Published online May 27, 2019. doi: 10.4240/wjgs.v11.i5.247
Peer-review started: April 2, 2019
First decision: April 20, 2019
Revised: May 9, 2019
Accepted: May 23, 2019
Article in press: May 23, 2019
Published online: May 27, 2019
Processing time: 56 Days and 22.5 Hours
With advanced age and chronic illness, the life expectancy of a patient with colorectal cancer (CRC) becomes less dependent on the malignant disease and more on their pre-morbid condition. Justifying major surgery for these elderly patients can be challenging. An accurate tool demonstrating post-operative survival probability would be useful for surgeons and their patients.
To integrate clinically significant prognostic factors relevant to elective colorectal surgery in the elderly into a validated pre-operative scoring system.
In this retrospective cohort study, patients aged 70 and above who underwent surgery for CRC at Singapore General Hospital between 1 January 2005 and 31 December 2012 were identified from a prospectively maintained database. Patients with evidence of metastatic disease, and those who underwent emergency surgery or had surgery for benign colorectal conditions were excluded from the analysis. The primary outcome was overall 3-year overall survival (OS) following surgery. A multivariate model predicting survival was derived and validated against an equivalent external surgical cohort from Kyungpook National University Chilgok Hospital, South Korea. Statistical analyses were performed using Stata/MP Version 15.1.
A total of 1267 patients were identified for analysis. The median post-operative length of stay was 8 [interquartile range (IQR) 6-12] d and median follow-up duration was 47 (IQR 19-75) mo. Median OS was 78 (IQR 65-85) mo. Following multivariate analysis, the factors significant for predicting overall mortality were serum albumin < 35 g/dL, serum carcinoembryonic antigen ≥ 20 µg/L, T stage 3 or 4, moderate tumor cell differentiation or worse, mucinous histology, rectal tumors, and pre-existing chronic obstructive lung disease. Advanced age alone was not found to be significant. The Korean cohort consisted of 910 patients. The Singapore cohort exhibited a poorer OS, likely due to a higher proportion of advanced cancers. Despite the clinicopathologic differences, there was successful validation of the model following recalibration. An interactive online calculator was designed to facilitate post-operative survival prediction, available at http://bit.ly/sgh_crc. The main limitation of the study was selection bias, as patients who had undergone surgery would have tended to be physiologically fitter.
This novel scoring system generates an individualized survival probability following colorectal resection and can assist in the decision-making process. Validation with an external population strengthens the generalizability of this model.
Core tip: Ageing results in a decreased functional reserve along with various comorbid diseases. Many elderly patients express age-related concerns when advised for operative intervention. This is the first predictive survival model specific for older patients planned for elective colorectal surgery and provides a visual guide to facilitate the counselling process.
Seow-En I, Tan WJ, Dorajoo SR, Soh SHL, Law YC, Park SY, Choi GS, Tan WS, Tang CL, Chew MH. Prediction of overall survival following colorectal cancer surgery in elderly patients. World J Gastrointest Surg 2019; 11(5): 0000-0000 URL: https://http://www.wjgnet.com/1948-9366/full/v11/i5/0000.htm DOI: https://dx.doi.org/10.4240/wjgs.v11.i5.0000
- Citation: Seow-En I, Tan WJ, Dorajoo SR, Soh SHL, Law YC, Park SY, Choi GS, Tan WS, Tang CL, Chew MH. Prediction of overall survival following colorectal cancer surgery in elderly patients. World J Gastrointest Surg 2019; 11(5): 247-260
- URL: https://www.wjgnet.com/1948-9366/full/v11/i5/247.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v11.i5.247
The world is facing a dramatic increase in the number and proportion of its elderly. Driven by remarkable improvements in life expectancy, the number of people aged 60 years and over is projected to grow from 901 million in 2015 to 1.4 billion in 2030, and more than double that in 2015 to 2.1 billion by 2050[1]. Whilst being a product of economic success and advancement in healthcare, an ageing population will suffer more death and disability from illnesses such as heart disease, diabetes, and cancer[2]. Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the fourth leading cause of cancer death globally, accounting for 1.4 million new cases and almost 700 000 deaths in 2012[3]. The incidence and mortality rates for CRC increases with age, with 90% of new cases and over 90% of deaths occurring at 50 years and beyond[4]. This raises genuine concern that CRC will result in a greater burden on healthcare with the shift towards an older demographic.
The normal physiological process of ageing reduces functional capacity and reserve, leading to a decreased ability to mount an adequate response to stress and resulting in a worse outcome should post-operative complications arise. Another problem is the increasing number and severity of comorbidities with age which may impact patient tolerance of anesthesia. With advanced age and chronic illness, the decision to undergo major surgery in the elderly patient can be challenging. Not infrequently, patients and their family members decline operative intervention due to age-related concerns. Even to the surgeon, the benefit of resection in certain individuals may not be so clear-cut. Moreover, the elderly are under-represented and under-prioritized in randomized trials[5], resulting in difficulty in generalizing existing data. Many clinicians now recognize that surgery in the elderly is different in terms of risks and meaningful outcomes[6].
We aimed to analyze our outcomes following major elective colorectal surgery in the elderly to determine factors significantly influencing mortality. A pre-operative scoring system predicting post-operative outcomes more objectively could then be derived, facilitating the decision-making process for both surgeons and patients.
Data for all patients aged 70 and above who underwent elective surgery for non-metastatic CRC at Singapore General Hospital (SGH) Department of Colorectal Surgery from 1 January 2005 to 31 December 2012 were obtained from hospital electronic records. Patients with evidence of distant disease, those who underwent emergency surgery or had surgery for benign colorectal conditions were excluded from the analysis. Instances of surgery for CRC recurrence occurring in the same patient over the study period were also excluded. Information for an equivalent group of elderly patients electively operated on at Kyungpook National University Chilgok Hospital (KNUCH), Daegu, South Korea, was retrieved over the same duration.
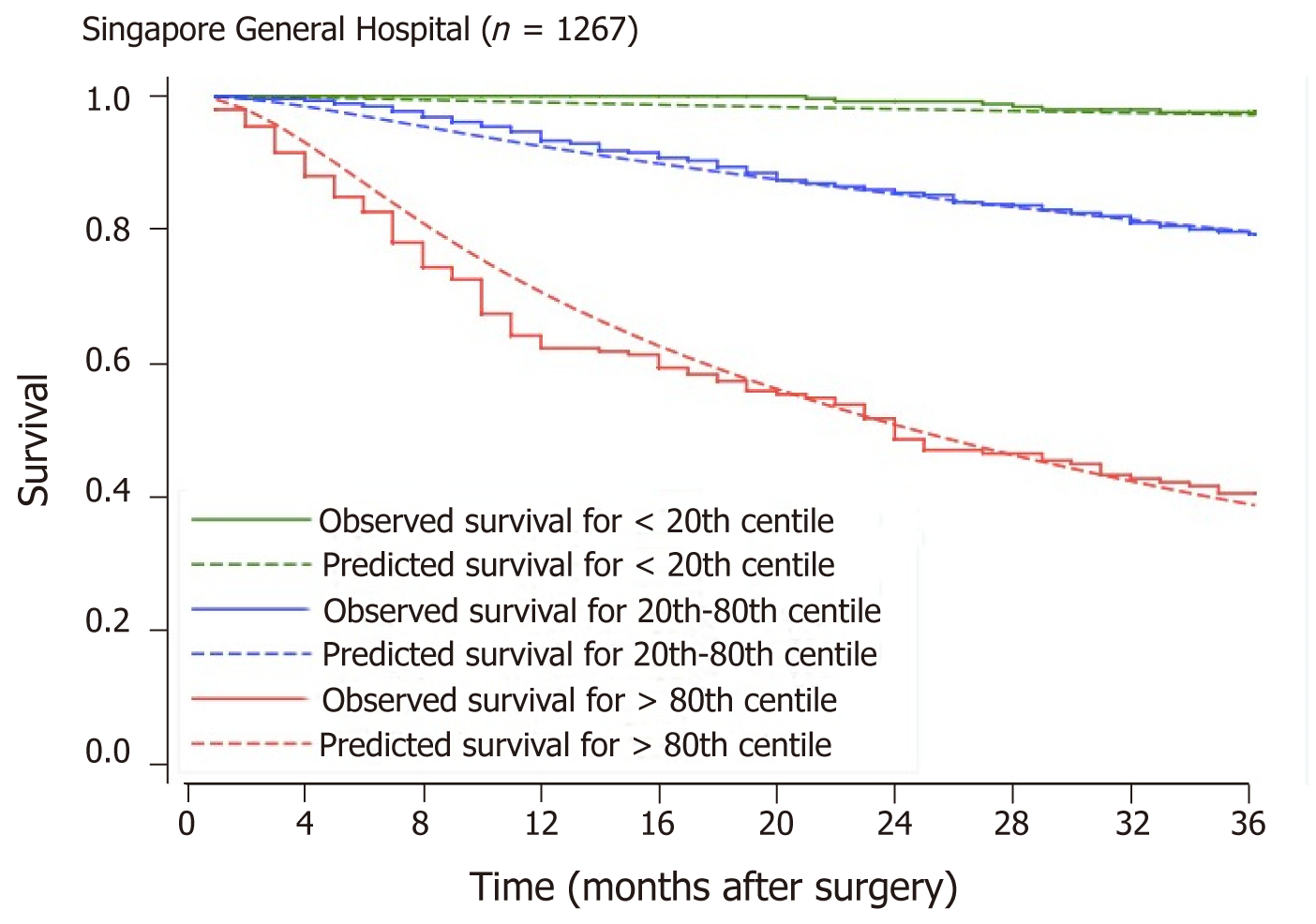
Missing data were filled using multiple imputation, performed via sequential imputation using chained equations with predictive mean matching. The variable with the highest proportion of missing values in the model derivation dataset was 11.5%. All variables, apart from the variable being imputed, were included in the imputation model to avoid bias. A total of 100 imputations were performed. To simplify the eventual prognostic scoring, the “minimum P value approach” was adopted to detect appropriate cut points for continuous variables[7,8]. Cut point selection was guided by upper and lower limits of normal laboratory values and an attempt was made to keep cut points to the nearest whole number, tens or fives to facilitate clinical ease of use. As Cox models do not provide a straightforward estimation of the baseline survival function required for predicting absolute survival probabilities, a flexible parametric model [Royston-Parmar (RP)] was constructed instead with all-cause mortality as the outcome[9]. All outcomes were truncated at three years from the time of surgery and patients who had not died were censored at three years. All independent variables were included in the multivariate regression and backward elimination was used to remove variables with P values greater than or equal to 0.05 until all the remaining variables had P values of < 0.05. A survival score was calculated for all patients using the final RP model’s beta coefficients. Patients were stratified by survival score and categorized into three arbitrary prognostic groups, defined using the 20th and 80th percentiles of the survival score. The observed survival profiles of the three prognostic groups were assessed using the Kaplan-Meier estimator and compared using the log-rank test. Calibration was evaluated by visually inspecting the agreement between observed and predicted 3-year survival by superimposing predicted survival profiles over the Kaplan-Meier curves.
The final RP model derived on the cohort from SGH to predict 3-year post-operative survival was applied on the group of patients from KNUCH. Discrimination and calibration were evaluated as previously described. Where evidence of model miscalibration was observed, recalibration was performed by fitting an RP model to the validation data using the linear predictor of the existing model on the log relative hazard scale. All statistical analyses were performed using Stata/MP Version 15.1 (College Station, TX, United States) and R Version 3.3.4 (http://www.r-project.org).
An online calculator was developed from our model to predict three-year survival profiles. The interactive calculator, generated via https://http://www.shinyapps.io/, facilitates individualized point-of-care survival probability and aids the visualisation of the predicted survival probability profile over time, given the unique combination of risk factors present[10]. The study protocol has been approved by the SingHealth Institutional Review Board (CIRB Ref No. 2015/2374).
A total of 1643 elective colorectal resections were performed for 1623 patients aged 70 and above over the study duration. One hundred and eighty-seven patients who underwent surgery for non-malignant conditions and 169 patients known to have distant metastasis at the time of surgery were excluded from the analysis. Twenty instances of repeat surgery for cancer recurrence performed for the same patient during the study period were also excluded. Analysis of 1267 resections for colorectal malignancy was performed. Clinical characteristics are shown in Table 1.
| Variable | SGH cohort (n = 1267) |
| Age, median (range), yr | 77 (70-102) |
| Gender | |
| Male | 658 (51.9) |
| Female | 609 (48.1) |
| Race | |
| Chinese | 1148 (90.6) |
| Malay | 50 (4.0) |
| Indian | 30 (2.4) |
| Others | 39 (3.1) |
| ECOG status | |
| 0 (asymptomatic) | 751 (59.3) |
| 1 | 401 (31.6) |
| 2 | 85 (6.7) |
| 3 | 23 (1.8) |
| 4 (bedbound) | 7 (0.2) |
| ASA score | |
| 1 | 178 (14.0) |
| 2 | 857 (67.6) |
| 3 | 225 (17.8) |
| 4 | 7 (0.6) |
| Tumor site | |
| Colon | 790 (62.3) |
| Rectum | 477 (37.6) |
| Surgical approach | |
| Open | 888 (70.1) |
| Laparoscopic | 379 (29.9) |
| Surgery | |
| High Anterior resection | 505 (40.0) |
| Low Anterior resection | 163 (12.9) |
| Ultra-Low Anterior resection | 150 (11.8) |
| Right Hemicolectomy | 286 (22.6) |
| Abdominoperineal resection | 58 (4.6) |
| Others | 105 (8.3) |
| TMN stagea | |
| 1 | 240 (18.9) |
| 2 | 416 (32.8) |
| 3 | 442 (34.9) |
| Tumor diameter, median (IQR), cm | 4.5 (3–6) |
| Number of lymph nodes harvested, median (IQR) | 15 (11–20) |
| Neoadjuvant therapy | 22 (1.8) |
| Adjuvant therapy | 219 (17.6) |
Outcome measures used were the 30-d post-operative complication rate, classified according to the Clavien-Dindo tool, and overall survival (OS). Complication details are summarized in Table 2. While just under one-quarter of patients experienced early complications, high grade complications of Clavien-Dindo III or higher only occurred in 82 patients (6.5%). These included 30 deaths within the initial 30 post-operative days; 21 were secondary to cardiorespiratory complications, eight were attributable to anastomotic leaks and the remaining one was due to a cerebrovascular accident.
| SGH cohort (n = 1267) | |
| Overall 30-d complications | 297 (23.4) |
| Clavien-Dindo classification | |
| I | 75 (5.9) |
| II | 140 (11.0) |
| III | 21 (1.7) |
| IV | 31 (2.4) |
| V (death) | 30 (2.4) |
| Type of complication | |
| Cardiac/CVA | 97 (7.7) |
| Respiratory | 28 (2.2) |
| Urinary | 24 (1.9) |
| Wound/stoma | 55 (4.3) |
| Anastomotic leak | 16 (1.3) |
| Others | 77 (6.1) |
The median post-operative length of stay was 8 (IQR 6-12) dand median follow-up duration was 47 (IQR 19-75) mo. Median OS was 78 (IQR 65-85) mo. Of 670 deaths occurring within the follow-up up period, 339 were attributable to CRC (51%), while 331 (49%) died of other causes. Disease recurrence occurred in 276 (22%), with a median time from surgery to recurrence of 13 (IQR 7-23) mo. Most cancer recurrences presented at distant locations only (65%), while locoregional recurrence without distant metastasis occurred in 30 patients (11%). Disease relapse in both local and distant organs accounted for a quarter of all recurrences (24%).
Univariate analysis of all suitable pre-operative variables is shown in Table 3. The final multivariate model for predicting OS is provided in Table 4 with the resultant survival curves generated from this model in Figure 1. The model stratifies OS reasonably well in terms of discrimination (separation of the three risk category curves) and calibration (agreement between observed and predicted survival curves for each risk category).
| Predictors | n | HR (95%CI) | P value |
| Gender | 1267 | 1.03 (0.81-1.31) | 0.807 |
| Age | 1267 | 1.00 (0.98-1.03) | 0.826 |
| Race | |||
| Chinese | 1148 | 1.00 (ref) | |
| Malay | 50 | 1.14 (0.60-2.14) | 0.682 |
| Indian | 30 | 1.43 (0.78-2.68) | 0.272 |
| Others | 39 | 0.67 (0.25-1.82) | 0.433 |
| Smoking status | |||
| Non-smoker | 1055 | 1.00 (ref) | |
| Smoker | 212 | 1.07 (0.78-1.47) | 0.692 |
| Primary lesion site | |||
| Colon | 790 | 1.00 (ref) | |
| Rectum | 477 | 1.40 (1.09-1.80) | 0.008 |
| Tumor stage | |||
| Tis/T1 | 97 | 1.00 (ref) | |
| T2 | 188 | 1.52 (0.31-7.41) | 0.605 |
| T3 | 752 | 10.8 (2.7-43.1) | 0.001 |
| T4a | 97 | 42.9 (10.5-173.5) | < 0.001 |
| T4b | 133 | 37.9 (9.38-153.2) | < 0.001 |
| Tumor grade | |||
| Well differentiated | 117 | 1.00 (ref) | |
| Moderately differentiated | 1009 | 3.85 (1.82-8.15) | < 0.001 |
| Poorly differentiated or mucinous | 141 | 8.59 (3.90-18.94) | < 0.001 |
| Past medical history | |||
| Diabetes Mellitus | 331 | 1.07 (0.81-1.41) | 0.655 |
| Hypertension | 416 | 1.17 (0.91-1.51) | 0.217 |
| End stage renal failure | 26 | 1.73 (0.54-5.53) | 0.354 |
| Previous myocardial infarction | 47 | 0.95 (0.50-1.81) | 0.881 |
| Previous PCI, cardiac surgery or angina | 140 | 1.36 (0.88-2.09) | 0.161 |
| Congestive heart failure | 41 | 1.18 (0.52-2.69) | 0.698 |
| Peripheral vascular disease | 30 | 1.14 (0.52-2.53) | 0.744 |
| Impaired sensorium, e.g., dementia | 14 | 2.77 (0.40-19.2) | 0.304 |
| Chronic obstructive pulmonary disease | 61 | 0.44 (0.29-0.68) | < 0.001 |
| Previous stroke or TIA | 90 | 1.08 (0.66-1.76) | 0.758 |
| Previous stroke with neurological deficits | 38 | 1.19 (0.53-2.69) | 0.675 |
| Dependent functional status | 60 | 0.81 (0.43-1.54) | 0.529 |
| ECOG | |||
| 0 | 751 | 1.00 (ref) | |
| 1 | 404 | 0.98 (0.76-1.28) | 0.903 |
| 2 or more | 116 | 1.13 (0.72-1.79) | 0.595 |
| ASA | |||
| 1 | 178 | 1.00 (ref) | |
| 2 | 857 | 0.75 (0.53-1.06) | 0.099 |
| 3 or more | 232 | 0.78 (0.51-1.19) | 0.250 |
| Laboratory parameters | |||
| Serum albumin (g/L) | 1119 | 0.94 (0.92-0.96) | < 0.001 |
| Carcinoembryonic antigen (µg/L) | 1196 | 1.01 (1.00-1.01) | < 0.001 |
| White blood cell count (× 109/L) | 1267 | 1.11 (1.06-1.15) | < 0.001 |
| Platelet count (× 103/L) | 1267 | 1.00 (1.00-1.00) | < 0.001 |
| Serum sodium (mmol/L) | 1247 | 0.95 (0.91-0.98) | 0.002 |
| Serum creatinine (µmol/L) | 1265 | 1.00 (0.99-1.00) | 0.244 |
| Serum potassium (mmol/L) | 1263 | 0.75 (0.56-0.99) | 0.049 |
| Serum urea (mmol/L) | 1264 | 0.98 (0.93-1.04) | 0.517 |
| Hemoglobin (g/dL) | 1243 | 0.85 (0.79-0.91) | 0.002 |
| Lesion size (cm) | 1267 | 2.03 (1.53-2.70) | < 0.001 |
| Dichotomized predictorsa | |||
| Age ≥ 80 yr | 1267 | 0.92 (0.70-1.21) | 0.540 |
| Serum albumin < 35 g/L | 1266 | 2.16 (1.69-2.76) | < 0.001 |
| Carcinoembryonic antigen ≥ 20 µg/L | 1247 | 4.33 (3.39-5.54) | < 0.001 |
| White blood cell count ≥ 8.5 × 109/L | 1265 | 1.78 (1.40-2.28) | 0.001 |
| Platelet count ≥ 450 × 103/L | 1263 | 2.36 (1.67-3.35) | < 0.001 |
| Serum sodium < 135 mmol/L | 1264 | 1.62 (1.20-2.20) | 0.002 |
| Serum creatinine ≥ 135 µmol/L | 1243 | 0.56 (0.31-1.02) | 0.057 |
| Serum potassium < 3.5 mmol/L | 1267 | 1.37 (1.01-1.85) | 0.043 |
| Serum urea ≥ 7 mmol/L | 1267 | 1.00 (0.71-1.40) | 0.999 |
| Hemoglobin < 11 g/dL | 1243 | 1.48 (1.16-1.89) | 0.002 |
| Lesion size ≥ 4 cm | 1267 | 2.03 (1.53-2.70) | < 0.001 |
| Variable | Hazard ratio | 2.5thpercentile | 97.5thpercentile | P value |
| Serum albumin < 35 g/dL | 1.41 | 1.08 | 1.83 | 0.011 |
| CEA ≥ 20 µg/L | 2.51 | 1.92 | 3.28 | < 0.001 |
| T stage | ||||
| T1/Tis | 1.00 (ref) | - | - | - |
| T2 | 1.11 | 0.23 | 5.41 | 0.894 |
| T3 | 6.18 | 1.55 | 24.6 | 0.010 |
| T4 | 17.9 | 4.45 | 72.1 | < 0.001 |
| Tumor grade | ||||
| Well differentiated | 1.00 (ref) | - | - | - |
| Moderately differentiated | 2.24 | 1.04 | 4.82 | 0.040 |
| Poorly differentiated or mucinous | 3.54 | 1.54 | 8.15 | 0.003 |
| Rectal lesion | 1.47 | 1.11 | 1.96 | 0.007 |
| Chronic obstructive lung disease | 1.87 | 1.11 | 3.17 | 0.019 |
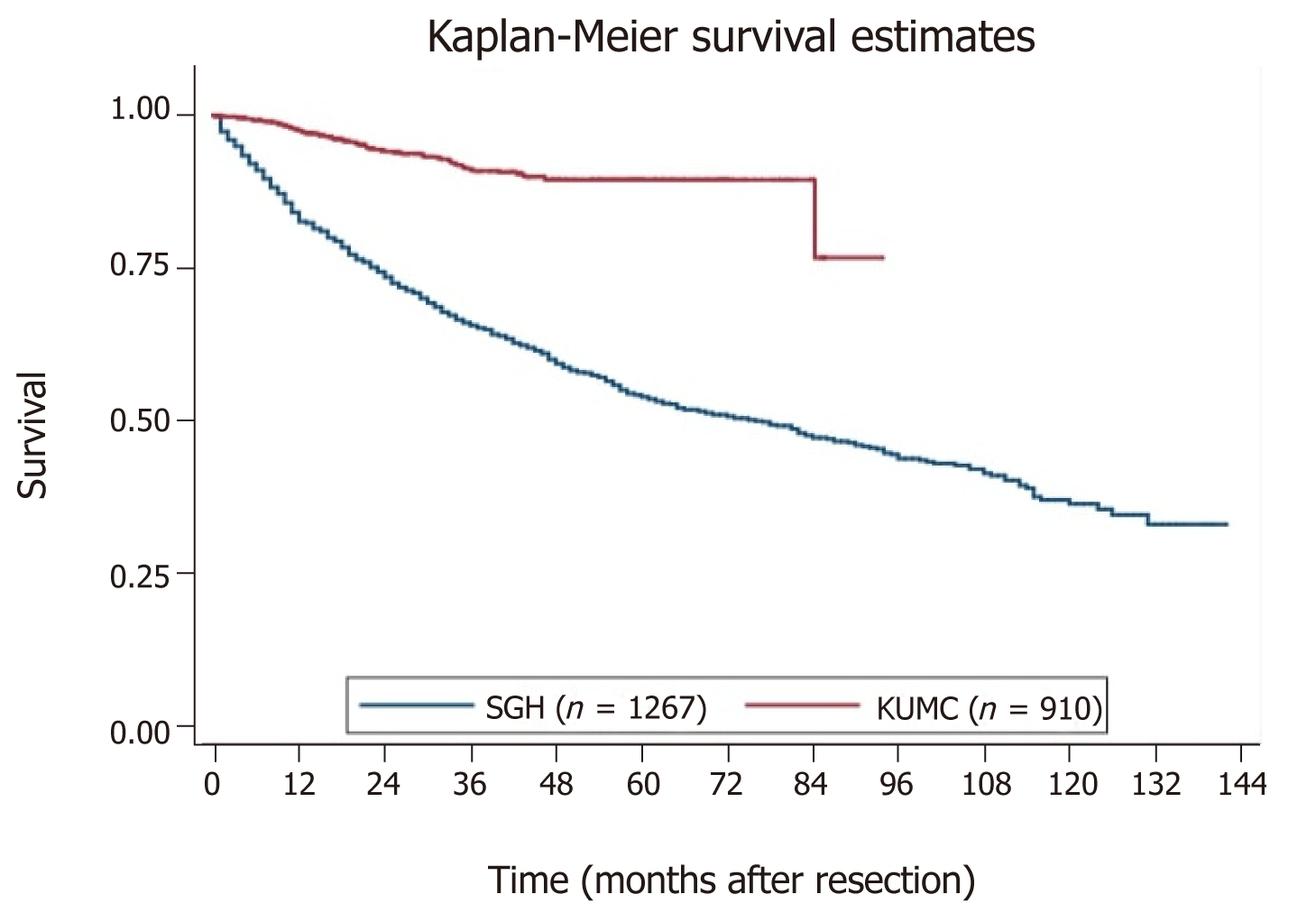
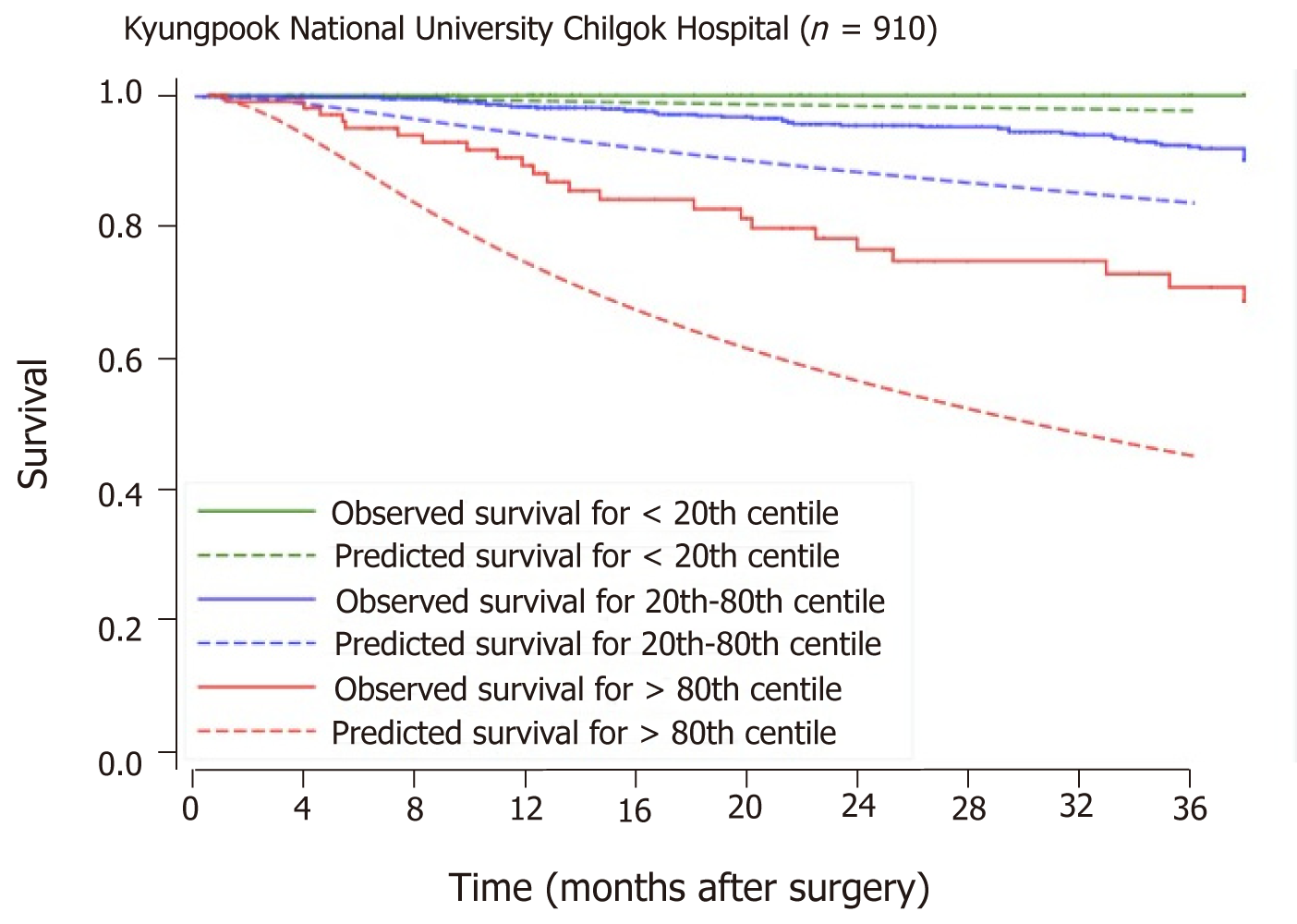
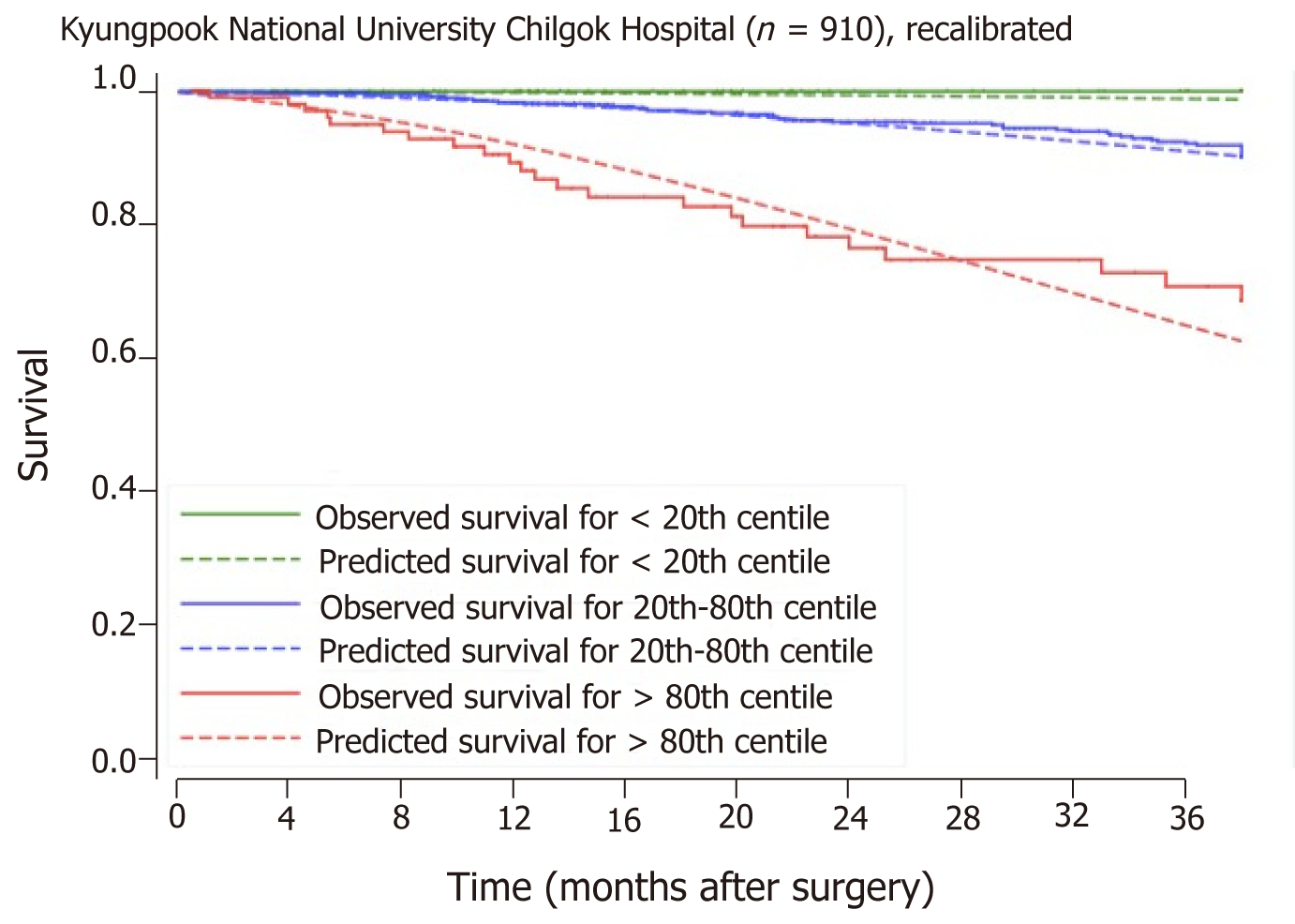
Baseline characteristics of patients from the Singapore and the Korean cohorts are compared head-to-head in Table 5. The SGH and KNUCH cohorts differ considerably in terms of survival, with the 80th percentile surviving 18 mo vs 85 mo respectively (Figure 2). Applying the model developed from the SGH cohort to stratify patients in the KNUCH dataset expectedly revealed model miscalibration. However, relative separation between the observed survival curves of patients in the three risk categories showed acceptable model discrimination (Figure 3). Model recalibration improved the agreement between the observed and predicted survival curves (Figure 4).
| Variable | SGH(n = 1267) | KNUCH(n = 910) | ||
| n | n | |||
| Age, median (range), yr | 1267 | 77 (70-102) | 910 | 75 (70-96) |
| Gender | ||||
| Male | 658 | (51.9) | 496 | (54.5) |
| Female | 609 | (48.1) | 414 | (45.5) |
| Lesion sites | ||||
| Colon | 790 | (62.3) | 540 | (59.3) |
| Rectum | 477 | (37.6) | 370 | (40.7) |
| Tumor grade | ||||
| Well differentiated | 117 | (9.0) | 29 | (3.2) |
| Moderately differentiated | 1009 | (79.6) | 809 | (88.9) |
| Poorly differentiated | 70 | (5.5) | 29 | (3.2) |
| Mucinous/signet ring cell | 71 | (5.6) | 43 | (4.7) |
| Laboratory parameters, mean (SD) | ||||
| Creatinine, µmol/L | 1267 | 88.9 (54.9) | 910 | 83.8 (38.6) |
| Urea, mmol/L | 1267 | 5.5 (2.7) | 910 | 2.7 (1.1) |
| Hemoglobin, g/dL | 1267 | 11.8 (1.8) | 910 | 12.0 (2.1) |
| WBC count, × 109/L | 1267 | 7.9 (2.6) | 908 | 8.0 (18.2) |
| Platelet count, × 109/L | 1267 | 300.6 (104.6) | 910 | 287.1 (95.3) |
| Serum albumin, g/L | 1119 | 33.7 (5.5) | 910 | 39.9 (4.6) |
| CEA, median (IQR), µg/L | 1196 | 4.3 (2.6–14.2) | 887 | 2.4 (1.5–4.8) |
| Comorbidities | ||||
| Diabetes | 331 | (26.1) | 167 | (18.4) |
| Hypertension | 416 | (32.8) | 415 | (45.6) |
| Ischemic heart disease | 47 | (3.7) | 14 | (1.5) |
| Congestive heart failure | 41 | (3.2) | 20 | (2.2) |
| PVD | 30 | (2.4) | 0 | (0.0) |
| COPD | 61 | (4.8) | 66 | (7.3) |
| Previous stroke | 90 | (7.1) | 50 | (5.5) |
| End stage renal disease | 26 | (2.1) | 6 | (0.7) |
| ASA | ||||
| 1 | 178 | (14.0) | 152 | (16.7) |
| 2 | 857 | (67.6) | 724 | (79.6) |
| 3 | 225 | (17.8) | 34 | (3.7) |
| 4 | 7 | (0.6) | 0 | (0.0) |
| T stagea | ||||
| Tis | 2 | (0.2) | 8 | (0.9) |
| T1 | 95 | (7.5) | 72 | (7.9) |
| T2 | 188 | (14.8) | 134 | (14.7) |
| T3 | 752 | (59.4) | 587 | (64.5) |
| T4a | 97 | (7.7) | 76 | (8.4) |
| T4b | 133 | (10.5) | 29 | (3.2) |
| ypCR | 0 | (0.0) | 4 | (0.4) |
| N stagea | ||||
| N0 | 688 | (54.3) | 577 | (63.4) |
| N1a | 148 | (11.7) | 124 | (13.6) |
| N1b | 185 | (14.6) | 109 | (12.0) |
| N1c | 4 | (0.3) | 1 | (0.1) |
| N2a | 99 | (7.8) | 58 | (6.4) |
| N2b | 143 | (11.3) | 41 | (4.5) |
| Short term outcomes | ||||
| Anastomotic leak | 16 | (1.3) | 44 | (4.8) |
| 30-d morbidity | 297 | (23.4) | 212 | (23.3) |
| 30-d mortality | 30 | (2.4) | 1 | (0.001) |
Following successful external validation, the model was productized as an interactive online calculator, available at http://bit.ly/sgh_crc.
One of the biggest challenges in healthcare is coping with an ageing population. In Singapore, the proportion of over 65-year-old has doubled from 6.0% in 1990 to 11.8% in 2015. With an annual increase of 0.5%-0.7% per year, this figure is expected to reach 20%-25% of the population by 2030. Life expectancy at birth in 2015 was 82.9 years (males 80.5 years, females 85.1 years), with the old age-dependency ratio climbing steadily to reach 16.2 per 100 residents (aged 15 to 64 years old)[11]. This census highlights not only the increasing proportion of the elderly but the potential strain on the rest of the working population. A similar trend can be observed in many developed nations worldwide.
In 2000, the Colorectal Cancer Collaborative Group published a systemic review of 28 studies consisting of more than 34000 patients, looking at outcomes post-colorectal surgery[12]. Patients were stratified by age group; less than 65 years, 65-74 years, 75-84 years, and above 84 years. The median post-operative mortality rates across these age groups were 3.0%, 6.4%, 8.6%, and 19.4% respectively. Median anastomotic leak rates were 4.0%, 5.0%, 4.0% and 3.0% respectively. The data does suggest that good surgical outcomes can be achieved in the elderly, but individualized evaluation of treatment goals and communication of realistic anticipated outcomes are essential[13].
Several risk stratification systems have been developed. Established scoring methods such as the American Society of Anesthesiologists score and Charlson Comorbidity Index are commonly used but have well described flaws; the former is too subjective with little specificity, and the latter was not designed to predict peri-operative risks in surgical patients. Colorectal surgery-specific scoring, such as the ColoRectal Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (Cr-POSSUM) and the Association of Coloproctology of Great Britain and Ireland scoring systems are validated as accurate predictors of 30-d post-operative mortality[14], but contain intraoperative or tumor staging parameters, which limit their use as a pre-operative optimization or counselling tool. This motivated us to develop a prognostic assessment tool to quantify the risk of mortality and predict survival after surgery in the elderly.
In the Singapore cohort, the rates of anastomotic leak, 30-d morbidity and 30-d mortality were 1.3%, 23.4% and 2.4% respectively. These outcomes are comparable to existing data on the elderly published during the past 10 years, with reported anastomotic leak rates ranging from 0.8%-5.9%, 30-d complications rates 17%-38% and 30-d mortality rates 0%-16%[15-22].
Only the OS prediction model was selected for validation with the external cohort, for several reasons. High grade morbidity of Clavien-Dindo grade III or IV accounted for less than one-fifth of the overall complications arising within 30 d. This limited the clinical applicability of a model predicting early clinically-relevant morbidity due to the small number of events. Moreover, the main causes of post-operative morbidity in elderly patients are known to be cardiovascular or pulmonary in nature[12,22], each of which already have existing specific risk assessment tools[23,24]. Compared to OS, predicting disease-free survival may also not be as practical to the geriatric patient with several life expectancy-limiting illnesses. Cancer-specific survival in the elderly has previously already been shown to be similar to that of the younger age group[12]. In the multivariate model, factors significant for predicting 3-year all-cause mortality were serum albumin < 35 g/dL, serum carcinoembryonic antigen ≥ 20 µg/L, T stage 3 or 4, tumor cell differentiation of moderate or worse, mucinous histology, rectal tumors, and the presence of existing chronic obstructive lung disease (COPD). As the model was intended to serve as a pre-operative patient counselling tool, intra-operative findings and information only available following final histopathological examination of the resected specimen were not included in the analysis.
Determination of the local stage of colorectal cancer can be difficult before surgery. A recent meta-analysis of 13 studies showed that computed tomography had good overall sensitivity of 90% at differentiating T1-T2 from T3-T4 colonic tumors[25], although a lower specificity estimate of 69% likely stemmed from radiologists interpreting benign pericolic desmoplastic reaction as tumor invasion to reduce the risk of understaging. Nodal stage prediction pre-operatively is even less precise and this was therefore not included in the univariate analysis.
Of all comorbidities analyzed (Table 3), only COPD remained significant for poorer OS on multivariate analysis. While COPD is known to confer a higher risk of early morbidity and mortality following abdominal surgery[26], longer term survival may be adversely influenced by associated pulmonary hypertension as well as the extra-pulmonary inflammatory effects of the disease[27]. Active smoking per se did not prove significant on univariate analysis.
Interestingly, age did not significantly influence OS on multivariate analysis, unlike in previous studies[12,22]. This demonstrates that advanced age alone without the presence of other predictors will not necessarily lead to a poorer outcome and should not be a contraindication to major resection.
The Singapore and Korean cohorts were similar in terms of patient age, cancer location and tumor differentiation. Disparity in survival between the populations was likely a result of a larger distribution of advanced cancers in the Singapore group; 18.2% vs 11.5% had T4 tumors, and 19.1% vs 10.9% had N2 disease. Median pre-operative CEA levels were also significantly higher in the SGH cohort (4.3 µg/L vs 2.4 µg/L). This may reflect a trend of elderly patients presenting later in the disease process in the Singapore population compared to Korean. The advent of national healthcare electronic records implemented in Singapore may also mean that mortality events are more readily captured even if they had discontinued follow-up at our institution.
Despite the differences in OS between the Singapore and Korea cohorts, observably distinct separation between survival curves was displayed when the Singapore model was applied to the external population (Figure 3). This suggests discriminatory capacity of the predictors for revealing relative survival differences amongst elderly patients who had undergone surgery for CRC. In absolute terms, however, the predicted survival probabilities generated did not match the observed survival profile of the external validation cohort and appeared miscalibrated. Regressing the original scores on observed survival in the external cohort allowed for the identification of an appropriate correction factor (beta coefficient). This was used to recalibrate the original scores following which successful validation was evident in the improved agreement between the observed and predicted survival curves (Figure 4).
Our study was subject to the limitations and bias inherent in observational retrospective research. The most important limitation was selection bias. Elderly patients who had already undergone elective surgery would have tended to be physiologically fitter based on traditional methods of patient evaluation. While it would have been ideal to compare our cohort with cancer patients who had not had surgery over the study duration, this information was unavailable. Measures of patient frailty or function, e.g., hand grip strength or ambulatory distance, while increasingly recognized as predictors of surgical morbidity and mortality in the elderly[28], was neither consistently recorded during the study duration nor has any part of current practice at our institution. The dissimilarity in survival between the cohorts may reflect the shortcomings of comparison between the populations of two distinct geographical locations, but eventual validation of the model notwithstanding these variations can be considered a strength. To ensure predictive accuracy of the model, further validation including re-identification of a correction factor with possible recalibration should be undertaken before use in separate populations.
In conclusion, while clinical decision-making for elderly patients with CRC can be challenging, advanced age per se is not a risk factor for poorer survival outcomes and patients should not be denied surgery based on age alone. However, there is a need for more objective pre-operative risk stratification in this vulnerable group of patients. Our novel scoring system predicting mortality following major resection uses parameters which are available before the surgery and can assist in the counselling and decision-making process between surgeons, their patients and families. Validation with an external Asian population strengthens the generalizability of this scoring method, although further validation may be necessary prior to adoption in differing patient cohorts.
Driven by remarkable improvements in life expectancy, the world is facing a dramatic increase in the number and proportion of its elderly. The incidence and mortality rates for colorectal cancer (CRC) increases with age, resulting in a greater burden on healthcare. Moreover, the life expectancy of an elderly patient with CRC may depend less on the malignant disease and more on their pre-morbid condition. Data shows that good surgical outcomes can be achieved in the elderly, but individualized evaluation of treatment goals and communication of realistic anticipated outcomes are essential.
With advanced age and chronic illness, the decision to undergo major surgery in the elderly patient can be challenging. Not infrequently, patients and their family members decline operative intervention due to age-related concerns. Even to the surgeon, the benefit of resection in certain individuals may not be so clear-cut. Moreover, the elderly are under-represented and under-prioritized in randomized trials, resulting in difficulty in generalizing existing data. Established risk stratification methods are commonly used but have well described flaws. This motivated us to develop a specific prognostic assessment tool to quantify the risk of mortality and predict survival after surgery in the elderly.
We aimed to analyze our outcomes following major elective colorectal surgery in the elderly to determine factors significantly influencing mortality. A pre-operative scoring system predicting post-operative outcomes more objectively could then be derived, facilitating the decision-making process for both surgeons and patients.
Data for all patients aged 70 and above who underwent elective surgery for non-metastatic CRC at Singapore General Hospital Department of Colorectal Surgery from 1 January 2005 to 31 December 2012 were obtained from hospital electronic records. Patients with evidence of distant disease, those who underwent emergency surgery or had surgery for benign colorectal conditions were excluded from the analysis. Instances of surgery for CRC recurrence occurring in the same patient over the study period were also excluded. Information for an equivalent group of elderly patients electively operated on at Kyungpook National University Chilgok Hospital, Daegu, South Korea, was retrieved over the same duration.
A total of 1267 patients were identified for analysis. The median post-operative length of stay was 8 [interquartile range (IQR) 6-12] d and median follow-up duration was 47 (IQR 19-75) mo. Median OS was 78 (IQR 65-85) mo. Following multivariate analysis, the factors significant for predicting overall mortality were serum albumin < 35 g/dL, serum carcinoembryonic antigen ≥ 20 µg/L, T stage 3 or 4, moderate tumor cell differentiation or worse, mucinous histology, rectal tumors, and pre-existing chronic obstructive lung disease. Advanced age alone was not found to be significant. The Korean cohort consisted of 910 patients. The Singapore cohort exhibited a poorer OS, likely due to a higher proportion of advanced cancers. Despite the clinicopathologic differences, there was successful validation of the model following recalibration. An interactive online calculator was designed to facilitate post-operative survival prediction, available at http://bit.ly/sgh_crc.
Advanced age per se is not a risk factor for poorer survival outcomes and patients should not be denied surgery based on age alone. However, there is a need for more objective pre-operative risk stratification in this vulnerable group of patients. Our novel scoring system predicting mortality following major resection uses parameters which are available before the surgery and can assist in the counselling and decision-making process between surgeons, their patients and families. Validation with an external Asian population strengthens the generalizability of this scoring method.
While it was not possible compare our cohort with cancer patients who had not had surgery over the study duration, this information should be considered for future studies. The dissimilarity in survival between the cohorts may reflect the shortcomings of comparison between the populations of two distinct geographical locations, but eventual validation of the model notwithstanding these variations can be considered a strength. To ensure predictive accuracy of the model, further validation including re-identification of a correction factor with possible recalibration should be undertaken before use in separate populations.
The authors thank Fung Joon Foo and Wan Qi Ng for their assistance in preparing the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: M’Koma AE, Uhlmann D S-Editor: Ji FF L-Editor: A E-Editor: Wang J
| 1. | United Nations,. Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2015 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP.241. Available from: URL: https://esa.un.org/unpd/wpp/publications/files/key_findings_wpp_2015.pdf. |
| 2. | World Health Organization. Projections of Mortality and Burden of Disease, 2004-2030. Available from: URL: http://www.who.int/healthinfo/global_burden_disease/projections/en/index.html. |
| 3. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |
| 4. | American Cancer Society. Colorectal Cancer Facts Figures 2014-2016. Atlanta: American Cancer Society 2014; Available from: URL: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2014-2016.pdf. |
| 5. | Broekhuizen K, Pothof A, de Craen AJ, Mooijaart SP. Characteristics of randomized controlled trials designed for elderly: a systematic review. PLoS One. 2015;10:e0126709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Deiner S, Westlake B, Dutton RP. Patterns of surgical care and complications in elderly adults. J Am Geriatr Soc. 2014;62:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using "optimal" cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 827] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 8. | Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1661] [Cited by in RCA: 1480] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 9. | Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9:265-290. |
| 10. | Dorajoo SR, Chan A. Implementing Clinical Prediction Models: Pushing the Needle Towards Precision Pharmacotherapy. Clin Pharmacol Ther. 2018;103:180-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Ministry of Health Singapore. Population and Vital Statistics. Available from: URL: https://www.moh.gov.sg/resources-statistics/singapore-health-facts/population-and-vital-statistics. |
| 12. | Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet. 2000;356:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 405] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 13. | Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA. 2014;311:2110-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 14. | Ferjani AM, Griffin D, Stallard N, Wong LS. A newly devised scoring system for prediction of mortality in patients with colorectal cancer: a prospective study. Lancet Oncol. 2007;8:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Nakamura T, Sato T, Miura H, Ikeda A, Tsutsui A, Naito M, Ogura N, Watanabe M. Feasibility and outcomes of surgical therapy in very elderly patients with colorectal cancer. Surg Laparosc Endosc Percutan Tech. 2014;24:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Tan KY, Kawamura Y, Mizokami K, Sasaki J, Tsujinaka S, Maeda T, Konishi F. Colorectal surgery in octogenarian patients--outcomes and predictors of morbidity. Int J Colorectal Dis. 2009;24:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Kazama K, Aoyama T, Hayashi T, Yamada T, Numata M, Amano S, Kamiya M, Sato T, Yoshikawa T, Shiozawa M, Oshima T, Yukawa N, Rino Y, Masuda M. Evaluation of short-term outcomes of laparoscopic-assisted surgery for colorectal cancer in elderly patients aged over 75 years old: a multi-institutional study (YSURG1401). BMC Surg. 2017;17:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Shalaby M, Di Lorenzo N, Franceschilli L, Perrone F, Angelucci GP, Quareisma S, Gaspari AL, Sileri P. Outcome of Colorectal Surgery in Elderly Populations. Ann Coloproctol. 2016;32:139-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Pawa N, Cathcart PL, Arulampalam TH, Tutton MG, Motson RW. Enhanced recovery program following colorectal resection in the elderly patient. World J Surg. 2012;36:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Rumstadt B, Guenther N, Wendling P, Engemann R, Germer CT, Schmid M, Kipfmueller K, Walz MK, Schwenk W. Multimodal perioperative rehabilitation for colonic surgery in the elderly. World J Surg. 2009;33:1757-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Keller DS, Lawrence JK, Nobel T, Delaney CP. Optimizing cost and short-term outcomes for elderly patients in laparoscopic colonic surgery. Surg Endosc. 2013;27:4463-4468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Devon KM, Vergara-Fernandez O, Victor JC, McLeod RS. Colorectal cancer surgery in elderly patients: presentation, treatment, and outcomes. Dis Colon Rectum. 2009;52:1272-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Goldman L, Caldera DL, Nussbaum SR, Southwick FS, Krogstad D, Murray B, Burke DS, O'Malley TA, Goroll AH, Caplan CH, Nolan J, Carabello B, Slater EE. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1895] [Cited by in RCA: 1501] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 24. | Gupta H, Gupta PK, Fang X, Miller WJ, Cemaj S, Forse RA, Morrow LE. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest. 2011;140:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Nerad E, Lahaye MJ, Maas M, Nelemans P, Bakers FC, Beets GL, Beets-Tan RG. Diagnostic Accuracy of CT for Local Staging of Colon Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2016;207:984-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 26. | Fields AC, Divino CM. Surgical outcomes in patients with chronic obstructive pulmonary disease undergoing abdominal operations: An analysis of 331,425 patients. Surgery. 2016;159:1210-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Lumb A, Biercamp C. Chronic obstructive pulmonary disease and anaesthesia. Cont Educ Anaesth Crit Care Pain. 2014;1:1–5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Farhat JS, Velanovich V, Falvo AJ, Horst HM, Swartz A, Patton JH, Rubinfeld IS. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72:1526-30; discussion 1530-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 409] [Article Influence: 31.5] [Reference Citation Analysis (0)] |