Published online Aug 27, 2019. doi: 10.4240/wjgs.v11.i8.334
Peer-review started: June 4, 2019
First decision: August 2, 2019
Revised: August 10, 2019
Accepted: August 13, 2019
Article in press: August 2, 2019
Published online: August 27, 2019
Processing time: 89 Days and 12.7 Hours
The anorectal leiomyosarcoma (LMS) is an aggressive malignant neoplasm. Owing to the rarity of LMSs, an optimal treatment modality has yet to be determined.
To collect all published data on anorectal LMS characteristics, explore current treatment options, and review recent cases of postradiation LMS.
A literature search of the PubMed electronic database was conducted using the MeSH terms “rectal neoplasms”, “anus neoplasms” and “gastrointestinal neoplasms” combined with “leiomyosarcoma”. The search was limited to English language and human studies. All available case reports and case series of anal or rectal LMSs that were published from the beginning of January 1996 to May 2017 were included if the diagnosis of LMS had been confirmed by histopathologic examination. Data were analyzed using simple statistics (mean, median, and standard deviation). Independent sample t-test was used to compare means for continuous variables.
A total of 27 articles reporting on 51 cases of anorectal LMS were identified. Among these cases, 11.7% had undergone previous pelvic radiotherapy (developing LMS at 13-35 years afterwards). Anorectal LMS affected the rectum in 92.2% of the cases, and no sex-based predominance was observed. Surgical resection with negative margins remains the mainstay of treatment, which can be accomplished with wide local excision or radical resection. The local recurrence rate was higher among cases who received wide local excision (30%), as compared to radical resection (20%); however, the overall rate of metastasis was 51.61% regardless of the treatment approach. The use of neoadjuvant radiation lowers the risk of local recurrence compared to adjuvant radiotherapy, and facilitates R0 resection of the tumor. Cases treated with adjuvant chemotherapy showed better rates of distant recurrence and overall survival. Nonetheless, multidisciplinary team discussion is necessary to determine the optimal management plan whilst considering patient- and disease-related factors.
A multidisciplinary team approach, considering the underlying patient- and disease- related factors, is necessary for optimal management of these complex tumors.
Core tip: The current mainstay treatment of anorectal leiomyosarcoma is surgical resection with negative margins. Based on the published case series and reports, sphincter-preserving surgery followed by radiotherapy yields local recurrence rates that are comparable to radical resection. Moreover, neoadjuvant radiation improves local recurrence rates, as compared to adjuvant radiation. Adjuvant chemotherapy significantly improves rates of distant recurrence and overall survival; however, the choice to use chemotherapy in this setting should be determined according to a multidisciplinary team consideration of patient-related factors and treatment toxicity. Since local and distant tumor recurrences are common, even years after resection, post-surgery long-term follow-up is needed.
- Citation: Nassif MO, Habib RA, Almarzouki LZ, Trabulsi NH. Systematic review of anorectal leiomyosarcoma: Current challenges and recent advances. World J Gastrointest Surg 2019; 11(8): 334-341
- URL: https://www.wjgnet.com/1948-9366/full/v11/i8/334.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v11.i8.334
Leiomyosarcomas (LMSs) are malignant neoplasms of smooth muscle origin. They rarely arise in the anorectum, having an estimated incidence of < 0.1% among all cases of anorectal malignancies[1]. Diagnosis of anorectal LMS relies on identification of a characteristic profile of histological features and immunohistochemical markers. Typically, these tumors express the smooth muscle markers of smooth muscle actin, muscle-specific actin, desmin, and h-caldesmon, and are negative for KIT (CD117), CD34, and DOG-1[2]. Such markers also serve to facilitate the differentiation of LMSs from gastrointestinal stromal tumors, since both tumors have similar histological appearance. Microscopically, LMSs appear as spindle cell tumors. The presence of cellular atypia and high mitotic activity [> 50 per 50 high power field (HPF)] further supports the diagnosis of LMS, and allows for differentiation from benign leiomyoma[3-5]. Different treatment approaches, including radical resection, sphincter-preserving surgery, and adjuvant treatments, have been reported. However, owing to the rarity of LMSs, the optimal treatment modality is yet to be determined[6].
Despite the unique characteristics of anorectal LMSs, their features, management, and outcomes are usually reported in conjunction with data on colonic or other gastrointestinal LMSs in the literature[7]. Hatch et al[8] have periodically published literature reviews of all cases of anorectal soft tissue tumors independently; the latest published review was in the year 2000. However, their studies described anorectal LMSs prior to the introduction of immunohistochemistry, and since that time no further reviews have been published to describe the post-immunohistochemistry recent cases of anorectal LMSs.
To supplement and carry on the work of Hatch et al[8], we designed this study to collect all characteristic data regarding LMSs of the anorectum, to explore current treatment options and their outcomes, and to provide an overview of the recent reports of radiation-induced LMSs of the anorectum.
The study was approved by the ethics committee of our institution (Ref. No. 255-16).
A literature search was conducted in the PubMed database using the MeSH terms “rectal neoplasms”, “anus neoplasms”, and “gastrointestinal neoplasms” combined with “leiomyosarcoma”. The search was restricted to articles published between January 1996 and end of May 2017, in the English language, and on humans. All case reports and case series of anal or rectal LMSs were considered, and additional studies were identified by manually searching the reference lists of the selected articles. Two authors, working independently, screened the titles and abstracts of each retrieved article, and those which were relevant were selected for full-text review and assessment for inclusion. Cases that were confirmed to be anorectal LMS by histopathologic examination were included.
Statistical analyses were performed using SPSS version 23 (The Statistical Package for the Social Sciences software; IBM Corp., Armonk, NY, United States). Due to heterogeneity of the reported mitotic rates, studies that reported the mitotic rate per 10 HPF were multiplied by 5 to unify the mitotic rate. This strategy was chosen after discussion with multiple renowned pathologists specialized in the field. Studies that reported the mitotic rate in < 10 HPF were excluded from calculation of the mean. Data were analyzed using simple statistics, such as by mean, median, and standard deviation. Independent sample t-test was used to compare the means for continuous variables. P value < 0.05 was considered significant.
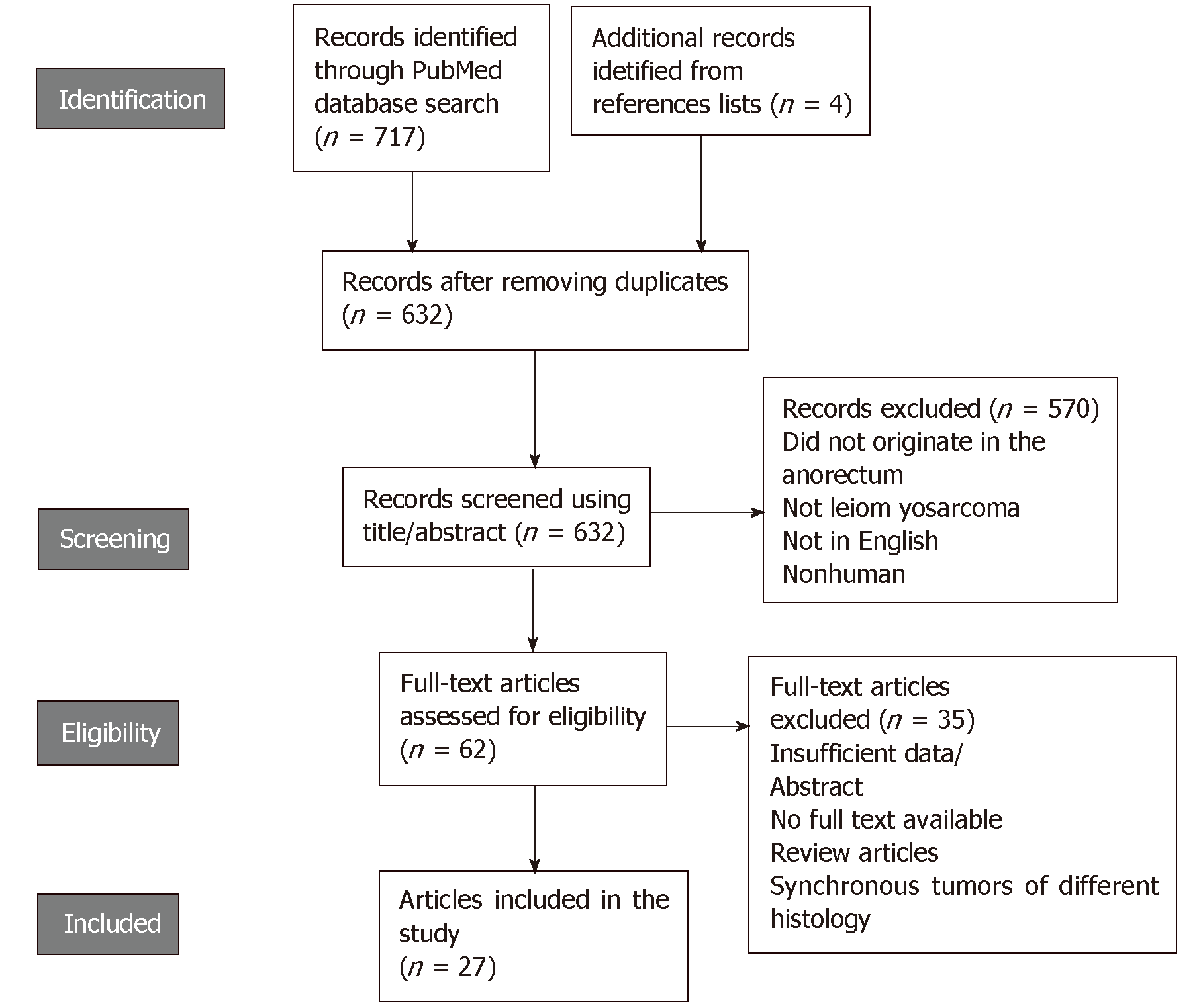
The literature search yielded 628 articles after removing duplicates, 570 of which were excluded because they did not meet the inclusion criteria (Figure 1). After a full-text review of the remaining 58 studies, 35 were further excluded and a total of 23 articles were compiled along with 4 additional articles identified by searching the reference lists. Finally, 27 articles were included in our review, reporting on a total of 51 cases of anorectal LMS. Of these cases, 47% (24/51) were confirmed by immunohistochemistry to be LMS, whereas the remaining were diagnosed by histopathologic examination alone. The tumors occurred mainly in the rectum 92% (47/51), and 8% (4/51) were located in the anal canal. Mean age at the time of diagnosis was 60 ± 17.1 years, affecting males and females equally. These tumors were commonly polypoid masses in appearance, with a median size of 6 cm [interquartile range (IQR) of 1.5-22 cm]. Moreover, 12% (6/51) of the patients reported a history of having undergone pelvic radiotherapy for tumors not related to anorectal LMSs. Rectal LMSs developed 13-35 years following the radiation. Additional clinicopathologic findings are summarized in Table 1.
| Characteristic | Data |
| Location, n = 51% | |
| Rectal | 47 (92.2) |
| Anal | 4 (7.8) |
| Sex, n = 51% | |
| Female | 26 (51) |
| Male | 25 (49) |
| Mean age ± standard deviation, n = 51 | 60 ± 17.1 yr |
| Median tumor size (IQR), n = 38 | 6 (1.5-22) cm |
| Mean mitotic rate ± standard deviation of mitoses/50 HPF, n = 21 | 68.1 ± 40.42 |
| Grade, n = 16% | |
| High | 10 (62.5) |
| Intermediate | 2 (12.5) |
| Low | 4 (25) |
| Symptoms, n = 35% | |
| Rectal bleeding | 17 (48.57) |
| Pain, rectal/abdominal | 13 (37.14) |
| Weight loss | 4 (11.43) |
| Constipation | 4 (11.43) |
| Altered bowel motion | 3 (8.57) |
| Protruding mass | 3 (8.57) |
| Asymptomatic | 3 (8.57) |
| Surgery, n = 45% | |
| Wide local excision | 11 (24.4) |
| Abdominoperineal resection | 14 (31.11) |
| Low anterior resection | 12 (26.7) |
| Others | 8 (17.8) |
| Outcome, n = 31% | |
| DOD | 13 (41.94) |
| ANED | 11 (35.48) |
| AWD | 4 (12.9) |
| DDD | 3 (9.68) |
Complete surgical resection with negative margins was the primary goal in the management of localized anorectal LMSs. The main surgical procedures performed were either wide local excision or radical excision (i.e., low anterior resection or abdominoperineal resection). Extensive surgical procedures, including en bloc resection and pelvic exenteration, had been required when tumor invasion into adjacent organs was present, as evidenced by preoperative imaging or intraoperative findings.
Local excision was performed in 24% (11/45) of cases, only 2 of which received postoperative radiotherapy, and the largest size of these tumors measured 7 cm. The status of tumor margin was not reported in all cases. Patients treated with local excision had higher rate of local recurrence (30%, 3/10) than radical resection (20%, 3/15); however, distant metastasis was higher in those who underwent radical resection (53.3%, 8/15 vs 20%, 2/10 for local excision). There was no significant difference found for tumor size between local excision (mean: 4.1 cm) and radical resection (mean: 6.2 cm, P = 0.1). These tumors demonstrated similar mitotic rates as well (local excision; mean of 50.4/ 50 HPF vs radical resection; mean of 58.6/ 50 HPF, P = 0.63). Lymphadenectomy was performed in 15 cases, and only 1 case was positive for lymph node metastasis, which demonstrated a high mitotic rate of 10/1 HPF.
Among the patients with the relevant data reported, adjuvant radiotherapy was given in 40% (8/20), either to decrease the risk of local recurrence following wide local excision, to address positive resection margins (1 case), or to address large tumor size. Local recurrence occurred in 1 patient after 111 mo, and distant metastasis developed in 62.5% (5/8) of patients after a median of 14.5 mo (IQR: 5-111 mo) of follow-up.
Regardless of the treatment approach, the rate of local recurrence of the LMSs was 29% (9/31) and that of secondary metastasis was 52% (16/31). The most common site of distant metastasis was the liver, followed by the lung. At a median follow-up period of 24 mo (IQR: 1-325 mo), 42% (13/31) of the patients died of the disease and 35% (11/31) were alive with no evidence of the disease.
The mainstay treatment of anorectal LMS is surgical resection with negative margins, which can be accomplished with local excision or radical resection. In the literature, wide local excision has been found to be associated with a higher rate of local recurrence (55%) compared to radical resection (24%), and the rate of distant metastasis was similar between the two operations[6]. Similarly, in our review the rate of local recurrence was not significantly different between the two operations (30% vs 20%), although a higher rate of distant metastasis was observed with radical resection, even though the tumors’ sizes and mitotic rates were similar between the two treatment approaches. This could be due to surgery selection bias as these cases would be more advanced locally and/or invading nearby structures, necessitating a radical excision.
Sphincter-preserving surgery followed by brachytherapy and/or external beam radiation has been investigated as an alternative to abdominoperineal resection. Grann et al[9] reported on 8 patients with tumors of 5 cm or less in size managed with this approach. The rate of local recurrence was 25% after 53 mo of follow up. These results were comparable to LMSs treated with abdominoperineal resection, where (19.5%) of patients developed local recurrence, and superior to those treated with wide local excision alone (67.5%) as described in another study. Although, it is important to note that tumor sizes ranged from 1 cm to 20 cm in that study[1]. There are a limited number of studies that have explored the outcomes of sphincter- preserving surgery that are nonrandomized and retrospective in nature, and have wide variation in histological grades and margin status across the reported cases[9-11]. Therefore, further randomized controlled trials (commonly known as RCTs) are needed to establish the benefit and criteria of patients eligible for this treatment approach.
Studies investigating the role of radiotherapy in anorectal LMS exclusively are lacking due the rarity of these tumors. However, the benefit of radiation therapy has been explored in retroperitoneal sarcomas by several studies who have reported improved local control rates after neoadjuvant radiotherapy. A meta-analysis including 11 studies with 1 RCT showed significantly improved local recurrence risk with preoperative compared to postoperative radiotherapy in resectable retroperitoneal sarcoma (odds ratio: 0.03, P = 0.02)[12]. Two prospective trials have reported favorable 5-year local recurrence- free survival rate of 60%, disease-free survival rate of 46%, and overall survival rate of 61% in patients with localized operable retroperitoneal sarcoma who underwent neoadjuvant radiotherapy[13]. Another RCT evaluating the benefit of neoadjuvant radiotherapy and complete surgical resection vs surgery alone in retroperitoneal sarcoma is underway (European Organisation for Research and Treatment of Cancer: EORTC 62092 STRASS Trial)[14].
LMSs rarely metastasize to lymph nodes, as shown in our study. Therefore, lymphadenectomy is not indicated unless regional lymph nodes are found to be enlarged in preoperative imaging. Leaving a positive margin should be avoided, since it is an independent predictor of local recurrence, and re-excision is indicated in cases of R1 or R2 resection whenever feasible[15]. Management of local or distant recurrence of LMSs is carried out by surgical resection or palliative chemoradiation. Surgical resection of liver metastases from a primary resectable colorectal LMS has been found to be associated with prolonged overall survival, with a median of 47 mo (range: 7-135 mo) in 5 patients[16].
Regarding the role of adjuvant chemotherapy, different regimens have been assessed in multiple trials; none of which, however, have been specific for abdominal LMSs. Doxorubicin-based regimens remain the standard first-line chemotherapy for metastatic or locally advanced soft tissue sarcoma, with an overall response rate of 14% (31/228) and a median overall survival of 12.8 mo[17]. For resectable soft tissue sarcomas, multiagent combination chemotherapy has shown promising results compared to single-agent regimens; this includes the combination of doxorubicin and ifosfamide, that resulted in significant reduction in distant recurrence rate (odds ratio of 0.61, 95% confidence interval of 0.41-0.92, P = 5.02) as well as reduced mortality with a hazard ratio of 0.56 (95% confidence interval of 0.36-0.85, P = 5.01). However, no significant changes were reported for local recurrence rates[18]. In addition, combination of doxorubicin and olaratumab showed significantly improved overall survival compared to doxorubicin alone (26.5 mo vs 14.7 mo, P = 0.003), having an acceptable safety profile[19]. Moreover, second-line agents that have been found to be effective against LMSs are trabectedin and pazopanib[20,21].
The prognosis of anorectal LMSs remains poor, even after surgical resection. Yeh et al[6] reported 5-year overall survival and disease-free survival rates of 75% and 46%, respectively, in 40 patients after tumor resection. A high mitotic rate (≥ 10/10 HPF), large tumor size (> 10 cm), and high tumor grade were found to be consistently associated with worse survival and higher risk of metastasis[6,15,22]. For radiation- induced sarcoma, a study[23] found that LMSs had a favorable outcome compared to other histological types, with 5-year disease-specific survival of 68%. However, that study included abdominal, extremity, and trunk LMSs. Moreover, LMSs developed after a median duration of 23.5 years following radiation, which was the longest latency period upon comparison to other sarcomas. Regardless of histological type, though, the 5-year disease-specific survival was significantly less in the radiation- induced sarcoma cases than in those of sporadic sarcoma. Margin status, tumor size, and histological type were independent predictors of disease-specific survival.
One of the limitations of our study is that it included cases of LMSs that were not proven by immunohistochemistry to be of smooth muscle origin. Also, there was wide variation in the reported follow-up periods and incomplete information in the included cases, both of which precluded survival analysis.
In conclusion, the current mainstay treatment of anorectal LMS is surgery. Neoadjuvant radiotherapy may improve local control after resection; however, local and distant recurrence are common, which may develop years after resection. Therefore, long- term follow-up is needed after the surgery.
Anorectal leiomyosarcomas (LMSs) are rare and complex tumors, known to present a therapeutic dilemma and having a high tumor recurrence risk after resection. Prior to application of immunohistochemistry to their diagnosis, LMSs were often misdiagnosed as gastrointestinal stromal tumors, which have a different treatment approach and prognosis. Additionally, owing to the rarity of anorectal LMSs, they are usually reported collectively with LMSs in other locations of the gastrointestinal tract.
To conduct a recent and comprehensive review of anorectal LMS in the time following the advent of immunohistochemistry use to highlight the tumor characteristics, treatment approach, role of adjuvant chemoradiation, and tumor prognosis as well as to review postradiation LMS of the anorectum.
To conduct a recent and comprehensive review of anorectal LMS in the time following the advent of immunohistochemistry use to highlight the tumor characteristics, treatment approach, role of adjuvant chemoradiation, and tumor prognosis as well as to review postradiation LMS of the anorectum.
A systematic literature search of the PubMed electronic database was conducted using the MeSH terms “rectal neoplasms”, “anus neoplasms” and “gastrointestinal neoplasms” combined with “leiomyosarcoma”. The search was limited to English language and studies on humans. All available case reports and case series of anorectal LMSs that were published from January 1996 to May 2017 were included if the diagnosis of LMS had been confirmed by histopathologic examination.
We identified a total of 27 articles, reporting on 51 cases of anorectal LMS. Of these, 6 reported on cases of previous pelvic radiotherapy who had developed LMS 13-35 years after the radiation. Anorectal LMS affected the rectum in 92.2% of the cases, and no sex-based predominance was observed. Surgical resection with negative margins remains the mainstay of treatment, which can be accomplished by wide local excision or radical resection. The rate of local recurrence was higher in wide local excision (30%) compared to radical resection (20%), and the overall rate of metastasis was 51.61% regardless of the treatment approach. Use of neoadjuvant radiation lowers the risk of local recurrence, as compared to adjuvant radiotherapy, and facilitates R0 resection of the tumor. The use of adjuvant chemotherapy has shown improvement in distant recurrence and overall survival rates; however, multidisciplinary team discussion is necessary to determine the optimal management plan whilst considering patient and disease-related factors.
The mainstay treatment of anorectal LMS is surgical resection with negative margins. Sphincter-preserving surgery followed by radiotherapy showed comparable local recurrence rates to radical resection based on case series and reports. Neoadjuvant radiation improved local recurrence rates compared to adjuvant radiation. Adjuvant chemotherapy showed significant improvement in distant recurrence and overall survival rates; however, use of chemotherapy in this setting should be assessed by a multidisciplinary team and with consideration to patient-related factors and treatment toxicity. Nevertheless, local and distant tumor recurrence are common and may develop years after the resection. Therefore, long-term follow-up is needed after surgery.
Anorectal LMSs are rare tumors and further randomized controlled trials are needed to outline the criteria for patients’ eligibility for sphincter-preserving surgery compared to radical resection. A multidisciplinary team approach is necessary for optimal management.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoki H S-Editor: Ma RY L-Editor: A E-Editor: Zhou BX
| 1. | Khalifa AA, Bong WL, Rao VK, Williams MJ. Leiomyosarcoma of the rectum. Report of a case and review of the literature. Dis Colon Rectum. 1986;29:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Nassif MO, Trabulsi NH, Bullard Dunn KM, Nahal A, Meguerditchian AN. Soft tissue tumors of the anorectum: rare, complex and misunderstood. J Gastrointest Oncol. 2013;4:82-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Yamaguchi U, Hasegawa T, Masuda T, Sekine S, Kawai A, Chuman H, Shimoda T. Differential diagnosis of gastrointestinal stromal tumor and other spindle cell tumors in the gastrointestinal tract based on immunohistochemical analysis. Virchows Arch. 2004;445:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Aggarwal G, Sharma S, Zheng M, Reid MD, Crosby JH, Chamberlain SM, Nayak-Kapoor A, Lee JR. Primary leiomyosarcomas of the gastrointestinal tract in the post-gastrointestinal stromal tumor era. Ann Diagn Pathol. 2012;16:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 325] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Yeh CY, Chen HH, Tang R, Tasi WS, Lin PY, Wang JY. Surgical outcome after curative resection of rectal leiomyosarcoma. Dis Colon Rectum. 2000;43:1517-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Hilal L, Barada K, Mukherji D, Temraz S, Shamseddine A. Gastrointestinal (GI) leiomyosarcoma (LMS) case series and review on diagnosis, management, and prognosis. Med Oncol. 2016;33:20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Hatch KF, Blanchard DK, Hatch GF, Wertheimer-Hatch L, Davis GB, Foster RS, Skandalakis JE. Tumors of the rectum and anal canal. World J Surg. 2000;24:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Grann A, Paty PB, Guillem JG, Cohen AM, Minsky BD. Sphincter preservation of leiomyosarcoma of the rectum and anus with local excision and brachytherapy. Dis Colon Rectum. 1999;42:1296-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Minsky BD, Cohen AM, Hajdu SI, Nori D. Sphincter preservation in rectal sarcoma. Dis Colon Rectum. 1990;33:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Minsky BD, Cohen AM, Hajdu SI. Conservative management of anal leiomyosarcoma. Cancer. 1991;68:1640-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Albertsmeier M, Rauch A, Roeder F, Hasenhütl S, Pratschke S, Kirschneck M, Gronchi A, Jebsen NL, Cassier PA, Sargos P, Belka C, Lindner LH, Werner J, Angele MK. External Beam Radiation Therapy for Resectable Soft Tissue Sarcoma: A Systematic Review and Meta-Analysis. Ann Surg Oncol. 2018;25:754-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Pawlik TM, Pisters PW, Mikula L, Feig BW, Hunt KK, Cormier JN, Ballo MT, Catton CN, Jones JJ, O'Sullivan B, Pollock RE, Swallow CJ. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006;13:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | European Organisation for Research and Treatment of Cancer - EORTC. Surgery With or Without Radiation Therapy in Untreated Nonmetastatic Retroperitoneal Sarcoma (STRASS). [Accessed on 6 August, 2019]. In ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01344018. |
| 15. | Gladdy RA, Qin LX, Moraco N, Agaram NP, Brennan MF, Singer S. Predictors of survival and recurrence in primary leiomyosarcoma. Ann Surg Oncol. 2013;20:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Faraj W, El-Kehdy J, Nounou GE, Deeba S, Fakih H, Jabbour M, Haydar A, El Naaj AA, Abou-Alfa GK, O'Reilly EM, Shamseddine A, Khalife M, Mukherji D. Liver resection for metastatic colorectal leiomyosarcoma: a single center experience. J Gastrointest Oncol. 2015;6:E70-E76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 17. | Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan J, Hohenberger P, Krarup-Hansen A, Alcindor T, Marreaud S, Litière S, Hermans C, Fisher C, Hogendoorn PC, dei Tos AP, van der Graaf WT; European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 831] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 18. | Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 662] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 19. | Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, Agulnik M, Cooney MM, Livingston MB, Pennock G, Hameed MR, Shah GD, Qin A, Shahir A, Cronier DM, Ilaria R, Conti I, Cosaert J, Schwartz GK. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 454] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 20. | van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP, Beppu Y, Le Cesne A, Gelderblom H, Judson IR, Araki N, Ouali M, Marreaud S, Hodge R, Dewji MR, Coens C, Demetri GD, Fletcher CD, Dei Tos AP, Hohenberger P; EORTC Soft Tissue and Bone Sarcoma Group; PALETTE study group. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1455] [Cited by in RCA: 1562] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 21. | Demetri GD, von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, Milhem M, Elias A, Ganjoo K, Tawbi H, Van Tine BA, Spira A, Dean A, Khokhar NZ, Park YC, Knoblauch RE, Parekh TV, Maki RG, Patel SR. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol. 2016;34:786-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 613] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 22. | Delaney TF, Kepka L, Goldberg SI, Hornicek FJ, Gebhardt MC, Yoon SS, Springfield DS, Raskin KA, Harmon DC, Kirsch DG, Mankin HJ, Rosenberg AE, Nielsen GP, Suit HD. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67:1460-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Gladdy RA, Qin LX, Moraco N, Edgar MA, Antonescu CR, Alektiar KM, Brennan MF, Singer S. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol. 2010;28:2064-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |