Published online Jan 27, 2020. doi: 10.4240/wjgs.v12.i1.1
Peer-review started: August 5, 2019
First decision: August 31, 2019
Revised: September 25, 2019
Accepted: November 7, 2019
Article in press: November 7, 2019
Published online: January 27, 2020
Processing time: 143 Days and 14.4 Hours
Portal hypertension (PH) is associated with changes in vascular structure and function of the portosplenomesenteric system (PSMS). This is referred to as portal hypertensive vasculopathy. Pathological abnormalities of PSMS has been described in the literature for cirrhotic patients. Raised portal pressure and hyperdynamic circulation are thought to be the underlying cause of this vasculopathy. In view of this, it is expected that pathological changes in splenic and portal vein similar to those reported in cirrhotic patients with PH may also be present in patients with non-cirrhotic PH (NCPH).
To investigate pathological abnormalities of splenic vein in patients with NCPH, and suggest its possible implications in the management of PH.
A prospective observational study was performed on 116 patients with NCPH [Extrahepatic portal vein obstruction (EHPVO): 53 and non-cirrhotic portal fibrosis (NCPF): 63] who underwent proximal splenorenal shunt (PSRS), interposition shunt or splenectomy with devascularization in JIPMER, Pondicherry, India, a tertiary level referral center, between 2011-2016. All patients were evaluated by Doppler study of PSMS, computed tomography porto-venogram and upper gastrointestinal endoscopy. An acoustic resonance forced impulse (ARFI) scan and abdomen ultrasound were done for all cases to exclude cirrhosis. Intraoperative and histopathological assessment of the harvested splenic vein was performed in all. The study group was divided into delayed and early presentation based on the median duration of symptoms (i.e. 108 mo).
The study group comprising of 116 patients [77 (66%) females and 39 (34%) males] with NCPH had a median age of 22 years. Median duration of symptoms was 108 mo. The most common presentation in both EHPVO and NCPF patients was upper gastrointestinal bleeding (hematemesis and melena). The ARFI scan revealed a median score of 1.2 (1.0-1.8) m/s for EHPVO and 1.5 (0.9-2.8) m/s for NCPF. PSRS was performed in 84 patients (two of whom underwent interposition PSRS using a 10 mm Dacron graft); splenoadrenal shunt in 9; interposition mesocaval shunt in 5; interposition 1st jejunal to caval shunt in 1 patient and devascularization with splenectomy in 17 patients. Median pre-splenectomy portal pressure was 25 (range: 15-51) mm Hg. In 77% cases, the splenic vein was abnormal upon intraoperative assessment. Under macroscopic examination, wall thickening was observed in 108 (93%), venous thrombosis in 32 (28%) and vein wall calcification in 27 (23%) cases. Upon examination under a surgical magnification loupe, 21 (18%) patients had intimal defects in the splenic vein. Histopathological examination of veins was abnormal in all cases. Medial hypertrophy was noted in nearly all patients (107/116), while intimal fibrosis was seen in 30%. Ninety one percent of patients with intimal fibrosis also had venous thrombosis. Vein wall calcification was found in 22%, all of whom had intimal fibrosis and venous thrombosis. The proportion of patients with pathological abnormalities in the splenic vein were significantly greater in the delayed presentation group as compared to the early presentation group.
Pathological changes in the splenic vein similar to those in cirrhotic patients with PH are noted in NCPH. We recommend that PH in NCPH be treated as systemic and pulmonary hypertension equivalent in the gastrointestinal tract, and that early aggressive therapy be initiated to reduce portal pressure and hemodynamic stress to avoid potential lethal effects.
Core tip: Portal hypertensive vasculopathy is well-investigated in cirrhotics. Raised portal pressure and hyperdynamic circulation are thought to be the underlying cause. Pathological changes in the splenic vein are similar in cirrhotic and non-cirrhotic portal hypertension (NCPH). They are not primarily due to venous degenerative changes, and are similar to those observed in the pulmonary vasculature in pulmonary hypertension. Portal hypertension in NCPH should be viewed as a systemic and pulmonary hypertension equivalent in the gastrointestinal tract. We show that these pathological venous changes in NCPH are observed in a greater proportion of patients in the delayed presentation group (P < 0.003). Interventions to reduce portal pressure should therefore be initiated at diagnosis of NCPH. Damage to the vasculature starts early and can be prevented from progressing to venous thrombosis and its sequelae if early surgical intervention is initiated to reduce portal pressure.
- Citation: Gupta S, Pottakkat B, Verma SK, Kalayarasan R, Chandrasekar A S, Pillai AA. Pathological abnormalities in splenic vasculature in non-cirrhotic portal hypertension: Its relevance in the management of portal hypertension. World J Gastrointest Surg 2020; 12(1): 1-8
- URL: https://www.wjgnet.com/1948-9366/full/v12/i1/1.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v12.i1.1
Portal hypertension (PH) is associated with changes in vascular structure and function of the portosplenomesenteric system (PSMS). This is referred to as portal hypertensive vasculopathy (PHV)[1]. Pathological abnormalities of PSMS have been described in the literature for cirrhotic patients[2,3]. Raised portal pressure and hyperdynamic circulation (HC) are thought to be the underlying cause of this vasculopathy[3]. In view of this, it is expected that pathological changes in splenic and portal vein similar to those reported in cirrhotic patients with PH may also be present in patients with non-cirrhotic PH (NCPH). To the best of our knowledge, studies on spleno-portomesenteric vasculopathy in patients with NCPH have not appeared in the literature. In this paper, we report on the pathological abnormalities in the splenic vein of patients with NCPH.
A prospective observational study was carried out on 116 consecutive patients with NCPH [extrahepatic portal vein obstruction (EHPVO): 53 and non-cirrhotic portal fibrosis (NCPF): 63] who underwent proximal splenorenal shunts, interposition shunts or splenectomy with devascularization in our Institute between February 2011–December 2016 after obtaining approval from the Institute Ethics Committee.
All patients were initially evaluated by Doppler study of PSMS, computed tomography porto-venogram and upper gastrointestinal endoscopy. Acoustic resonance forced impulse (ARFI) scans and abdomen ultrasounds were performed for all cases to exclude cirrhosis. Intra-operatively, portal pressure was assessed by measuring pressure in the omental vein using a 22G venous cannula and a transducer. Macroscopic appearance of the splenic vein wall, e.g., presence of calcification, thrombosis, thickening (global or focal) were assessed. Examination under a surgical magnification loupe was performed to look for focal aneurysmal dilatations and intimal defects. A frozen section analysis of the vein wall was performed in all cases. Specimen of the splenic vein and artery were obtained after retrieving the spleen, and a segment of each was sent for histopathological examination.
All vessel wall specimens were processed and stained with Hematoxylin-Eosin (HE) and examined under a light microscope. Frozen specimen sections were stained with rapid HE stain after appropriate processing. Characteristics of the splenic venous wall e.g., presence of medial hypertrophy, wall thickening, intimal fibrosis, adventitial attenuation along with evidence of thrombus formation in the splenic vein and vein wall calcification, were assessed using both histopathology and frozen section analyses. The study group was divided into delayed and early presentation based on the median duration of symptoms (i.e. 108 mo).
The splenic arterial wall was also assessed for medial hypertrophy and intimal thickening. Intra-operatively, trucut and wedge liver biopsies were taken in all cases to exclude cirrhosis. Specimens of the splenic vein and artery in cases with immune thrombocytopenic purpura (ITP, n = 22) and hemolytic anemia (n = 10) who underwent splenectomy were sent for histopathological examination for comparison as assumed normal controls.
Nonparametric variables were expressed as medians (range). Frequency of occurrence were expressed as proportions. Statistical analysis was performed using the statistical program GraphPad INSTAT version 3 (GraphPad Software, Inc., La Jolla, CA, United States). Proportions were compared using Fischer's exact tests.
In the study group comprised of 116 patients (77, 66% females and 39, 34% males) with NCPH, the median age was 22 (range: 12-55) years. They presented with symptoms for a median duration of 108 (1-240) mo. Sixty-seven patients had the disease for more than 108 mo. While both EHPVO and NCPF are more common in females (65% and 85%, respectively); EHPVO was seen more frequently (60%) in the younger age group (< 25 years), while NCPF was more common (54%) in older (> 40 years) patients. Cirrhosis was excluded in the study group by ARFI scan, which revealed a median score of 1.2 (range: 1.0-1.8) m/s for EHPVO and 1.5(range: 0.9-2.8) m/s for NCPF. The distribution of shunt surgeries performed are given in Table 1. Median pre-splenectomy portal pressure was 25 (range: 15-51) mm Hg.
| Surgical procedure | n (%) | |
| 1 | Proximal splenorenal shunt (including two interposition proximal splenorenal shunts using 10 mm Dacron graft) | 84 (72.4) |
| 2 | Splenoadrenal shunt | 9 (7.7) |
| 3 | Interposition mesocaval shunt | 5 (4.3) |
| 4 | Interposition first jejunal to caval shunt | 1 (0.9) |
| 5 | Devascularization and splenectomy | 17 (14.6) |
| Total | 116 (100) |
Intra-operatively, upon macroscopic examination, wall thickening was observed in 108 (93%), venous thrombosis in 32 (28%) and vein wall calcification in 27 (23%) cases. Upon examination under surgical magnification loupe, 21 (18%) patients had intimal defects in the splenic vein. In 89/116 (77%) cases, the splenic vein was found to be abnormal under intraoperative assessment (based on the presence of one or more of the following features: wall thickening, wall calcification, presence of thrombus, and intimal defects). On histopathological examination of veins, however, splenic veins in all patients were found to be abnormal (based on the presence of one or more of the pathological characteristics mentioned above). The study group was divided into delayed and early presentation based on the median duration of symptoms (i.e. 108 mo) (Table 2). The proportion of patients with pathological abnormalities in the splenic vein were significantly more in the delayed presentation group compared to the early presentation group (Table 2). While the incidence of thrombosis at the anastomotic end was more in the delayed presentation group, the difference was not statistically significant. All patients in the delayed presentation group had Grade III/IV esophageal varices in endoscopy. Of these 67 patients, 47 (70%) had NCPF.
| Pathological abnormalities | Delayed presentation group, n = 67 | Early presentation group, n = 49 | P value |
| Medial hypertrophy, n (%) | 67 (100) | 40 (81.6) | 0.003 |
| Wall thickening, n (%) | 67 (100) | 40 (81.6) | 0.003 |
| Intimal fibrosis, n (%) | 32 (47.8) | 3 (6.1) | < 0.001 |
| Adventitial attenuation, n (%) | 30 (44.8) | 5 (10.2) | < 0.001 |
| Thrombosis, n (%) | 32 (47.8) | 0 (0) | < 0.001 |
| Vein wall calcification, n (%) | 26 (38.8) | 0 (0) | < 0.001 |
| Thrombosis at anastomotic end, n (%) | 13 (19.4) | 3 (6.1) | 0.0556 |
Pathological examination of the splenic vein of patients without any PH who underwent splenectomy (open and laparoscopic) was also performed for comparison as assumed normal controls. This group included 22 patients (median age 20 years; 95% females) with ITP and ten patients (median age 26 years; 90% females) with hemolytic anemia and splenomegaly. The splenic veins of patients with ITP was found to be normal, whereas medial hypertrophy and intimal fibrosis was seen in patients with splenomegaly and hemolytic anemia.
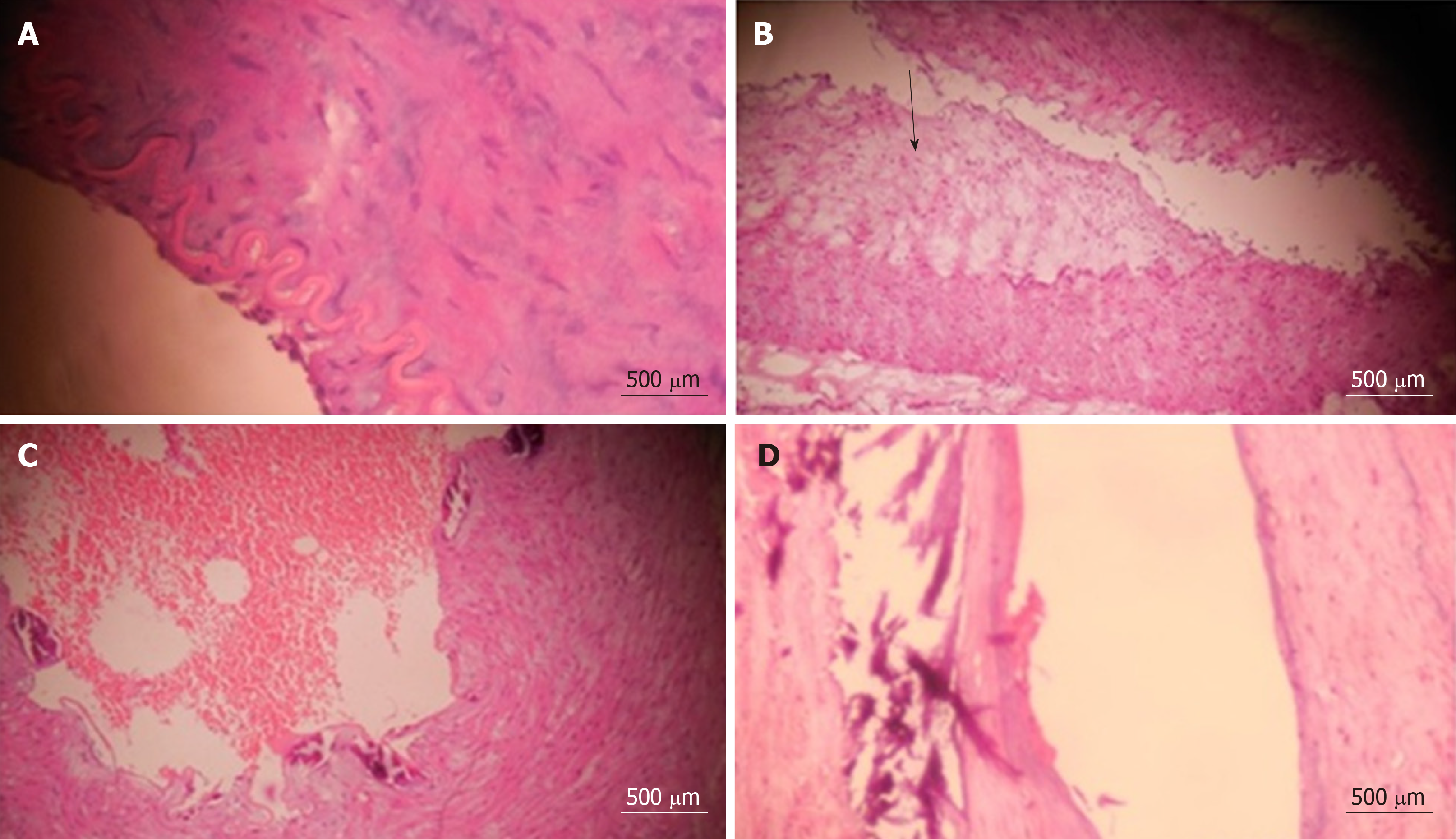
Figure 1 shows typical histopathological appearances of all the characteristics of abnormal veins. Frozen section analysis of the splenic venous wall corroborated with histopathological assessment in all but two cases, i.e. 98%. A splenic venous aneurysm was found in one case, while a splenic artery aneurysm was found in 11 patients. Histopathological examination of the splenic artery was performed in all cases. Eighty nine percent (103/116) of patients had medial hypertrophy, of whom 6% (8) had intimal thickening. Liver biopsies taken intraoperatively showed normal liver histology in all cases with EHPVO, 30/63 (48%) cases with NCPF, and both mild periportal inflammatory changes and periportal fibrosis in the rest of the NCPF cases.
Damage to the intima of visceral veins and contractile structures in the visceral arterial wall, termed as PHV, has been observed in patients with cirrhosis and PH[1-3]. In several studies, different mechanisms of pathogenesis of PHV have been proposed. PHV is most likely primarily related to hemodynamic changes in the portal system, particularly to high pressure perfusion of veins in cirrhotics. Other proposed contributing factors may include immune response, gene modulation, vasoactive substances, and intrahepatic blood flow resistance[3,4].
Increased pressure in the portal system compresses feeding vessels, called vasa vasorum, and reduces partial pressure of oxygen in them. This results in ischemic damage and consequent nutritional depletion of vascular intima. Increased pressure with stiffening of walls (and medial layer hypertrophy) leads to displacement of vessel wall architecture and consequent shear strain on the adventitial layer, which further lodges the vasa vasorum. Injury to the vasa vasorum leads to ischemia of the vein wall with degeneration of internal elastic lamina and media. Ischemia leads to the release of growth factors and seepage of extracellular matrix into media with a resultant change in the medial smooth muscle phenotype and hypertrophy. In the venous system, intimal changes are associated with luminal thrombosis due to activation of platelets and fibrin.
Proliferative intima, thrombus adherent to the venous wall, smooth muscle hypertrophy, and increased extracellular matrix was found in both the splenic and gastric coronary veins of cirrhotic patients[3]. In cirrhotic patients, vessels are reported to have a higher sensitivity to Angiotensin II, and this has been ascribed to injury of vascular endothelial cells and basement membrane by portal HC. Intimal damage probably influences contractile function of the vessels[5-7]. Smooth muscle cells of the vasculature are found to be predominantly of the synthetic type in them.
Examination of the splenic and portal veins in a series of patients with cirrhosis has led to a classification of the changes, which occur in successive stages[4]. The different stages give an indication of the extent or duration of the congestion. Thrombosis has been reported to be present in the group that showed the most significant changes in the vein walls. Intimal changes with small sub-endothelial muscle bundles arranged longitudinally were reported to succeed medial muscle hypertrophy in the portal vein. As a result of fibrous tissues replacing these muscles, intima appeared thickened and fibrotic. No evidence of any degenerative or atheromatous changes were reported. Since veins were found with medial muscular hypertrophy without intimal thickening but not the other way round, it appears reasonable to assume that the changes in the intima succeed those in the medial muscle. Changes in the splenic and portal vein were similar; the extent of change was of a lesser degree in the former. Extensive intimal thrombosis is reported to be a culmination of this chain of events[5]. Calcification in the wall of sclerotic veins has been reported to occur only in the late stages of evolution of these changes. Venous wall inflammation has been proposed to be one of the factors playing a vital role in the pathogenesis of phlebosclerosis and in the eventual development of wall calcification[5].
In our study of pathological features of splenic veins, we encounter features similar to those reported for cirrhotic patients. We have found the presence of medial hypertrophy and wall thickening in nearly all splenic veins (107/116; 92%). Intimal fibrosis was found in 35 (30%) patients, all of whom had medial hypertrophy. We noted that 32 of 35 (91%) patients with intimal fibrosis also had venous thrombosis. Vein wall calcification, which occurs in the late stage of PHV as noted above, was found in 26 (22%) patients, all of whom had intimal fibrosis and venous thrombosis.
Medial hypertrophy and intimal fibrosis was observed in patients without PH who underwent splenectomy for hemolytic anemia and had splenomegaly. This is probably a result of the presence of raised portal pressure and HC in this subgroup of patients. On the other hand, the splenic veins of all patients with ITP who had normal sized spleen and did not have any were found to be normal.
These changes in PSMS are similar to the ones observed in the pulmonary vasculature in cases of pulmonary artery hypertension[8]. Pulmonary arteriolar remodelling occurs in pulmonary hypertension (defined as mean pulmonary arterial pressure ≥ 25 mmHg). Histopathological changes including medial hypertrophy, intimal and adventitial fibrosis, thickening of the alveolar-capillary membrane, and luminal occlusion in small pulmonary arterioles have been reported. The mechanisms underlying the thickening of the pulmonary vascular medial layer have been linked mostly to cell proliferation and, more recently, to inhibition of cell apoptosis[9-11]. Raised mean pulmonary arterial pressure (≥ 25 mmHg) is in the same range as the raised portal pressure in our patients with NCPH (median portal pressure of 25 mmHg; range: 15-51 mmHg). We suggest that the pathological changes that we report in splenic vasculature in PH are the result of a raised portal pressure and HC, similar to the effect of raised pulmonary artery pressure on pulmonary vasculature.
Splenic artery in the presence of PH also shows pathological abnormalities. Microscopic examination of splenic arterial walls in cirrhotics is reported to undergo considerable thickening with disrupted endothelial cells. Disturbed smooth muscle cell layers with disrupted internal elastic lamina were also noted[3]. This injury is perhaps a result of increased pressure and flow, as well as increased cytokines, growth factors, shearing force and oxygen stress. In our study, 96% of patients with NCPH had abnormal splenic arteries upon histopathological examination (103/116; 89% had medial hypertrophy while 8/116; 7% had intimal thickening).
The similarity of the pathological features in the vasculature of patients with cirrhosis, NCPH with PHV and pulmonary hypertension leads us to conclude that the features we observe in NCPH are caused by raised pressure and HC, and not due to any degenerative disease of the veins. The same is true for splenic veins of patients with splenomegaly and hemolytic anemia who also have HC. Venous thrombosis has been reported to be the terminal event of all pathological changes in the venous wall of cirrhotics with PHV[5]. The raised pressure in the PSMS in both cirrhotic and non-cirrhotic PH could thus potentially result in the development of mesenteric venous thrombosis and intestinal gangrene. A reduction in portal pressure can prevent the development of this condition. The role of non-selective beta blockers in this context should be reassessed. Since the lifespan of patients with NCPH is compared to that of cirrhotics, compliance to lifelong drug therapy is unlikely. It is difficult to predict the clinical value and cost effectiveness of such treatment in preventing deaths from variceal bleeding, as well as preventing complications related to mesenteric venous thrombosis[12]. According to a meta-analysis, haemodynamic response rate to non-selective beta blockers is only 46%[13]. In view of these, a prophylactic shunt may be justified to reduce portal pressure and prevent complications.
We observed that the proportion of patients with pathological abnormalities in the splenic vein were significantly greater in the delayed presentation group as compared to the early presentation group. On this basis, we propose that interventions to reduce portal pressure must be initiated at the diagnosis of NCPH instead of waiting for the patient to be symptomatic before the initiation of therapy. The damage to the vasculature starts early and can be prevented from progressing to venous thrombosis and its sequelae if early surgical intervention is initiated to reduce portal pressure. The complete reversibility of the pathological changes in the veins, once established, is uncertain.
Pathological changes in splenic vein similar to those in cirrhotic patients have been observed in patients with NCPH. They reflect the effect of HC and increased pressure in the PSMS, and are not primarily due to any venous degenerative changes. These changes are similar to those observed in pulmonary vasculature in pulmonary hypertension. We recommend that PH in NCPH be treated as systemic and pulmonary hypertension equivalent in the gastrointestinal tract. We show that these pathological venous changes in NCPH are observed in a greater proportion of patients in the delayed presentation group (P < 0.003). It would therefore be interesting to further explore the utility of early aggressive intervention to reduce portal pressure and hemodynamic stress to avoid potential lethal effects of mesenteric venous thrombosis and its sequelae on the intestine, liver and pancreas.
Portal hypertension (PH) is known to be associated with changes in vascular structure and function of the portosplenomesenteric system (PSMS, portal hypertensive vasculopathy). Pathological abnormalities of PSMS has been described in the literature only for cirrhotic patients. This vasculopathy is believed to be related to raised portal pressure and hyperdynamic circulation (HC).
In view of similar circulatory changes in PSMS, it is anticipated that pathological changes in the splenic and portal veins, which are similar to those reported in cirrhotic patients with PH, may also be present in patients with non-cirrhotic PH (NCPH). Venous thrombosis is the terminal event of this vasculopathy. Early shunt surgery in NCPH may prevent the development of mesenteric venous thrombosis and its sequelae.
In this study, we aimed to study the possible association of long-term exposure of PSMS to raised pressure and HC, portal hypertensive vasculopathy (PHV) and resultant mesenteric venous thrombosis and its sequelae.
A prospective observational study was performed on 116 patients with NCPH (extrahepatic portal vein obstruction: 53 and non-cirrhotic portal fibrosis: 63) who underwent proximal splenorenal shunt, interposition shunt or splenectomy with devascularization in JIPMER, Pondicherry, India, a tertiary level referral center, between 2011-2016. All patients were evaluated by Doppler study of PSMS, computed tomography porto-venogram and upper gastrointestinal endoscopy. Acoustic resonance forced impulse scans and abdomen ultrasounds were done for all cases to exclude cirrhosis. Intraoperative and histopathological assessment of the harvested splenic vein was performed in all. The study group was divided into delayed and early presentation based on the median duration of symptoms (i.e. 108 mo).
Upon histopathological examination of veins, splenic veins in all patients with NCPH were found to be abnormal (based on the presence of one or more pathological characteristics studied).
There is no report in the literature on PHV in NCPH. PHV involving the splenic vein is similar in cirrhotic as well as non-cirrhotic portal hypertensive patients. They reflect the effect of HC and increased pressure in the PSMS. We show that these pathological venous changes in NCPH are observed in a greater proportion of patients in the delayed presentation group (P < 0.003). It would therefore be interesting to explore the utility of early aggressive intervention to reduce portal pressure and hemodynamic stress to avoid potential lethal effects of mesenteric venous thrombosis and its sequelae on the intestine, liver and pancreas.
It would be significant to investigate the role of early shunt surgery in NCPH to prevent the worsening of PHV and the development of mesenteric venous thrombosis and its sequelae in these patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): C
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caronna R, Dragoteanu MN, Isik A S-Editor: Yan JP L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Li T, Ni JY, Qi YW, Li HY, Zhang T, Yang Z. Splenic vasculopathy in portal hypertension patients. World J Gastroenterol. 2006;12:2737-2741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Li T, Yang Z. Research progress of vasculopathy in portal hypertension. World J Gastroenterol. 2005;11:6079-6084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Yang Z, Zhang L, Li D, Qiu F. Pathological morphology alteration of the splanchnic vascular wall in portal hypertensive patients. Chin Med J (Engl). 2002;115:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Wilson JB. Changes in the portal and splenic veins in portal hypertension and their relation to splenomegaly. Gut. 1961;2:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Li PL. Adaptation in veins to increased intravenous pressure, with special reference to the portal system and inferior vena cava. J Path Bact. 1940;50:121-136. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | McCarthy AL, Woolfson RG, Raju SK, Poston L. Abnormal endothelial cell function of resistance arteries from women with preeclampsia. Am J Obstet Gynecol. 1993;168:1323-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 182] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Hosokawa H, Aiuchi S, Kambe T, Hagiwara Y, Kubo T. Mechanical stretch-induced mitogen-activated protein kinase activation is mediated via angiotensin and endothelin systems in vascular smooth muscle cells. Biol Pharm Bull. 2002;25:1588-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiéry JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37:942-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 442] [Cited by in RCA: 495] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 9. | Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28:23-42, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Yi ES, Kim H, Ahn H, Strother J, Morris T, Masliah E, Hansen LA, Park K, Friedman PJ. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension. A morphometric and immunohistochemical study. Am J Respir Crit Care Med. 2000;162:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Palevsky HI, Schloo BL, Pietra GG, Weber KT, Janicki JS, Rubin E, Fishman AP. Primary pulmonary hypertension. Vascular structure, morphometry, and responsiveness to vasodilator agents. Circulation. 1989;80:1207-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 129] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Sarin SK, Kapoor D. Non-cirrhotic portal fibrosis: current concepts and management. J Gastroenterol Hepatol. 2002;17:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Albillos A, Bañares R, González M, Ripoll C, Gonzalez R, Catalina MV, Molinero LM. Value of the hepatic venous pressure gradient to monitor drug therapy for portal hypertension: a meta-analysis. Am J Gastroenterol. 2007;102:1116-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |