Published online Jun 27, 2019. doi: 10.4240/wjgs.v11.i6.287
Peer-review started: May 10, 2019
First decision: May 31, 2019
Revised: June 13, 2019
Accepted: June 20, 2019
Article in press: June 21, 2019
Published online: June 27, 2019
Processing time: 49 Days and 14.6 Hours
Hepatocellular carcinoma is one of the leading malignancies worldwide. Early detection of hepatocellular carcinoma and its management in the form of liver transplantation offers an attractive treatment option. The Milan criteria, proposed by Mazzaferro et al, have been the standard for selecting patients with hepatocellular carcinoma for transplantation. Recently, several studies have shown that even patients selected outside the Milan criteria can undergo transplantation with a relatively good outcome. This article examines the currently existing criteria other than the Milan criteria and also evaluates use of alpha-fetoprotein and positron emission tomography scans to predict the chance of recurrence.
Core tip: The Milan criteria have been used extensively worldwide to select patients with hepatocellular carcinoma for liver transplantation. Over the years, it has been questioned whether the Milan criteria are too restrictive and whether patients outside the Milan criteria could benefit from liver transplantation. Several other criteria have been proposed and validated, and latest is the hepatocellular carcinoma Metroticket concept. This minireview evaluates the various current criteria that exist for liver transplantation for hepatocellular carcinoma cases.
- Citation: Mullath A, Krishna M. Hepatocellular carcinoma – time to take the ticket. World J Gastrointest Surg 2019; 11(6): 287-295
- URL: https://www.wjgnet.com/1948-9366/full/v11/i6/287.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v11.i6.287
Hepatocellular carcinoma (HCC) is one of the leading malignant diseases worldwide. It ranks third in terms of cancer-related mortality, and the incidence of HCC is on the rise[1]. A range of management options are available for HCC and include excision, radiofrequency ablation, transarterial chemo embolization (TACE), and use of biological agents.
Liver transplantation offers a very unique treatment for HCC. Specifically, along with the lesion, it removes the tissue that is at risk for developing malignancy. However, there are certain risk factors associated with recurrence of tumor in the transplanted liver and development of metastatic disease at a later date. Hence, not all cases of HCC are compatible with liver transplantation. Mazzaferro et al[2] came up with criteria to select patients with HCC for liver transplantation. These criteria, which are widely known as the Milan criteria, suggest that patients with a single 5-cm tumor or with up to 3 tumors (each tumor not larger than 3 cm) can be eligible for liver transplantation. According to the Milan criteria, the outcome of transplantation is highly favorable, with an overall survival rate of 70%[3].
The rising incidence of HCC and a relatively easy availability of organs due to the living donor liver transplantation (often referred to as LDLT) have led to the question of whether the Milan criteria are too strict. Can patients outside the Milan criteria also benefit from liver transplantation? This problem has been addressed in two ways. First, advanced HCC patients are down-staged using locoregional therapy to fit into the Milan criteria. Second, the criteria for transplantation were expanded to include patients outside the Milan criteria.
Majno et al[4] was the first to test the concept of HCC down-grading before trans-plantation. Preoperative TACE was applied in a cohort of 111 patients before orthotopic liver transplant (OLT). Majno et al[4] concluded that there was no beneficial effect of preoperative TACE on recurrence-free survival after OLT. Another study by Graziadei et al[5] investigated a cohort of 15 patients with an HCC stage exceeding the T2 criteria, who underwent preoperative TACE. Among this cohort, 10 patients underwent OLT ultimately, and the 5-year survival rate after OLT was 41%. Though the initial studies painted a dismal picture, the limiting factor in these studies was the absence of well-defined criteria to select the patients for down-staging.
The seminal paper by Yao et al[6] looked into this problem. Those researchers developed the modified UCSF down-staging inclusion criteria (Table 1). Initially, the study looked at a cohort of 30 patients, and the later follow-up paper expanded this cohort to 61 patients. Successful down-staging was achieved in 70% of the cases, and among those who underwent transplantation, the 4-year posttransplant survival rate was 92%.
| Inclusion criteria |
| a) 1 lesion > 5 cm and ≤ 8 cm |
| b) 2 or 3 lesions, at least one > 3 cm but ≤ 5 cm + the total tumor diameter ≤ 8 cm |
| c) 4 or 5 lesions, all ≤ 3 cm + the total tumor diameter ≤ 8 cm |
| d) No vascular invasion on imaging |
| Criteria for successful down-staging |
| Tumor size and number need to satisfy the UNOS T2 criteria |
| Complete tumor necrosis without contrast enhancement to suggest residual tumor, equivalent to obliteration of the tumor irrespective of the tumor size |
| Additional guidelines |
| 1) A minimum observation period of 3 mo after down-staging is required before deceased donor liver transplantation, and if imaging studies meet the above-mentioned criteria for successful down-staging |
| 2) Patients can undergo live donor liver transplantation at 3 mo after down-staging, and if imaging studies satisfy the UCSF criteria |
| 3) Those with acute hepatic decompensation after the down-staging procedure are not eligible for liver transplantation unless they satisfy the abovementioned criteria |
Lei et al[7] compared the overall survival and tumor-free survival in patients who underwent transplantation according to the Milan criteria and those who were down-staged before transplantation. Out of the 112 patients included in the study, 58 patients were outside the Milan criteria. The modified UCSF down-staging inclusion criteria were used to include advanced HCC patients. TACE, ‘RAF’, ‘HIFU’, resection, etc were used as the down-staging therapies. The overall survival rate in patients who underwent down-staging was 70.7% compared to 74.1% in patients who initially met the Milan criteria. The tumor recurrence rate was 20.7% in the down-staging group and 20.5% in the Milan criteria group.
The latest American Association for the Study of Liver Diseases (commonly known as AASLD) guidelines[8] recommend that patients outside the Milan criteria should be considered for liver transplantation after successful down-staging. Though the highest overall survival was noted for patients undergoing multimodal therapy, the optimal form of therapy is not known. TACE with stereotactic radiation therapy was initially used as a modality for treating inoperable HCC. Due to the treatment effectiveness, the same approach was extrapolated for use as a down-staging tool before transplantation. A limited case series of 12 patients, who underwent down-staging with TACE plus stereotactic radiation therapy, was published by Jacob et al[9]. Six patients among the twelve total underwent liver transplantation. There were no difficulties during the procedure, secondary to the effects of radiation. The explant pathology showed no viable tumor deposits at the 10 treated HCC sites in 6 patients.
The data from the Global investigation of therapeutic decision in HCC and of treatment with sorafenib (referred to as ‘GIDEON’)[10] have shown that the combination of TACE and sorafenib has a beneficial effect on overall survival in patients with advanced disease. The START trial[11] also supports the above-mentioned finding. The SPACE trial[12] tried this combination on the Barcelona stage B HCC and did not find any difference compared to using TACE alone. Even though few of the latest studies[13] have shown benefit, further investigation is required, and feasibility of this combination for down-staging needs to be evaluated.
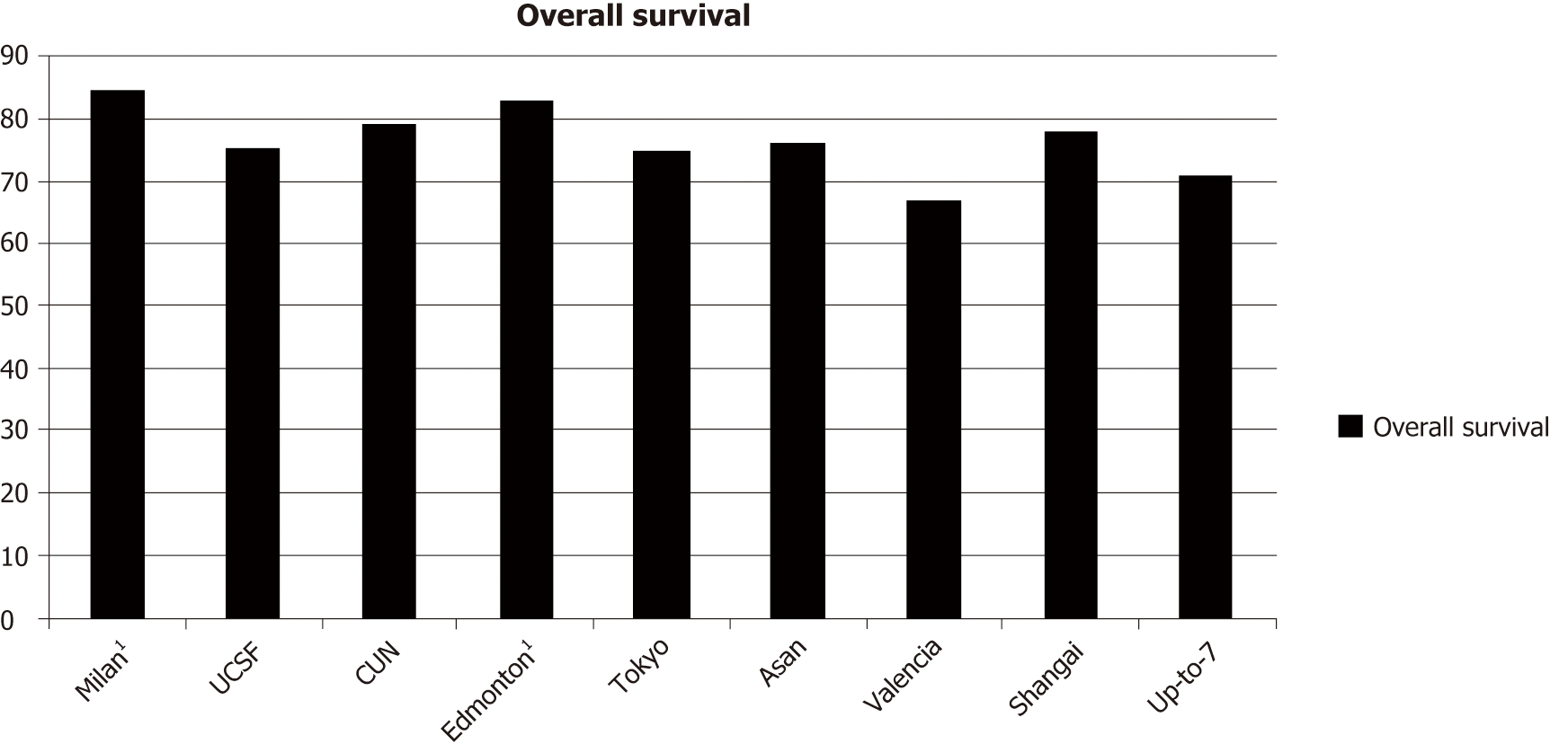
The currently widely used Milan criteria were proposed by Mazzaferro et al[2] in 1996. Then, several studies ratified the utility of the Milan criteria by reporting 5-year survival rates after liver transplantation ranging from 71% to 75%. This cemented the Milan criteria as a tool to select patients for OLT. However, the concept of limiting transplantation based only on tumor size and number of nodules was originally drawn from clinical experience. It is not known whether these are the ideal criteria. Several studies have shown that expanding the criteria in terms of the number of nodules and size of lesion offers survival rates that are comparable to those of the Milan criteria. A list of currently used extended criteria (Table 2) and the related overall survival (Figure 1) is presented below.
| Extended criteria | |
| UCSF[28] | Single tumor ≤ 6.5 cm in diameter or no more than 3 lesions ≤ 4.5 cm in diameter, and total tumor diameter ≤ 8 cm |
| Asan criteria[29] | ≤ 6 tumors, all ≤ 5 cm in diameter, and no gross vascular invasion |
| Valencia criteria[30] | 1-3 tumors ≤ 5 cm and cumulative tumor burden ≤ 10 cm |
| Up-to-7 criteria[14] | Sum of the sizes of the largest tumor (in cm) and the number of tumors ≤ 7 |
| CUN criteria[31] | Single tumor ≤ 6 cm or up to 3 nodules ≤ 5 cm |
| Mount-Sinai[32] | Any number of lesions, each is 5-7 cm in diameter |
| Edmonton[33] | Number of single tumors ≤ 7, tumor ≤ 5 cm in diameter |
| Dallas[34] | Single tumor diameter ≤ 6 cm or 2-4 tumors each ≤ 5 cm in diameter |
| Tokyo[35] | ≤ 5 tumors not exceeding 5 cm in diameter |
| Shanghai[36] | Single tumor ≤ 9 cm in diameter or no more than 3 nodules with the largest nodule ≤ 5 cm in diameter, overall tumor diameter ≤ 9 cm without extrahepatic metastasis, lymph node or macrovascular invasion |
In 2009, Mazzaferro et al[14], who originally suggested the Milan criteria, developed an extended criteria, termed the Up-to-7 criteria (UTSC). Since then, the UTSC have been utilized in several studies. According to the UTSC, the 5-year survival rate after a transplant is 71.2%. One of the latest studies, by Diaz et al [15], reviewed the trans-plantations performed at their unit by dividing these transplantations into three groups: patients within the Milan criteria; patients outside the Milan criteria but within the UTSC; and patients outside the UTSC. Out of the total 91 patients, the maximum number of patients were within the Milan criteria (n = 74) and only 12 patients were outside the Milan criteria but within the UTSC. The 5-year survival was found to be 58.3% for the patients within the UTSC. Though this survival rate was significantly lower than that in the earlier studies, the rate was still high compared to the patients outside the UTSC.
However, the question remains as to how far can we push the envelope? Are we causing more harm than good by expanding these criteria? What is the limit? In the past, a 5-year post transplant survival of 50% was considered to be a cut-off. A study conducted by the University of Michigan Health System compared the benefits in patients with HCC undergoing transplantation beyond the Milan criteria versus the harm done to other patients on the organ waiting list[16]. The study concluded that the posttransplant 5-year survival rate should be at least 61% to avoid harm to other patients on the waiting list.
The advantage of OLT for HCC is that it removes precancerous tissue and also helps prevent recurrence. However, in approximately 20% of patients, there is recurrence of HCC[16]. These patients have to undergo salvage transplantation or other treatments. An assessment to help estimate the risk of recurrence will help with better selection of candidates for transplantation.
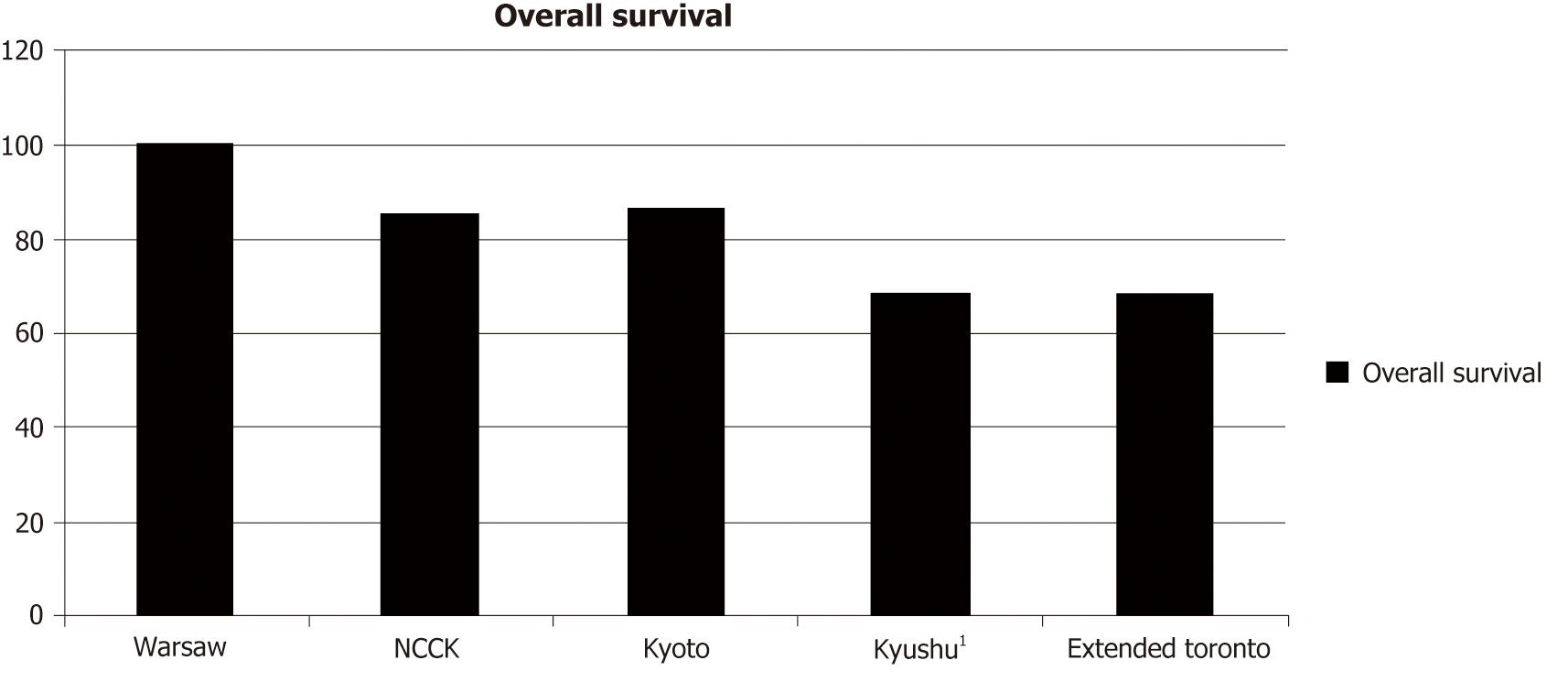
Tumor biology helps with better prediction of the risk of recurrence. The gold standard remains biopsy of the tumor with histopathological examination. However, this invasive procedure is often fraught with dangers, such as bleeding and needle track seeding. In addition, ascites in patients with decompensated cirrhosis is another contraindication for biopsy. To overcome this problem, other biomarkers are being used to predict tumor behavior. These biomarkers include alpha-fetoprotein (AFP), des-gamma carboxy prothrombin, and positron emission tomography (PET) scan. A list of criteria that use biomarkers (Table 3) and the associated survival rates (Figure 2) are presented below.
| Biomarker criteria | |
| TTV/AFP[18] | Total tumor volume ≤ 115 cm3 and AFP ≤ 400 ng/mL |
| AFP-TTD[37] | Total tumor diameter ≤ 8 cm and AFP ≤ 400 ng/mL |
| Warsaw[38] | Outside the Milan criteria but within the UCSF/ Up-to-7 criteria with AFP < 100 ng/mL |
| NCCK[39] | Negative PET/CT findings and the total tumor size < 10 cm |
| Kyoto[19] | ≤ 10 tumors, all of which ≤ 5 cm in diameter, and serum DCP ≤ 400 mAU/mL |
| Kyushu university[40] | Tumor diameter ≤ 5 cm and serum DCP ≤ 300 mAU/mL |
| Extended Toronto[41] | No size-number limitation, no vascular invasion, no extrahepatic disease, no cancer-related symptoms, biopsy of the largest tumor not poorly differentiated |
AFP is one of the earliest tumor markers for HCC. With the advent of better imaging, the role of AFP has diminished. However, several studies have shown that preoperative levels of AFP can help predict recurrence. Several criteria have evolved to include AFP for selecting patients for transplantation. A study conducted by Toso et al[18] reviewed 6478 patients who underwent liver transplantation for HCC. According to this study, only the total tumor volume and AFP levels can predict patient survival. Then, a composite score was created with a cut-off of total tumor volume > 115 cm3 and AFP > 400 ng/mL. Patients who did not meet the score requirement had a 5-year survival rate below 50% at 3 years. Other similar AFP-based criteria include the “AFP-TTD” and Warsaw criteria.
Another study conducted by Ito et al[19] analyzed the results of 125 patients who underwent liver transplantation for HCC, to determine the optimal criteria outside the Milan criteria. In their multivariate analysis, PIVKA-II level < 400 mAU/mL was found to significantly correlate with 5-year recurrence rates. A similar study conducted by Kim et al[20] studied the factors involved in recurrence of HCC following the adult LDLT. Out of the cohort of 461 patients, 77 patients had a recurrence. The study concluded that PIVKA-II level > 100 mAU/mL and AFP level > 150 ng/mL were among the important deciding factors for tumor recurrence.
PET is being increasingly used in oncology treatment worldwide. This functional scan is based on the utilization of glucose by metabolically active tissues, such as tumors. The most commonly used tracer is 18-fluorodeoxyglucose (FDG). Other tracers that have been used include 11-C-acetate and 18-F-choline. The sensitivity of 18FDG in HCC ranges from 50% to 70%[21]. Because hepatocytes have a physiological uptake of glucose, the ability to contrast between a well-differentiated tumor and normal liver cells is difficult. Okazumi et al[22] have classified liver tumors based on the FDG uptake pattern into three types: Type I - greater accumulation of tracer compared to normal liver tissue; Type II - similar to normal liver tissue accumulation of tracer; and Type III – lower accumulation compared to normal liver tissue.
There have been several publications describing investigations into the utility of PET scan in managing HCC. An article by Campos et al[23] reviewed the use of FDG-PET in HCC patients undergoing transplantation. It was observed that the amount of tracer uptake had a significant association with the outcome after surgery. The tracer uptake value is denoted by SUVmax, and tumors with a low tracer uptake had better prognosis after transplantation even if tumors were outside the Milan criteria. Another value that is commonly used in studies is the ratio between SUVmax of the tumor and the normal liver tissue denoted as TSUVmax/LSUVmax. Studies have shown that TSUVmax/LSUVmax < 1.15 indicates a disease-free survival of 97% at 2 years compared to 42% in patients with a value > 1.15[24]. A recent study by Song et al[25] evaluated 123 HCC patients who underwent partial liver transplant. The study showed that the PET-transarterial chemolipiodolization and AFP levels, when used together, had a better chance at predicting recurrence than the Milan criteria. The cut-off for the AFP level was 200 and the PET positivity was set at TSUVmax/LSUVmax > 1.1.
HCC is one of the leading malignancies worldwide. HCC is also a leading cause of mortality. Surgical management of HCC in the form of liver transplantation has a very good outcome when performed in the right candidates. The latest studies support the “HCC-Metroticket” concept[26] in which the criteria fulfilling a 5-year survival of 60%-80% are acceptable. The Metroticket project has launched a website (http://www.hcc-olt-metroticket.org/), on which you can calculate the preoperative 5-year survival rates using parameters such as the maximum tumor size, number of nodules, and AFP level.
Ultimately, all of these protocols and criteria were created because we do not have a complete understanding of the genetic alterations that lead to carcinogenesis and the way that different mutations affect cancer biology. In the future, having a better understanding of the genetic makeup of tumors and using new markers, such as long noncoding RNAs[27], can make these criteria obsolete. Until then, we can strive to achieve the best result possible without causing harm to other patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang XY, Zhu H S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Liu JH
| 1. | Rossi L, Zoratto F, Papa A, Iodice F, Minozzi M, Frati L, Tomao S. Current approach in the treatment of hepatocellular carcinoma. World J Gastrointest Oncol. 2010;2:348-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5309] [Article Influence: 183.1] [Reference Citation Analysis (0)] |
| 3. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 706] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 4. | Majno P, Giostra E, Mentha G. Management of hepatocellular carcinoma on the waiting list before liver transplantation: time for controlled trials? Liver Transpl. 2007;13:S27-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, Hirose R, Fidelman N, Kerlan RK, Roberts JP. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 370] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 7. | Lei J, Wang W, Yan L. Downstaging advanced hepatocellular carcinoma to the Milan criteria may provide a comparable outcome to conventional Milan criteria. J Gastrointest Surg. 2013;17:1440-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3025] [Article Influence: 432.1] [Reference Citation Analysis (3)] |
| 9. | Jacob R, Saddekni S, Dover L, DuBay DA. Successful hepatocellular carcinoma downstaging with transarterial chemoembolization followed by stereotactic radiotherapy. Liver Transpl. 2016;22:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Geschwind JF, Gholam PM, Goldenberg A, Mantry P, Martin RC, Piperdi B, Zigmont E, Imperial J, Babajanyan S, Foreman PK, Cohn A. Use of Transarterial Chemoembolization (TACE) and Sorafenib in Patients with Unresectable Hepatocellular Carcinoma: US Regional Analysis of the GIDEON Registry. Liver Cancer. 2016;5:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY, Chao Y. Interim analysis of START: Study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. Int J Cancer. 2013;132:2448-2458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, Luca A, Del Arbol LR, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 543] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 13. | Ren B, Wang W, Shen J, Li W, Ni C, Zhu X. Transarterial Chemoembolization (TACE) Combined with Sorafenib <i>versus</i> TACE Alone for Unresectable Hepatocellular Carcinoma: A Propensity Score Matching Study. J Cancer. 2019;10:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1572] [Article Influence: 92.5] [Reference Citation Analysis (1)] |
| 15. | Leon Diaz FJ, Perez Daga JA, Sanchez Perez B, Fernandez Aguilar JL, Montiel Casado C, Aranda Narvaez JM. Up-to-7 Criteria for Hepatocellular Carcinoma Liver Transplantation: A Retrospective Analysis of Experiences. Transplant Proc. 2016;48:2969-72:[27932121]:[10.1016/j.transproceed.2016.08.035]. |
| 16. | Yao FY. Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria. Am J Transplant. 2008;8:1982-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Yan WT, Quan B, Xing H, Wu MC, Yang T. Time to recurrence, but not recurrence-free survival, should be the endpoint used to predict early recurrence after HCC resection. J Hepatol. 2019;70:570-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 19. | Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F, Ogawa K, Sakamoto S, Ogura Y, Egawa H, Tanaka K, Uemoto S. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl. 2007;13:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Kim DY, Paik YH, Ahn SH, Youn YJ, Choi JW, Kim JK, Lee KS, Chon CY, Han KH. PIVKA-II is a useful tumor marker for recurrent hepatocellular carcinoma after surgical resection. Oncology. 2007;72 Suppl 1:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Cho KJ, Choi NK, Shin MH, Chong AR. Clinical usefulness of FDG-PET in patients with hepatocellular carcinoma undergoing surgical resection. Ann Hepatobiliary Pancreat Surg. 2017;21:194-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Okazumi S, Isono K, Enomoto K, Kikuchi T, Ozaki M, Yamamoto H, Hayashi H, Asano T, Ryu M. Evaluation of liver tumors using fluorine-18-fluorodeoxyglucose PET: characterization of tumor and assessment of effect of treatment. J Nucl Med. 1992;33:333-339. [PubMed] |
| 23. | Cascales-Campos PA, Romero PR, Schneider MA, Lopez-Lopez V, Navarro JL, Frutos L, Pons Miñano JA, Paricio PP. Positron emission tomography/computed tomography in patients with hepatocellular carcinoma undergoing liver transplantation. Useful, necessary or irrelevant? Eur J Radiol. 2017;91:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Lee JW, Paeng JC, Kang KW, Kwon HW, Suh KS, Chung JK, Lee MC, Lee DS. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med. 2009;50:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 25. | Song MJ, Bae SH, Yoo IeR, Park CH, Jang JW, Chun HJ, Choi BG, Lee HG, Choi JY, Yoon SK. Predictive value of ¹⁸F-fluorodeoxyglucose PET/CT for transarterial chemolipiodolization of hepatocellular carcinoma. World J Gastroenterol. 2012;18:3215-3222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 26. | Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 665] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 27. | Krishna M, Mullath A. Role of lncRNAs in GI Cancer. J Cancer Therapy. 2018;9:281-298. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1695] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 29. | Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, Ko GY, Park KM, Ha TY, Song GW. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 30. | Silva M, Moya A, Berenguer M, Sanjuan F, López-Andujar R, Pareja E, Torres-Quevedo R, Aguilera V, Montalva E, De Juan M, Mattos A, Prieto M, Mir J. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl. 2008;14:1449-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Herrero JI, Sangro B, Quiroga J, Pardo F, Herraiz M, Cienfuegos JA, Prieto J. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, Miller CM, Schwartz ME. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 318] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 33. | Kneteman NM, Oberholzer J, Al Saghier M, Meeberg GA, Blitz M, Ma MM, Wong WW, Gutfreund K, Mason AL, Jewell LD, Shapiro AM, Bain VG, Bigam DL. Sirolimus-based immunosuppression for liver transplantation in the presence of extended criteria for hepatocellular carcinoma. Liver Transpl. 2004;10:1301-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Onaca N, Davis GL, Goldstein RM, Jennings LW, Klintmalm GB. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2007;13:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25:310-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 36. | Fan J, Yang GS, Fu ZR, Peng ZH, Xia Q, Peng CH, Qian JM, Zhou J, Xu Y, Qiu SJ, Zhong L, Zhou GW, Zhang JJ. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. J Cancer Res Clin Oncol. 2009;135:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Lai Q, Avolio AW, Manzia TM, Sorge R, Agnes S, Tisone G, Berloco PB, Rossi M. Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation. Clin Transplant. 2012;26:E125-E131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Grąt M, Kornasiewicz O, Lewandowski Z, Hołówko W, Grąt K, Kobryń K, Patkowski W, Zieniewicz K, Krawczyk M. Combination of morphologic criteria and α-fetoprotein in selection of patients with hepatocellular carcinoma for liver transplantation minimizes the problem of posttransplant tumor recurrence. World J Surg. 2014;38:2698-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Lee SD, Lee B, Kim SH, Joo J, Kim SK, Kim YK, Park SJ. Proposal of new expanded selection criteria using total tumor size and (18)F-fluorodeoxyglucose - positron emission tomography/computed tomography for living donor liver transplantation in patients with hepatocellular carcinoma: The National Cancer Center Korea criteria. World J Transplant. 2016;6:411-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Aishima S, Terashi T, Shimada M, Maehara Y. Extended indication for living donor liver transplantation in patients with hepatocellular carcinoma. Transplantation. 2007;83:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, Cleary SP, Lilly L, Cattral MS, Marquez M, Selzner M, Renner E, Selzner N, McGilvray ID, Greig PD, Grant DR. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology. 2016;64:2077-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (0)] |