications for staging laparoscopy in pancreatic cancer. HPB (Oxford). 2016;18:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Neuzillet C, Gaujoux S, Williet N, Bachet JB, Bauguion L, Colson Durand L, Conroy T, Dahan L, Gilabert M, Huguet F, Marthey L, Meilleroux J, de Mestier L, Napoléon B, Portales F, Sa Cunha A, Schwarz L, Taieb J, Chibaudel B, Bouché O, Hammel P; Thésaurus National de Cancérologie Digestive (TNCD); Société Nationale Française de Gastroentérologie (SNFGE); Fédération Francophone de Cancérologie Digestive (FFCD); Groupe Coopérateur multidisciplinaire en Oncologie (GERCOR); Fédération Nationale des Centres de Lutte Contre le Cancer (UNICANCER); Société Française de Chirurgie Digestive (SFCD); Société Française d’Endoscopie Digestive (SFED); Société Française de Radiothérapie Oncologique (SFRO); Association de Chirurgie Hépato-Bilio-Pancréatique et Transplantation (ACHBT); Association Française de Chirurgie (AFC). Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC). Dig Liver Dis. 2018;50:1257-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 23. | Heger U, Sun H, Hinz U, Klaiber U, Tanaka M, Liu B, Sachsenmaier M, Springfeld C, Michalski CW, Büchler MW, Hackert T. Induction chemotherapy in pancreatic cancer: CA 19-9 may predict resectability and survival. HPB (Oxford). 2020;22:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Boone BA, Steve J, Zenati MS, Hogg ME, Singhi AD, Bartlett DL, Zureikat AH, Bahary N, Zeh HJ 3rd. Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol. 2014;21:4351-4358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 25. | Macedo FI, Ryon E, Maithel SK, Lee RM, Kooby DA, Fields RC, Hawkins WG, Williams G, Maduekwe U, Kim HJ, Ahmad SA, Patel SH, Abbott DE, Schwartz P, Weber SM, Scoggins CR, Martin RCG, Dudeja V, Franceschi D, Livingstone AS, Merchant NB. Survival Outcomes Associated With Clinical and Pathological Response Following Neoadjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel Chemotherapy in Resected Pancreatic Cancer. Ann Surg. 2019;270:400-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 26. | Tsai S, George B, Wittmann D, Ritch PS, Krepline AN, Aldakkak M, Barnes CA, Christians KK, Dua K, Griffin M, Hagen C, Hall WA, Erickson BA, Evans DB. Importance of Normalization of CA19-9 Levels Following Neoadjuvant Therapy in Patients With Localized Pancreatic Cancer. Ann Surg. 2020;271:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 27. | Humphris JL, Chang DK, Johns AL, Scarlett CJ, Pajic M, Jones MD, Colvin EK, Nagrial A, Chin VT, Chantrill LA, Samra JS, Gill AJ, Kench JG, Merrett ND, Das A, Musgrove EA, Sutherland RL, Biankin AV; NSW Pancreatic Cancer Network. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012;23:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 28. | Berger AC, Meszoely IM, Ross EA, Watson JC, Hoffman JP. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2004;11:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 29. | Distler M, Pilarsky E, Kersting S, Grützmann R. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas - a retrospective tumor marker prognostic study. Int J Surg. 2013;11:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Poruk KE, Gay DZ, Brown K, Mulvihill JD, Boucher KM, Scaife CL, Firpo MA, Mulvihill SJ. The clinical utility of CA 19-9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Curr Mol Med. 2013;13:340-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 31. | Reni M, Cereda S, Balzano G, Passoni P, Rognone A, Fugazza C, Mazza E, Zerbi A, Di Carlo V, Villa E. Carbohydrate antigen 19-9 change during chemotherapy for advanced pancreatic adenocarcinoma. Cancer. 2009;115:2630-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Li J, Li Z, Kan H, Sun Z, Xing J, Cheng Y, Bai C. CA19-9 elevation as an indication to start salvage treatment in surveillance after pancreatic cancer resection. Pancreatology. 2019;19:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Cao S, Hu Y, Gao X, Liao Q, Zhao Y. Serum Carbohydrate Antigen 19-9 in Differential Diagnosis of Benign and Malignant Pancreatic Cystic Neoplasms: A Meta-Analysis. PLoS One. 2016;11:e0166406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Jan IS, Chang MC, Yang CY, Tien YW, Jeng YM, Wu CH, Chen BB, Chang YT. Validation of Indications for Surgery of European Evidence-Based Guidelines for Patients with Pancreatic Intraductal Papillary Mucinous Neoplasms. J Gastrointest Surg. 2020;24:2536-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 901] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 36. | van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 384] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 37. | Nagashio Y, Hijioka S, Mizuno N, Hara K, Imaoka H, Bhatia V, Niwa Y, Tajika M, Tanaka T, Ishihara M, Shimizu Y, Hosoda W, Yatabe Y, Yamao K. Combination of cyst fluid CEA and CA 125 is an accurate diagnostic tool for differentiating mucinous cystic neoplasms from intraductal papillary mucinous neoplasms. Pancreatology. 2014;14:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Snozek CL, Mascarenhas RC, O'Kane DJ. Use of cyst fluid CEA, CA19-9, and amylase for evaluation of pancreatic lesions. Clin Biochem. 2009;42:1585-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Maire F, Voitot H, Aubert A, Palazzo L, O'Toole D, Couvelard A, Levy P, Vidaud M, Sauvanet A, Ruszniewski P, Hammel P. Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol. 2008;103:2871-2877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Chan EE, Shelat VG. Pancreaticopleural Fistula Causing Massive Right Hydrothorax and Respiratory Failure. Case Rep Surg. 2016;2016:8294056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Yan T, Ke Y, Chen Y, Xu C, Yu C, Li Y. Serological characteristics of autoimmune pancreatitis and its differential diagnosis from pancreatic cancer by using a combination of carbohydrate antigen 19-9, globulin, eosinophils and hemoglobin. PLoS One. 2017;12:e0174735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver. 2017;11:13-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 43. | Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 285] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 45. | Tao LY, Cai L, He XD, Liu W, Qu Q. Comparison of serum tumor markers for intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Am Surg. 2010;76:1210-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Acharya A, Markar SR, Matar M, Ni M, Hanna GB. Use of Tumor Markers in Gastrointestinal Cancers: Surgeon Perceptions and Cost-Benefit Trade-Off Analysis. Ann Surg Oncol. 2017;24:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Scarpa M, Noaro G, Saadeh L, Cavallin F, Cagol M, Alfieri R, Plebani M, Castoro C. Esophageal cancer management: preoperative CA19.9 and CEA serum levels may identify occult advanced adenocarcinoma. World J Surg. 2015;39:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Tokunaga R, Imamura Y, Nakamura K, Uchihara T, Ishimoto T, Nakagawa S, Iwatsuki M, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N, Oyama S, Shono T, Naoe H, Saeki H, Oki E, Watanabe M, Sasaki Y, Maehara Y, Baba H. Carbohydrate antigen 19-9 is a useful prognostic marker in esophagogastric junction adenocarcinoma. Cancer Med. 2015;4:1659-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 49. | Song YX, Huang XZ, Gao P, Sun JX, Chen XW, Yang YC, Zhang C, Liu HP, Wang HC, Wang ZN. Clinicopathologic and Prognostic Value of Serum Carbohydrate Antigen 19-9 in Gastric Cancer: A Meta-Analysis. Dis Markers. 2015;2015:549843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 50. | Shimada H, Noie T, Ohashi M, Oba K, Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 367] [Article Influence: 33.4] [Reference Citation Analysis (1)] |
| 51. | Suenaga Y, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, Tanaka C, Kobayashi D, Fujiwara M, Murotani K, Kodera Y. Prognostic significance of perioperative tumor marker levels in stage II/III gastric cancer. World J Gastrointest Oncol. 2019;11:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Duffy MJ, van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R, Nilsson O, Sturgeon C, Topolcan O. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer. 2003;39:718-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 53. | Stiksma J, Grootendorst DC, van der Linden PW. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clin Colorectal Cancer. 2014;13:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 54. | Stojkovic Lalosevic M, Stankovic S, Stojkovic M, Markovic V, Dimitrijevic I, Lalosevic J, Petrovic J, Brankovic M, Pavlovic Markovic A, Krivokapic Z. Can preoperative CEA and CA19-9 serum concentrations suggest metastatic disease in colorectal cancer patients? Hell J Nucl Med. 2017;20:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 55. | Yu Z, Chen Z, Wu J, Li Z, Wu Y. Prognostic value of pretreatment serum carbohydrate antigen 19-9 level in patients with colorectal cancer: A meta-analysis. PLoS One. 2017;12:e0188139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Rosen LS, Jacobs IA, Burkes RL. Bevacizumab in Colorectal Cancer: Current Role in Treatment and the Potential of Biosimilars. Target Oncol. 2017;12:599-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 57. | Narita Y, Taniguchi H, Komori A, Nitta S, Yamaguchi K, Kondo C, Nomura M, Kadowaki S, Takahari D, Ura T, Andoh M, Muro K. CA19-9 level as a prognostic and predictive factor of bevacizumab efficacy in metastatic colorectal cancer patients undergoing oxaliplatin-based chemotherapy. Cancer Chemother Pharmacol. 2014;73:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Thomsen M, Skovlund E, Sorbye H, Bolstad N, Nustad KJ, Glimelius B, Pfeiffer P, Kure EH, Johansen JS, Tveit KM, Christoffersen T, Guren TK. Prognostic role of carcinoembryonic antigen and carbohydrate antigen 19-9 in metastatic colorectal cancer: a BRAF-mutant subset with high CA 19-9 level and poor outcome. Br J Cancer. 2018;118:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 59. | Pearce S, Thornes H, Carr D, Tanner A. Diagnostic pitfall; interpretation of CA 19-9 concentrations in the presence of hepatic dysfunction. Gut. 1994;35:707-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Milkiewicz P, Chilton AP, Hubscher SG, Elias E. Antidepressant induced cholestasis: hepatocellular redistribution of multidrug resistant protein (MRP2). Gut. 2003;52:300-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Akimoto S, Banshodani M, Nishihara M, Nambu J, Kawaguchi Y, Shimamoto F, Dohi K, Sugino K, Ohdan H. Acute Cholecystitis with Significantly Elevated Levels of Serum Carbohydrate Antigen 19-9. Case Rep Gastroenterol. 2016;10:410-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Tsen A, Barbara M, Rosenkranz L. Dilemma of elevated CA 19-9 in biliary pathology. Pancreatology. 2018;18:862-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 63. | Doğan ÜB, Gümürdülü Y, Gölge N, Kara B. Relationship of CA 19-9 with choledocholithiasis and cholangitis. Turk J Gastroenterol. 2011;22:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Vickers AJ, Savage C, O'Brien MF, Lilja H. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol. 2009;27:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 65. | Hernandez JM, Cowgill SM, Al-Saadi S, Collins A, Ross SB, Cooper J, Villadolid D, Zervos E, Rosemurgy A. CA 19-9 velocity predicts disease-free survival and overall survival after pancreatectomy of curative intent. J Gastrointest Surg. 2009;13:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Rieser CJ, Zenati M, Hamad A, Al Abbas AI, Bahary N, Zureikat AH, Zeh HJ 3rd, Hogg ME. CA19-9 on Postoperative Surveillance in Pancreatic Ductal Adenocarcinoma: Predicting Recurrence and Changing Prognosis over Time. Ann Surg Oncol. 2018;25:3483-3491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 67. | Chan KS, Ho BCS, Shelat VG. A pilot study of estrogen receptor (ER) expression in pancreatic ductal adenocarcinoma (PDAC). Transl Gastroenterol Hepatol. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Shelat VG. Role of inflammatory indices in management of hepatocellular carcinoma-neutrophil to lymphocyte ratio. Ann Transl Med. 2020;8:912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 69. | Weitzenfeld P, Bournazos S, Ravetch JV. Antibodies targeting sialyl Lewis A mediate tumor clearance through distinct effector pathways. J Clin Invest. 2019;129:3952-3962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 70. | Gupta S, McDonald JD, Ayabe RI, Khan TM, Gamble LA, Sinha S, Hannah C, Blakely AM, Davis JL, Hernandez JM. Targeting CA 19-9 with a humanized monoclonal antibody at the time of surgery may decrease recurrence rates for patients undergoing resections for pancreatic cancer, cholangiocarcinoma and metastatic colorectal cancer. J Gastrointest Oncol. 2020;11:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Kiat TTJ, Gunasekaran SK, Junnarkar SP, Low JK, Woon W, Shelat VG. Are traditional scoring systems for severity stratification of acute pancreatitis sufficient? Ann Hepatobiliary Pancreat Surg. 2018;22:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Engle DD, Tiriac H, Rivera KD, Pommier A, Whalen S, Oni TE, Alagesan B, Lee EJ, Yao MA, Lucito MS, Spielman B, Da Silva B, Schoepfer C, Wright K, Creighton B, Afinowicz L, Yu KH, Grützmann R, Aust D, Gimotty PA, Pollard KS, Hruban RH, Goggins MG, Pilarsky C, Park Y, Pappin DJ, Hollingsworth MA, Tuveson DA. The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice. Science. 2019;364:1156-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 73. | Shelat VG. Sulfasalazine induced acute pancreatitis in a patient with prior cholecystectomy. Postgrad Med J. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Ding T, Xu J, Wang F, Shi M, Zhang Y, Li SP, Zheng L. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009;40:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 75. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients with Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1274] [Article Influence: 141.6] [Reference Citation Analysis (2)] |
| 76. | Lu LH, Zhang YF, Wei W, Shi M, Guo RP. Preoperative Carbohydrate Antigen 19-9: Its Neglected Role in Alpha-Fetoprotein-Negative Hepatocellular Carcinoma Patients. J Gastrointest Surg. 2017;21:2025-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Liu SL, Song ZF, Hu QG, Shan D, Hu SB, Li J, Zheng QC. Serum carbohydrate antigen (CA) 19-9 as a prognostic factor in cholangiocarcinoma: a meta-analysis. Front Med China. 2010;4:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Hu HJ, Mao H, Tan YQ, Shrestha A, Ma WJ, Yang Q, Wang JK, Cheng NS, Li FY. Clinical value of preoperative serum CA 19-9 and CA 125 levels in predicting the resectability of hilar cholangiocarcinoma. Springerplus. 2016;5:551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Huang XW, Huang Y, Chen LD, Wang Z, Yang Z, Liu JY, Xie XY, Lu MD, Shen SL, Wang W. Potential diagnostic performance of contrast-enhanced ultrasound and tumor markers in differentiating combined hepatocellular-cholangiocarcinoma from hepatocellular carcinoma and cholangiocarcinoma. J Med Ultrason (2001). 2018;45:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Li R, Yang D, Tang CL, Cai P, Ma KS, Ding SY, Zhang XH, Guo DY, Yan XC. Combined hepatocellular carcinoma and cholangiocarcinoma (biphenotypic) tumors: clinical characteristics, imaging features of contrast-enhanced ultrasound and computed tomography. BMC Cancer. 2016;16:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 81. | Sachan A, Saluja SS, Nekarakanti PK, Nimisha, Mahajan B, Nag HH, Mishra PK. Raised CA19-9 and CEA have prognostic relevance in gallbladder carcinoma. BMC Cancer. 2020;20:826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 82. | Wang YF, Feng FL, Zhao XH, Ye ZX, Zeng HP, Li Z, Jiang XQ, Peng ZH. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol. 2014;20:4085-4092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 83. | Kwon W, Jang JY, Lee SE, Hwang DW, Kim SW. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci. 2009;24:481-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 84. | Chen Z, Liu Z, Zhang Y, Wang P, Gao H. Combination of CA19-9 and the Neutrophil-to-Lymphocyte Ratio for the Differential Diagnosis of Gallbladder Carcinoma. Cancer Manag Res. 2020;12:4475-4482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Yu H, Yu TN, Cai XJ. Tumor biomarkers: help or mislead in the diagnosis of xanthogranulomatous cholecystitis? Chin Med J (Engl). 2013;126:3044-3047. [PubMed] |
| 86. | Zhuang PY, Zhu MJ, Wang JD, Zhou XP, Quan ZW, Shen J. Xanthogranulomatous cholecystitis: a clinicopathological study of its association with gallbladder carcinoma. J Dig Dis. 2013;14:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 87. | Liu F, Wang JK, Ma WJ, Yang Q, Hu HJ, Li FY. Clinical value of preoperative CA19-9 levels in evaluating resectability of gallbladder carcinoma. ANZ J Surg. 2019;89:E76-E80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Yu T, Yu H, Cai X. Preoperative prediction of survival in resectable gallbladder cancer by a combined utilization of CA 19-9 and carcinoembryonic antigen. Chin Med J (Engl). 2014;127:2299-2303. [PubMed] |
| 89. | Xu WY, Zhang HH, Yang XB, Bai Y, Lin JZ, Long JY, Xiong JP, Zhang JW, Sang XT, Zhao HT. Prognostic significance of combined preoperative fibrinogen and CA199 in gallbladder cancer patients. World J Gastroenterol. 2018;24:1451-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 90. | Lee JW, Kim YT, Lee SH, Son JH, Kang JW, Ryu JK, Jang DK, Paik WH, Lee BS. Tumor Marker Kinetics as Prognosticators in Patients with Unresectable Gallbladder Adenocarcinoma Undergoing Palliative Chemotherapy. Gut Liver. 2018;12:102-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21:11567-11583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 189] [Cited by in RCA: 255] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 92. | Bertino G, Ardiri AM, Calvagno GS, Malaguarnera G, Interlandi D, Vacante M, Bertino N, Lucca F, Madeddu R, Motta M. Carbohydrate 19.9 antigen serum levels in liver disease. Biomed Res Int. 2013;2013:531640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Liu F, Kong X, Dou Q, Ye J, Xu D, Shang H, Xu K, Song Y. Evaluation of tumor markers for the differential diagnosis of benign and malignant ascites. Ann Hepatol. 2014;13:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 94. | Sari R, Yildirim B, Sevinc A, Bahceci F, Hilmioglu F. The importance of serum and ascites fluid alpha-fetoprotein, carcinoembryonic antigen, CA 19-9, and CA 15-3 levels in differential diagnosis of ascites etiology. Hepatogastroenterology. 2001;48:1616-1621. [PubMed] |
| 95. | Kaleta EJ, Tolan NV, Ness KA, O'Kane D, Algeciras-Schimnich A. CEA, AFP and CA 19-9 analysis in peritoneal fluid to differentiate causes of ascites formation. Clin Biochem. 2013;46:814-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Gu W, Tong Z. Serum Carbohydrate Antigen 199 as a Biomarker for Evaluating Patients with Choledocholithiasis. Gastroenterol Res Pract. 2020;2020:2739612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 97. | Hegele A, Mecklenburg V, Varga Z, Olbert P, Hofmann R, Barth P. CA19.9 and CEA in transitional cell carcinoma of the bladder: serological and immunohistochemical findings. Anticancer Res. 2010;30:5195-5200. [PubMed] |
| 98. | Wang QH, Ji ZG, Chen ZG, Li HZ, Fan H, Fan XR, Shi BB, Fang Y. Serum CA 19-9 as a good prognostic biomarker in patients with bladder cancer. Int J Surg. 2015;15:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 99. | Pal K, Roy S, Mondal SA, Chatterjee U, Tiwari P, Bera M. Urinary level of CA19-9 as a tumor marker in urothelial carcinoma of the bladder. Urol J. 2011;8:203-208. [PubMed] |
| 100. | Hashim H, Woodhouse CRJ. Ureteropelvic Junction Obstruction. Eur Urol Suppl. 2012;11:25-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 101. | Nabavizadeh B, Khorramirouz R, Amini E, Pishgar F, Hojjat A, Kajbafzadeh AM. Value of urinary carbohydrate antigen 19-9 to predict failure of conservative management in children with ureteropelvic junction obstruction. J Pediatr Surg. 2019;54:1650-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Alizadeh F, Taefnia AM, Haghdani S. Urinary carbohydrate antigen 19-9/creatinine ratio: A non-invasive marker for follow-up of unilateral ureteropelvic junction obstruction in children. J Pediatr Urol 2018; 14: 62.e1-62. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Miranda EP, Duarte RJ, de Bessa J Jr, Lopes RI, Srougi V, Andrade HS, Bandeira RAST, Arap MA, Mitre AI, Viana NI, Reis ST, Leite KRM, Srougi M. The role of urinary KIM-1, NGAL, CA19-9 and β2-microglobulin in the assessment of ureteropelvic junction obstruction in adults. Biomarkers. 2017;22:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 104. | Banerjee I, Tomar V, Yadav SS, Vyas N, Yadav S, Sathian B. Role of Urinary and Serum Carbohydrate Antigen 19-9 as a Biomarker in Diagnosis of Adult Giant Hydronephrosis. J Clin Diagn Res. 2016;10:PC08-PC11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 105. | Neuville MF, Krzesinski JM, Jouret F. Serum levels of carbohydrate antigen 19-9 do not systematically increase in case of liver cyst infection in patients with autosomal dominant polycystic kidney disease. Clin Kidney J. 2020;13:482-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Kanaan N, Goffin E, Pirson Y, Devuyst O, Hassoun Z. Carbohydrate antigen 19-9 as a diagnostic marker for hepatic cyst infection in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2010;55:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Tsoukalas N, Kostakis ID, Giaginis C, Tolia M, Galanopoulos M, Kiakou M, Aravantinou-Fatorou E, Tsapakidis K, Baxevanos P, Litos I, Tzouda V, Tzovaras A, Kyrgias G, Tsiambas E, Theocharis S. Carcinoembryonic antigen and carbohydrate antigen 19-9 serum levels in non-small cell lung cancer. J BUON. 2017;22:1390-1394. [PubMed] |
| 108. | Sato Y, Fujimoto D, Uehara K, Shimizu R, Ito J, Kogo M, Teraoka S, Kato R, Nagata K, Nakagawa A, Otsuka K, Hamakawa H, Takahashi Y, Imai Y, Tomii K. The prognostic value of serum CA 19-9 for patients with advanced lung adenocarcinoma. BMC Cancer. 2016;16:890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 109. | Wang WJ, Tao Z, Gu W, Sun LH. Clinical observations on the association between diagnosis of lung cancer and serum tumor markers in combination. Asian Pac J Cancer Prev. 2013;14:4369-4371. [PubMed] |
| 110. | Ghosh I, Bhattacharjee D, Das AK, Chakrabarti G, Dasgupta A, Dey SK. Diagnostic Role of Tumour Markers CEA, CA15-3, CA19-9 and CA125 in Lung Cancer. Indian J Clin Biochem. 2013;28:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 111. | Ma S, Shen L, Qian N, Chen K. The prognostic values of CA125, CA19.9, NSE, and SCC for stage I NSCLC are limited. Cancer Biomark. 10:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Isaksson S, Jönsson P, Monsef N, Brunnström H, Bendahl PO, Jönsson M, Staaf J, Planck M. CA 19-9 and CA 125 as potential predictors of disease recurrence in resectable lung adenocarcinoma. PLoS One. 2017;12:e0186284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 113. | Ren X, Zhang Y, Lyu Y, Jin B, Guo H, Wu J, Li X, Liu X. Lactate dehydrogenase and serum tumor markers for predicting metastatic status in geriatric patients with lung adenocarcinoma. Cancer Biomark. 2019;26:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 114. | Xie X, Chen L, Zeng J, Qin C, Cheng D, Wei X, Liang Z. Clinical features and biological markers of lung cancer-associated stroke. J Int Med Res. 2016;44:1483-1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 115. | Feng M, Zhu J, Liang L, Zeng N, Wu Y, Wan C, Shen Y, Wen F. Diagnostic value of tumor markers for lung adenocarcinoma-associated malignant pleural effusion: a validation study and meta-analysis. Int J Clin Oncol. 2017;22:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 116. | Maher TM, Oballa E, Simpson JK, Porte J, Habgood A, Fahy WA, Flynn A, Molyneaux PL, Braybrooke R, Divyateja H, Parfrey H, Rassl D, Russell AM, Saini G, Renzoni EA, Duggan AM, Hubbard R, Wells AU, Lukey PT, Marshall RP, Jenkins RG. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir Med. 2017;5:946-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 117. | Hong JY, Jang SH, Kim SY, Chung KS, Song JH, Park MS, Kim YS, Kim SK, Chang J, Kang YA. Elevated serum CA 19-9 levels in patients with pulmonary nontuberculous mycobacterial disease. Braz J Infect Dis. 2016;20:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 118. | Bian J, Sun X, Li B, Ming L. Clinical Significance of Serum HE4, CA125, CA724, and CA19-9 in Patients with Endometrial Cancer. Technol Cancer Res Treat. 2017;16:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 119. | Nisenblat V, Bossuyt PM, Shaikh R, Farquhar C, Jordan V, Scheffers CS, Mol BW, Johnson N, Hull ML. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016: CD012179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 120. | Prodromidou A, Pandraklakis A, Loutradis D, Haidopoulos D. Is There a Role of Elevated CA 19-9 Levels in the Evaluation of Clinical Characteristics of Mature Cystic Ovarian Teratomas? Cureus. 2019;11:e6342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 121. | Cho HY, Kim K, Jeon YT, Kim YB, No JH. CA19-9 elevation in ovarian mature cystic teratoma: discrimination from ovarian cancer - CA19-9 level in teratoma. Med Sci Monit. 2013;19:230-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 122. | Bozkurt M, Yumru AE, Aral I. Evaluation of the importance of the serum levels of CA-125, CA15-3, CA-19-9, carcinoembryonic antigen and alpha fetoprotein for distinguishing benign and malignant adnexal masses and contribution of different test combinations to diagnostic accuracy. Eur J Gynaecol Oncol. 2013;34:540-544. [PubMed] |
| 123. | Zhang W, Wang L, Xin Z. Combination of serum CA19-9 and CA125 levels and contrast-enhanced ultrasound parametric data facilitates to differentiate ovarian serous carcinoma from ovarian malignant epithelial cancer. Medicine (Baltimore). 2018;97:e0358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 124. | Elisei R, Lorusso L, Piaggi P, Torregrossa L, Pellegrini G, Molinaro E, Agate L, Bottici V, Pani F, Cacciato Insilla A, Casella F, Ciampi R, Tognetti I, Materazzi G, Basolo F, Romei C. Elevated level of serum carbohydrate antigen 19.9 as predictor of mortality in patients with advanced medullary thyroid cancer. Eur J Endocrinol. 2015;173:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 125. | Alencar R, Kendler DB, Andrade F, Nava C, Bulzico D, Cordeiro de Noronha Pessoa C, Corbo R, Vaisman F. CA19-9 as a Predictor of Worse Clinical Outcome in Medullary Thyroid Carcinoma. Eur Thyroid J. 2019;8:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 126. | Vargas CVF, Ceolin L, Scheffel RS, Benini AF, Graudenz MS, Maia AL. The tissue expression pattern of CA 19.9 is associated with oncological features in medullary thyroid carcinoma. Endocrine. 2020;70:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 127. | Milman S, Arnold JL, Price M, Negassa A, Surks MI, Fleischer N, Whitney KD. Medullary thyroid cancer that stains negative for CA 19-9 has decreased metastatic potential. Endocr Pract. 2015;21:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 128. | Ogawa M, Hori H, Hirayama M, Kobayashi M, Shiraishi T, Watanabe Y, Komada Y. Anaplastic transformation from papillary thyroid carcinoma with increased serum CA19-9. Pediatr Blood Cancer. 2005;45:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 129. | Yamaguchi E, Makino Y, Sato T, Uchida M, Harada Y, Maruyama R. CA19-9-producing lung metastasis after surgery for papillary thyroid carcinoma: report of a case. Surg Today. 2014;44:2157-2161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 130. | Luo G, Fan Z, Cheng H, Jin K, Guo M, Lu Y, Yang C, Fan K, Huang Q, Long J, Liu L, Xu J, Lu R, Ni Q, Warshaw AL, Liu C, Yu X. New observations on the utility of CA19-9 as a biomarker in Lewis negative patients with pancreatic cancer. Pancreatology. 2018;18:971-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |