Published online Oct 27, 2020. doi: 10.4240/wjgs.v12.i10.435
Peer-review started: June 30, 2020
First decision: July 30, 2020
Revised: August 13, 2020
Accepted: September 14, 2020
Article in press: September 14, 2020
Published online: October 27, 2020
Processing time: 118 Days and 11.6 Hours
Mass lesions located in the wall of the stomach (and also of the bowel) are referred to as “intramural.” The differential diagnosis of such lesions can be challenging in some cases. As such, it may occur that an inconclusive fine needle aspiration (FNA) result give way to an unexpected diagnosis upon final surgical pathology. Herein, we present a case of an intramural gastric nodule mimicking a gastric gastrointestinal stromal tumor (GIST).
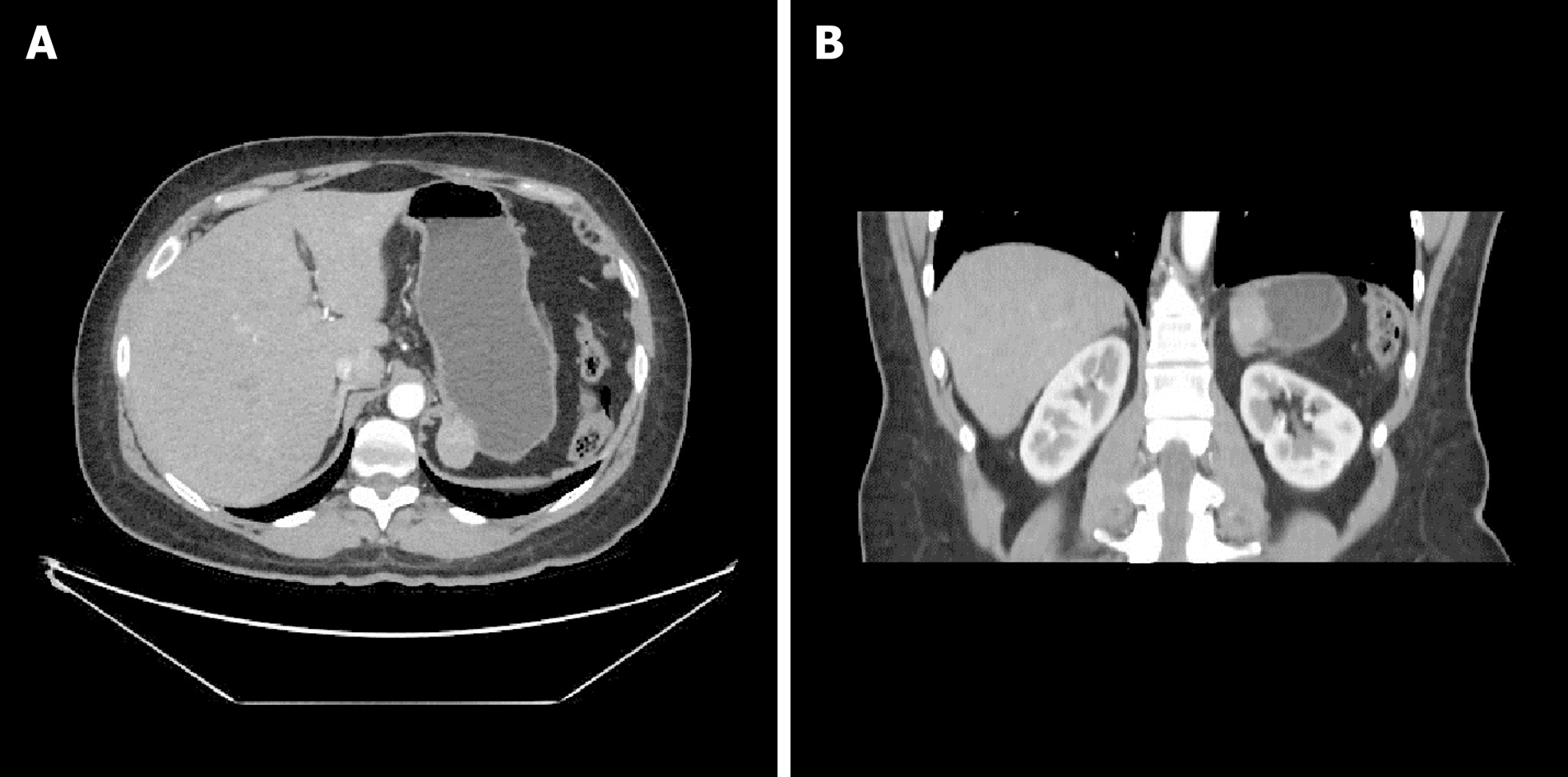
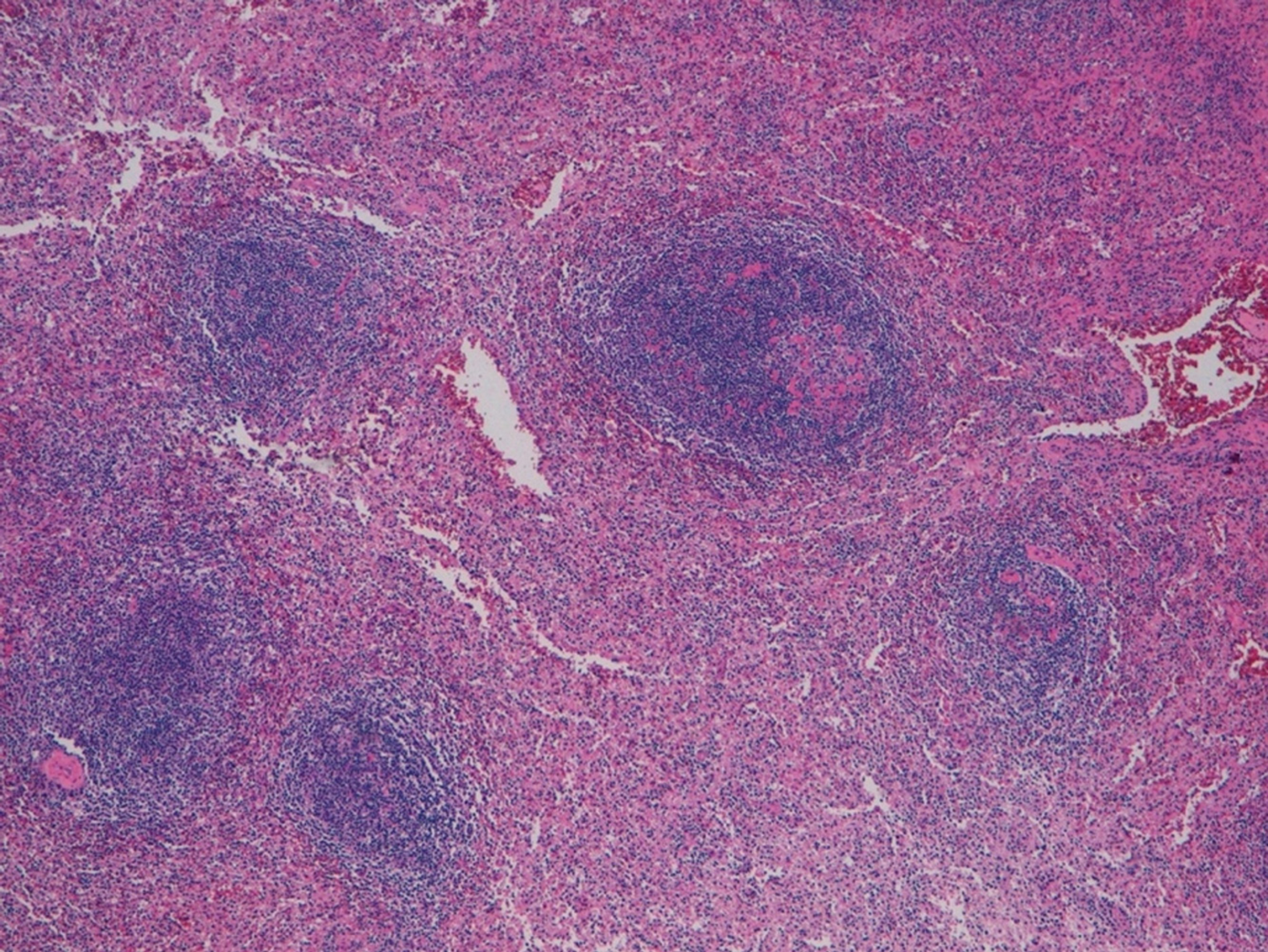
A 47-year-old Caucasian woman, who had undergone splenectomy for trauma at the age of 16, underwent gastroscopy for long-lasting epigastric pain and dyspepsia. It revealed a 15 mm submucosal nodule bulging into the gastric lumen with smooth margins and normal overlying mucosa. A thoraco-abdominal computed tomography scan showed in the gastric fundus a rounded mass (30 mm in diameter) with an exophytic growth and intense enhancement after administration of intravenous contrast. Endoscopic ultrasound scan showed a hypoechoic nodule, and fine needle FNA was inconclusive. Gastric GIST was considered the most probable diagnosis, and surgical resection was proposed due to symptoms. A laparoscopic gastric wedge resection was performed. The postoperative course was uneventful, and the patient was discharged on the seventh postoperative day. The final pathology report described a rounded encapsulated accumulation of lymphoid tissue of about 4 cm in diameter consistent with spleen parenchyma implanted during the previous splenectomy.
Splenosis is a rare condition that should always be considered as a possible diagnosis in splenectomized patients who present with an intramural gastric nodule.
Core Tip: Intramural gastric nodules are rare, but all differential diagnoses must always be considered. If feasible, a preoperative fine needle aspiration can help the surgeon in selecting the best treatment option. Splenosis is uncommon in the general population, but it must be considered in each patient with a history of splenectomy (especially after trauma). In this specific cluster it is reasonable to insist on ruling out splenosis even making a second histologic sampling after a first failure.
- Citation: Isopi C, Vitali G, Pieri F, Solaini L, Ercolani G. Gastric splenosis mimicking a gastrointestinal stromal tumor: A case report. World J Gastrointest Surg 2020; 12(10): 435-441
- URL: https://www.wjgnet.com/1948-9366/full/v12/i10/435.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i10.435
The masses arising from the wall of the stomach are referred to as “intramural”. In these cases the endoscopic and radiologic features may lead to several differential diagnoses because several overlapping characteristics have been shown to exist among the various gastric masses. Intramural lesions can be benign or malignant, and the most common diagnosis is gastrointestinal stromal tumors (GISTs).
Only a preoperative sampling allows planning the best therapeutic approach, but when the nature of the nodule cannot be preoperatively determined, an assessment about size, possible diagnoses, patient’s characteristics and clinical symptoms should be done before considering an upfront surgical approach.
Herein, we present a case of an intramural gastric nodule mimicking gastric gastrointestinal stromal tumor, whose nature could be defined only after surgery.
A 47-year-old Caucasian woman was referred to our unit for an intragastric nodule detected during a gastroscopy.
The gastroscopy was performed for long lasting epigastric pain and dyspepsia.
Patient’s past medical history included: Asthma, hypothyroidism, migraine and a splenectomy for trauma.
No family histories were identified.
The patient was in good general condition and slightly overweight (body mass index: 25.6). There were no abdominal mass and no pain on palpation.
Routine laboratory tests revealed no abnormalities.
Endoscopy showed a 15 mm submucosal nodule bulging into the gastric lumen with smooth margins and macroscopically normal overlying mucosa. Biopsies were negative for malignancy and showed superficial chronic gastritis.
Consequently, a thoraco-abdominal computed tomography scan (Figure 1A and 1B) and an endoscopic ultrasound with a fine needle aspiration were planned. Those investigations found a roundish formation on the gastric fundus of about 30 mm in diameter with an exophytic development. The mass was in close contiguity with the left adrenal gland and the left pillar of the diaphragm with no signs of infiltration. The ultrasound appearance was of a solid mass with well-defined margins with a homogeneous and well vascularized internal texture in the absence of calcified or necrotic areas. The fine needle aspiration (FNA) was performed without complications, but the result was nondiagnostic due to inadequate tissue yield.
Our main diagnostic suspect remained a gastric GIST and the symptoms could be related to the location of the mass. After a careful evaluation of the risks and benefits and according to the European Society for Medical Oncology guidelines[1], the surgical excision was planned.
The laparoscopic resection was performed with a three trocars technique (10 mm supraumbilical and right hypochondrium and 5 mm left hypochondrium). After a careful lysis of the adhesions related to the previous splenectomy, the exophytic mass of the fundus was identified. The perigastric vessels were dissected in order to expose the nodule; the resection was performed with a linear stapler.
The postoperative course was uneventful, and the patient was discharged on the seventh postoperative day. The final pathology of the specimen did not confirm our hypothesis but reported a rounded encapsulated accumulation of lymphoid tissue of 4 cm in diameter consistent with spleen parenchyma probably implanted during the previous splenectomy (Figure 2).
At the 6 mo follow-up the patient was symptom free.
Ectopic splenic tissue can be found in the body as accessory spleens and splenosis[2]. The former is congenital and receives blood supply from the splenic artery. The latter is a benign condition caused by the spillage upon the peritoneal surface of cells from the spleen after splenic trauma or surgical procedures.
Splenosis is usually considered to be a rare phenomenon, but its real prevalence is difficult to define. Pearson et al[3] showed that recurrent splenic activity after urgent splenectomy is frequent, and according to Sikov et al[4], its incidence could be as high as 76% in patients who had undergone splenectomy for trauma.
Splenosis is a benign condition, usually found incidentally and unless symptomatic surgery is not indicated[5]. In some cases the implantation could be responsible for serious conditions like gastrointestinal hemorrhage, pain from compression of the abdominal structures and bowel obstruction[6]. Splenosis may resemble several abdominal malignancies. As such several studies reported cases of splenosis mimicking a pancreatic mass[7], lymphomas[8], neuroendocrine tumors[9], intramural colonic masses[10], liver masses[11,12] and GISTs[13-16]. For this variability, the diagnosis of splenosis may be challenging. On a peripheral smear the absence of Howell-Jolly and Heinz bodies and siderocytes despite a history of splenectomy could mildly suggest the presence of a splenosis[17]. Imaging may not be accurate in defining this condition[18]. Differential diagnoses between benign[19-26] and malignant[27-32] forms and the radiologic features of intramural gastric masses[33,34] are presented in the Table 1.
| Location in the stomach | CT special features | Special features | |
| Benign lesions | |||
| Lipoma[19] | Antrum | Attenuation values -70 HU to -120 HU | Solitary, fibrous capsulated, soft (change in size and shape with peristalsis), no vessels |
| Leiomyoma[24] | Cardia | Low attenuation, endoluminal growth pattern | Negative for c-kit, positive for desmin and smooth muscle actin |
| Schwannoma[21] | Body | Minimal enhancement on the arterial phase | Absence of calcification, hemorrhage, necrosis; not encapsulated; positive for S-100 |
| Glomus tumor[20] | Antrum | Strong enhancement on early-phase | Highly vascular; positive for calponin and smooth muscle actin |
| Inflammatory fibroid polyp[22] | Antrum | Enhancement on arterial phase | Positive for CD34 and vimentin |
| Hemangioma[25] | - | Strong enhancement on early-phase | Phleboliths are pathognomonic |
| Plexiform fibromyxoma[23] | Antrum | Myxoid tissue interspersed with vessels | Unique to the stomach, size from 2 cm to 15 cm |
| Ectopic pancreas[26] | Greater curvature | Similar to normal pancreas | - |
| Splenosis[5] | - | Enhancement on arterial phase | Splenectomized patients |
| Malignant lesions | |||
| GIST[18] | Body | Smoothly circumscribed, bullseye sign | Positive for c-kit or dog-1; 50% greater than 2 cm |
| Non-GIST sarcoma (liposarcoma, leiomyosarcoma, unclassified sarcoma)[27] | - | Usually large, heterogeneous enhancement | Positive for desmin and smooth muscle actin, negative for c-kit |
| Lymphoma[33] | - | Wall thickening | Distant (more than close) and large adenopathy |
| Carcinoid[29] | - | Multiple small lesions | Reactive to synaptophysin and chromogranin A, hypergastrinemia related symptoms |
| Inflammatory myofibroblastic tumor[28] | - | Heterogeneously enhancing tumor (malignant appearance) | Borderline tumor, more frequent in young adults and children; reactivity for ALK |
| Metastasis[30-32] | - | - | “Homomorphic” endoscopic features; dyschromic lesions |
Nowadays, there is a general consensus that the mainstay for the diagnosis of splenosis is the noninvasive scintigraphy using technetium-99m-labeled heat damaged red blood cell or indium 111-labeled platelets[35]. However, it must be highlighted that the real critical point in diagnosing splenosis is thinking about it in a suggestive past medical history.
During the assessment of a gastric intramural nodule, mass biopsy may help solving the diagnostic dilemma. However, in our case preoperative diagnosis was not possible, and the patient was submitted to surgery according to her symptoms and the most probable diagnosis.
Splenosis is a rare condition that should always be considered as a possible diagnosis in patients who had undergone splenectomy. If feasible, a preoperative FNA may be the best preoperative investigation to rule out other diagnoses and to plan the most appropriate treatment.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Alshomimi S, Gong N, Tabibian JH, Tian YT, Zhang XF S-Editor: Wang JL L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY; ESMO Guidelines Committee and EURACAN. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv68-iv78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 289] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 2. | Varga I, Galfiova P, Adamkov M, Danisovic L, Polak S, Kubikova E, Galbavy S. Congenital anomalies of the spleen from an embryological point of view. Med Sci Monit. 2009;15:RA269-RA276. [PubMed] |
| 3. | Pearson HA, Johnston D, Smith KA, Touloukian RJ. The born-again spleen. Return of splenic function after splenectomy for trauma. N Engl J Med. 1978;298:1389-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 241] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Sikov WM, Schiffman FJ, Weaver M, Dyckman J, Shulman R, Torgan P. Splenosis presenting as occult gastrointestinal bleeding. Am J Hematol. 2000;65:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Fremont RD, Rice TW. Splenosis: a review. South Med J. 2007;100:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Ksiadzyna D, Peña AS. Abdominal splenosis. Rev Esp Enferm Dig. 2011;103:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Mascioli F, Ossola P, Esposito L, Iascone C. A rare case of pancreatic splenosis and a literature review. Ann Ital Chir. 2020;9 . [PubMed] |
| 8. | Priola AM, Picciotto G, Priola SM. Diffuse abdominal splenosis: a condition mimicking abdominal lymphoma. Int J Hematol. 2009;90:543-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Matsubayashi H, Bando E, Kagawa H, Sasaki K, Ishiwatari H, Ono H. A Multinodular Mass of Abdominal Splenosis: Case Report of Uncommon Images of a Rare Disease. Diagnostics (Basel). 2019;9:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Obokhare ID, Beckman E, Beck DE, Whitlow CB, Margolin DA. Intramural colonic splenosis: a rare case of lower gastrointestinal bleeding. J Gastrointest Surg. 2012;16:1632-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kang KC, Cho GS, Chung GA, Kang GH, Kim YJ, Lee MS, Kim HK, Park SJ. Intrahepatic splenosis mimicking liver metastasis in a patient with gastric cancer. J Gastric Cancer. 2011;11:64-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Luo X, Zeng J, Wang Y, Min Y, Shen A, Zhang Y, Deng H, Gong N. Hepatic splenosis: Rare yet important - A case report and literature review. J Int Med Res. 2019;47:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Wang W, Li W, Sun Y, Zhao Y, Zhu R, Li J, Zhang H. Intra-gastric Ectopic Splenic Tissue. J Gastrointest Surg. 2016;20:218-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Xiao SM, Xu R, Tang XL, Ding Z, Li JM, Zhou X. Splenosis with lower gastrointestinal bleeding mimicking colonical gastrointestinal stromal tumour. World J Surg Oncol. 2017;15:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Li B, Huang Y, Chao B, Zhao Q, Hao J, Qin C, Xu H. Splenosis in gastric fundus mimicking gastrointestinal stromal tumor: a report of two cases and review of the literature. Int J Clin Exp Pathol. 2015;8:6566-6570. [PubMed] |
| 16. | Guan B, Li XH, Wang L, Zhou M, Dong ZW, Luo GJ, Meng LP, Hu J, Jin WY. Gastric fundus splenosis with hemangioma masquerading as a gastrointestinal stromal tumor in a patient with schistosomiasis and cirrhosis who underwent splenectomy: A case report and literature review. Medicine (Baltimore). 2018;97:e11461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Tavakkoli A. The Spleen. In: Zinner MJ, Ashley SW. Maingot’s Abdominal Operations, 12th Edition. New York: Mc Gray Hill, 2013: 1239-1269. |
| 18. | Kang HC, Menias CO, Gaballah AH, Shroff S, Taggart MW, Garg N, Elsayes KM. Beyond the GIST: mesenchymal tumors of the stomach. Radiographics. 2013;33:1673-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Maderal F, Hunter F, Fuselier G, Gonzales-Rogue P, Torres O. Gastric lipomas--an update of clinical presentation, diagnosis, and treatment. Am J Gastroenterol. 1984;79:964-967. [PubMed] |
| 20. | Harig BM, Rosen Y, Dallemand S, Farman J. The radiology corner*: glomus tumor of the stomach. Am J Gastroenterol. 1975;63:423-428. [PubMed] |
| 21. | Li R, Gan H, Ni S, Fu Y, Zhu H, Peng W. Differentiation of Gastric Schwannoma From Gastric Gastrointestinal Stromal Tumor With Dual-Phase Contrast-Enhanced Computed Tomography. J Comput Assist Tomogr. 2019;43:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Stolte M, Sticht T, Eidt S, Ebert D, Finkenzeller G. Frequency, location, and age and sex distribution of various types of gastric polyp. Endoscopy. 1994;26:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 136] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Miettinen M, Makhlouf HR, Sobin LH, Lasota J. Plexiform fibromyxoma: a distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol. 2009;33:1624-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 24. | Lee MJ, Lim JS, Kwon JE, Kim H, Hyung WJ, Park MS, Kim MJ, Kim KW. Gastric true leiomyoma: computed tomographic findings and pathological correlation. J Comput Assist Tomogr. 2007;31:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Levy AD, Abbott RM, Rohrmann CA Jr, Frazier AA, Kende A. Gastrointestinal hemangiomas: imaging findings with pathologic correlation in pediatric and adult patients. AJR Am J Roentgenol. 2001;177:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Attwell A, Sams S, Fukami N. Diagnosis of ectopic pancreas by endoscopic ultrasound with fine-needle aspiration. World J Gastroenterol. 2015;21:2367-2373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Aggarwal G, Sharma S, Zheng M, Reid MD, Crosby JH, Chamberlain SM, Nayak-Kapoor A, Lee JR. Primary leiomyosarcomas of the gastrointestinal tract in the post-gastrointestinal stromal tumor era. Ann Diagn Pathol. 2012;16:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 621] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 29. | Levy AD, Sobin LH. From the archives of the AFIP: Gastrointestinal carcinoids: imaging features with clinicopathologic comparison. Radiographics. 2007;27:237-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Weigt J, Malfertheiner P. Metastatic Disease in the Stomach. Gastrointest Tumors. 2015;2:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Januszewicz W, Corrie P, Liu H, Chan J, Fitzgerald RC, di Pietro M. A sinister black finding in the stomach. Lancet. 2019;393:1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Weissman S, Mehta TI, Zhornitskiy A, Tondon R, Tabibian JH. "Homomorphic" Tumor Metastases as an Endodiagnostic Clue: A Case Series of Renal-Cell Carcinoma Metastatic to the Stomach. Gastrointest Tumors. 2019;6:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Fishman EK, Urban BA, Hruban RH. CT of the stomach: spectrum of disease. Radiographics. 1996;16:1035-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Park SH, Han JK, Kim TK, Lee JW, Kim SH, Kim YI, Choi BI, Yeon KM, Han MC. Unusual gastric tumors: radiologic-pathologic correlation. Radiographics. 1999;19:1435-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Hagman TF, Winer-Muram HT, Meyer CA, Jennings SG. Intrathoracic splenosis: superiority of technetium Tc 99m heat-damaged RBC imaging. Chest. 2001;120:2097-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |